Abstract
Neurons in the subthalamic nucleus occupy a pivotal position in the circuitry of the basal ganglia. They receive direct excitatory input from the cerebral cortex and the intralaminar nuclei of the thalamus, and directly excite the inhibitory basal ganglia output neurons in the internal segment of the globus pallidus and the substantia nigra. They are also engaged in a reciprocal synaptic arrangement with inhibitory neurons in the external segment of the globus pallidus. Although once viewed as a simple relay of extrinsic input to the basal ganglia, physiological studies of subthalamic neurons have revealed that activity in these neurons does not directly reflect their pattern of extrinsic excitation. Subthalamic neurons are autonomously active at rates comparable to those observed in vivo, and they generate complex patterns of intrinsic activity arising from the interactions between voltage sensitive ion channels on the somatodendritic and axonal membranes. Extrinsic synaptic excitation does not create the firing pattern of the subthalamic neuron, but rather controls the timing of action potentials generated intrinsically. The dopaminergic innervation of the subthalamic nucleus, although moderate, can directly influence firing patterns by acting both on synaptic transmission and voltage-sensitive ion channels responsible for intrinsic properties. Furthermore, chronic dopamine depletion in Parkinson’s disease may modify both synaptic transmission and integration in the subthalamic nucleus, in addition to its effects on other regions of the basal ganglia.
Keywords: Parkinson’s disease, dopamine, oscillations, spontaneous firing, basal ganglia
The subthalamic nucleus (STN) is unique among forebrain basal ganglia nuclei, because it is excitatory. The projection neurons of the other parts of the basal ganglia, the striatum, both the external and internal segments of the globus pallidus (GPe and GPi, respectively) and the substantia nigra pars reticulata (SNr), all are GABAergic, and inhibitory. Subthalamic neurons all use glutamate as their neurotransmitter and are excitatory at all of their targets. When the excitatory nature of the STN neurons was originally discovered, it seemed to solve a fundamental problem that had haunted thinking about the pallidum: why the cells fired at all. The GPe, GPi and also SNr exhibit high frequency firing that is only transiently inhibited, but most of their afferent connections, among each other and from the large afferent system arising in the striatum, are inhibitory. In an influential review of the evidence for its excitatory influence, Kitai and Kita (1987) proposed that the STN was “the driving force of the basal ganglia”. Since that time, it has become clear that background firing in the GPe, GPi and SNr is autonomous in nature, and does not require an external source of excitation for its maintenance (e.g. Surmeier et al 2005), but the unique role of the STN as the main excitatory input to those structures remains. The STN is also an alternative entry point for excitation from the cortex and thalamus influencing the basal ganglia. Because of its short latency responses to cortical stimuli, the first effects of those stimuli on the basal ganglia are mediated by the STN, with striatal-derived influences arriving later. For the same reason, it should also be the fastest route for thalamic excitation to influence the basal ganglia. For this reason, the cortico-subthalamo-pallidal pathway is sometimes called the “hyperdirect” pathway, and is said to occupy a special position within the cortico-basal ganglia loop (Nambu, 2004).
The STN is also unique in that it participates in a prominent recurrent loop with the GPe. Most basal ganglia connections are feed-forward in nature. For example, the cortex projects to the striatum, but there are no striato-cortical projections. This pattern is largely repeated through the GPe, GPi and SNr. Except for a small ascending connection from a subset of GPe cells to striatal interneurons (Bevan et al. 1998), the powerful reciprocal loop between the STN and GPe are the only exception to this feed-forward arrangement. This nominates the STN and GPe as a likely source of persistent activity in the basal ganglia, and particularly oscillations (Plenz and Kitai 1999; Terman et al. 2002). The emergence of rhythmic bursting in the STN and its targets in Parkinson’s disease (PD; Bergman et al. 1994) has raised much interest in the potential importance of the recurrent subthalamo-pallidal network in the pathophysiology of that disease. The STN is also an important source of excitatory input to the dopaminergic neurons of the substantia nigra, pars compacta, and one of the candidates for the source of the signal responsible for their reward-related bursts (Smith and Grace, 1992).
Firing Patterns of STN Neurons
STN neurons recorded from awake, resting monkeys fire irregularly at average rates varying among studies from 18 to 28 spikes/s (Wichmann et al. 1994; Georgeopoulos et al. 1983; Isoda and Hikosaka 2008; Matusmura et al. 1992). In anesthetized rats (Hollerman and Grace, 1992; Fujimota and Kita, 1993; Ryan and Clark, 1992), the cells fire more slowly, averaging 7–12 spikes/s, which is consistent with a substantial contribution of tonic excitation from the cortex to the baseline firing rate. On the other hand, studies of STN slices in rats (in which afferent activity from the cortex is greatly reduced or absent), report spontaneous firing rates nearly as high as those seen in intact, anesthetized animals, as shown in Figure 1 (also see Nakanishi et al. 1987; Abbott et al. 1997; Bevan and Wilson, 1999; Beurrier et al. 1999). Thus, the influences controlling the firing rate of STN neurons are more complex than the balance between cortical excitation and pallidal inhibition that is usually suggested by box and arrow diagrams.
Figure 1.
Spontaneous activity of STN neurons in slices.
A. photomicrograph of a biocytin-filled subthalamic neuron in coronal section. B. Spontaneous activity and interspike interval trajectory. C. Voltage clamp recording of persistent ionic currents evoked in an STN neuron during a slow (1 s.) depolarizing ramp. The control current (black) does not show an equilibrium potential in the subthreshold range. After ttx treatment (1 μM), the persistent inward current is abolished and the cell acquires a resting potential near −55 mV (blue). The ttx-sensitive current (red) activates over the voltage range visited by the membrane potential during the interspike interval trajectory. Average of 25 traces. The voltage protocol is shown in the inset.
In vivo, three classes of firing patterns have been described: rhythmic, irregular and bursty. About 12% in the STN of humans with essential tremor (Steigerweld, et al. 2008), 22% of cells in chloral hydrate anesthetized rat (Hollerman and Grace, 1992), 22% of cells in the urethane-anesthetized guinea pig (Overton and Greenfield, 1995) and 26% of cell in the urethane anesthetized rat (Ryan et al. 1992) fire in a regular single spiking pattern, that is rhythmic enough to create multiple peaks in the autocorrelogram. This firing pattern is identified by a mildly skewed interspike interval histogram which in the most regularly-firing cells is approximately symmetric. The second pattern seen in vivo is an irregular pattern dominated by occasional long interspike intervals and sporadic bouts of rapid firing, usually forming doublets and triplets. This pattern dominates the STN in humans with essential tremor (52%, Steigerweld, et al. 2008), urethane-anesthetized guinea pigs (61%, Overton and Greenfield, 1995), urethane anesthetized rats (71%, Ryan et al. 1992) and in chloral hydrate anesthetized rats (42%, Hollerman and Grace, 1992). It has also been described in awake behaving monkeys (Wichman et al. 1994), although we do not know what proportion of cells fire in each pattern in this preparation. The irregular firing pattern is associated with a highly skewed interspike interval histogram. The third pattern is a bursting pattern, consisting of irregularly-occurring or rhythmic bursts of firing at high frequency. In humans with essential tremor, these cells account for 36% of all cells. They account for 17% of cells in the urethane-anesthetized guinea pig, 3% of cells in urethane-anesthetized rats (Ryan et al. 1992) and 29% of cells in the chloral hydrate anesthetized rat (Hollerman and Grace, 1992). This pattern is characterized by a highly skewed interspike interval histogram, which can be bimodal when bursts occur rhythmically.
In humans with PD, monkeys made parkinsonian by MPTP treatment, or rats treated with 6-hydroxydopamine, the number of bursting STN cells increases dramatically, with a corresponding decrease in the regular single spiking pattern (Steigerwald et al., 2008; Bergman et al. 1994; Hollerman and Grace, 1992; Magill et al., 2001). In addition, bursts become shorter and more rhythmic, especially at frequencies < 30 Hz (Bergman et al., 1994; Wichmann and Soares, 2006; Weinberger et al., 2006; Steigerwald et al., 2008).
The origin of spontaneous activity in the STN
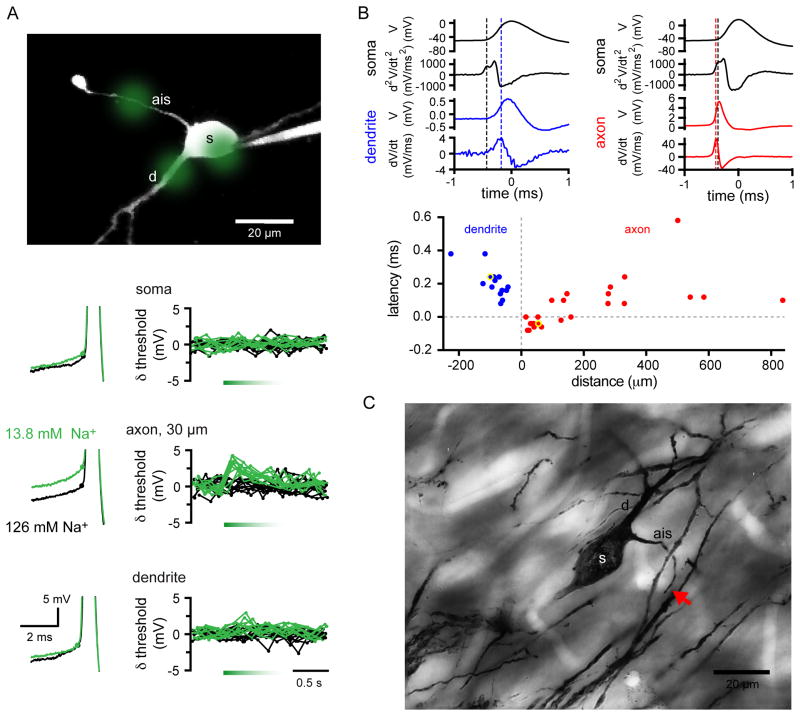
In subthalamic slices, the regular single spiking pattern dominates, but with a small but variable-sized group of cells firing in the bursty pattern (Nakanishi et al., 1987; Abbott et al. 1997; Beurrier et al. 1999; Bevan and Wilson, 1999). These two firing patterns are similar to patterns seen in vivo, but the irregular pattern, which is the most common firing pattern see in vivo, is not seen in slices. The firing patterns seen in most slice preparations are independent of fast glutamatergic and GABAergic synaptic inputs, being unaltered by treatment with antagonists for those receptors (Bevan and Wilson 1999). The regular single spiking pattern is also seen in dissociated STN neurons (Do and Bean, 2003). In slices, action potentials are initiated autonomously at the axon initial segment and then propagate orthodromically along the myelinated axon and antidromically over the soma and dendrites, as shown in Figure 2 (see also Atherton et al. 2008).
Figure 2.
Contribution of axonal Nav channels to autonomous STN activity. A. Effects of 50 ms applications of low [Na+] artificial cerebrospinal fluid (ACSF) (green) to the soma, axon initial segment (ais) and dendrite (d) of a STN neuron. Upper panel, sites of low [Na+] application (green circles). The ais is distinguished by its fine caliber and retraction ball at the point of transection. Left lower panels, action potentials at threshold (dots) under control conditions (black) and following low [Na+] ACSF application (green). Right lower panels, changes in action potential threshold (relative to mean threshold) under control conditions (black; 5 trials) and following low [Na+] ACSF application (green; 5 trials). Application of low [Na+] ACSF to the ais 30 μm from the soma consistently elevated action potential threshold compared to the effects of somatic or dendritic application. B. Upper panels, spike-triggered averages of 100 autonomous action potentials recorded with somatic whole-cell (black traces) and dendritic (blue traces) or axonal (red traces) loose-seal cell-attached electrodes. In order to discriminate between the axonal and somatodendritic action potential components the second derivative of the whole-cell record is displayed. Since the loose-seal cell-attached recording approximates the first derivative of the membrane potential that signal was further differentiated to be consistent with the second derivative of the somatic whole-cell record. Thus, the peak of the first temporal derivative of the dendritic loose-seal recording (blue dotted line) follows the first peak of the second temporal derivative of the whole-cell record (black dotted line), whereas the peak of the first temporal derivative of the axonal loose-seal recording (red dotted line) precedes the first peak of the second temporal derivative of the whole-cell record. Lower panel, latency of the first peak of the second derivative of the somatic action potential relative to the peak of the first derivative of the cell-attached record plotted against the distance of the loose-seal cell-attached recording electrode from the soma. Distances of cell-attached recordings from the soma are plotted as negative and positive for dendrites (n = 14) and axons (n = 24), respectively. The points drawn from the upper examples are highlighted. Note that action potentials were first detected in the proximal axon. C. A Golgi labeled STN neuron. The axon is labeled up to ~ 40 μm from the soma (red arrow), which corresponds to the non-myelinated ais, where action potentials are first initiated.
During rhythmic single spiking, inward currents remain slightly larger than outward currents throughout most of the interspike interval (Bevan and Wilson, 1999; Beurrier et al. 2000; Farries et al. 2010). When slowed by small constant currents, the neurons can sustain rhythmic firing at arbitrarily low rates (Bevan and Wilson, 1999). The balance of currents favoring depolarization is thus owed to currents that adapt slowly compared to the interspike interval, and the mechanism of the oscillation is evident in the steady-state I–V curve (Fig 1C; Farries et al. 2010). The most significant inward current during the interspike interval is a persistent, TTX-sensitive voltage-dependent Na+ (Nav) channel current, which begins to activate as the membrane potential recovers from the spike afterhyperpolarization, and increases continuously up to the time of spike generation (Bevan and Wilson; Beurrier et al. 2000; Do and Bean, 2003). Approximately half of the persistent Nav channel current is absent in mice with a null Nav1.6 mutation, but the remaining current is sufficient for maintaining spontaneous firing (Do and Bean, 2004). The ability of this current to maintain rhythmic firing at very low rates is owed to its long inactivation time constant, which is about 5 seconds (Do and Bean 2003; Barraza et al. 2009). The persistent Nav channel current is opposed by persistent K+ currents also active in the same voltage range, and the balance between these results in a near-zero slope conductance over much of the interspike interval (Farries et al. 2010). This means that the charge placed on the membrane by voltage-sensitive or synaptic currents is not rapidly dissipated through the membrane resistance, but will be integrated up to the point of action potential generation. The main determinants of the interspike interval of the STN neuron during spontaneous firing are the size of the net inward current responsible for depolarization between action potentials, and the depth of the hyperpolarization generated after each action potential.
STN neurons have brief afterhyperpolarization currents during single spiking, the longest of which is generated by small-conductance apamin-sensitive Ca2+-dependent K+ (SK) channels. SK current is activated by Ca2+ entry triggered by action potentials; there is practically no Ca2+ entry that can be traced to subthreshold-activated calcium currents during the interspike interval (Bevan and Wilson 1999; Do and Bean 2003; Hallworth et al. 2003; Ramanthan et al., 2008; Teagarden et al. 2008). Because of rapid diffusion of Ca2+ away from the plasma membrane after each action potential, SK current decays rapidly over a period of about 30 ms after each action potential during spontaneous activity (Teagarden et al. 2008). The Ca2+ influx responsible for SK current is primarily through Cav2.2 channels, and is blocked by the specific Ca2+ channel blocker ω-conotoxin GVIA (Hallworth et al. 2003; Ramanathan et al., 2008). The afterhyperpolarization generated by the SK current returns the neuron to a consistent initial state of the membrane potential, and Nav channel inactivation after each action potential, and thus is responsible for maintaining the regularity of firing. After blockade of SK current, firing is much less regular (Bevan and Wilson, 1999; Hallworth et al. 2003). Under these conditions, a briefer voltage-sensitive afterhyperpolarization current is revealed (Wigmore and Lacey, 2000; Teagarden et al. 2008). Blockade of SK channels increases the excitability of STN neurons when measured using depolarizing current pulses, but it has less effect on the resting firing rate (Hallworth et al. 2003; Wilson et al. 2004).
Driven firing and control of average firing rate
The average firing rate of STN neurons has been thought to be an important component of the state of the basal ganglia, and changes in mean rate have been suggested to partly underlie the pathophysiology of PD (e.g. Wichmann and DeLong, 1996). Changes in average firing rate could arise from changes in the volume of the excitatory synaptic barrage from the cortex and thalamus, or the inhibitory input from the GPe, all of which are continuously active in vivo. To understand the responses of STN neurons to changes in the average synaptic current it has been useful to study their responses to constant currents applied by intracellular electrodes. Early studies showed that the cells were capable of sustained firing at very high rates (up to 500 spikes/s) in response to 100–1000 ms duration current pulses (Nakanishi et al. 1987; Bevan and Wilson 1999). This was consistent with high frequency firing seen to occur transiently during movements, which likewise can exceed 250 spikes/s (Matsumura et al., 1992; Isoda and Hikosaka, 2008). As shown in Figure 3, frequency-intensity curves for current pulses are sigmoidal in shape, with the steepest section of the curve occurring when the cell is firing between 50 and 150 spikes/s. Firing of STN neurons often accelerates at the beginning of responses to depolarizing steps, and for moderate stimuli, firing frequency can increase by a factor of two over the first 100 ms of the response (Hallworth, et al. 2003; Wilson et al. 2004; Barraza et al. 2009). After reaching a maximum firing rate, rates are stable or fall slightly during the rest of a 1 s. depolarizing current pulse. This absence of spike frequency adaptation over the short time period occurs because the afterhyperpolarization currents generated by individual action potentials do not accumulate over time (Teagarden et al. 2008). The fast afterhyperpolarization current that precedes the SK channel-mediated one is reduced in amplitude with repetitive firing, and this reduction occurs concurrently with the initial speedup of firing (Teagarden et al 2008). Current pulses longer than one second reveal a much more profound spike frequency adaptation that develops with a time course of about 20 seconds. That slow spike frequency adaptation is primarily caused by the accumulation of a K+ current (Barraza et al. 2009). The current responsible for the slow adaptation is triggered by action potentials, but each action potential contributes only a tiny amount of current that cannot be detected unless the cell fires at a high rate for a sustained period of time. With sustained firing, it can accumulate to be much larger than the currents that drive spontaneous activity. This slow adaptation prevents STN cells from firing at high rates in response to sustained changes in inputs. It also can produce a very powerful inhibition of STN neurons lasting seconds after prolonged periods of faster than spontaneous firing.
Figure 3.
Firing rate dependence on constant current. A. 1 s current pulses produce high frequency firing with relatively little spike frequency adaptation. After high frequency firing, there is a pause in spontaneous firing. B. Frequency-intensity relationship for the same cell shown in A. The mean firing rate vs current relationship for 1 s current pulses is sigmoidal in shape (blue) with a maximum sensitivity between 100 and 150 Hz firing rates. The rate defined by the first interspike interval in the response to the current pulse is shown in red. The deviation between this curve and the mean rate curve indicates that firing accelerated during the response. C. Instantaneous firing rate during the traces shown in A, showing the acceleration in firing at currents beyond 200 pA. D. Slow spike frequency adaptation in response to a much longer current pulse than used in A. After the initial acceleration, spike frequency very gradually decreases to a fraction of the initial rate. After cessation of the current pulse, spontaneous activity is silenced for about 20 seconds, and then gradually returns.
Rebounds, plateau potentials, and rhythmic bursting
STN neurons express low-threshold class 3 voltage dependent Ca2+ (Cav3) or t-type calcium channels, which can generate large rebound depolarizations and trigger bursts after prolonged hyperpolarizations with current pulses or repetitive synaptic inhibition (Nakanishi et al., 1987; Overton and Greenfield, 1995; Beurrier et al. 1999; Bevan et al, 1999; Bevan et al. 2002; Hallworth et al. 2003). These currents do not contribute significantly to spontaneous single spiking activity, being largely inactivated over the entire interspike interval, but may be very influential in the STN cell’s response to inhibitory synaptic input, (Bevan et al. 2002; Hallworth and Bevan 2005; Beurrier et al. 2000). Likewise, STN neurons have a substantial complement of somatodendritic hyperpolarization activated cyclic nucleotide gated (HCN) channels that can readily be detected when the neurons are hyperpolarized by current pulses or synaptic inhibition (Nakanishi et al. 1987; Bevan and Wilson, 1999; Atherton et al. 2010; Beurrier, 2000). Like the Cav3-channel current, this is minimally activated during spontaneous firing in slices, and contributes little to the rhythmic single spiking firing pattern (Bevan and Wilson 1999; Beurrier et al. 2000; Do and Bean 2003; Atherton et al. 2010). The influence of these channels is seen when the spontaneous firing pattern is interrupted by inhibition sustained enough to remove inactivation from Cav3 channels or activate the slowly-activating HCN channels (Bevan et al. 2002; Hallworth et al. 2005). Because they both require sustained hyperpolarization below the voltage range visited during spontaneous single spiking, these Ca2+ and non-specific cation currents often occur together. They both contribute to prominent rebound bursts seen in all STN neurons after prolonged hyperpolarizations (Beurrier et al. 1999; Hallworth et al. 2003; Hallworth and Bevan, 2005; Atherton et al. 2010). The long duration of those rebounds, which can last for seconds, also rely on a contribution from persistent calcium currents from the Cav1 and Cav2 families (Beurrier 1999; Song et al. 2000; Otsuka et al. 2001; Hallworth et al. 2003).
The set of currents contributing to prolonged rebound bursts after hyperpolarizations are also contributors to plateau potentials and spontaneous burst firing sometimes seen in STN neurons, especially when slightly hyperpolarized with constant current (e.g. Figure 4, also see Nakanishi et al. 1987; Beurrier 1999; 2000, Otsuka et al. 2001; Kass and Mintz 2006). Some proportion of STN cells exhibit spontaneous bursting, even in slices as shown in Figure 4C (Beurrier 1999;2000, Otsuka et al. 2001; Kass and Mintz 2006), again, usually when slightly hyperpolarized with constant current. The proportion of cells exhibiting this property varies from study to study, but all STN cells will exhibit strong rebound bursts following hyperpolarizing current pulses (Hallworth et al. 2003). It is therefore likely that spontaneous bursting is not a discrete property of a limited subset of STN neurons, but a functional state that could be entered by any cell, in which the rebound burst mechanism becomes self-repeating. Studies so far have not revealed the conditions required to induce natural bursting, although it has been shown to be promoted by blockade of SK channels with apamin (Hallworth et al. 2003). Of course, treatment of the cells with NMDA agonists can induce plateau potentials and rhythmic bursting (Zhu et al. 2004; Loucif et al. 2005), as it does in many cells. Bursts are driven by underlying plateau potentials, which do not rely on action potentials and persist after treatment with TTX (Beurrier et al. 1999; 200; Kass and Mintz 2005). Plateau potentials without action potentials can last for seconds, and may be of arbitrary length, requiring a hyperpolarizing stimulus for their termination (Kass and Mintz 2005).
Figure 4.
Plateau potentials and bursting occasionally seen in STN neurons. A. Prolonged high frequency firing triggered by a rebound burst after a hyperpolarizing pulse. Firing rate is elevated for more than 1 s following the rebound. B. Plateau potential and high frequency firing following the offset of a depolarizing current pulse, which gradually decays to baseline levels. C. Rhythmic bursting during hyperpolarization caused by recovery from high frequency prolonged firing as in Figure 3D.
Excitatory synaptic input to the STN
The STN receives excitatory glutamatergic inputs from both the ipsilateral cerebral cortex and intralaminar nuclei of the thalamus. Despite the small size of the STN, both cortical and thalamic afferents display a highly topographic distribution (Afsharpour, 1985; Groenewegen and Berendse, 1990; Bevan et al. 1995; Nambu et al., 1996; Takada et al. 2001). The cortico-subthalamic pathway arises primarily from widespread regions of the frontal cortex, including both motor and nonmotor areas (Afsharpour, 1985; Groenewegen and Berendse, 1990; Nambu et al. 1996). Cortical and thalamic inputs make similar synaptic connections on STN neurons, except that cortical inputs tend to be on smaller dendrites, suggesting a more distal synaptic location than thalamic inputs (Bevan et al. 1995). Responses of the STN to stimulation of the motor cortex have been thoroughly studied in intact animals. In intracellular recordings from rats, Kitai and Deniau (1981) observed an initial short latency monosynaptic excitatory synaptic potential. The monosynaptic EPSP produces a large increase in firing probability in STN cells, as seen in peristimulus histograms from rats and monkeys (Fujimoto and Kita, 1993; Kita et al. 2005; Kolomiets et al. 2001; Magill et al 2004; Nambu et al. 2000; Ryan and Clark 1992). That response is followed by a profound but very brief (10–20 ms) pause and a second excitatory period that lasts for 20–30 ms. These responses are followed by a weaker and longer lasting inhibition. Only the earliest component of this response is certain to be a direct monosynaptic effect of cortical excitation. The rest of the response reflects the interaction between excitatory and inhibitory polysynaptic responses via the globus pallidus and other structures (Kita et al. 2005). Similar response sequences have also been observed following brief electrical stimulation of the parafascicular thalamic nucleus. Since the initial monosynaptic excitation is the only survivor of transection of fibers arising from the cortex or GPe, the response sequence originates directly from excitation of the thalamus (Mouroux et al., 1995).
In STN tissue slices, glutamatergic synaptic potentials are readily observed after local stimulation or stimulation of the cerebral peduncle nearby (e.g. Nakanishi et al. 1988). These likely include both cortical and thalamic (and possibly brainstem and midbrain (Bevan and Bolam, 1995; Coizet et al., 2009) components, and differences between them have not been studied. The synaptic currents consist of both AMPA and NMDA-receptor mediated components (Nakanishi et al 1988; Farries et al. 2010). The effectiveness of excitatory synaptic transmission is dependent on the history of membrane potential changes preceding synaptic activation. Hyperpolarization, caused either by synaptic inhibition (Baufreton et al. 2005) or by the afterhyperpolarization of a preceding action potential (Farries et al. 2010) leads to a negative shift in action potential threshold and increased excitability in large part due to the removal of inactivation from Nav channels (Baufreton et al. 2005). Likewise, rapid depolarizations caused by EPSPs late in the interspike interval cause a negative shift in threshold that helps to sharpen the early part of the peristimulus histogram, by increasing the reliability of spike timing for responses to strong synaptic input (Farries et al. 2010). For this reason and others, the effect of synaptic inputs on the ongoing firing pattern of subthalamic nucleus neurons is sensitive to the prior history of firing, as shown in Figure 5.
Figure 5.
Resetting of spontaneous firing by excitatory and inhibitory synaptic stimulation. A. (top) Superimposition of 50 trials applying a strong stimulation of the internal capsule at the arrow, in the presence of picrotoxin (150 μM) and CGP-55845 (2 μM) to block GABAA and GABAB receptors respectively. The resulting EPSP triggers action potentials on nearly every trial, but with variable latency. The evoked action potential is followed by resumption of spontaneous firing at its normal interspike interval, thus resetting rhythmic firing. (bottom) Three trials are superimposed, showing the phase-sensitivity of firing latency of in response to the stimulus. A trial in which the EPSP arrives near the end of the interspike interval (red trace) results in an almost immediate action potential, whereas a trial in which the stimulus arrives near the beginning of the interspike interval (blue trace ) produces a longer-latency response. A trial in which the stimulus falls at an intermediate latency (black trace) produces a response at intermediate latency. These phase relationships are preserved in the next cycle of spontaneous firing. B. (top) Superimposition of 50 trials applying a similar strong stimulus, but in the presence of DNQX (20 μM) and APV (50 μM) to block AMPA and NMDA receptors respectively. A strong IPSP hyperpolarizes the neuron on each trial, and produces a pause in firing approximately the same duration as the spontaneous interspike interval. (bottom) Three trials are superimposed, showing the phase sensitivity of the interspike interval following inhibition. A trial in which the IPSP arrives almost at the end of the interspike interval (red trace) produces the smallest delay in the resumption of firing. A trial in which the stimulus arrives near the beginning of the interspike interval (blue trace) produces a longer subsequent interspike interval. An intermediate stimulus arrival time (black) produces an intermediate result. These phase relationships will be preserved on subsequent cycles.
GPe-STN inhibition
The monosynaptic excitatory response of STN neurons to cortical or thalamic stimulation in vivo is followed by a brief period of strong inhibition, which is reduced by lesion or inactivation of the GPe (Ryan and Clark, 1992; Fujimoto and Kitai, 1993; Mouroux et al., 1995; Maurice et al. 1998; Paz et al., 2005; Farries et al. 2010). GPe neurons also have early excitatory responses to cortical or thalamic stimulation that are dependent upon the STN (Mouroux et al., 1995; Nambu et al. 2000). Thus the early fast inhibitory phase of the response of STN neurons to cortical or thalamic stimulation probably results from direct short latency GPe-STN inhibition evoked by STN-GPe excitation.
The GPe sends a prominent highly topographic GABAergic projection to the STN. Thus motor, associative and limbic GPe inputs terminate heavily in the lateral, middle and medial thirds of the nucleus, respectively (Smith et al., 1990; Shink et al., 1996; Karachi et al., 2005). Given the extensive nature of their dendrites relative to the dimensions of the nucleus, STN neurons may, to a certain extent, integrate functionally diverse GPe inputs (Hammond and Yelnik, 1983; Bevan et al., 1997). At the synaptic level GPe inputs terminate mostly (~70%) on the somata and large diameter (putative proximal) dendrites of STN neurons with fewer inputs (~30%) to small diameter (putative distal) dendrites (Smith et al., 1990; Shink et al., 1996; Bevan et al., 1997). The axon initial segment of STN neurons, which is the primary site of autonomous action potential initiation, is however largely devoid of synaptic input from the GPe or other sources (Atherton et al., 2008).
It is thought that the majority of GPe neurons project to the STN (Kita and Kitai, 1994; Bevan et al., 1998; Sato et al., 2000). In rats, it has been estimated that there are 46,000 GPe neurons and 13,600 STN neurons (Oorschot et al., 1996) and each GPe-STN neuron contributes ~ 250 synaptic terminals to the STN (Baufreton et al., 2009). If each of these terminals made synapses with a different STN neuron then individual GPe neurons could innervate up to 2% of STN neurons. However, individual GPe-STN terminal fields are arranged in sparsely distributed clusters and many of these clusters correspond to multiple terminations onto individual STN neurons. Thus individual GPe-STN neurons innervate an even smaller fraction of the STN.
Estimates of the total number of GPe-STN synapses support the view that STN neurons receive input from a restricted number of GPe neurons. Thus, the total number of rat GPe-STN synapses is ~ 12 million (Baufreton et al., 2009). If these synapses were distributed equally between 13,600 STN neurons each STN neuron would receive approximately 880 synaptic inputs from the GPe. If each of these 880 synapses arose from a different GPe neuron each STN neuron would therefore receive input from up to 2% of GPe neurons. However, as GPe neurons often form multisynaptic contacts with individual STN neurons (see above) the proportion of GPe neurons innervating each STN neuron is considerably smaller. Taken together these data indicate that GPe-STN connectivity is considerably more sparse and selective than might otherwise have been thought. This feature of GP-STN circuitry may contribute fundamentally to the decorrelated nature of the GPe and STN activity under normal conditions.
The GPe-STN input predominantly influences the STN through the activation of synaptic GABAA receptors, although GABAB receptors may also be recruited under certain circumstances (Hallworth and Bevan, 2005; see below). Electrophysiological recordings confirm that individual GPe neurons influence STN neurons through multiple synaptic connections because the mean amplitude of the unitary GABAA receptor mediated conductance is ~ 10 X the mean amplitude of the miniature (univesicular) synaptic conductance (0.7 nS) (Baufreton et al., 2009). Furthermore, simultaneous recordings from STN neurons during stimulation of single GPe-STN axons confirm that (even) neighboring STN neurons share few inputs from common GPe neurons (Baufreton et al., 2009). Given that individual STN neurons receive ~ 880 GPe-STN synaptic inputs and each input contributes a conductance of ~ 0.7 nS, the total potential conductance arising from GPe-STN transmission is > 600 nS. GABAergic synaptic inputs may interact with the intrinsic membrane properties of STN neurons in a variety of complex ways because GABAA receptor-mediated inhibitory postsynaptic potentials (IPSPs) are unusually hyperpolarizing, i.e. the equilibrium potential of GABAA receptor current is below −80 mV (Bevan et al., 2000, 2002). Chloride, the principal permeant ion of the GABAA receptor is therefore powerfully extruded by STN neurons, presumably by the K-Cl cotransporter KCC2, which is highly expressed in the STN (Kanaka et al., 2001).
In order to further appreciate the impact of GPe-STN transmission on STN activity a range of synthetic GPe-STN synaptic conductances have been injected into these cells using the dynamic clamp technique. This approach suggests that synchronous transmission at < 4% of GPe-STN synapses is sufficient to inhibit STN neurons, reset the phase of their autonomous oscillation and thus synchronize their activity (Baufreton et al., 2009). Furthermore, replay of GPe-STN IPSPs as voltage clamp waveforms confirms that GPe-STN inputs reset autonomous activity by deactivating Nav channels (Baufreton et al., 2005), which contribute the subthreshold persistent, resurgent and transient Na+ currents that underlie autonomous firing (Bevan and Wilson, 1999; Beurrier et al., 2000; Do and Bean, 2003). A fraction of STN Nav channels are inactivated by autonomous activity and GPe-STN IPSPs can transiently enhance the responsiveness of STN neurons to subsequent excitatory inputs by deinactivating these inactivated channels (Baufreton et al., 2005).
In vitro preparations that maintain spontaneous GPe-STN transmission confirm that GPe-STN inputs inhibit autonomous STN activity largely through the phasic activation of synaptic GABAA receptors (Hallworth and Bevan, 2005). Tonic currents due to activation of extrasynaptic GABAA receptors are apparently not prominent in STN neurons, presumably due to the tight regulation of extrasynaptic GABA by the neuronal and glial GABA transporters GAT-1 and GAT-3, respectively, which are highly expressed in the STN (Wang and Onn, 1999; Ng et al, 2000) and the low affinity of STN GABAA receptors which are mainly comprised of α1, β2 and γ2 subunits (Goetz et al., 2007). GABAA receptor-mediated inhibition of autonomous activity is partially opposed by activation of postsynaptic somatodendritic HCN channels, which activate at ~ −70 mV and contribute a non-selective cation conductance that limits the amplitude and timecourse of IPSPs (Atherton et al., 2010). Modeling suggests that the uniform somatodendritic distribution of STN HCN channels more effectively counteracts GABAergic inhibition than somatically distributed channels alone.
Synchronous, high-frequency bursts of GPe-STN transmission can generate sufficient hyperpolarization for the deinactivation of postsynaptic low-voltage activated Ca2+ (Cav1 and Cav3) channels, which at the offset of inhibition underlie a rebound burst of activity (Bevan et al., 2002; Hallworth and Bevan, 2005). Although GABAA receptor-mediated inhibition is sufficiently powerful and hyperpolarizing to generate rebound burst firing in isolation, rebound activity may be powerfully augmented by the additional activation of GABAB receptors. In STN neurons activation of these receptors generates a slow IPSP that reverses close to the K+ equilibrium potential and is therefore presumably mediated by the activation Kir/GIRK channels. In some cases GABAB receptor-mediated inhibition alone is sufficient to generate rebound burst activity following pharmacological blockade of GABAA receptors. GABAB receptors located presynaptically on GPe-STN axon terminals also act as autoreceptors by regulating the probability of GABA release (Shen and Johnson, 2001; Chen and Yung, 2005).
Dopamine in the STN
The STN is innervated by dopaminergic axons arising from midbrain dopamine neurons including those in the substantia nigra that are susceptible to degeneration in PD (Hassani et al., 1997; François et al., 2000; Prensa et al., 2000; Cragg et al., 2004). This innervation has, until recently, been overlooked due to its modest nature compared to that found in the striatum. STN dopaminergic axons exhibit both en passant and terminal varicosities. At the ultrastructural level both varicose and non-varicose portions of dopaminergic axons form conventional symmetrical synaptic contacts with the somata and dendrites of STN neurons (Figure 6A, B; Cragg et al., 2004). Fast-scan cyclic voltammetry has been used to confirm that dopaminergic axons in the STN release dopamine in a Ca2+- and action potential-dependent manner and that the spatiotemporal profile of released dopamine is regulated by the dopamine transporter (Figure 6C, Cragg et al., 2004). It is not known whether dopamine is also released at non-synaptic sites.
Figure 6.
Dopaminergic neuromodulation of the STN. A. Tyrosine hydroxylase (TH)-immunoreactive (putative dopaminergic) axon terminals in a sagittal section of the STN. B. Example of a dopamine-immunoreactive axon terminal (asterisk) in the STN that forms a conventional symmetrical synaptic contact (arrow) with the soma (s) of a STN neuron. C. Local 50 Hz electrical stimulation for 1 second (black bar) leads to the release of dopamine in the STN, as measured by fast-scan cyclic voltammetry. The level of extracellular dopamine was enhanced by the blockade of the dopamine transporter with GBR12909 (grey trace) compared to control conditions (black trace). Inset, representative voltammograms. Peak potentials are indicated (dotted lines) for dopamine oxidation (*) and reduction (**). D. Application of the D2-like receptor agonist quinpirole (10 μM) to a STN neuron led to depolarization and an increase in the frequency and variability of autonomous firing. E. D2 receptor-mediated neuromodulation reduced Cav(2.2) channel current that was evoked by an action potential voltage clamp waveform. F. D2 receptor-mediated inhibition of Cav2.2 channels led to a reduction in SK channel-mediated spike afterhyperpolarization current that was evoked by a 5 ms voltage clamp step from −60 mV to 20 mV.
Dopamine acts on the STN through a variety of sites/receptors and signaling pathways. STN neurons express both D1-like (D5) and D2-like (D2 and D3) receptors (Flores et al., 1999; Baufreton et al., 2003; Ramanathan et al., 2008) and D2-like receptors are expressed by the axon terminals of STN afferents (Shen and Johnson, 2000; Baufreton and Bevan, 2008). Activation of postsynaptic dopamine receptors depolarizes STN neurons by several mV and increases the frequency of their autonomous activity. However, the receptors and signaling pathways underlying the actions of dopamine are controversial. In the majority of studies dopamine’s actions are completely mimicked by D2-like receptor agonists and largely abolished by D2-like receptor antagonists. In general D2-like receptor activation leads to a reduction in whole-cell K+ conductance and thus an increase in excitability (Zhu et al., 2002a,b; Ramanathan et al., 2008). One study demonstrated that D2-like receptor activation led to a G-protein-mediated reduction in voltage-independent K+ channel conductance of less than 1 nS (Zhu et al., 2002a). Another demonstrated that D2/3 receptor activation inhibited Cav2.2 channels via direct binding of Gβγ subunits (Ramanathan et al., 2008). As described above, Cav2.2 channels are functionally coupled to SK channels, which underlie a component of action potential afterhyperpolarization (Figure 6; Hallworth et al., 2003). Inhibition of Cav2.2 channels therefore reduces action potential afterhyperpolarization, which in addition to increasing autonomous activity (and excitability) also reduces the precision of firing (Fig. 6D–F). This latter effect is due, at least in part, to a reduction in the availability of Nav channels, as evidenced by a marked elevation in action potential threshold and a reduction in the maximum rate of rise of action potentials (Ramanathan et al., 2008).
Another group reported that dopamine increased autonomous firing via activation of both D2-like dopamine receptors (but with an unusual pharmacology) and D1-like dopamine receptors (Tofighy et al., 2003; Loucif et al., 2008). D1-like receptor activation stimulated the adenylate cyclase-cAMP-PKA cascade, which increased firing through an increase of ~ 1.5 nS in non-selective cyclic nucleotide-gated (CNG) cation channel conductance. However, no effect of D1-like receptor activation on HCN channels was observed implying that the difference between the level of cAMP in STN neurons in the absence and presence of D1-like receptor activation is insufficient to modulate HCN channel gating. D1-like receptor activation of the adenylate cyclase-cAMP-PKA signaling pathway has also been shown to enhance the conductance of Cav1 channels (Baufreton et al., 2003), which underlie a long duration component of rebound activity in a subset of STN neurons. Together these data demonstrate that dopamine modulates a variety of postsynaptic targets and processes in STN neurons. However, the most influential targets appear to be Cav channels because these channels are particularly critical for the firing behavior of STN neurons (Beurrier et al., 1999; Bevan and Wilson, 1999; Otsuka et al., 2001; Baufreton et al., 2003; Hallworth et al., 2003; Kass and Mintz, 2055) and are associated with and coupled to relatively sizable conductances compared to leak K+ and CNG channels.
Dopamine also influences the patterning of STN neuron activity through activation of presynaptic D2-like dopamine receptors. D2-like receptor activation reduces the initial probability of transmitter release at GABAergic synapses, as evidenced by an increase in paired-pulse ratio and a reduction in the frequency but not amplitude of miniature transmission (Shen and Johnson, 2000; Baufreton and Bevan, 2008). Since the vast majority of GABAergic axon terminals in the STN arise from the GPe it can be reasonably assumed that the primary target of modulation is GPe-STN transmission. The receptors mediating this modulation are D2/3 because D2/3 but not D4 receptor-selective drugs are effective. By reducing the initial release probability of GABA dopaminergic neuromodulation retards the onset but does not prevent steady state depression at GPe-STN synapses. Thus phasic bursts of GPe-STN transmission are specifically reduced in transmission strength by dopamine, which reduces their capability to reset/synchronize autonomous activity and generate rebound burst activity (Baufreton and Bevan, 2008).
Dopamine acting at D2-like receptors also reduces the initial probability of excitatory glutamatergic synaptic transmission in STN (Shen and Johnson, 2000). However since major glutamatergic afferents arise from cortex, thalamus and midbrain/brainstem (Afsharpour, 1985: Groenewegen and Berendse, 1990: Bevan and Bolam, 1995; Bevan et al., 1995; Nambu et al., 1996; Takada et al. 2001; Coizet et al., 2009) we do not know which of these excitatory afferents are the targets of dopaminergic neuromodulation. Given the multiple locations, types and signaling pathways of dopamine receptors in the STN it is perhaps not surprising that local application of dopamine receptor selective drugs in vivo has produced mixed effects. However, most studies concur that local application of D2 receptor agonists or dopamine (acting at D2 receptors) increases the firing rate of STN neurons (MIntz et al., 1986; Hassani and Féger, 1999). Furthermore, selective lesions of the dopaminergic innervation of the STN lead to a significant reduction in the firing rate of STN neurons (Ni et al., 2001a).
STN activity in PD
Under normal conditions the activities of the STN, GPe and GPi are irregular, arrhythmic and interspersed by sporadic bursts. Although activity within and between the nuclei is largely uncorrelated it exhibits somatotopic specificity, as expected from the highly topographic nature of afferent inputs. In PD both the frequency and pattern of activity are profoundly altered. Thus, GPe activity may be reduced by 30% and STN and GPi activity may be elevated by 40% and 25%, respectively (reviewed by Galvan and Wichmann, 2008). Such firing rate changes are consistent with the predictions of the classical direct, indirect pathway model and imply that hypoactivity of the GPe underlies hyperactivity of the STN, which in turn drives hyperactivity of the GPi, excessive inhibition of basal ganglia targets and motor dysfunction (Albin et al., 1989; DeLong et al., 1990). The relationship between the frequency of activity and motor symptoms are however less robust than the models predictions: 1) the mean rates of activity in PD and Huntington’s disease are similar in some (Tang et al., 2005) but not all (Starr et al., 2008) studies; 2) symptomatic parkinsonian individuals may or may not exhibit rate changes (Chesselet and Delfs, 1996); 3) both elevations in GPi activity generated through deep brain stimulation of the STN (Hashimoto et al., 2003) and reductions in GPi activity by STN or GPi lesions (Walter and Vitek, 2004) ameliorate symptoms. As a result activity pattern changes are now also thought critical for motor dysfunction. Thus, in PD STN, GPe and GPi activity is rhythmic at 4–7 Hz (theta), 8–12 Hz (delta) and 13–30 Hz (beta) frequencies and the proportion of action potentials discharging in bursts is greatly elevated (interestingly, in the non-human primate model of PD rhythmic activity is relatively prevalent in the delta band compared to PD patients and rodent models of PD; Galvan and Wichmann, 2008). Furthermore, activity within and between the STN, GPe and GPi is highly correlated and somatotopic specificity disintegrates. 8–12 Hz (delta) and 13–30 Hz activity is correlated with akinesia, bradykinesia and rigidity, whereas 4–7 Hz activity is often associated with resting tremor (Brown, 2007; Galvan and Wichmann, 2008; Weinberger et al., 2009; Zaidel et al., 2009). These activity patterns and their associated symptoms may result from distinct processes. 8–12 Hz and 13–30 Hz rhythms are relatively synchronous and continuous compared to 4–7 Hz activity, which is often asynchronous and transient. Furthermore, akinesia, bradykinesia and rigidity are more closely related to the degree of dopamine neuron loss than tremor and occur in its absence (Zaidel et al., 2009).
Exaggerated beta band activity appears profoundly antikinetic. Under normal conditions cortical (and STN) beta band activity increases during steady contractions and during movement prevention in Go, NoGo tasks but declines transiently during the preparation and execution of movement (Engel and Fries, 2010). The amelioration of PD akinesia, bradykinesia and rigidity by dopaminergic medication is also correlated with the suppression of beta band activity (Brown, 2007). Similarly, electrical stimulation of the STN at frequencies > 60 Hz reduces the strength of beta band activity and ameliorates PD symptoms, whereas stimulation at frequencies < 30 Hz worsens symptoms (Benabid, 2003; Eusebio and Brown, 2009). The mechanisms underlying both hypersynchronous delta and beta band activity in PD are unknown but may result from the abnormal hypersensitivity of the dopamine-depleted STN-GPe-GPi network to rhythmic cortical inputs (Bevan et al., 2006; Brown, 2007; Mallet et al., 2008a,b), which in turn leads to amplification and resonance of rhythmic activity throughout the cortico-basal ganglia-thalamocortical loop. According to this hypothesis rhythmic cortical activity is directly imparted to the STN and then relayed to the GPe and GPi through feedforward excitation. Feedback inhibition from the GPe may further amplify the cortical patterning of STN activity through the rhythmic deactivation and de-inactivation of STN Nav channels, as described above (Baufreton et al., 2005). Cortical driving of ~8 Hz rhythmic STN and GPe activity has been directly demonstrated in genetic absence epilepsy rats during cortical seizures although in this case feedback inhibition from the GPe appears was sufficiently powerful to generate rebound burst activity in the STN (Paz et al., 2005). It has also been suggested that hyperactivity of the striatal-GPe inputs may contribute to the pathological activity pattern of the STN, GPe, GPi network in PD by altering the mode of interaction between the STN and GPe (Terman et al., 2002). Another possibility is that that the dopamine-depleted STN-GPe network is the primary source of pathological activity but thus far evidence in support of this view is from organotypic (Plenz and Kitai, 1999) rather than native preparations (Magill et al., 2001).
The loss of extrastriatal dopaminergic neuromodulation may contribute to pathological hypersynchronous, low-frequency (<30 Hz), rhythmic, burst activity. As described above dopamine may decorrelate neuronal activity through several mechanisms: 1) acting via postsynaptic D2-like and possibly D1-like receptors dopamine depolarizes STN neurons and decreases the regularity of their intrinsic activity thereby reducing/altering the efficacy and impact of synaptic inputs (Zhu et al., 2002; Loucif et al., 2008; Ramanathan et al., 2008); 2) acting via presynaptic D2-like receptors dopamine reduces the probability of efferent transmission arising from the STN and GPe (Shen and Johnson, 2000; Hernández et al., 2006; Ibañez-Sandoval et al, 2006; Baufreton and Bevan, 2008; de Jesús Aceves et al., 2011) thus reducing the tendency for correlated firing in the STN, GPe and GPi; 3) acting at presynaptic D2-like receptors dopamine may also reduce the probability of cortical-STN transmission and thus its capability to pattern STN, GPe and GPi activity (Shen and Johnson, 2000).
The gradual emergence of pathological activity in the STN-GPe-GPi network following dopamine depletion (Ni et al., 2001b; Mallet et al., 2008b) further suggests that (mal)adaptive changes in the strength of synaptic connections and neuronal properties may contribute to its emergence. Indeed, following dopamine depletion, there is an increase in the expression of GABAA and GABAB receptors and the ratio of AMPA to NMDA receptors, as evidenced by changes in whole-cell currents evoked by exogenous agonist application (Shen and Johnson, 2005).
The autonomous activity of GPe and STN neurons also declines following depletion of dopamine (Chan et al., 2011; Wilson et al., 2006; Zhu et al., 2002b). In GPe neurons this is due to reduced expression of HCN channels. Furthermore, both autonomous activity and pathological burst firing of GPe neurons in vivo were corrected by viral vector-mediated rescue of GPe HCN channels (Chan et al., 2011). The mechanisms underlying the reduction in autonomous STN activity have yet to be defined. In summary, both the loss of direct dopaminergic modulation together with chronic changes in intrinsic and synaptic properties may contribute fundamentally to pathological activity in the STN, GPe and GPi in PD by increasing the influence of synaptic inputs on action potential generation at the expense of intrinsic mechanisms (Holgardo et al., 2010).
Highlights.
Subthalamic neurons are autonomously active cells
Spontaneous activity in STN neurons depends on persistent sodium current
The autonomous activity of the STN cells shapes their responses to inputs
The dopaminergic innervation controls STn intrinsic and synaptic properties.
Acknowledgments
Supported by NIH/NINDS grants NS047085 (CJW and MDB) and NS041280. Thanks to David Barraza and Michael Farries for providing data used in Figures 3 and 5 and to Jeremy Atherton for data in Figure 4.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott A, Wigmore MA, Lacey MG. Excitation of rat subthalamic nucleus neurones in vitro by activation of a group I metabotropic glutamate receptor. Brain Res. 1997;766:162–167. doi: 10.1016/s0006-8993(97)00550-7. [DOI] [PubMed] [Google Scholar]
- Afsharpour S. Topographical projections of the cerebral cortex to the subthalamic nucleus. J Comp Neurol. 1985;236:14–28. doi: 10.1002/cne.902360103. [DOI] [PubMed] [Google Scholar]
- Atherton JF, Kitano K, Baufreton J, Fan K, Wokosin D, Tkatch T, Shigemoto R, Surmeier DJ, Bevan MD. Selective participation of somatodendritic HCN channels in inhibitory but not excitatory synaptic integration in neurons of the subthalamic nucleus. J Neurosci. 2010;30:16025–16040. doi: 10.1523/JNEUROSCI.3898-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atherton JF, Wokosin DL, Ramanathan S, Bevan MD. Autonomous initiation and propagation of action potentials in neurons of the subthalamic nucleus. J Physiol. 2008;586:5679–5700. doi: 10.1113/jphysiol.2008.155861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Baufretan J, Atherton JF, Surmeier DJ, Bevan MD. Enhancement of excitatory synaptic integration by GABAergic inhibition in the subthalamic nucleus. J Neurosci. 2005;25:8505–8517. doi: 10.1523/JNEUROSCI.1163-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baufreton J, Bevan MD. D2-like dopamine receptor-mediated modulation of activity-dependent plasticity at GABAergic synapses in the subthalamic nucleus. J Physiol. 2008;586:2121–2142. doi: 10.1113/jphysiol.2008.151118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baufreton J, Kirkham E, Atherton JF, Menard A, Magill PJ, Bolam JP, Bevan MD. Sparse but selective and potent synaptic transmission from the globus pallidus to the subthalamic nucleus. J Neurophysiol. 2009;102:532–545. doi: 10.1152/jn.00305.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baufreton J, Garret M, Rivera A, de la Calle A, Gonon F, Dufy B, Bioulac B, Taupignon A. D5 (not D1) dopamine receptors potentiate burst-firing in neurons of the subthalamic nucleus by modulating an L-type calcium conductance. J Neurosci. 2003;23:816–825. doi: 10.1523/JNEUROSCI.23-03-00816.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benabid AL. Deep brain stimulation for Parkinson’s disease. Curr Opin Neurobiol. 13:696–706. doi: 10.1016/j.conb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Bergman H, Wichmann T, Karmon B, DeLong MR. The primate subthalamic nucleus. II Neuronal activity in the MPTP model of Parkinsonism. J Neurophysiol. 1994;72:507–520. doi: 10.1152/jn.1994.72.2.507. [DOI] [PubMed] [Google Scholar]
- Beurrier C, Congar P, Biolac B, Hammond C. Subthalamic nucleus neurons switch from single-spike activity to burst-firing mode. J Neurosci. 1999;19:599–609. doi: 10.1523/JNEUROSCI.19-02-00599.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurrier C, Bioulac B, Hammond C. Slowly inactivating sodium current (I(NaP)) underlies single-spike activity in rat subthalamic neurons. J Neurophysiol. 2000;83:1951–1957. doi: 10.1152/jn.2000.83.4.1951. [DOI] [PubMed] [Google Scholar]
- Bevan MD, Atherton JF, Baufreton J. Cellular principles underlying normal and pathological activity in the subthalamic nucleus. Curr Opin Neurobiol. 2006;16:621–628. doi: 10.1016/j.conb.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Bevan MD, Bolam JP. Cholinergic, GABAergic, and glutamate-enriched inputs from the mesopontine tegmentum to the subthalamic nucleus in the rat. J Neurosci. 1995;15:7105–7120. doi: 10.1523/JNEUROSCI.15-11-07105.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan MD, Booth PA, Eaton SA, Bolam JP. Selective innervation of neostriatal interneurons by a subclass of neuron in the globus pallidus of the rat. J Neurosci. 1998;18:9438–9452. doi: 10.1523/JNEUROSCI.18-22-09438.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan MD, Clarke NP, Bolam JP. Synaptic integration of functionally diverse pallidal information in the entopeduncular nucleus and subthalamic nucleus in the rat. J Neurosci. 1997;17:308–324. doi: 10.1523/JNEUROSCI.17-01-00308.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan MD, Francis CM, Bolam JP. The glutamate-enriched cortical and thalamic input to neurons in the subthalamic nucleus of the rat: convergence with GABA-positive terminals. J Comp Neurol. 1995;361:491–511. doi: 10.1002/cne.903610312. [DOI] [PubMed] [Google Scholar]
- Bevan MD, Magill PJ, Hallworth NE, Bolam JP, Wilson CJ. Regulation of the timing and pattern of action potential generation in rat subthalamic neurons in vitro by GABA-A IPSPs. J Neurophysiol. 2002;87:1348–1362. doi: 10.1152/jn.00582.2001. [DOI] [PubMed] [Google Scholar]
- Bevan MD, Wilson CJ. Mechanisms underlying spontaneous oscillation and rhythmic firing in rat subthalamic neurons. J Neurosci. 1999;19:7617–7628. doi: 10.1523/JNEUROSCI.19-17-07617.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan MD, Wilson CJ, Bolam JP, Magill PJ. Equilibrium potential of GABA(A) current and implications for rebound burst firing in rat subthalamic neurons in vitro. J Neurophysiol. 2000;83:3169–3172. doi: 10.1152/jn.2000.83.5.3169. [DOI] [PubMed] [Google Scholar]
- Brown P. Abnormal oscillatory synchronisation in the motor system leads to impaired movement. Curr Opin Neurobiol. 2007;17:656–664. doi: 10.1016/j.conb.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Chen L, Yung WH. Tonic activation of presynaptic GABA(B) receptors on rat pallidosubthalamic terminals. Acta Pharmacol Sin. 2005;26:10–16. doi: 10.1111/j.1745-7254.2005.00012.x. [DOI] [PubMed] [Google Scholar]
- Chesselet MF, Delfs JM. Basal ganglia and movement disorders: an update. Trends Neurosci. 1996;19:417–22. doi: 10.1016/0166-2236(96)10052-7. [DOI] [PubMed] [Google Scholar]
- Coizet V, Graham JH, Moss J, Bolam JP, Savasta M, McHaffie JG, Redgrave P, Overton PG. Short-latency visual input to the subthalamic nucleus is provided by the midbrain superior colliculus. J Neurosci. 2009;29:5701–5709. doi: 10.1523/JNEUROSCI.0247-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg SJ, Baufreton J, Xue Y, Bolam JP, Bevan MD. Synaptic release of dopamine in the subthalamic nucleus. Eur J Neurosci. 2004;20:1788–802. doi: 10.1111/j.1460-9568.2004.03629.x. [DOI] [PubMed] [Google Scholar]
- DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- Do MT, Bean BP. Subthreshold sodium currents and pacemaking of subthalamic neurons: modulation by slow inactivation. Neuron. 2003;39:109–20. doi: 10.1016/s0896-6273(03)00360-x. [DOI] [PubMed] [Google Scholar]
- Do MT, Bean BP. Sodium currents in subthalamic nucleus neurons from Nav1.6-null mice. J Neurophysiol. 2004;92:726–733. doi: 10.1152/jn.00186.2004. [DOI] [PubMed] [Google Scholar]
- Farries MA, Kita H, Wilson CJ. Dynamic spike threshold and zero membrane slope conductance shape the response of subthalamic neurons to cortical input. J Neurosci. 2010;30:13180–13191. doi: 10.1523/JNEUROSCI.1909-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto K, Kita H. Response characteristics of subthalamic neurons to the stimulation of the sensorimotor cortex in the rat. Brain Res. 1993;609:185–192. doi: 10.1016/0006-8993(93)90872-k. [DOI] [PubMed] [Google Scholar]
- Engel AK, Fries P. Beta-band oscillations-signalling the status quo? Curr Opin Neurobiol. 2010;20:156–65. doi: 10.1016/j.conb.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Eusebio A, Brown P. Synchronisation in the beta frequency-band--the bad boy of parkinsonism or an innocent bystander? Exp Neurol. 2009;217:1–3. doi: 10.1016/j.expneurol.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores G, Liang JJ, Sierra A, Martínez-Fong D, Quirion R, Aceves J, Srivastava LK. Expression of dopamine receptors in the subthalamic nucleus of the rat: characterization using reverse transcriptase-polymerase chain reaction and autoradiography. Neuroscience. 1999;91:549–56. doi: 10.1016/s0306-4522(98)00633-2. [DOI] [PubMed] [Google Scholar]
- François C, Savy C, Jan C, Tande D, Hirsch EC, Yelnik J. Dopaminergic innervation of the subthalamic nucleus in the normal state, in MPTP-treated monkeys, and in Parkinson’s disease patients. J Comp Neurol. 2000;425:121–129. doi: 10.1002/1096-9861(20000911)425:1<121::aid-cne10>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Galvan A, Wichmann T. Pathophysiology of parkinsonism. Clin Neuropysiol. 2008;119:1459–1474. doi: 10.1016/j.clinph.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos AP, DeLong MR, Crutcher MD. Relations between parameters of step-tracking movements and single cell discharge in the globus pallidus and subthalamic nucleus of the behaving monkey. J Neurosci. 1983;3:1586–1598. doi: 10.1523/JNEUROSCI.03-08-01586.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz T, Arslan A, Wisden W, Wulff P. GABA(A) receptors: structure and function in the basal ganglia. Prog Brain Res. 2007;160:21–41. doi: 10.1016/S0079-6123(06)60003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, Berendse HW. Connections of the subthalamic nucleus with ventral striatopallidal parts of the basal ganglia in the rat. J Comp Neurol. 1990;294:607–22. doi: 10.1002/cne.902940408. [DOI] [PubMed] [Google Scholar]
- Hallworth NE, Wilson CJ, Bevan MD. Apamin-sensitive small conductance calcium-activated potassium channels, through their selective coupling to voltage-gated calcium channels, are critical determinants of the precision, pace, and pattern of action potential generation in rat subthalamic nucleus. J Neurosci. 2003;23:7525–7542. doi: 10.1523/JNEUROSCI.23-20-07525.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallworth NE, Bevan MD. Globus pallidus neurons dynamically regulate the activity pattern of subthalamic nucleus neurons through the frequency-dependent activation of postsynaptic GABAA and GABAB receptors. J Neurosci. 2005;25:6304–6315. doi: 10.1523/JNEUROSCI.0450-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond C, Yelnik J. Intracellular labelling of rat subthalamic neurones with horseradish peroxidase: computer analysis of dendrites and characterization of axon arborization. Neuroscience. 1983;8:781–90. doi: 10.1016/0306-4522(83)90009-x. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Elder CM, Okun MS, Patrick SK, Vitek JL. Stimulation of the subthalamic nucleus changes the firing pattern of pallidal neurons. J Neurosci. 2003;23:1916–23. doi: 10.1523/JNEUROSCI.23-05-01916.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassani OK, Féger J. Effects of intrasubthalamic injection of dopamine receptor agonists on subthalamic neurons in normal and 6-hydroxydopamine-lesioned rats: an electrophysiological and c-Fos study. Neuroscience. 1999;92:533–543. doi: 10.1016/s0306-4522(98)00765-9. [DOI] [PubMed] [Google Scholar]
- Hassani OK, François C, Yelnik J, Féger J. Evidence for a dopaminergic innervation of the subthalamic nucleus in the rat. Brain Res. 1997;749:88–94. doi: 10.1016/s0006-8993(96)01167-5. [DOI] [PubMed] [Google Scholar]
- Hernández A, Ibáñez-Sandoval O, Sierra A, Valdiosera R, Tapia D, Anaya V, Galarraga E, Bargas J, Aceves J. Control of the subthalamic innervation of the rat globus pallidus by D2/3 and D4 dopamine receptors. J Neurophysiol. 2006;96:2877–2888. doi: 10.1152/jn.00664.2006. [DOI] [PubMed] [Google Scholar]
- Holgado AJ, Terry JR, Bogacz R. Conditions for the generation of beta oscillations in the subthalamic nucleus-globus pallidus network. J Neurosci. 2010;30:12340–52. doi: 10.1523/JNEUROSCI.0817-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollerman JR, Grace AA. Subthalamic nucleus cell firing in the 6-OHDA-treated rat: basal activity and response to haloperidol. Brain Res. 1992;590:291–299. doi: 10.1016/0006-8993(92)91108-q. [DOI] [PubMed] [Google Scholar]
- Ibañez-Sandoval O, Hernández A, Florán B, Galarraga E, Tapia D, Valdiosera R, Erlij D, Aceves J, Bargas J. Control of the subthalamic innervation of substantia nigra pars reticulata by D1 and D2 dopamine receptors. J Neurophysiol. 2006;95:1800–1811. doi: 10.1152/jn.01074.2005. [DOI] [PubMed] [Google Scholar]
- Isoda M, Hikosaka O. Role for subthalamic nucleus neurons in switching from automatic to controlled eye movement. J Neurosci. 2008;28:7218–7209. doi: 10.1523/JNEUROSCI.0487-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jesús Aceves J, Rueda-Orozco PE, Hernández R, Plata V, Ibañez-Sandoval O, Galarraga E, Bargas J. Dopaminergic presynaptic modulation of nigral afferents: its role in the generation of recurrent bursting in substantia nigra pars reticulata neurons. Front Syst Neurosci. 2011;5:6. doi: 10.3389/fnsys.2011.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaka C, Ohno K, Okabe A, Kuriyama K, Itoh T, Fukuda A, Sato K. The differential expression patterns of messenger RNAs encoding K-Cl cotransporters (KCC1,2) and Na-K-2Cl cotransporter (NKCC1) in the rat nervous system. Neuroscience. 2001;104:933–946. doi: 10.1016/s0306-4522(01)00149-x. [DOI] [PubMed] [Google Scholar]
- Karachi C, Yelnik J, Tandé D, Tremblay L, Hirsch EC, François C. The pallidosubthalamic projection: an anatomical substrate for nonmotor functions of the subthalamic nucleus in primates. Mov Disord. 2005;20:172–180. doi: 10.1002/mds.20302. [DOI] [PubMed] [Google Scholar]
- Kass JI, Mintz IM. Silent plateau potentials, rhythmic bursts, and pacemaker firing: Three patterns of activity that coexist in quadristable subthalamic neurons. PNAS. 2005;103:183–188. doi: 10.1073/pnas.0506781102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita H, Kitai ST. The morphology of globus pallidus projection neurons in the rat: an intracellular staining study. Brain Res. 1994;636:308–319. doi: 10.1016/0006-8993(94)91030-8. [DOI] [PubMed] [Google Scholar]
- Kita H, Tachibana Y, Nambu A, Chiken S. Balance of monosynaptic excitatory and disynaptic inhibitory responses of the globus pallidus induced after stimulation of the subthalamic nucleus in the monkey. J Neurosci. 2005;23:8611–8619. doi: 10.1523/JNEUROSCI.1719-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitai ST, Deniau JM. Cortical inputs to the subthalamus: intracellular analysis. Brain Res. 1981;214:411–415. doi: 10.1016/0006-8993(81)91204-x. [DOI] [PubMed] [Google Scholar]
- Kitai ST, Kita H. Anatomy and physiology of the subthalamic nucleus: a driving force of the basal ganglia. In: Carpenter MB, Jayaraman A, editors. The Basal Ganglia II. Plenum; 1987. pp. 357–373. [Google Scholar]
- Kolomiets BP, Deniau JM, Mailly P, Ménétrey A, Glowinski J, Thierry AM. Segregation and convergence of information flow through cortico-subthalamic pathways. J Neurosci. 2001;21:5764–5772. doi: 10.1523/JNEUROSCI.21-15-05764.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loucif AJ, Woodhall GL, Sehirli US, Stanford IM. Depolarisation and suppression of burst firing activity in the mouse subthalamic nucleus by dopamine D1/D5 receptor activation of a cyclic-nucleotide gated non-specific cation conductance. Neuropharmacology. 2008;55:94–105. doi: 10.1016/j.neuropharm.2008.04.025. [DOI] [PubMed] [Google Scholar]
- Magill PJ, Bolam JP, Bevan MD. Dopamine regulates the impact of the cerebral cortex on the subthalamic nucleus-globus pallidus network. Neuroscience. 2001;106:313–30. doi: 10.1016/s0306-4522(01)00281-0. [DOI] [PubMed] [Google Scholar]
- Magill PJ, Sharott A, Bevan MD, Brown P, Bolam JP. Synchronous unit activity and local field potentials evoked in the subthalamic nucleus by cortical stimulation. J Neurophysiol. 2004;92:700–714. doi: 10.1152/jn.00134.2004. [DOI] [PubMed] [Google Scholar]
- Mallet N, Pogosyan A, Márton LF, Bolam JP, Brown P, Magill PJ. Parkinsonian beta oscillations in the external globus pallidus and their relationship with subthalamic nucleus activity. J Neurosci. 2008a;28:14245–14258. doi: 10.1523/JNEUROSCI.4199-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet N, Pogosyan A, Sharott A, Csicsvari J, Bolam JP, Brown P, Magill PJ. Disrupted dopamine transmission and the emergence of exaggerated beta oscillations in subthalamic nucleus and cerebral cortex. J Neurosci. 2008b;28:4795–4806. doi: 10.1523/JNEUROSCI.0123-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura M, Kojima J, Gardiner TW, Hikosaka O. Visual and oculomotor functions of monkey subthalamic nucleus. J Neurophysiol. 1992;67:1615–1632. doi: 10.1152/jn.1992.67.6.1615. [DOI] [PubMed] [Google Scholar]
- Maurice N, Deniau J-M, Glowinski J, Thierry A-M. Relationships between the prefrontal cortex and the basal ganglia in the rat: Physiology of the corticosubthalamic circuits. J Neurosci. 1998;18:9539–9546. doi: 10.1523/JNEUROSCI.18-22-09539.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouroux M, Hassani OK, Féger J. Electrophysiological study of the excitatory parafascicular projection to the subthalamic nucleus and evidence for ipsi- and contralateral controls. Neuroscience. 1995;67:399–407. doi: 10.1016/0306-4522(95)00032-e. [DOI] [PubMed] [Google Scholar]
- Mintz I, Hammond C, Féger J. Excitatory effect of iontophoretically applied dopamine on identified neurons of the rat subthalamic nucleus. Brain Res. 1986;375:172–5. doi: 10.1016/0006-8993(86)90971-6. [DOI] [PubMed] [Google Scholar]
- Nakanishi H, Kita H, Kitai ST. Electrical membrane properties of rat subthalamic neurons in an in vitro slice preparation. Brain Res. 1987;437:35–44. doi: 10.1016/0006-8993(87)91524-1. [DOI] [PubMed] [Google Scholar]
- Nakanishi H, Kita H, Kitai ST. An n-methyl-d-aspartate receptor medicated excitatory postsynaptic potential evoked in subthalamic neurons in an in vitro slice preparation of the rat. Neurosci Lett. 1988;95:130–136. doi: 10.1016/0304-3940(88)90645-3. [DOI] [PubMed] [Google Scholar]
- Nambu A. A new dynamic model of the cortico-basal ganglia loop. Prog Brain Res. 2004;143:461–466. doi: 10.1016/S0079-6123(03)43043-4. [DOI] [PubMed] [Google Scholar]
- Nambu A, Takada M, Inase M, Tokuno H. Dual somatotopical representations in the primate subthalamic nucleus: Evidence for ordered but reversed body-map transformations from the primary motor cortex and the supplementary motor area. J Neurosci. 1996;16:2671–2683. doi: 10.1523/JNEUROSCI.16-08-02671.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambu A, Tokuno H, Hamada I, Kita H, Imanishi M, Akazawa T, Ikeuchi Y, Hasegawa N. Excitatory cortical inputs to pallidal neurons via the subthalamic nucleus in the monkey. J Neurophysiol. 2000;84:289–300. doi: 10.1152/jn.2000.84.1.289. [DOI] [PubMed] [Google Scholar]
- Ng CH, Wang XS, Ong WY. A light and electron microscopic study of the GABA transporter GAT-3 in the monkey basal ganglia and brainstem. J Neurocytol. 2000;29:595–603. doi: 10.1023/a:1011076219493. [DOI] [PubMed] [Google Scholar]
- Ni Z, Bouali-Benazzouz R, Gao D, Benabid AL, Benazzouz A. Intrasubthalamic injection of 6-hydroxydopamine induces changes in the firing rate and pattern of subthalamic nucleus neurons in the rat. Synapse. 2001a;40:145–53. doi: 10.1002/syn.1036. [DOI] [PubMed] [Google Scholar]
- Ni ZG, Bouali-Benazzouz R, Gao DM, Benabid AL, Benazzouz A. Time-course of changes in firing rates and firing patterns of subthalamic nucleus neuronal activity after 6-OHDA-induced dopamine depletion in rats. Brain Res. 2001b;899:142–7. doi: 10.1016/s0006-8993(01)02219-3. [DOI] [PubMed] [Google Scholar]
- Otsuka T, Murakami F, Song WJ. Excitatory postsynaptic potentials trigger a plateau potential in rat subthalamic neurons at hyperpolarized states. J Neurophysiol. 2001;86:1816–25. doi: 10.1152/jn.2001.86.4.1816. [DOI] [PubMed] [Google Scholar]
- Overton PG, Greenfield SA. Determinants of neuronal firing pattern in the guinea-pig subthalamic nucleus: an in vivo and in vitro comparison. J Neural Transm. 1995;10:41–54. doi: 10.1007/BF02256628. [DOI] [PubMed] [Google Scholar]
- Paz JT, Deniau JM, Charpier S. Rhythmic bursting in the cortico-subthalamo-pallidal network during spontaneous genetically determined spike and wave discharges. J Neurosci. 2005;25:2092–101. doi: 10.1523/JNEUROSCI.4689-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plenz D, Kitai ST. A basal ganglia pacemaker formed by the subthalamic nucleus and external globus pallidus. Nature. 1999;400:677–682. doi: 10.1038/23281. [DOI] [PubMed] [Google Scholar]
- Prensa L, Cossette M, Parent A. Dopaminergic innervation of human basal ganglia. J Chem Neuroanat. 2000;20:207–213. doi: 10.1016/s0891-0618(00)00099-5. [DOI] [PubMed] [Google Scholar]
- Ramanathan S, Tkatch T, Atherton JF, Wilson CJ, Bevan MD. D2-like dopamine receptors modulate SKCa channel function in subthalamic nucleus neurons through inhibition of Cav2.2 channels. J Neurophysiol. 2008;99:442–459. doi: 10.1152/jn.00998.2007. [DOI] [PubMed] [Google Scholar]
- Ryan LJ, Sanders DJ, Clark KB. Auto- and cross-correlation analysis of subthalamic nucleus neuronal activity in neostriatal- and globus pallidal-lesioned rats. Brain Res. 1991;583:253–261. doi: 10.1016/s0006-8993(10)80031-9. [DOI] [PubMed] [Google Scholar]
- Ryan LJ, Clark KB. Alteration of neuronal responses in the subthalamic nucleus following globus pallidus and neostriatal lesions in rats. Brain Res Bulletin. 1992;29:319–327. doi: 10.1016/0361-9230(92)90063-4. [DOI] [PubMed] [Google Scholar]
- Sato F, Lavallée P, Lévesque M, Parent A. Single-axon tracing study of neurons of the external segment of the globus pallidus in primate. J Comp Neurol. 2000;417:17–31. [PubMed] [Google Scholar]
- Shink E, Bevan MD, Bolam JP, Smith Y. The subthalamic nucleus and the external pallidum: two tightly interconnected structures that control the output of the basal ganglia in the monkey. Neuroscience. 1996;73:335–357. doi: 10.1016/0306-4522(96)00022-x. [DOI] [PubMed] [Google Scholar]
- Shen KZ, Johnson SW. Presynaptic dopamine D2 and muscarine M3 receptors inhibit excitatory and inhibitory transmission to rat subthalamic neurones in vitro. J Physiol. 2000;525:331–341. doi: 10.1111/j.1469-7793.2000.00331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen KZ, Johnson SW. Presynaptic GABA(B) receptors inhibit synaptic inputs to rat subthalamic neurons. Neuroscience. 2001;108:431–6. doi: 10.1016/s0306-4522(01)00424-9. [DOI] [PubMed] [Google Scholar]
- Smith ID, Grace AA. Role of the subthalamic nucleus in the regulation of nigral dopamine neuron activity. Synapse. 1992;12:287–303. doi: 10.1002/syn.890120406. [DOI] [PubMed] [Google Scholar]
- Smith Y, Bolam JP, Von Krosigk M. Topographical and Synaptic Organization of the GABA-Containing Pallidosubthalamic Projection in the Rat. Eur J Neurosci. 1990;2:500–511. doi: 10.1111/j.1460-9568.1990.tb00441.x. [DOI] [PubMed] [Google Scholar]
- Song W-J, Baba Y, Otsuka T, Murakami F. Characterization of Ca2+ channels in rat subthalamic neurons. J Neurophysiol. 2000;84:2630–2637. doi: 10.1152/jn.2000.84.5.2630. [DOI] [PubMed] [Google Scholar]
- Starr PA, Kang GA, Heath S, Shimamoto S, Turner RS. Pallidal neuronal discharge in Huntington’s disease: support for selective loss of striatal cells originating the indirect pathway. Exp Neurol. 2008;211:227–33. doi: 10.1016/j.expneurol.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steigerwald F, Pötter M, Herzog J, Pinsker M, Kopper F, Medhorn H, Deuschl G, Volkmann J. Neuronal activity in the human subthalamic nucleus in the parkinsonian and nonparkinsonian state. J Neurophysiol. 2008;100:2515–2524. doi: 10.1152/jn.90574.2008. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Mercer JN, Chang CS. Autonomous pacemakers in the basal ganglia: who needs excitatory synapses anyway? Curr Opin Neurobiol. 2005;15:312–318. doi: 10.1016/j.conb.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Takada M, Tokuno H, Hamada I, Inase M, Ito Y, Imanishi M, Hasegawa N, Akazawa T, Hatanaka N, Nambu A. Organization of inputs from cingulate motor areas to basal ganglia in macaque monkey. Eur J Neurosci. 2001;14:1633–1650. doi: 10.1046/j.0953-816x.2001.01789.x. [DOI] [PubMed] [Google Scholar]
- Tang JK, Moro E, Lozano AM, Lang AE, Hutchison WD, Mahant N, Dostrovsky JO. Firing rates of pallidal neurons are similar in Huntington’s and Parkinson’s disease patients. Exp Brain Res. 166:230–236. doi: 10.1007/s00221-005-2359-x. [DOI] [PubMed] [Google Scholar]
- Teagerden M, Atherton JF, Bevan MD, Wilson CJ. Accumulation of cytoplasmic calcium, but not apamin-sensitive afterhyperpolarization current, during high frequency firing in rat subthalamic neurons. J Physiol. 2008;586:817–833. doi: 10.1113/jphysiol.2007.141929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terman D, Rubin JE, Yew AC, Wilson CJ. Activity patterns in a model for the subthalamopallidal network of the basal ganglia. J Neurosci. 2002;22:2963–2976. doi: 10.1523/JNEUROSCI.22-07-02963.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tofighy A, Abbott A, Centonze D, Cooper AJ, Noor E, Pearce SM, Puntis M, Stanford IM, Wigmore MA, Lacey MG. Excitation by dopamine of rat subthalamic nucleus neurones in vitro-a direct action with unconventional pharmacology. Neuroscience. 2003;116:157–166. doi: 10.1016/s0306-4522(02)00546-8. [DOI] [PubMed] [Google Scholar]
- Walter BL, Vitek JL. Surgical treatment for Parkinson’s disease. Lancet Neurol. 2004;3:719–28. doi: 10.1016/S1474-4422(04)00934-2. [DOI] [PubMed] [Google Scholar]
- Wang XS, Ong WY. A light and electron microscopic study of GAT-1 in the monkey basal ganglia. J Neurocytol. 1999;28:1053–1061. doi: 10.1023/a:1007056608820. [DOI] [PubMed] [Google Scholar]
- Weinberger M, Mahant N, Hutchison WD, Lozano AM, Moro E, Hodaie M, Lang AE, Dostrovsky JO. Beta oscillatory activity in the subthalamic nucleus and its relation to dopaminergic response in Parkinson’s disease. J Neurophysiol. 2006;96:3248–56. doi: 10.1152/jn.00697.2006. [DOI] [PubMed] [Google Scholar]
- Weinberger M, Hutchison WD, Dostrovsky JO. Pathological subthalamic nucleus oscillations in PD: can they be the cause of bradykinesia and akinesia. Exp Neurol. 2009;219:58–61. doi: 10.1016/j.expneurol.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Wichmann T, Bergman H, DeLong MR. The primate subthalamic nucleus. I Functional properties. J Neurophysiol. 1994;72:494–506. doi: 10.1152/jn.1994.72.2.494. [DOI] [PubMed] [Google Scholar]
- Wichmann T, DeLong MR. Functional and pathophysiological models of the basal ganglia. Cur Opinion in Neurobiol. 1996;6:751–758. doi: 10.1016/s0959-4388(96)80024-9. [DOI] [PubMed] [Google Scholar]
- Wichmann T, DeLong MR. Basal ganglia discharge abnormalities in Parkinson’s disease. J Neural Transm Suppl. 2006:21–25. doi: 10.1007/978-3-211-45295-0_5. [DOI] [PubMed] [Google Scholar]
- Wichmann T, Soares J. Neuronal firing before and after burst discharges in the monkey basal ganglia is predictably patterned in the normal state and altered in parkinsonism. J Neurophysiol. 2006;95:2120–33. doi: 10.1152/jn.01013.2005. [DOI] [PubMed] [Google Scholar]
- Wigmore MA, Lacey MG. A Kv3-like persistent, outwardly rectifying, CS+-permeable, K+ current in rat subthalamic neurones. J Physiol. 2000;527:493–506. doi: 10.1111/j.1469-7793.2000.t01-1-00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CJ, Weyrick A, Terman D, Hallworth NE, Bevan MD. A model of reverse spike frequency adaptation and repetitive firing of subthalamic nucleus neurons. J Neurophysiol. 2004;91:1963–1980. doi: 10.1152/jn.00924.2003. [DOI] [PubMed] [Google Scholar]
- Wilson CL, Cash D, Galley K, Chapman H, Lacey MG, Stanford IM. Subthalamic nucleus neurones in slices from 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mice show irregular, dopamine-reversible firing pattern changes, but without synchronous activity. Neuroscience. 2006;143:565–572. doi: 10.1016/j.neuroscience.2006.07.051. [DOI] [PubMed] [Google Scholar]
- Zaidel A, Arkadir D, Israel Z, Bergman H. Akineto-rigid vs. tremor syndromes in Parkinsonism. Curr Opin Neurol. 2009;22:387–93. doi: 10.1097/WCO.0b013e32832d9d67. [DOI] [PubMed] [Google Scholar]
- Zhu Z-T, Munhall A, Shen K-Z, Johnson SW. Calcium dependent-subthreshold oscillations determine bursting activity induced by N-methyl-D-aspartate in rat subthalamic neurons in vitro. Eur J Neurosci. 2004;19:1296–1304. doi: 10.1111/j.1460-9568.2004.03240.x. [DOI] [PubMed] [Google Scholar]
- Zhu ZT, Shen KZ, Johnson SW. Pharmacological identification of inward current evoked by dopamine in rat subthalamic neurons in vitro. Neuropharmacology. 2002a;42:772–781. doi: 10.1016/s0028-3908(02)00035-7. [DOI] [PubMed] [Google Scholar]
- Zhu Z, Bartol M, Shen K, Johnson SW. Excitatory effects of dopamine on subthalamic nucleus neurons: in vitro study of rats pretreated with 6-hydroxydopamine and levodopa. Brain Res. 2002b;945:31–40. doi: 10.1016/s0006-8993(02)02543-x. [DOI] [PubMed] [Google Scholar]