Abstract
Objective
picardial adipose tissue is associated with coronary artery disease, however the causal relationship between perivascular adipose tissue and local atherogenesis is unclear.
Methods and Results
Apolipoprotein E deficient (ApoE−/−) mice underwent transplantation of visceral or subcutaneous adipose tissue immediately adjacent to the right common carotid artery. Carotid arteries with fat transplants were analyzed for atherosclerosis by surface oil-red-O staining and cross-sectional analysis. Vascular function of the carotid arteries was assessed using pressure myography. Visceral fat transplants were also performed to ApoE−/− mice with neutralization of P-selectin glycoprotein ligand-1 (Psgl-1). Atherosclerosis surface area and lesion thickness were greater in mice receiving the perivascular visceral fat compared to the subcutaneous fat. Mice with visceral fat transplants also displayed more complicated atherosclerotic lesions with evidence of atherothrombosis. Serum Mcp-1 was higher in mice receiving visceral fat transplants compared to subcutaneous transplants. Visceral fat transplantation also caused impaired endothelial-dependent relaxation of the carotid artery. Psgl-1 deficiency or neutralization of Psgl-1 with an anti-Psgl-1 antibody was protective against perivascular visceral adipose tissue-induced atherosclerosis and was associated with reduced Mcp-1 levels.
Conclusions
Perivascular visceral fat leads to endothelial dysfunction and accelerated atherosclerosis. This proatherogenic effect of perivascular adipose tissue is blocked by neutralization of Psgl-1.
Keywords: atherosclerosis, adipocyte, cytokine, visceral obesity
INTRODUCTION
Excessive visceral adipose tissue is independently associated with cardiovascular events 1. In addition to triggering a systemic inflammatory state 2, visceral adipose tissue may also exert effects on local, adjacent tissues. For example, increases in the volume of the adipose tissue that overlays the coronary arteries, the epicardial fat, is associated with increased presence of noncalcified coronary plaques 3, 4 and total coronary occlusions 5. This association is independent of total visceral fat mass, hypertension, dyslipidemia, and diabetes, suggesting there may be a direct local effect of the epicardial fat on coronary atherosclerosis. However, the causal role of perivascular fat on the progression of atherosclerosis in arteries directly underlying the adipose tissue is unknown.
We have previously shown that transplantation of adipose tissue can be used as a tool to study effects of inflammatory fat (i.e. fat with increased macrophage content compared to lean endogenous fat) on clinically relevant endpoints without other confounding metabolic effects of obesity 6. To determine whether perivascular, inflammatory adipose tissue is sufficient to cause endothelial dysfunction and atherosclerosis, we performed transplantation of visceral and subcutaneous fat to the common carotid artery of atherosclerosis-prone, apolipoprotein E deficient (ApoE−/−) mice. We also examined the effect of P-selectin glycoprotein ligand-1 (Psgl-1) deficiency on the effect of perivascular transplanted fat on atherosclerosis since deficiency of Psgl-1 has been shown to be protective against both visceral adipose tissue inflammation and atherosclerosis 7, 8.
MATERIALS AND METHODS
Mice
10 week old male ApoE−/− and combined ApoE−/−,Psgl-1−/− mice were used, all on the C57BL/6J background strain. Original breeding pairs were purchased from Jackson Laboratory (Bar Harbor, ME). ApoE−/−,Psgl-1−/− mice were generated by first crossing ApoE−/− mice to Psgl-1−/− mice and then intercrossing ApoE+/−,Psgl-1+/− breeders to produce ApoE−/−,Psgl-1−/− and ApoE−/−,Psgl-1+/+ littermates. Mice were housed in specific pathogen-free facilities and were fed a normal chow diet (Laboratory Rodent Diet 5001, 5% fat, LabDiet, New Brunswick, NJ) prior to and 4 wks following the surgery (to facilitate healing), after which they were switched to Western diet (Rodent Western Diet #D12079B, Research Diet, New Brunswick, NJ) for 4 wks. Mice were sacrificed 8 wks post-operatively.
All procedures conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) and were approved by the University of Michigan Committee on Use and Care of Animals.
Adipose Transplantation to Carotid Artery
All recipient mice were anesthetized with intraperitoneal (i.p.) injection of sodium pentobarbital (67 mg/kg). Sixty mg of visceral (epididymal) adipose tissue was removed from ApoE−/− donors, and implanted adjacent to the right common carotid artery of the recipient ApoE−/− and ApoE−/−,Psgl-1−/− mice. Control ApoE−/− mice received 60 mg of subcutaneous inguinal fat. Sham-operated ApoE−/− mice underwent the same surgery without a fat transplant. Skin was sutured with 6-0 nylon filament.
Measurements of Mcp-1, insulin, glucose and cholesterol
Blood samples from mice were collected by retro-orbital bleeding using capillary tubes (Kimble Chase, Vineland, NJ). Commercially available murine ELISA kits were used to measure monocyte chemoattractant protein-1 (Mcp-1) (R&D Systems, Minneapolis, MN) and fasting insulin (Crystal Chem Inc., Downers Grove, IL). Glucose was measured after overnight fasting with a glucometer using test strips (Ascensia ® Contour, Bayer HealthCare LLC, Mishawaka, IN) and total cholesterol was measured with a colorimetric assay (Wako Chemicals USA, Inc., Richmond, VA).
Atherosclerosis Quantification
Eight weeks after the operation, mice were euthanized with sodium pentobarbital (67 mg/kg) and blood was collected via cardiac puncture. Animals were perfused with PBS at physiologic pressure, and then fixed using formalin with a 25-gauge needle inserted into the left ventricle, at a rate of 1 ml/min. The carcass was fixed in formalin and the arterial tree was then dissected and placed in 70% ethanol. After removing the connective tissue from the arterial trees, the right common carotid arteries were left intact, stained with oil-red-O and pinned on wax. Atherosclerotic lesion area was assessed as % coverage of the right carotid tube. To quantitate lesion thickness, right carotid arteries were analyzed by cross sections. Sections of the paraffin embedded right common carotid arteries were cut at 5 μm intervals at the site of the adipose transplant. 3 different areas were sampled along the carotid artery spaced 100 μm apart. All images were analyzed with Image-Pro Plus software (Media Cybernetics).
Immunohistochemistry
Immunohistochemistry was performed to characterize lesion composition. Carotid artery cross sections were stained with hematoxylin & eosin (H&E), monoclonal antibodies to Mac-3 (BD Biosciences, San Jose, CA), and α-smooth muscle actin (Millipore (Chemicon), Billerica, MA) as previously described 9 to quantify macrophages and smooth muscle cells, respectively. A goat anti-mouse fibrin(ogen) polyclonal antibody (Nordic Immunological Laboratories, The Netherlands), and Masson Trichrome staining (Sigma-Aldrich, St Louis, MO) were used to quantify fibrin(ogen) and connective tissue elements in the plaque, respectively. Transplanted adipose tissue was removed from the dissected artery at the time of sacrifice, fixed in formalin and embedded in paraffin. Adipose tissue cross sections were cut at 5 μm intervals and stained with the Mac-3 monoclonal antibody (BD Biosciences, San Jose, CA) for macrophages and rabbit polyclonal antibody to CD31 (PECAM-1) (Abcam, Cambridge, MA) for endothelium. Three fields (40x magnification) were studied per slide.
Pressure myography
Carotid artery fat transplantation was performed to 10 week old ApoE−/− mice with visceral and subcutaneous adipose tissue. Mice were fed with standard chow for one week post-operatively followed by Western diet for 1.5 weeks. Mice were sacrificed earlier at 2.5 weeks following transplantation in attempts to avoid development of severe lesions in the transplanted carotid artery, which might confound the myograph procedure. Following anesthesia with sodium pentobarbital (67 mg/kg, i.p.) and exsanguination via right ventricle phelobotomy, a segment of the right common carotid artery distal to the fat transplant site was removed and placed into a silastic-elastomer lined petridish filled with Physiological Salt Solution (PSS) (equilibrated with 5% CO2- 95% O2) containing (in mmol/L): NaCl 120, KCl 4.7, MgSO4 1.18, CaCl2 2.5, KH2PO4 1.18, NaHCO3 25, glucose 5.5, EDTA 0.026, pH 7.4. The surrounding connective tissue was gently cleared and a vessel segment 3 mm in length was mounted onto glass cannulas of the pressure myograph (Living Systems, VT). One cannula was adjusted with axial direction of vessel until walls of vessel were parallel without any stretch. Vessels were then equilibrated in PSS at 37°C (60 minutes, 80 mmHg intraluminal pressure). The real-time dimension of the vessel wall was detected and analyzed with a video dimension analyzer (Living Systems, VT). Vascular activity was tested under no-flow condition. After equilibration, vascular contraction was assessed by measuring the constrictive response of lumen diameter to cumulatively applied phenylephrine (PE) (Sigma-Aldrich, St. Louis, MO) (10−9 to 10−3 mol/L). After washing and equilibration for one hour, endothelium-dependent relaxation was assessed by measuring the dilatory response to acetylcholine (Ach) (Sigma-Aldrich, St. Louis, MO) (10−9 to 10−5 mol/L) in PE precontracted vessels (10−5 mol/L).
Antibody injections to animals
Starting two weeks post-operation, a rat anti-mouse Psgl-1 antibody (4RA10) or control IgG k isotype (BD Pharmingen, Franklin Lakes, NJ) was injected via tail vein (50μg in 200μl 1xPBS) into transplanted mice once a week for 3 weeks. Mice were fed standard chow for 2 weeks post-operatively, followed by 6 weeks of Western diet. Mice were sacrificed 8 weeks post-operatively.
Statistical Analysis
The statistical significance of differences between groups was determined by 1-way ANOVA followed by Tukey’s Studentized Range (HSD) post-hoc test for multiple comparisons, or Student’s t test when comparisons were made between 2 groups. Values are expressed as mean ± SEM. P<0.05 are considered significant.
RESULTS
Perivascular inflammatory adipose tissue increases local atherosclerosis
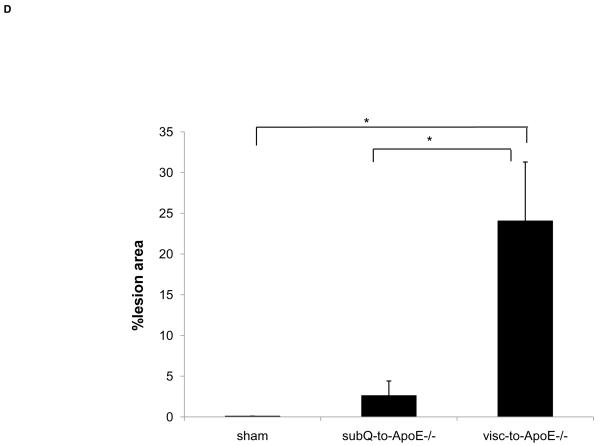
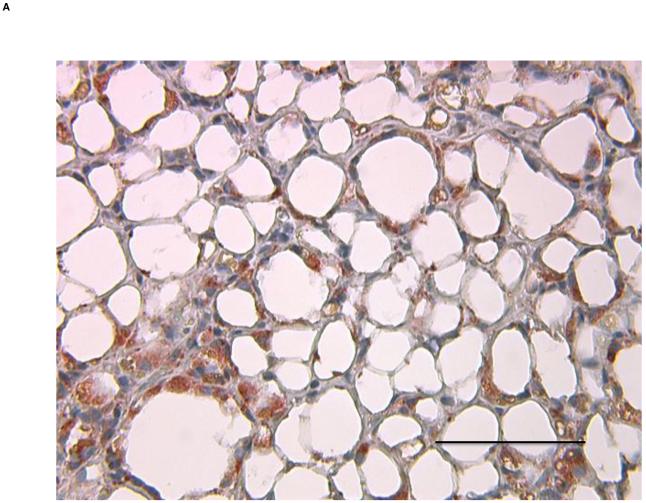
To first determine if an adipose tissue transplant overlying the right common carotid artery would lead to formation of a local atherosclerotic plaque, ApoE−/− mice underwent visceral fat transplant or sham operation to the mid right common carotid artery, a site that typically does not develop spontaneous atherosclerosis. Eight weeks after the transplantation, mice receiving the visceral fat transplant (n=5) had large lipid-rich atherosclerotic lesions of the right common carotid artery while sham operated (n=4) mice did not display any lesions (Fig. 1A, 1B). To next determine whether the type of fat affected the local lesion formation, another experiment was performed transplanting ApoE−/− mice with subcutaneous fat (n=5). Mice with subcutaneous adipose transplants had significantly smaller lipid-rich lesion area than mice with visceral fat transplants, while there was no significant difference compared to sham operated animals (Fig. 1C). To determine whether the effect of the different types of fat on atherosclerosis was related to macrophage content of the transplanted fat, Mac3 immunostaining was performed on transplanted fat and endogenous fat depots. Transplanted visceral adipose tissue revealed marked increase in macrophages compared to the endogenous visceral (epididymal) depot (38.1±2.2 vs. 7.5±2.0%, p<3.7×10−6) (Fig. 2), while there was no difference between transplanted visceral and transplanted subcutaneous fat (38.1±2.2 vs. 33.6±2.0%, p=NS). Transplanted fat was also stained with antibody to CD31 (PECAM-1) to determine vascularization of the tissue. There was no difference in the number of vessels between visceral and subcutaneous fat transplants (1.5±0.5 vs 1.6±0.1 vessels per 40x field, p=NS).
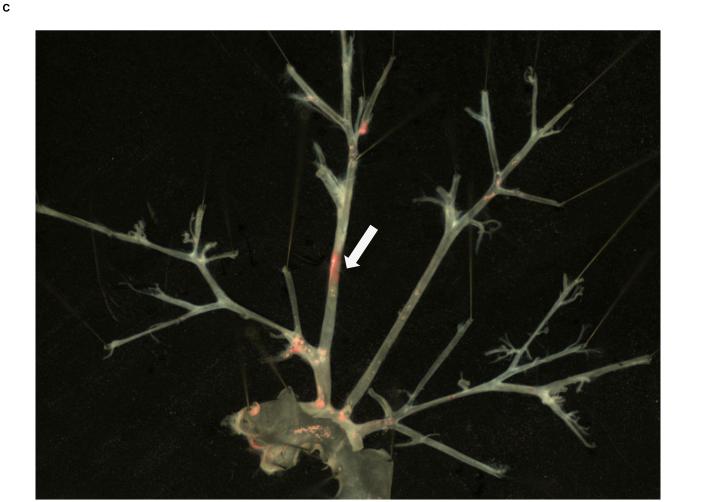
Figure 1. Local atherosclerosis increased in mice with perivascular visceral fat transplantation.
Oil-red-o stained aortic arch and major branches of ApoE−/− mouse transplanted with A) visceral (visc-to-ApoE−/−) adipose tissue, B) sham operated ApoE−/− mouse and C) ApoE−/− mouse transplanted with subcutaneous (subQ-to-ApoE−/−) adipose tissue. D) Lesion surface area in operated ApoE−/− mice. *p<0.05. Arrows point to the site of fat transplantation on the right common carotid arteries.
Figure 2. Inflammatory infiltrate in transplanted adipose tissue.
A) Transplanted visceral adipose tissue and B) endogenous visceral adipose tissue stained with Mac3 antibody. Magnification 40x, scale bar =100 μm.
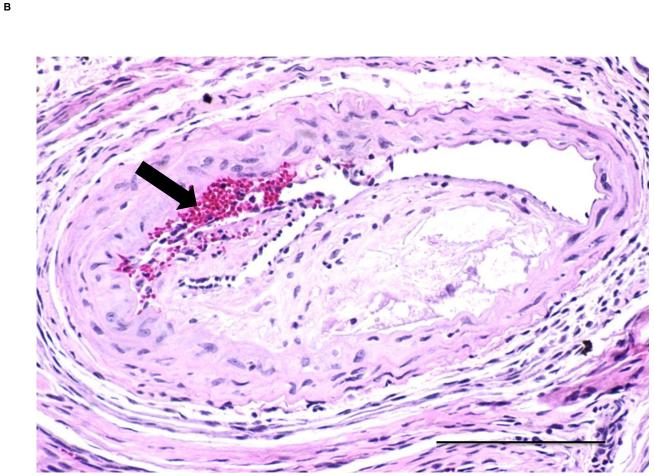
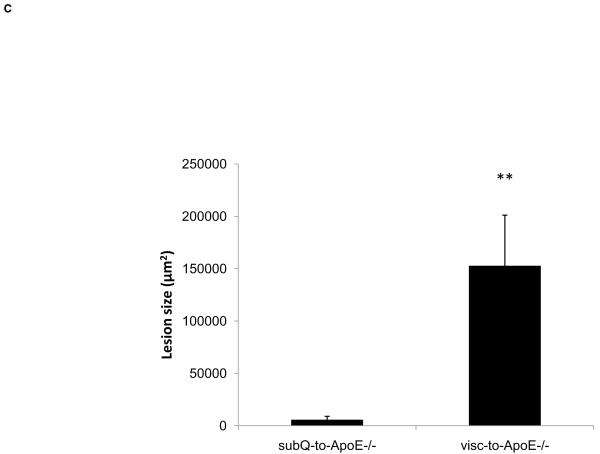
In order to further characterize the atherosclerotic lesions triggered by the fat transplant, new groups of ApoE−/− mice were transplanted with visceral (n=9) and subcutaneous (n=9) adipose tissue to the right carotid artery for cross-sectional analysis of lesion thickness and composition. Mice receiving visceral fat had greater lesion area compared to mice receiving subcutaneous fat transplants (152446±48691 vs. 5349±3617 μm2, p<0.008) (Fig. 3) and higher intima/media ratio (0.6±0.2 vs. 0.02±0.01, p<0.02). There was no difference in the medial area between ApoE−/− mice with visceral fat and ApoE−/− mice with subcutaneous fat transplants (268902±28899 vs. 227659±26969 μm2, p=NS).
Figure 3. Perivascular visceral adipose tissue increases lesion thickness.
H&E stained cross sections of the right common carotid artery from ApoE−/− mice with A) subcutaneous and B) visceral adipose tissue transplant. Arrow pointing to a potential intraplaque hemorrhage. Scale bar = 100 μm, magnification 40x. C) Lesion size in operated ApoE−/− mice. **p<0.01.
There were no differences in body weight (29.9±0.6 vs. 30.3±0.5 g, p=NS), fasted insulin (1.5±0.8 vs. 1.0±0.3 ng/ml, p=NS), fasted glucose (115.8±15.1 vs. 127.4±13.5 mg/dl, p=NS) and total cholesterol levels (222.1±13.1 vs. 205.8±29.1 mg/dl, p=NS) between ApoE−/− mice with visceral versus subcutaneous fat transplants.
Perivascular visceral adipose tissue increases serum Mcp-1 and triggers more complicated lesion formation
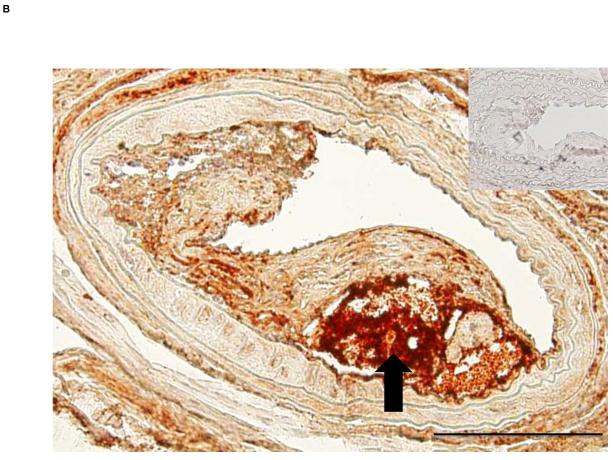
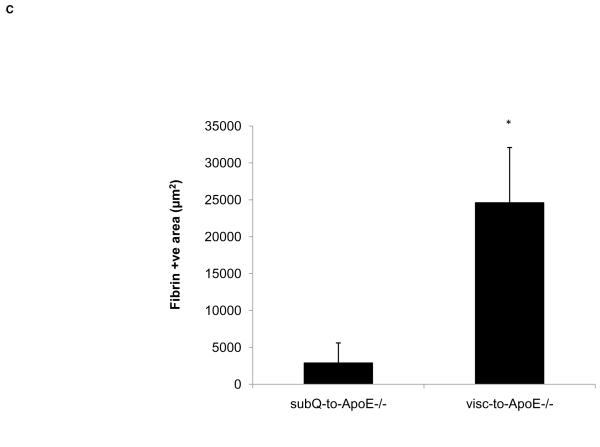
Since Mcp-1 is a marker of adverse vascular effects related to visceral fat inflammation, circulating Mcp-1 was measured from recipient mice 8 weeks post-operation. ApoE−/− mice with visceral fat transplants had significantly higher serum Mcp-1 compared to ApoE−/− mice with subcutaneous fat transplants (66.0±5.6 vs. 46.7±2.0 pg/ml, p<0.006). Comparison of serum Mcp-1 levels of the fat transplanted ApoE−/− mice to age-matched control ApoE−/− mice without fat transplantation (n=7) revealed no difference between the control mice and mice with subcutaneous fat transplants (48.9±5.1 vs. 46.7±2.0 pg/ml, p=NS, respectively). Mice with visceral fat transplants had significantly higher Mcp-1 compared to non-transplanted control mice (66.0±5.6 vs. 48.9±5.1, p<0.05, respectively). Cross sectional analysis of carotid artery atherosclerotic lesions revealed more area occupied by macrophages (41.7±14.6 vs. 4.6±0.9 μm2, p<0.05) and more extensive fibrin deposition (24610±7474 vs. 2903±2698 μm2, p<0.05) in mice with visceral fat transplants compared to subcutaneous transplants, respectively. Visceral fat transplanted mice showed large subocclusive plaques with areas of intense fibrin staining suggestive of intraplaque hemorrhage (Fig. 4), a finding not observed in mice with subcutaneous transplants. There were no differences between ApoE−/− mice with visceral fat compared to ApoE−/− mice with subcutaneous fat in the lesion content of connective tissue (18.4±10.3 vs. 9.0±7.7 μm2, p=NS) or smooth muscle cells (11.4±5.3 vs. 5.4±3.1 μm2, p=NS). Cross sections from sham-operated ApoE−/− mice did not display any lesions in the same area of the right common carotid artery.
Figure 4. ApoE−/− mice transplanted with visceral fat display complicated lesions.
A) Cross section of carotid artery stained with fibrin(ogen) antibody from ApoE−/− mice transplanted with A) subcutaneous and B) visceral fat. Inset shows negative control without primary antibody. Arrow points to necrotic core of the lesion. Scale bar = 100 μm, magnification 40x. C) Fibrin-positive area in lesions in operated mice. *p<0.05.
Perivascular visceral adipose tissue impairs endothelial function
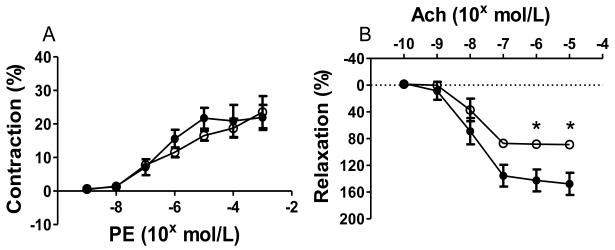
To study the effect of perivascular adipose tissue on vascular function of carotid arteries from ApoE−/− mice transplanted with visceral or subcutaneous adipose tissue, pressure myography was performed with segments of carotid arteries immediately distal to the fat transplant. These studies were performed at an earlier time point (18 days), prior to development of severe lesions. Vasoconstrictor responses to PE were similar between mice receiving visceral fat (n=4) and those receiving subcutaneous fat (n=5) (Fig. 5A). However, endothelial relaxation responses to Ach were significantly reduced in mice receiving visceral fat compared to those receiving subcutaneous fat (p<0.05) (Fig. 5B).
Figure 5. Perivascular visceral fat transplant leads to endothelial dysfunction.
A) Vasoconstriction responses to phenylephrine (PE). B) Relaxation responses to acetylcholine (Ach). ApoE−/− mice with visceral (○) and subcutaneous (●) fat transplants. *p<0.05.
Deficiency of Psgl-1 is protective against perivascular inflammatory fat-induced local atherosclerosis
Since Psgl-1 deficiency has been shown to be protective against fat inflammation triggered by obesity 8, we tested the role of Psgl-1 deficiency on the development of atherosclerosis in this model. Visceral adipose tissue was therefore transplanted to the right carotid artery of ApoE−/−,Psgl-1−/− mice (n=5). Eight weeks post-operatively, there was no significant difference in total cholesterol (222.1±13.1 vs. 169.0±30.4 mg/dl, p=NS) or fasted insulin (1.2±0.5 vs. 1.5±0.7 ng/ml, p=NS) between ApoE−/−,Psgl-1−/− mice and ApoE−/−,Psgl-1+/+ mice with visceral fat. The macrophage content of transplanted visceral adipose tissue in ApoE−/−,Psgl-1−/− mice was significantly lower compared to visceral transplants harvested from ApoE−/−,Psgl-1+/+ mice (23.5±1.3 vs. 38.1±2.2 %, p<0.0006). ApoE−/−,Psgl-1−/− mice had no detectable lipid-rich atherosclerotic plaques in the right carotid artery while ApoE−/−,Psgl-1+/+ mice with visceral fat transplants all had plaques (0.0±0.0% vs 24.1±7.2% of total area, p=0.01). Circulating levels of Mcp-1 were lower in ApoE−/−,Psgl-1−/− mice compared to ApoE−/−,Psgl-1+/+ mice (26.8±4.2 vs. 66.0±5.6 pg/ml, p=0.003).
To test a potential therapeutic strategy, the effect of an anti-Psgl-1 antibody on the proatherogenic effect of transplanted fat was determined. Carotid fat transplantation was performed with visceral adipose tissue to ApoE−/− mice, and mice were then injected once a week with either anti-Psgl-1 antibody (n=8) or control IgG k isotype (n=10) for 3 consecutive weeks post-operatively. Eight weeks after the transplantation, ApoE−/− mice injected with anti-Psgl-1 antibody had significantly lower serum Mcp-1 compared to controls (54.1±3.3 vs. 81.1±7.4 pg/ml, p=0.007). Cross sections of right common carotid artery at the location of the fat transplant were analyzed for lesion thickness. Mice injected with anti-Psgl-1 antibody had significantly smaller lesion size (23314±12764 vs. 63124±14386 μm2, p=0.03) and intima/media ratio (0.2±0.1 vs. 0.9±0.3, p=0.03) compared to controls.
DISCUSSION
Obesity is a risk factor for cardiovascular events and mortality from all causes 10. The mechanism(s) by which obesity increases vascular risk is unclear. Visceral adiposity in particular appears to be most strongly associated with features of the metabolic syndrome and vascular risk 1, 11. In addition to systemic proinflammatory effects, visceral adipose tissue may exert local effects on adjacent blood vessels 12, 13. Consistent with this hypothesis, clinical studies have demonstrated that epicardial adipose tissue volume is correlated with total visceral adipose tissue mass and metabolic syndrome14, 15, and is also independently associated with the presence of noncalcified coronary plaques3, 4 and total coronary occlusions5. Inflammatory epicardial fat, in particular, independently correlates with the presence of coronary atherosclerosis 3, 16. Both human and animal studies have shown that obesity17, 18, high-fat feeding19 and hypercholesterolemia without obesity20 can increase inflammation in perivascular adipose tissue, which may contribute to the effects of these manipulations on atherosclerosis. Inflammatory perivascular adipose tissue may in turn secrete proatherogenic cytokines12, and cause local endothelial dysfunction 17, 21, thus contributing to progression of systemic and local vascular disease.
Epidemiologic and preclinical data support a strong association between perivascular adipose tissue with local atherosclerosis, however, a direct causal relationship remains to be established. One way to address this is by the perivascular addition of adipose tissue using fat transplantation techniques. Since the process of fat transplantation is associated with chronic inflammation in the transplanted depot 6, this also provides a model of inflammatory fat which may mimic the perivascular inflammation that occurs in the obese state 17. Although the mechanism(s) by which inflammation occurs in the setting of fat transplantation compared to endogenous obesity are likely to be different, the downstream adverse effects of the inflammatory fat may be similar. We have previously shown that transplantation of 400 mg of visceral adipose tissue to the dorsal aspect of the mouse leads to a viable graft capable of secreting adipokines such as leptin for at least 1 year 6. Although the transplant becomes revascularized, as shown in this study, it is associated with chronic inflammation with features similar to those observed in visceral depots in the setting of obesity 6. This visceral fat transplantation protocol to ApoE−/− mice is sufficient to accelerate atherosclerosis systemically, throughout the vascular tree 6.
In the current study, we transplanted a much smaller amount of fat (only 60 mg) to a site immediately adjacent to the common carotid artery to determine if inflammatory fat in a perivascular location could affect vascular function and trigger formation of a local atherosclerotic lesion. The mid carotid artery site was chosen since this site typically remains free of atherosclerosis in ApoE−/− mice at the ages we were studying.
The transplanted perivascular visceral adipose tissue led to local impaired vascular relaxation in our model. The important role of perivascular adipose tissue in regulation of vascular tone has been previously reported 22, 23 and is likely the precursor to subsequent atherosclerotic lesion formation. In obesity, endothelial dysfunction is likely secondary to the inflammation and hypoxia-induced changes in periadventitial fat 24. Activated macrophages in perivascular fat may be particularly important towards the loss of anticontractile activity 25. Transplanted adipose tissue is similar to obese adipose tissue in regards to inflammation and hypoxic damage to tissue and our results support the recent human studies which have shown abnormalities in endothelium-dependent vasodilatation involving small arteries from obese patients 17. As we expected, mice subjected to a sham operation did not show evidence of atherosclerosis in the mid common carotid arteries. However, severe complex lesions occurred in mice in which a piece of visceral fat was placed adjacent to the mid common carotid. These lesions showed frequent evidence of necrotic, fibrin-positive cores representing either plaque rupture with thrombosis or plaque hemorrhage. Perivascular fat may also be triggering local thrombus formation which could serve as a nidus for plaque growth. However, in a group of mice sacrificed 1 week after the transplant, there was no evidence of thrombus or lesion suggesting acute thrombus formation related to the procedure was not responsible for lesion formation. Because there were some plaques without thrombus, we suspect that plaque formation preceded the thrombosis or hemorrhage. This is a particularly interesting finding since human studies have shown an association between epicardial adipose tissue volume and unstable coronary plaques4. Although we cannot completely rule out hemodynamic effects from the fat transplant on lesion formation in the adjacent carotid artery, there was no obvious mechanical extrinsic obstruction apparent at the time of fat transplantation, or later at the time of sacrifice. In addition, transplantation of subcutaneous fat of identical mass did not produce the same effect as visceral fat, despite a similar inflammatory infiltrate. We cannot rule out a greater effect of visceral adipose tissue on characteristics of the local carotid adventitia as accurate quantitation of the adventitial was not possible in this study. The difference between visceral and subcutaneous fat on local atherosclerosis suggests that an interaction between visceral adipocytes and inflammatory cells is playing an important role toward the proatherogenic effect of perivascular fat. This effect is not due to inflammatory fat effects on insulin resistance since no differences in glucose or insulin levels were noted between the groups of transplanted mice. Interestingly, Mcp-1, which may be a biomarker of accelerated vascular disease induced by inflammatory fat 9, was elevated in the presence of visceral compared to subcutaneous perivascular fat transplantation.
Therapeutic targeting of the macrophage infiltration into visceral fat may reduce the subsequent adverse vascular effects of perivascular inflammatory adipose tissue. Psgl-1 deficiency has been shown to reduce obesity-induced visceral fat macrophage infiltration by reducing the adhesive characteristics of the endothelium induced by obesity 8. To determine if Psgl-1 deficiency in the recipient ApoE−/− mice would be sufficient to attenuate the proatherogenic effect of visceral fat, ApoE−/−,Psgl-1−/− mice were generated. ApoE−/−,Psgl-1−/− were completely protected from the effects of the visceral fat transplant with no evidence of atherosclerosis at the site of fat transplant. Antibody blockade of Psgl-1 with weekly injections of anti-Psgl-1 antibody for only 3 weeks following the fat transplant was also effective in reducing the proatherogenic effect of the transplanted fat and was associated with reduced levels of Mcp-1. It has been previously shown that a single injection of this anti-Psgl-1 antibody is capable of reducing neointima formation 28 days following injury to the carotid artery in ApoE−/− mice 26, thus interference with Psgl-1 may have long term effects, especially in the setting of acute or subacute injury.
In conclusion, transplanted perivascular visceral adipose tissue induces endothelial dysfunction and triggers formation of local complex atherosclerotic plaques. Inhibition of the leukocyte ligand, Psgl-1, may provide a therapeutic approach to attenuate the effects of inflammatory visceral adipose tissue on atherosclerosis.
ACKNOWLEDGEMENTS
This work was supported by the National Institutes of Health (HL57346, HL073150 to D.T.E.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
None.
REFERENCES
- 1.See, R., Abdullah SM, McGuire DK, Khera A, Patel MJ, Lindsey JB, Grundy SM, de Lemos JA. The association of differing measures of overweight and obesity with prevalent atherosclerosis: The dallas heart study. Journal of the American College of Cardiology. 2007;50:752–759. doi: 10.1016/j.jacc.2007.04.066.
- 2.Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96:939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- 3.Konishi M, Sugiyama S, Sugamura K, Nozaki T, Ohba K, Matsubara J, Matsuzawa Y, Sumida H, Nagayoshi Y, Nakaura T, Awai K, Yamashita Y, Jinnouchi H, Matsui K, Kimura K, Umemura S, Ogawa H. Association of pericardial fat accumulation rather than abdominal obesity with coronary atherosclerotic plaque formation in patients with suspected coronary artery disease. Atherosclerosis. 2010;209:573–578. doi: 10.1016/j.atherosclerosis.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 4.Alexopoulos N, McLean DS, Janik M, Arepalli CD, Stillman AE, Raggi P. Epicardial adipose tissue and coronary artery plaque characteristics. Atherosclerosis. 2010;210:150–154. doi: 10.1016/j.atherosclerosis.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 5.Ueno K, Anzai T, Jinzaki M, Yamada M, Jo Y, Maekawa Y, Kawamura A, Yoshikawa T, Tanami Y, Sato K, Kuribayashi S, Ogawa S. Increased epicardial fat volume quantified by 64-multidetector computed tomography is associated with coronary atherosclerosis and totally occlusive lesions. Circulation Journal. 2009;73:1927–1933. doi: 10.1253/circj.cj-09-0266. [DOI] [PubMed] [Google Scholar]
- 6.Ohman MK, Shen Y, Obimba CI, Wright AP, Warnock M, Lawrence DA, Eitzman DT. Visceral adipose tissue inflammation accelerates atherosclerosis in apolipoprotein e-deficient mice. Circulation. 2008;117:798–805. doi: 10.1161/CIRCULATIONAHA.107.717595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.An G, Wang H, Tang R, Yago T, McDaniel JM, McGee S, Huo Y, Xia L. P-selectin glycoprotein ligand-1 is highly expressed on ly-6chi monocytes and a major determinant for ly-6chi monocyte recruitment to sites of atherosclerosis in mice. Circulation. 2008;117:3227–3237. doi: 10.1161/CIRCULATIONAHA.108.771048. Epub 2008 Jun 3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Russo HM, Wickenheiser KJ, Luo W, Ohman MK, Franchi L, Wright AP, Bodary PF, Nunez G, Eitzman DT. P-selectin glycoprotein ligand-1 regulates adhesive properties of the endothelium and leukocyte trafficking into adipose tissue. Circ Res. 2010;107:388–397. doi: 10.1161/CIRCRESAHA.110.218651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohman MK, Wright AP, Wickenheiser KJ, Luo W, Russo HM, Eitzman DT. Monocyte chemoattractant protein-1 deficiency protects against visceral fat-induced atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30:1151–1158. doi: 10.1161/ATVBAHA.110.205914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pischon T, Boeing H, Hoffmann K, Bergmann M, Schulze MB, Overvad K, van der Schouw YT, Spencer E, Moons KGM, Tjonneland A, Halkjaer J, Jensen MK, Stegger J, Clavel-Chapelon F, Boutron-Ruault MC, Chajes V, Linseisen J, Kaaks R, Trichopoulou A, Trichopoulos D, Bamia C, Sieri S, Palli D, Tumino R, Vineis P, Panico S, Peeters PHM, May AM, Bueno-de-Mesquita HB, van Duijnhoven FJB, Hallmans G, Weinehall L, Manjer J, Hedblad B, Lund E, Agudo A, Arriola L, Barricarte A, Navarro C, Martinez C, Quiros JR, Key T, Bingham S, Khaw KT, Boffetta P, Jenab M, Ferrari P, Riboli E. General and abdominal adiposity and risk of death in europe. N Engl J Med. 2008;359:2105–2120. doi: 10.1056/NEJMoa0801891. [DOI] [PubMed] [Google Scholar]
- 11.Lakka HM, Lakka TA, Tuomilehto J, Salonen JT. Abdominal obesity is associated with increased risk of acute coronary events in men. Eur Heart J. 2002;23:706–713. doi: 10.1053/euhj.2001.2889. [DOI] [PubMed] [Google Scholar]
- 12.Henrichot E, Juge-Aubry CE, Pernin A, Pache J-C, Velebit V, Dayer J-M, Meda P, Chizzolini C, Meier CA. Production of chemokines by perivascular adipose tissue: A role in the pathogenesis of atherosclerosis? Arterioscler Thromb Vasc Biol. 2005;25:2594–2599. doi: 10.1161/01.ATV.0000188508.40052.35. [DOI] [PubMed] [Google Scholar]
- 13.Ouwens DM, Sell H, Greulich S, Eckel J. The role of epicardial and perivascular adipose tissue in the pathophysiology of cardiovascular disease. Journal of Cellular and Molecular Medicine. 2010;14:2223–2234. doi: 10.1111/j.1582-4934.2010.01141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iacobellis G, Ribaudo MC, Assael F, Vecci E, Tiberti C, Zappaterreno A, Di Mario U, Leonetti F. Echocardiographic epicardial adipose tissue is related to anthropometric and clinical parameters of metabolic syndrome: A new indicator of cardiovascular risk. J Clin Endocrinol Metab. 2003;88:5163–5168. doi: 10.1210/jc.2003-030698. [DOI] [PubMed] [Google Scholar]
- 15.Wang T-D, Lee W-J, Shih F-Y, Huang C-H, Chang Y-C, Chen W-J, Lee Y-T, Chen M-F. Relations of epicardial adipose tissue measured by multidetector computed tomography to components of the metabolic syndrome are region-specific and independent of anthropometric indexes and intraabdominal visceral fat. J Clin Endocrinol Metab. 2009;94:662–669. doi: 10.1210/jc.2008-0834. [DOI] [PubMed] [Google Scholar]
- 16.Cheng KH, Chu CS, Lee KT, Lin TH, Hsieh CC, Chiu CC, Voon WC, Sheu SH, Lai WT. Adipocytokines and proinflammatory mediators from abdominal and epicardial adipose tissue in patients with coronary artery disease. Int J Obes. 2007;32:268–274. doi: 10.1038/sj.ijo.0803726. [DOI] [PubMed] [Google Scholar]
- 17.Greenstein AS, Khavandi K, Withers SB, Sonoyama K, Clancy O, Jeziorska M, Laing I, Yates AP, Pemberton PW, Malik RA, Heagerty AM. Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circulation. 2009;119:1661–1670. doi: 10.1161/CIRCULATIONAHA.108.821181. [DOI] [PubMed] [Google Scholar]
- 18.Police SB, Thatcher SE, Charnigo R, Daugherty A, Cassis LA. Obesity promotes inflammation in periaortic adipose tissue and angiotensin ii-induced abdominal aortic aneurysm formation. Arterioscler Thromb Vasc Biol. 2009;29:1458–1464. doi: 10.1161/ATVBAHA.109.192658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chatterjee TK, Stoll LL, Denning GM, Harrelson A, Blomkalns AL, Idelman G, Rothenberg FG, Neltner B, Romig-Martin SA, Dickson EW, Rudich S, Weintraub NL. Proinflammatory phenotype of perivascular adipocytes: Influence of high-fat feeding. Circ Res. 2009;104:541–549. doi: 10.1161/CIRCRESAHA.108.182998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lohmann C, Schäfer N, von Lukowicz T, Sokrates Stein MA, Borén J, Rütti S, Wahli W, Donath MY, Lüscher TF, Matter CM. Atherosclerotic mice exhibit systemic inflammation in periadventitial and visceral adipose tissue, liver, and pancreatic islets. Atherosclerosis. 2009;207:360–367. doi: 10.1016/j.atherosclerosis.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Bhagat K, Vallance P. Inflammatory cytokines impair endothelium-dependent dilatation in human veins in vivo. Circulation. 1997;96:3042–3047. doi: 10.1161/01.cir.96.9.3042. [DOI] [PubMed] [Google Scholar]
- 22.Verlohren S, Dubrovska G, Tsang S-Y, Essin K, Luft FC, Huang Y, Gollasch M. Visceral periadventitial adipose tissue regulates arterial tone of mesenteric arteries. Hypertension. 2004;44:271–276. doi: 10.1161/01.HYP.0000140058.28994.ec. [DOI] [PubMed] [Google Scholar]
- 23.Yudkin JS, Eringa E, Stehouwer CDA. “Vasocrine” signalling from perivascular fat: A mechanism linking insulin resistance to vascular disease. The Lancet. 2005;365:1817–1820. doi: 10.1016/S0140-6736(05)66585-3. [DOI] [PubMed] [Google Scholar]
- 24.Marchesi C, Ebrahimian T, Angulo O, Paradis P, Schiffrin EL. Endothelial nitric oxide synthase uncoupling and perivascular adipose oxidative stress and inflammation contribute to vascular dysfunction in a rodent model of metabolic syndrome. Hypertension. 2009;54:1384–1392. doi: 10.1161/HYPERTENSIONAHA.109.138305. [DOI] [PubMed] [Google Scholar]
- 25.Withers SB, Agabiti-Rosei C, Livingstone DM, Little MC, Aslam R, Malik RA, Heagerty AM. Macrophage activation is responsible for loss of anticontractile function in inflamed perivascular fat. Arterioscler Thromb Vasc Biol. 31:908–913. doi: 10.1161/ATVBAHA.110.221705. [DOI] [PubMed] [Google Scholar]
- 26.Phillips JW, Barringhaus KG, Sanders JM, Hesselbacher SE, Czarnik AC, Manka D, Vestweber D, Ley K, Sarembock IJ. Single injection of p-selectin or pselectin glycoprotein ligand-1 monoclonal antibody blocks neointima formation after arterial injury in apolipoprotein e-deficient mice. Circulation. 2003;107:2244–2249. doi: 10.1161/01.CIR.0000065604.56839.18. [DOI] [PubMed] [Google Scholar]