Abstract
Objective
To examine prospectively the association of total and high molecular weight (HMW) adiponectin, and HMW/total adiponectin ratio with risk of incident coronary heart disease (CHD) in women, and to examine to what extent adjustment for potentially intermediary variables would explain this association.
Methods and Results
Among 30,111 women from the Nurses’ Health Study, 468 women developed non-fatal myocardial infarction or fatal CHD during 14 years of follow-up. Using risk set sampling, controls were selected 2:1 matched on age, smoking, and date of blood draw. Adjusted for matching factors, parental history of myocardial infarction, hormone replacement therapy, alcohol consumption, physical activity, body mass index, hypertension, and low-density lipoprotein cholesterol levels, the relative risk in the highest versus lowest quintile was 0.50 (95%-CI 0.33-0.75; p trend=0.001) for total adiponectin, 0.53 (95%-CI 0.35-0.80; p trend=0.004) for HMW adiponectin, and 0.63 (95%-CI 0.43-0.93; p trend=0.03) for HMW/total adiponectin ratio. After adjustment for diabetes, HDL-cholesterol, HbA1c, and CRP these associations were attenuated and no longer significant (RRs, 0.84; 95%-CI 0.53-1.33; p trend=0.62; 0.95; 95%-CI 0.60-1.52; p trend=0.98; 0.97; 95%-CI 0.64-1.47; p trend=0.80).
Conclusions
High levels of total and HMW adiponectin, and HMW/total adiponectin ratio are associated with a lower risk of CHD among women. HMW adiponectin and HMW/total adiponectin ratio are not more closely related to risk than total adiponectin. These associations are largely mediated by parameters related to glucose and lipid metabolism and inflammation, especially HDL-cholesterol levels.
Keywords: adiponectin, cohort study, coronary disease, epidemiology, risk factors
Introduction
Adiponectin is an adipose tissue-derived collagen-like protein that beneficially affects many pathways that may be relevant for the development of atherosclerosis, including glucose and lipid metabolism, inflammation, endothelial function, as well as thrombogenesis, and it may therefore potentially protect from coronary heart disease (CHD).1 However, results from prospective studies in humans provide inconsistent results, with only some showing significant inverse associations between adiponectin and risk of CHD.2-11 The basis for these inconsistent results are likely due to over adjustment for other biological markers, including glucose, high density lipoprotein cholesterol (HDL-C) and C-reactive protein (CRP), thought to be in the causal pathway between adiponectin and CHD. Further, most prior studies have been conducted among predominantly male populations, and thus, limited information exists for women. In a previous analysis from the Health Professionals Follow-up Study we found a statistically significant inverse association between plasma adiponectin levels and risk of myocardial infarction (MI) among men.2 In that analysis, adjustment for history of diabetes or plasma levels of hemoglobin A1c (HbA1c) or CRP had little impact, whereas adjustment for HDL-C modestly attenuated the relationship, although it remained statistically significant.
The effects of adiponectin may depend on its quaternary structure in plasma. It was suggested that high molecular weight (HMW) adiponectin may be a better measure of metabolically active adiponectin and therefore more closely related to insulin sensitivity and risk of type 2 diabetes.12, 13 However, in the only report published thus far on HMW adiponectin and risk of incident CHD (including 167 cases and 333 controls over a follow-up period of 4 years), the association was not statistically significant.14
The aim of the present study was therefore to examine the association of total and HMW adiponectin, and HMW/total adiponectin ratio with risk of incident CHD in the Nurses’ Health Study (NHS), a well described cohort study of women. Because animal studies suggest that adiponectin affects several downstream metabolic pathways that may be relevant for cardiovascular risk, we were particularly interested to examine to what extent adjustment for these potentially intermediary variables related to glucose (HbA1c, history of diabetes) and lipid metabolism (HDL-C) and inflammation (CRP) would explain the inverse association between adiponectin and CHD in a human population.
Methods
Study population
The NHS is a prospective cohort investigation involving 121,700 female U.S. registered nurses who were 30-55 years old at baseline in 1976. Information about anthropometry, health and disease is assessed biennially, and information about diet is obtained every four years using self-administered questionnaires.15 The questionnaires and the validity and reproducibility of measurements have been described previously.16 From 1989-1990, a blood sample was requested from all participants in the NHS, and 32,826 women provided one. Participants who provided blood samples were similar to those who did not. Among the 30,111 women without cardiovascular disease (CVD) or cancer prior to 1990, we identified 468 women with incident non-fatal MI or fatal CHD between date of blood drawing and June 2004. Using risk-set sampling,17 we randomly selected controls in a 2:1 ratio who were individually matched for age, smoking status, fasting status and date of blood sampling from participants free of CVD at the time of diagnosis of the index case. Study physicians blinded to the participant’s exposure status confirmed the diagnosis of MI on the basis of the criteria of the World Health Organization (symptoms plus either diagnostic electrocardiographic changes or elevated levels of cardiac enzymes). Deaths were identified from state vital records and the National Death Index or reported by the participant’s next of kin or the postal system. Fatal CHD was confirmed by an examination of hospital or autopsy records, by the listing of CHD as the cause of death on the death certificate, if CHD was the underlying and most plausible cause, and if evidence of previous CHD was available. The study protocol was approved by the review boards of Brigham and Women’s Hospital and Harvard School of Public Health.
Measurement of biochemical variables
Blood samples were collected in liquid sodium heparin tubes, placed on ice packs, stored in Styrofoam containers, returned to our laboratory by overnight courier, centrifuged, and divided into aliquots for storage in liquid-nitrogen freezers (−130°C or colder). Total (TC), low-density lipoprotein (LDL-C) cholesterol, HDL-C, and triglycerides (TG) were measured with standard methods using reagents from Roche Diagnostics and Genzyme with coefficients of variation (CVs) of 1.7%, 2.5%, 1.8%, and 3.1%, respectively. CRP and HbA1c levels were quantified using an immunoturbidimetric technique on the Hitachi 911 analyzer, with a CV of 1.4%, and 2.6%, respectively. Total and HMW adiponectin levels were measured using a commercially available enzyme linked immunosorbent assay method from ALPCO Diagnostics Inc. (Salem, NH), with CVs of less than 15%. The laboratory used is certified by the NHLBI/CDC Lipid Standardization program. Total and HMW adiponectin levels were available for 455 cases and 911 controls. For covariates when information was missing, we assigned the median level within the cohort (TC, n=18; LDL-C, n=37; HDL-C, n=18; TG, n=98; CRP, n=34; HbA1c, n=28).
In a reliability study among 20 men from the Health Professionals Follow-up Study (a cohort study similar to the NHS) with 2 blood measurements taken 1 year apart, within-person plasma HMW adiponectin levels tended to decrease over the 1-year period from geometric mean plasma levels of 2.86 mg/L (95%-confidence interval 2.21-3.69 mg/L) to 2.56 mg/L (95%-CI 1.93-3.39 mg/L; p based on Student’s paired t-test, 0.04); however, there was excellent reliability in the 2 measurements (intraclass correlation coefficient based on log-transformed levels, 0.91; 95%-CI 0.79-0.96). Total adiponectin had similar excellent reliability and was not substantially affected by transport conditions.18
Statistical analysis
Plasma levels of total and HMW adiponectin, and HMW/total adiponectin ratio were categorized into quintiles based on control participants, and unconditional logistic regression adjusted for matched variables (age, 5-year categories; smoking status, never, past, or current; fasting status, yes or no; and month of blood draw, 5 categories) was used to investigate the association with and incidence of CHD. Linear trend tests across categories were conducted using median log-transformed adiponectin levels among controls. We also estimated the multivariable-adjusted relative risk associated with a difference in log-transformed continuous adiponectin levels of log(2), which corresponds to 2-fold higher adiponectin levels on the original scale. In multivariable models, we further adjusted for family history of MI before age 60 years (yes/no), alcohol intake (nondrinker; 0.1-4.9, 5.0-14.9, 15.0-29.9, or ≥30.0 g/d; or missing), body mass index (BMI, <20, 20-24, 25-29, 30-34, or ≥35 kg/m2), history of hypertension (yes/no), physical activity (quintiles), hormone replacement therapy use (yes/no), and LDL-C levels (quintiles). We examined the impact of potential intermediate biomarkers by adding history of diabetes (yes/no), or plasma levels of HDL-C, HbA1c, or CRP (all in quintiles) separately and in combination to our models. Conditional logistic regression provided essentially the same results and hence, are not reported. With risk-set sampling, the odds ratio derived from logistic regression directly estimates the incidence (hazard) rate ratio, and, therefore, the relative risk.17, 19 Additional analyses were stratified by alcohol intake (non-drinkers versus moderate drinkers), BMI (<25 versus ≥25 kg/m2), and age (<60 versus ≥60 years), and interactions tested using cross product terms.
P values presented are 2-tailed and P<0.05 was considered statistically significant. Analyses were performed using SAS software, version 8.2 (SAS Institute Inc, Cary, NC).
Results
Cases had a significantly higher BMI and were more likely to have a history of hypertension or diabetes and to use cholesterol lowering drugs (Table 1). Alcohol consumption was significantly lower among cases. Cases had significantly lower plasma levels of total and HMW adiponectin, and a lower HMW/total adiponectin ratio. TC, LDL-C, TG, CRP, and HbA1c levels were significantly higher and HDL-C levels significantly lower among cases than among controls.
Table 1.
Baseline characteristics of cases with incident coronary heart disease and matched controls during 14 years of follow-up in the Nurses’ Health Study
| Characteristics | Cases | Controls | p |
|---|---|---|---|
| N | 455 | 911 | |
| Age*, mean ± SD, years | 60.0 ± 6.5 | 59.9 ± 6.5 | 0.87 |
| Smoking status*, % | 0.97 | ||
| Current smoker | 26.8 | 26.2 | |
| Past smoker | 37.6 | 37.8 | |
| Never smoker | 35.6 | 36.0 | |
| BMI, mean ± SD, kg/m2 | 26.7 ± 5.4 | 25.2 ± 4.3 | <0.0001 |
| Parental history of MI, % | 20.9 | 12.6 | <0.0001 |
| Postmenopausal, % | 89.3 | 88.2 | 0.55 |
| HRT use among postmenopausal women, % |
36.7 | 41.5 | 0.12 |
| History of hypertension, % | 51.4 | 27.9 | <0.0001 |
| History of diabetes, % | 15.2 | 6.3 | <0.0001 |
| Aspirin use, % | 19.3 | 23.2 | 0.11 |
| Cholesterol lowering drugs, % | 4.8 | 2.7 | 0.046 |
| Alcohol consumption, median (IQR) g/d |
0.9 (0-5.3) | 1.8 (0-8.1) | 0.003 |
| Physical activity, median (IQR), MET- hrs/wk |
11.1 (3.9-25.7) | 12.3 (5.2-25.2) | 0.08 |
| Total adiponectin, mg/L | |||
| mean ± SD | 8.17 ± 4.13 | 9.33 ± 3.96 | <0.0001 |
| median (IQR) | 7.52 (5.35-10.09) | 8.67 (6.52-11.43) | <0.0001 |
| HMW adiponectin, mg/L | |||
| mean ± SD | 4.78 ± 3.28 | 5.67 ± 3.15 | <0.0001 |
| median (IQR) | 4.17 (2.49-6.25) | 5.03 (3.40-7.32) | <0.0001 |
| HMW/total adiponectin | |||
| mean ± SD | 0.545 ± 0.129 | 0.575 ± 0.110 | <0.0001 |
| median (IQR) | 0.550 (0.462- 0.627) |
0.578 (0.507-0.649) | <0.0001 |
| TC, mean ± SD, mg/dL | 233.6 ± 40.8 | 226.8 ± 40.1 | 0.003 |
| LDL-C, mean ± SD, mg/dL | 143.8 ± 36.6 | 135.5 ± 37.1 | <0.0001 |
| HDL-C, mean ± SD, mg/dL | 52.1 ± 14.9 | 59.6 ± 16.9 | <0.0001 |
| TG, median (IQR), mg/dL | 118.0 (89.0-173.0) | 110.0 (79.0-144.0) | <0.0001 |
| Fasting TG, median (IQR), mg/dL† | 126.0 (87.0-181.0) | 106.5 (75.0-144.0) | <0.0001 |
| CRP, median (IQR), mg/L | 2.71 (1.20-5.90) | 1.92 (0.80-3.87) | 0.009 |
| HbA1c, median (IQR), % | 5.58 (5.26-6.01) | 5.44 (5.18-5.72) | <0.0001 |
Abbreviations: HRT, hormone replacement therapy; IQR, interquartile range; MET-hrs metabolic equivalent-hours; SD, standard deviation
P values for the difference between cases and controls (unadjusted) were determined by Student’s t-test for variables expressed as means ±SD, by Wilcoxon’s rank-sum test for variables expressed as medians, and by the chi-square test for variables expressed as percentages.
Age and smoking status were among the matching criteria
971 women provided fasting blood samples (329 cases, 642 controls)
Plasma levels of total and HMW adiponectin were highly correlated (Table 2). Total and HMW adiponectin levels and HMW/total adiponectin ratio were significantly inversely related at a similar magnitude to BMI, TG, CRP, HbA1c and LDL-C levels (although the latter inverse correlation was rather weak), and positively related to HDL-C levels.
Table 2.
Age-adjusted Spearman partial correlation coefficients at baseline of total and HMW adiponectin levels and HMW/total adiponectin ratio with selected cardiovascular risk factors among controls (n=911)
| Total adiponectin |
HMW adiponectin |
HMW/total adiponectin |
||||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| Age* | 0.06 | 0.07 | 0.04 | 0.24 | −0.01 | 0.78 |
| HMW adiponectin | 0.97 | <0.0001 | - | - | 0.83 | <0.0001 |
| HMW/total adiponectin | 0.68 | <0.0001 | - | - | - | - |
| BMI | −0.38 | <0.0001 | −0.39 | <0.0001 | −0.33 | <0.0001 |
| TC | −0.01 | 0.74 | −0.01 | 0.71 | −0.004 | 0.91 |
| LDL-C | −0.13 | 0.0001 | −0.12 | 0.0002 | −0.08 | 0.02 |
| HDL-C | 0.49 | <0.0001 | 0.50 | <0.0001 | 0.40 | <0.0001 |
| TG | −0.38 | <0.0001 | −0.40 | <0.0001 | −0.35 | <0.0001 |
| TG (fasting)† | −0.39 | <0.0001 | −0.41 | <0.0001 | −0.35 | <0.0001 |
| CRP | −0.29 | <0.0001 | −0.30 | <0.0001 | −0.26 | <0.0001 |
| HbA1c | −0.19 | <0.0001 | −0.21 | <0.0001 | −0.21 | <0.0001 |
The Spearman correlation coefficient for age is not adjusted for age.
restricted to 642 women who provided a fasting blood sample
Total and HMW adiponectin levels and HMW/total adiponectin ratio were significantly inversely related to 14-year risk of CHD (Table 3). After adjustment for matching variables, family history of MI, BMI, alcohol consumption, physical activity, hormone replacement therapy use, hypertension, and LDL-C levels, participants in the highest compared with the lowest quintile of total, HMW adiponectin levels, or HMW/total adiponectin ratio had a relative risk (RR) of CHD of 0.50 (95%-confidence interval [CI] 0.33-0.75; p for trend on a log scale=0.001), 0.53 (95%-CI 0.35-0.80; p trend=0.004), or 0.63 (95%-CI 0.43-0.93; p trend=0.03), respectively. On a continuous scale, 2-fold higher levels in total adiponectin, HMW adiponectin, or the HMW/total adiponectin ratio were associated with a 0.70-fold (95%-CI 0.57-0.85), 0.78-fold (95%-CI 0.67-0.90), or 0.63-fold (95%-CI 0.43-0.92) RR of CHD, respectively.
Table 3.
Relative risk of CHD during 14 years of follow-up according to baseline plasma levels of total or HMW adiponectin or HMW/total adiponectin ratio
| Quintile |
Continuously (per doubling) |
||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | p trend* | ||
| Total adiponectin | |||||||
| Median (range), mg/L† | 4.83 (1.57-5.97) | 6.99 (5.97-7.88) | 8.67 (7.89-9.53) | 10.81 (9.54-12.37) | 14.69 (12.39-28.45) | ||
| Cases, no. | 153 | 96 | 67 | 84 | 55 | ||
| Controls, no. | 182 | 182 | 182 | 182 | 183 | ||
| Relative risk (95%-CI) | |||||||
| Adjusted for matching factors‡ | 1 | 0.62 (0.45-0.87) | 0.43 (0.30-0.61) | 0.52 (0.37-0.73) | 0.35 (0.24-0.51) | <0.0001 | 0.57 (0.47-0.68) |
| Multivariable adjusted§ | 1 | 0.80 (0.56-1.14) | 0.53 (0.36-1.78) | 0.76 (0.52-1.12) | 0.50 (0.33-0.75) | 0.001 | 0.70 (0.57-0.85) |
| HMW adiponectin | |||||||
| Median (range), mg/L† | 2.21 (0.46-3.00) | 3.75 (3.01-4.32) | 5.03 (4.33-5.79) | 6.65 (5.79-7.95) | 9.83 (7.96-23.87) | ||
| Cases, no. | 152 | 88 | 83 | 76 | 56 | ||
| Controls, no. | 182 | 182 | 182 | 182 | 183 | ||
| Relative risk (95%-CI) | |||||||
| Adjusted for matching factors‡ | 1 | 0.57 (0.41-0.80) | 0.53 (0.38-0.75) | 0.48 (0.33-0.67) | 0.35 (0.24-0.51) | <0.0001 | 0.67 (0.59-0.76) |
| Multivariable adjusted§ | 1 | 0.77 (0.54-1.11) | 0.69 (0.48-1.00) | 0.71 (0.48-1.05) | 0.53 (0.35-0.80) | 0.004 | 0.78 (0.67-0.90) |
| HMW/total adiponectin ratio | |||||||
| Median (range)† | 0.44 (0.10-0.49) | 0.52 (0.49-0.55) | 0.58 (0.55-0.60) | 0.63 (0.60-0.66) | 0.71 (0.67-0.93) | ||
| Cases, no. | 151 | 76 | 75 | 85 | 68 | ||
| Controls, no. | 182 | 181 | 182 | 182 | 183 | ||
| Relative risk (95%-CI) | |||||||
| Adjusted for matching factors‡ | 1 | 0.50 (0.36-0.71) | 0.48 (0.34-0.68) | 0.55 (0.39-0.77) | 0.44 (0.31-0.62) | <0.0001 | 0.41 (0.29-0.59) |
| Multivariable adjusted§ | 1 | 0.62 (0.43-0.90) | 0.63 (0.43-0.91) | 0.73 (0.51-1.06) | 0.63 (0.43-0.93) | 0.03 | 0.63 (0.43-0.92) |
P values for trend are based on the median plasma levels in quintiles of the controls.
Quintiles, medians, and ranges are based on controls only.
Adjusted for matching factors, including age, smoking status, date of blood draw, fasting status, and reported problems with blood draw
Multivariable models are adjusted for matching factors, parental history of myocardial infarction, hormone replacement therapy use, alcohol consumption, physical activity, BMI, history of hypertension, and LDL-C levels
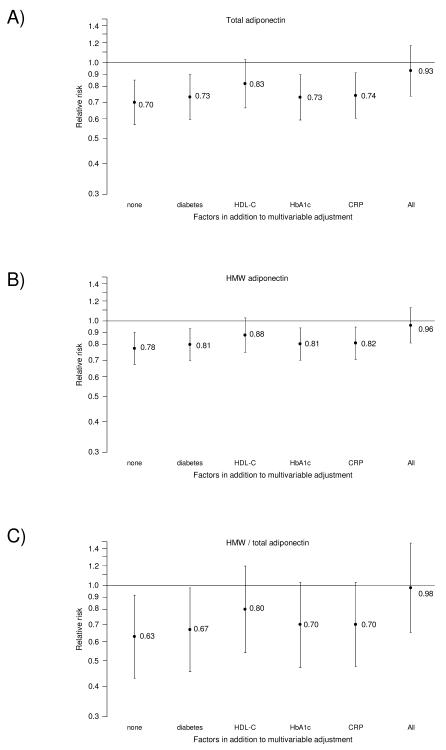
We next examined the impact of entering potential intermediary biomarkers separately or in combination to our model (Figure 1, Supplementary Tables 1 and 2). When added separately, the inverse association for the adiponectin measures was most strongly attenuated (and no longer statistically significant) after adjustment for HDL-C. By comparison, the other potential intermediary factors when added individually did not appreciably attenuate the RRs. When all potential intermediary variables were added simultaneously to the multivariable adjusted model the associations were substantially attenuated and no longer significant (highest versus lowest quintile, total adiponectin, RR=0.84; 95%-CI 0.53-1.33; p trend=0.62; HMW adiponectin, RR=0.95; 95%-CI 0.60-1.52; p trend=0.98; HMW/total adiponectin, RR=0.97; 95%-CI 0.64-1.47; p trend=0.80). On a continuous scale, the RRs associated with 2-fold higher levels in total adiponectin, HMW adiponectin, or the HMW/total adiponectin ratio were 0.93 (95%-CI 0.74-1.17), 0.96 (95%-CI 0.82-1.13), or 0.97 (95%-CI 0.65-1.47) (Figure 1, Supplementary Table 1).
Figure 1.
Multivariable-adjusted estimated relative risk of coronary heart disease during 14 years of follow-up associated with a doubling in total adiponectin, HMW adiponectin, or HMW/total adiponectin ratio with and without adjustment for potentially intermediate variables. Relative risks calculated using log-transformed adiponectin level as a continuous variable, adjusted for matching factors (age, smoking status, date of blood draw, fasting status, and reported problems with blood draw), and additional adjustment for parental history of MI, hormone replacement therapy use, alcohol consumption, physical activity, BMI, history of hypertension, and LDL-C levels, with and without additional separate or combined adjustment for diabetes, HDL-C, HbA1c, CRP as indicated.
BMI is among the major determinants of circulating adiponectin levels; however, lifestyle factors, particularly moderate alcohol consumption may increase adiponectin concentrations. In general, the inverse associations of adiponectin with risk of CHD within strata of alcohol consumption were slightly stronger among moderate drinkers than among non-drinkers, and also among non-overweight than overweight participants (Table 4, Supplementary Table 3); however, these differences were not statistically significant. The association between adiponectin and CHD tended to be stronger among younger (<60 years) compared to older women (≥60 years) but these differences also were not statistically significant.
Table 4.
Multivariable-adjusted estimated relative risk of CHD during 14 years of follow-up associated with a doubling in total or HMW adiponectin or HMW/total adiponectin ratio in subgroups*
| Total adiponectin |
HMW adiponectin |
HMW/total adiponectin |
||||||
|---|---|---|---|---|---|---|---|---|
| cases | controls | RR (95%-CI) | p | RR (95%-CI) | p | RR (95%-CI) | p | |
| Alcohol intake†‡ | ||||||||
| Non-drinkers | 193 | 331 | 0.98 (0.71-1.35) | 0.44 | 0.94 (0.75-1.19) | 0.62 | 0.72 (0.39-1.35) | 0.31 |
| moderate drinkers | 247 | 536 | 0.56 (0.42-0.74) | <0.0001 | 0.69 (0.56-0.84) | 0.0003 | 0.58 (0.35-0.96) | 0.04 |
| Test for interaction§ | 0.10 | 0.25 | 0.97 | |||||
| Overweight | ||||||||
| No (BMI<25) | 211 | 524 | 0.66 (0.49-0.89) | 0.007 | 0.73 (0.59-0.91) | 0.006 | 0.51 (0.28-0.95) | 0.03 |
| Yes (BMI≥25) | 244 | 387 | 0.75 (0.56-0.99) | 0.05 | 0.83 (0.68-1.01) | 0.06 | 0.73 (0.45-1.19) | 0.20 |
| Test for interaction§ | 0.24 | 0.40 | 0.75 | |||||
| Age | ||||||||
| <60 years | 194 | 388 | 0.56 (0.40-0.80) | 0.001 | 0.70 (0.55-0.89) | 0.003 | 0.56 (0.30-1.05) | 0.07 |
| ≥60 years | 261 | 523 | 0.79 (0.61-1.02) | 0.07 | 0.84 (0.69-1.01) | 0.06 | 0.67 (0.41-1.10) | 0.12 |
| Test for interaction§ | 0.06 | 0.12 | 0.46 | |||||
Relative risk estimates and p values were calculated using log-transformed adiponectin level as a continuous variable, adjusted for matching factors (age, smoking status, date of blood draw, fasting status, and reported problems with blood draw), and additional adjustment for parental history of myocardial infarction, hormone replacement therapy use, alcohol consumption, physical activity, BMI, history of hypertension, and LDL-C levels
Not adjusted for the stratification variable
Non-drinkers include participants who reported no consumption of alcohol; moderate drinkers includes participants who reported 0.1-29.9 g/d alcohol consumption. Heavy drinkers (≥30 g/d alcohol) were excluded from this subgroup analysis.
P values for interaction were calculated using dichotomous variables for alcohol intake (non-drinkers versus moderate drinkers) or continuous variables for BMI or age, and log-transformed adiponectin levels for main effects and interaction terms.
The multivariable adjusted association with adiponectin was similar for non-fatal MI and for fatal CHD: a 2-fold higher level of total adiponectin was associated with a 0.71-fold (95%-CI 0.57-0.89) RR of non-fatal MI, and a 0.63-fold (95%-CI 0.41-0.97) RR of fatal CHD. These RRs were 0.79 (95%-CI 0.68-0.93) and 0.71 (95%-CI 0.53-0.96), respectively, for HMW adiponectin, and 0.66 (95%-CI 0.44-0.99) and 0.49 (95%-CI 0.23-1.04), respectively, for HMW/total adiponectin ratio. Similar to the main analysis, these RRs were attenuated after adjustment for HDL-C. The association of adiponectin with risk of CHD tended to be stronger for earlier events than later events during follow-up: In the multivariable adjusted model, 2-fold higher levels of total adiponectin were associated with a 0.62-fold (95%-CI 0.46-0.83) RR during the first 8 years, and a 0.82-fold (95%-CI 0.60-1.11) RR after 8 years of follow-up; similar results were found for HMW adiponectin HMW/total adiponectin ratio. Tests for interaction, however, were not significant (p=0.12 for total adiponectin, p=0.12 for HMW adiponectin, and p=0.12 for HMW/total adiponectin ratio).
Discussion
In this prospective nested case-control study, higher total or HMW adiponectin levels, or higher HMW/total adiponectin ratios were associated with a lower risk of CHD over a follow-up period of 14 years among women without CVD at baseline. These associations appeared to be largely explained by potential intermediary variables, including parameters related to glucose and lipid metabolism and inflammation, especially HDL-C levels.
Experimental in vitro and animal studies have shown that adiponectin beneficially affects many pathways that may be related to the development of CVD. Administration of adiponectin in animal models improves insulin sensitivity and may have anti-atherogenic and anti-inflammatory properties.20, 21 In humans, individuals with CHD or cerebrovascular disease have lower adiponectin levels than healthy controls.1, 20, 21 This association generally follows a “dose-response” relationship, with lower adiponectin levels in more severe forms of CHD.1 However, prospective studies of the association of circulating adiponectin levels with risk of CHD have provided inconsistent results, and most published studies have been conducted among male populations. In the Health Professionals Follow-up Study, men who were free of diagnosed CVD and who were in the highest compared to the lowest quintile of adiponectin levels had a significantly 44% decreased risk of subsequent CHD over 6-year follow-up, independent of other cardiovascular risk factors.2 These observations were confirmed among diabetic men in this cohort,3 and by an 8-year follow-up study of men reported by Frystik et al.4 Consistent with these findings, low plasma adiponectin levels have been shown to predict the progression of coronary calcification.22 The Strong Heart Study, including individuals of American Indian heritage with a very high prevalence of type 2 diabetes, and the male British Regional Heart Study also observed inverse relationships between adiponectin and risk of CHD; however, these associations were no longer statistically significant after adjustment for other risk factors.5-7 Kuller et al. reported no significant association with risk of fatal CHD among men with the metabolic syndrome during 18 years of follow-up.23 The MONICA/KORA study in Germany did not find a significant inverse association between adiponectin and risk of CHD.8, 24
In women the data are sparser. The British Women’s Heart and Health Study found a nonsignificant inverse relationship between adiponectin and CHD.6 In that study, HMW adiponectin and the HMW/total adiponectin ratio were also not significantly related to risk of CHD.14 However, the sample size in that study was rather limited, including 167 cases and 333 controls. Further, their inclusion of diverse clinical endpoints (angina pectoris, coronary artery bypass surgery, or angioplasty) in the definition of nonfatal CHD may have blurred the findings because risk factors may differ between revascularization endpoints compared with MIs, with invasive coronary procedures being strongly dependent on access to the medical care system or potentially to patient lifestyle characteristics (including BMI).25 In the 20-year prospective analysis of the Rancho Bernardo Study, high plasma adiponectin levels were related to a significantly lower risk of non-fatal CHD among men only, whereas adiponectin was not significantly associated with risk of fatal CHD in either sex.26 Non-fatal events among women were not available in that report.26 In the KORA study, adiponectin levels were not related to risk of CHD among women 24.
Since experimental studies suggest that adiponectin may be involved in glucose and lipid metabolisms as well as in inflammation,27 our findings indicate that among women any protective effect of adiponectin on the cardiovascular system (if causal) may be explained by these intermediary mechanisms. Interestingly, experimental studies suggest that adiponectin may accelerate reverse cholesterol transport by increasing HDL assembly in the liver through increased expression and secretion of apolipoprotein A1 and ATP-binding cassette transporter 1 (ABCA1) in the liver.28, 29 Further, in macrophages adiponectin leads to increased HDL-mediated cholesterol efflux, which may partly be explained by adiponectin induced up-regulation of the expression of ABCA1 in these cells.30, 31 Although adiponectin also inhibits the uptake of oxidized LDL into macrophages, there is no substantial correlation of adiponectin with LDL-C in humans.31 Taken together, these findings together with our observations support the hypothesis that a large proportion of the effects of adiponectin on the vascular system may be mediated via HDL-C metabolism.
HMW adiponectin was reported to be more strongly related to insulin sensitivity and to risk of type 2 diabetes than other circulating forms.13, 20 The only report on the association of HMW adiponectin with risk of CHD we are aware of came from the British Women’s Heart and Health Study and found no significant association (see above).14 In our study, total and HMW adiponectin, and HMW/total adiponectin ratio were significantly inversely associated with risk of CHD to a similar extent in the multivariable adjusted model, and attenuated toward the null after adjustment for potential intermediary variables. Our findings therefore do not support the hypothesis that HMW adiponectin or HMW/total adiponectin ratio is more strongly inversely related to risk of CHD than total adiponectin.
We examined the association of adiponectin with risk of CHD in various low and high cardiovascular risk groups in our study population. Although the associations tended to be stronger among women with low versus high BMI, among moderate drinkers versus non-drinkers, and among younger versus women, none of these differences was statistically significant at the 5%-level.
Our cohort does not represent a random sample of the US population, which may limit the generalizability of our results. However the biological relationship between risk factors and cardiovascular outcomes found in this study should be similar to healthy women within the studied age-range in general. It was suggested that the association of adiponectin with cardiovascular outcomes may depend on the presence of preexisting diseases or advanced ageing. 32 Thus, while in more general healthy populations, adiponectin is inversely related to obesity, insulin resistance and CVD risk factors, high adiponectin levels were observed in subjects with liver cirrhosis, inflammatory bowel diseases, rheumatoid arthritis, and old age. In the presence of these conditions, adiponectin may be a marker of underlying wasting processes related to poor cardiovascular outcomes, rather than a true risk factor; a phenomenon described as “reverse epidemiology”.32 Since our study included middle-aged women from a relatively healthy population our findings may not be applicable to elderly individuals or subjects with preexisting diseases.
Although a single biomarker assessment may be susceptible to short-term variation, which would bias the results toward the null, we found in our reliability studies intraindividual total and HMW adiponectin levels to be reasonably stable over time.18 The inclusion of highly correlated measures in regression analyses may yield to imprecise or instable risk estimates and complicate the interpretation of results. However, the correlation of adiponectin with most markers was modest and the width of the confidence intervals did not appreciably change when we combined adiponectin with other markers. In addition, we obtained similar results when we adjusted our analysis for a biomarker score that was based on combination of quintiles of the potentially intermediary biomarkers (data not shown).
In conclusion, high levels of total or HMW adiponectin, or HMW/total adiponectin ratio are associated with a lower risk of CHD among women without previous CVD. These associations can be explained in part by potential intermediate variables related to lipid and glucose metabolism and inflammation, especially HDL-C levels. Although all measures of adiponectin were strongly inversely associated with CHD among women, our data do not support the hypothesis that HMW adiponectin or HMW/total adiponectin ratio is more closely related to risk of CHD than total adiponectin.
Supplementary Material
Acknowledgments
This study was supported by research grants HL34594 and AA11181 from the National Institutes of Health. Additional support was provided by Merck Research Laboratories (MRL) but MRL had no access to the data and the academic institutions had full and final right to publish. Dr. Girman is an employee of Merck & Co., Inc, which manufactures or is developing pharmaceutical products for the treatment of cardiovascular disease and diabetes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pischon T. Adiponectin: a biomarker of obesity? Current Cardiovascular Risk Reports. 2008;2:150–155. [Google Scholar]
- 2.Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. Jama. 2004;291:1730–1737. doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- 3.Schulze MB, Shai I, Rimm EB, Li T, Rifai N, Hu FB. Adiponectin and future coronary heart disease events among men with type 2 diabetes. Diabetes. 2005;54:534–539. doi: 10.2337/diabetes.54.2.534. [DOI] [PubMed] [Google Scholar]
- 4.Frystyk J, Berne C, Berglund L, Jensevik K, Flyvbjerg A, Zethelius B. Serum adiponectin is a predictor of coronary heart disease: a population-based 10-year follow-up study in elderly men. J Clin Endocrinol Metab. 2007;92:571–576. doi: 10.1210/jc.2006-1067. [DOI] [PubMed] [Google Scholar]
- 5.Lindsay RS, Resnick HE, Zhu J, Tun ML, Howard BV, Zhang Y, Yeh J, Best LG. Adiponectin and coronary heart disease: the Strong Heart Study. Arterioscler Thromb Vasc Biol. 2005;25:e15–16. doi: 10.1161/01.ATV.0000153090.21990.8c. [DOI] [PubMed] [Google Scholar]
- 6.Lawlor DA, Smith G. Davey, Ebrahim S, Thompson C, Sattar N. Plasma adiponectin levels are associated with insulin resistance, but do not predict future risk of coronary heart disease in women. J Clin Endocrinol Metab. 2005;90:5677–5683. doi: 10.1210/jc.2005-0825. [DOI] [PubMed] [Google Scholar]
- 7.Sattar N, Wannamethee G, Sarwar N, Tchernova J, Cherry L, Wallace AM, Danesh J, Whincup PH. Adiponectin and coronary heart disease: a prospective study and meta-analysis. Circulation. 2006;114:623–629. doi: 10.1161/CIRCULATIONAHA.106.618918. [DOI] [PubMed] [Google Scholar]
- 8.Koenig W, Khuseyinova N, Baumert J, Meisinger C, Lowel H. Serum concentrations of adiponectin and risk of type 2 diabetes mellitus and coronary heart disease in apparently healthy middle-aged men: results from the 18-year follow-up of a large cohort from southern Germany. J Am Coll Cardiol. 2006;48:1369–1377. doi: 10.1016/j.jacc.2006.06.053. [DOI] [PubMed] [Google Scholar]
- 9.Soderberg S, Stegmayr B, Stenlund H, Sjostrom LG, Agren A, Johansson L, Weinehall L, Olsson T. Leptin, but not adiponectin, predicts stroke in males. J Intern Med. 2004;256:128–136. doi: 10.1111/j.1365-2796.2004.01351.x. [DOI] [PubMed] [Google Scholar]
- 10.Matsumoto M, Ishikawa S, Kajii E. Association of adiponectin with cerebrovascular disease: a nested case-control study. Stroke. 2008;39:323–328. doi: 10.1161/STROKEAHA.107.497552. [DOI] [PubMed] [Google Scholar]
- 11.Ingelsson E, Riserus U, Berne C, Frystyk J, Flyvbjerg A, Axelsson T, Lundmark P, Zethelius B. Adiponectin and risk of congestive heart failure. Jama. 2006;295:1772–1774. doi: 10.1001/jama.295.15.1772-c. [DOI] [PubMed] [Google Scholar]
- 12.Whitehead JP, Richards AA, Hickman IJ, Macdonald GA, Prins JB. Adiponectin--a key adipokine in the metabolic syndrome. Diabetes Obes Metab. 2006;8:264–280. doi: 10.1111/j.1463-1326.2005.00510.x. [DOI] [PubMed] [Google Scholar]
- 13.Heidemann C, Sun Q, van Dam RM, Meigs JB, Zhang C, Tworoger SS, Mantzoros CS, Hu FB. Total and high-molecular-weight adiponectin and resistin in relation to the risk for type 2 diabetes in women. Ann Intern Med. 2008;149:307–316. doi: 10.7326/0003-4819-149-5-200809020-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sattar N, Watt P, Cherry L, Ebrahim S, Smith G. Davey, Lawlor DA. High molecular weight adiponectin is not associated with incident coronary heart disease in older women: a nested prospective case-control study. J Clin Endocrinol Metab. 2008;93:1846–1849. doi: 10.1210/jc.2007-2603. [DOI] [PubMed] [Google Scholar]
- 15.Colditz GA, Manson JE, Hankinson SE. The Nurses’ Health Study: 20-year contribution to the understanding of health among women. J Womens Health. 1997;6:49–62. doi: 10.1089/jwh.1997.6.49. [DOI] [PubMed] [Google Scholar]
- 16.Willett W. Nutritional Epidemiology. Oxford University Press; New York: 1998. [Google Scholar]
- 17.Prentice RL, Breslow NE. Retrospective studies and failure time models. Biometrika. 1978;65:153–158. [Google Scholar]
- 18.Pischon T, Hotamisligil GS, Rimm EB. Adiponectin: Stability in Plasma over 36 Hours and Within-Person Variation over 1 Year. Clin Chem. 2003;49:650–652. doi: 10.1373/49.4.650. [DOI] [PubMed] [Google Scholar]
- 19.Rothman KJ, Greenland S. Case-control studies. In: Rothman KJ, Greenland S, editors. Modern epidemiology. Lippincott-Raven; Philadelphia: 1998. pp. 93–114. [Google Scholar]
- 20.Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116:1784–1792. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szmitko PE, Teoh H, Stewart DJ, Verma S. Adiponectin and cardiovascular disease: state of the art? Am J Physiol Heart Circ Physiol. 2007;292:H1655–1663. doi: 10.1152/ajpheart.01072.2006. [DOI] [PubMed] [Google Scholar]
- 22.Maahs DM, Ogden LG, Kinney GL, Wadwa P, Snell-Bergeon JK, Dabelea D, Hokanson JE, Ehrlich J, Eckel RH, Rewers M. Low plasma adiponectin levels predict progression of coronary artery calcification. Circulation. 2005;111:747–753. doi: 10.1161/01.CIR.0000155251.03724.A5. [DOI] [PubMed] [Google Scholar]
- 23.Kuller LH, Grandits G, Cohen JD, Neaton JD, Prineas R. Lipoprotein particles, insulin, adiponectin, C-reactive protein and risk of coronary heart disease among men with metabolic syndrome. Atherosclerosis. 2006 doi: 10.1016/j.atherosclerosis.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karakas M, Zierer A, Herder C, Baumert J, Meisinger C, Koenig W, Thorand B. Leptin, adiponectin, their ratio and risk of Coronary Heart Disease: results from the MONICA/KORA Augsburg Study 1984-2002. Atherosclerosis. 2010;209:220–225. doi: 10.1016/j.atherosclerosis.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 25.Quatromoni J, Jones R. Inequalities in socio-economic status and invasive procedures for coronary heart disease: a comparison between the USA and the UK. Int J Clin Pract. 2008;62:1910–1919. doi: 10.1111/j.1742-1241.2008.01943.x. [DOI] [PubMed] [Google Scholar]
- 26.Laughlin GA, Barrett-Connor E, May S, Langenberg C. Association of adiponectin with coronary heart disease and mortality: the Rancho Bernardo study. Am J Epidemiol. 2007;165:164–174. doi: 10.1093/aje/kwk001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang ZV, Scherer PE. Adiponectin, cardiovascular function, and hypertension. Hypertension. 2008;51:8–14. doi: 10.1161/HYPERTENSIONAHA.107.099424. [DOI] [PubMed] [Google Scholar]
- 28.Matsuura F, Oku H, Koseki M, Sandoval JC, Yuasa-Kawase M, Tsubakio-Yamamoto K, Masuda D, Maeda N, Tsujii K, Ishigami M, Nishida M, Hirano K, Kihara S, Hori M, Shimomura I, Yamashita S. Adiponectin accelerates reverse cholesterol transport by increasing high density lipoprotein assembly in the liver. Biochem Biophys Res Commun. 2007;358:1091–1095. doi: 10.1016/j.bbrc.2007.05.040. [DOI] [PubMed] [Google Scholar]
- 29.Oku H, Matsuura F, Koseki M, Sandoval JC, Yuasa-Kawase M, Tsubakio-Yamamoto K, Masuda D, Maeda N, Ohama T, Ishigami M, Nishida M, Hirano K, Kihara S, Hori M, Shimomura I, Yamashita S. Adiponectin deficiency suppresses ABCA1 expression and ApoA-I synthesis in the liver. FEBS Lett. 2007;581:5029–5033. doi: 10.1016/j.febslet.2007.09.038. [DOI] [PubMed] [Google Scholar]
- 30.Tsubakio-Yamamoto K, Matsuura F, Koseki M, Oku H, Sandoval JC, Inagaki M, Nakatani K, Nakaoka H, Kawase R, Yuasa-Kawase M, Masuda D, Ohama T, Maeda N, Nakagawa-Toyama Y, Ishigami M, Nishida M, Kihara S, Shimomura I, Yamashita S. Adiponectin prevents atherosclerosis by increasing cholesterol efflux from macrophages. Biochem Biophys Res Commun. 2008;375:390–394. doi: 10.1016/j.bbrc.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 31.Tian L, Luo N, Klein RL, Chung BH, Garvey WT, Fu Y. Adiponectin reduces lipid accumulation in macrophage foam cells. Atherosclerosis. 2009;202:152–161. doi: 10.1016/j.atherosclerosis.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rathmann W, Herder C. Adiponectin and cardiovascular mortality: evidence for “reverse epidemiology”. Horm Metab Res. 2007;39:1–2. doi: 10.1055/s-2007-958630. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.