Abstract
Angiogenesis is thoroughly balanced and regulated in health; however, it is dysregulated in many diseases including cancer, age-related macular degeneration, cardiovascular diseases such as coronary and peripheral artery diseases and stroke, abnormal embryonic development, and abnormal wound healing. In addition to angiogenesis, lymphangiogenesis is pivotal for maintaining the immune system, homeostasis of body fluids and lymphoid organs; dysregulated lymphangiogenesis may cause inflammatory diseases and lymph node mediated tumor metastasis. Anti-angiogenic or anti-lymphangiogenic small peptides may play an important role as therapeutic agents normalizing angiogenesis or lymphangiogenesis in disease conditions. Several novel endogenous peptides derived from proteins containing a conserved somatotropin domain have been previously identified with the help of our bioinformatics-based methodology. These somatotropin peptides were screened for inhibition of angiogenesis and lymphangiogenesis using in vitro proliferation, migration, adhesion and tube formation assays with blood and lymphatic endothelial cells. We found that the peptides have the potential for inhibiting both angiogenesis and lymphangiogenesis. Focusing the study on the inhibition of lymphangiogenesis, we found that a peptide derived from the somatotropin conserved domain of transmembrane protein 45A human was the most potent lymphangiogenesis inhibitor, blocking lymphatic endothelial cell migration, adhesion, and tube formation.
Keywords: Lymphatic endothelial cell, Blood endothelial cell, Endogenous somatotropin peptides, Transmembrane protein 45A human
1. Introduction
Angiogenesis is the process of new blood vessel formation from pre-existing blood vasculature (Folkman and Klagsbrun, 1987). Angiogenesis is an important process occurring in both health and disease. Appropriate balance between angiogenic stimulators and inhibitors is fundamental for regulating and maintaining angiogenesis in health. Disturbed homeostasis in angiogenesis is associated with many diseases including cancer, age-related macular degeneration (AMD), diabetes, rheumatoid arthritis, psoriasis and cardiovascular diseases such as coronary and peripheral artery diseases and stroke (Carmeliet and Jain, 2011).
Lymphangiogenesis, the process of new lymphatic vessel formation from pre-existing lymphatics, is important for functioning of the immune system and lymphoid organs, tissue fluid homeostasis and absorption of dietary fats (Stacker et al., 2002). Dysregulated lymphangiogenesis can result in pathological conditions such as lymphedema, abnormal fat metabolism, hypertension, inflammatory diseases and lymph node mediated tumor metastasis (Tammela and Alitalo, 2010; Norrmén et al., 2011).
A number of therapeutic angiogenesis inhibitors have been developed. These include FDA approved monoclonal antibodies bevacizumab and ranibizumab targeting vascular endothelial growth factor (VEGF), small molecule tyrosine kinase inhibitors involved in angiogenesis-related signal transduction (erlotinib, sunitinib, sorafenib, pazopanib), and mammalian target of rapamycin (mTOR) inhibitors (temsirolimus and everolimus) (Li et al., 2008). Many peptide angiogenesis inhibitors are in preclinical development or clinical trials (Rosca et al., 2011).
In contrast, there are relatively few effective inhibitors of lymphangiogenesis compared to those of angiogenesis. This is because molecular studies in lymphatic biology have only been conducted since the late 1990s after lymphatic endothelial cell (EC) markers including vascular endothelial growth factor receptor 3 (VEGFR-3) (Kaipainen et al., 1995), lymphatic vessel endothelial hyaluronan receptor 1 (LYVE-1) (Banerji et al., 1999), prospero homeobox protein 1 (Prox-1) (Wigle and Oliver, 1999), neuropilin 2 (NRP-2) (Yuan et al., 2002) and podoplanin (Schacht et al., 2003) were identified. VEGFC/D (Joukov et al., 1996; Achen et al., 1998), VEGFR3 (He et al., 2005), cyclooxygenase 2 (COX-2) (Timoshenko et al., 2006), chemokine receptors CCR7 (Forster et al., 1999) and matrix metalloproteinase 2/9 (MMP-2/9) (Daniele et al., 2010) have been proposed as potential molecular targets for regulating lymphangiogenesis. Large proteins or antibodies including a VEGF-D neutralizing antibody (Roberts et al., 2006), a soluble VEGFR-3 fusion protein (a VEGF-C/D trap) (Lin et al., 2005) and a neuropilin-2 antibody (Caunt et al., 2008) were reported to inhibit lymphangiogenesis in vitro and in vivo. However no FDA approved anti-lymphangiogenic agent has yet been developed and to our knowledge no anti-lymphangiogenic peptide agents have been identified.
Here we investigate anti-lymphangiogenic and anti-angiogenic activity of novel endogenous 14-mer somatotropin domain-derived peptides; to our knowledge, these are the first short peptide agents with anti-lymphangiogenic activity exhibiting a potency of inhibiting lymphatic endothelial cell (LEC) proliferation, migration, adhesion and tube formation. Using bioinformatics, our laboratory has previously identified over 100 anti-angiogenic peptides derived from conserved domains of several classes of proteins: type IV collagen, CXC chemokines, thrombospondin 1 (TSP1) repeat-containing proteins, somatotropins and serpins (Karagiannis and Popel, 2008). The basis for this analysis was homology to known anti-angiogenic protein fragments that allowed us to identify several anti-angiogenic motifs in the human proteome.
Among these novel peptides, we tested 14-mer peptides derived from the somatotropin conserved domain on lymphatic and blood endothelial cells in vitro. The tested peptides are derived from the endogenous proteins interleukin 17 (IL-17) receptor C (the peptide denoted SP5001, its sequence is RLRLLTLQSWLL), brush border myosin-1 (SP5028, LMRKSQILISSWF), neuropeptide FF receptor 2 (SP5029, LLIVALLFILSWL), chorionic somatomammotropin (SP5030, LLRLLLLIESWLE), transmembrane protein 45A (SP5031, LLRSSLILLQGSWF), chorionic somatomammotropin-like 1 (SP5032, LLHISLLLIESRLE) and placental lactogen (SP5033, LLRISLLLIESWLE). We performed proliferation, migration, adhesion and tube formation assay on lymphatic and blood endothelial cells to identify anti-lymphangiogenic and anti-angiogenic peptides. Lymphangiogenesis or angiogenesis involves multiple steps including lymphatic or blood endothelial cell attachment and adhesion to the extracellular matrix, cell migration, cell proliferation, tube formation as well as matrix remodeling. When a peptide inhibits any one or more of these steps, it can serve as a prototype for anti-lymphangiogenic or anti-angiogenic drug development.
2. Materials and Methods
2.1. Peptide synthesis and handling
The peptides were produced by a commercial manufacturer (Bachem, Torrance, CA) using a solid-phase synthesis technique (Table 1). HPLC and MS analyses of each peptide were provided by the manufacturer to demonstrate greater than 95% purity. The peptides were stored at −80°C and thawed at room temperature just before use. For preparation of peptide stock solutions, dimethyl sulfoxide (DMSO) was used as a solvent at a maximum concentration of 5 or 10% (vol/vol) in PBS due to their hydrophobic profile. We verified the final concentration of DMSO was less than 0.6% when the peptide stock solutions were more diluted in media for in vitro experiments. Experimental groups including controls and peptide treated groups were controlled as they contained same amount of DMSO to eliminate solvent effects.
Table 1.
The amino acid sequences of the tested somatotropin peptides.
| Peptide name | Peptide origin | Accession # | Peptide sequence |
|---|---|---|---|
| SP5001 | IL-17 receptor C | NP703191 (376–387) | RLRLLTLQSWLL |
| SP5028 | Brush border myosin-1 | AAD31189 (719–731) | LMRKSQILISSWF |
| SP5029 | Neuropeptide FF receptor 2 | AAG41398 (276–288) | LLIVALLFILSWL |
| SP5030 | Chorionic somatomammotropin | AAA52116 (101–113) | LLRLLLLIESWLE |
| SP5031 | Transmembrane protein 45A | NP060474 (181–194) | LLRSSLILLQGSWF |
| SP5032 | Chorionic somatomammotropin-like 1 | Q14406 (83–96) | LLHISLLLIESRLE |
| SP5033 | Placental lactogen | AAA98621 (101–114) | LLRISLLLIESWLE |
2.2. Cell culture
Human umbilical vein endothelial cells (HUVEC), microvascular endothelial cells (MEC) and lymphatic endothelial cells (LEC) were purchased from Lonza (Walkersville, MD). HUVEC were grown and maintained according to the vendor’s recommendation using Endothelial Basal Media (EBM-2) supplemented with the Bullet Kit (EGM-2) from Lonza. The MEC and LEC were propagated in Microvascular Endothelial Cell Growth Medium-2 (EGM-2MV, Lonza). Breast cancer cells, MDA-MB-231 were supplied by Dr. Zaver Bhujwalla (JHMI, Radiology and Oncology). MDA-MB-231 were propagated in RPMI-1640 medium (Gibco, Carlsbad, CA) supplemented with 10% FBS and antibiotics (1% penicillin/streptomycin). Cells were maintained under standard conditions of 37°C and 5% CO2 and the passage numbers of all cells were between 3 and 9.
2.3. Specific staining for LEC marker
Western blotting and an immunohistochemistry assay using antibodies against the lymphendothelium specific markers podoplanin and LYVE-1 were performed on LEC and HUVEC to confirm the lineage of LEC. HUVEC were used as controls. In the western blotting assay cell extracts were prepared from HUVEC and LEC by incubation of cells in cell lysis buffer (150mM NaCl, 1mM EDTA, 100 μL/mL Protease Inhibitors (Sigma), 10 μL/mL Phosphatase inhibitors (Sigma) and 0.1% Triton) for 2 h. Extracts were centrifuged (14,000 g for 15 min) and protein concentration was assessed by Bradford Assay (BioRad, CA). The extracts were separated by gel electrophoresis and transferred to nitrocellulose membrane for antibody staining. A typical immunohistochemistry protocol was followed for staining using the LEC specific anti-podoplanin antibody D2-40 (Covance, IN).
In the immunohistochemistry assay each well of an 8-well plate was coated with 0.1% gelatin solution and incubated at room temperature for 2 h. 35,000 HUVEC or LEC were seeded and the plate was incubated at 37°C in a tissue culture incubator for 20 h to allow cells to adhere. Media was removed and 100 μL of 1:4 dilution of BD Cytofix fixation buffer (BD Bioscience, CA) was added and incubated at room temperature for 15 min. The fixation buffer was removed and 100 μL of binding media (EBM-2, 1mM 1,10-phenanthroline, 200 μg/mL bacitracin, 0.5 μg/ml leupeptin and 3% BSA) was added for 1 h to prevent non-specific binding. LYVE-1 antibody (Fitzgerald) (4 μg/ml) was added and the plate was incubated at 4°C for 20 h. After rinsing three times with 200 μL/well washing buffer (0.1 L 10xTris buffer + 0.9 L distilled water + 10 mL 10% tween per 1L buffer), anti-mouse IgG Fab 2 labeled with Alexa fluor 488 molecular probes (1:1000 dilution, Cell Signaling) and 5 μg/ml of 4′,6-diamidino-2-phenylindole (DAPI, Roche, IN) were added and the plate was incubated at room temperature for 1 h. After 3 rinses with 200 μL/well washing buffer (for 10 min each time), 200 μL of PBS with Ca++/Mg++ was added and the cells were imaged using a Nikon microscope (Nikon Instruments Inc.).
2.4. Proliferation assay
Proliferation assays in the presence or absence of the peptides were performed using the WST-1 reagent (Roche). Pale yellow colored tetrazolium salt WST-1 can be reduced by mitochondrial dehydrogenases and transformed into dark yellow colored Formazan. In detail, HUVEC was seeded in 96-well plates at a density of 2,000 cells/well in 100 μL of EGM-2 media (100 μL of EGM-2MV was used in MEC and LEC proliferation). The plates were incubated at 37°C in a tissue culture incubator for 20 h to allow cells to adhere. The prepared peptide solutions were added and the plates were incubated for 72 h. Media was removed and 100 μL of WST-1 solution (1:10 dilution of WST-1 in serum free EBM media) was added. After incubation for 4 h, the absorbance at 450 nm was measured using a Victor V (Perkin Elmer, Waltham, MA). IC50 values of each peptide were calculated by using GraphPAD Prism 5 (GraphPad Software Inc.).
2.5. Cytotoxicity and apoptosis assay
Peptide cytotoxicity was determined using CytoTox-ONE Homogeneous Membrane Integrity assay (Promega, WI). This assay is for detecting the lactate dehydrogenase (LDH) released from cells with damaged membranes. HUVEC, MEC and LEC were plated at 5,000 cells per well in 96-well plates and the plates were incubated overnight to allow cells to adhere. Then media were replaced with normal media with or without peptide. After 72 h the CytoTox-ONE substrate (100 μL/well) was added. After incubating cells for 10 min at room temperature the stop solution was added to each well and fluorescence was measured with a Victor V plate reader (Perkin Elmer, MA). Serum-free media (EBM, Lonza), which can be cytotoxic to cells was used as a control.
Apoptosis was detected by using a Caspase-Glo 3/7 apoptosis detection assay kit (Promega, WI). HUVEC, MEC and LEC were plated at 10,000 cells per well in opaque 96-well plates and incubated overnight to allow cells to adhere. Then the media was replaced with complete media with or without peptide. After 72 h the Caspase-Glo chemiluminescent substrate (100 μL/well) was added and the plates were incubating 1.5 h at room temperature. Luminescence was determined with a Victor V plate reader (Perkin Elmer, MA). Serum-free media (EBM, Lonza) was used as a control.
2.6. Migration assay
HUVEC, MEC, LEC and MDA-MB-231 migration was assessed using the ACEA Real-Time Cell Analysis (RTCA) system (ACEA Biosciences Inc.) and the Cell Invasion and Migration plates 16 (CIM-plates-16) from Roche Diagnostics (Mannheim, Germany). The ACEA RTCA system measures cell electrical impedance expressed as cell index in real time. The cell index indicates the extent of cell migration from the top compartment of a two-chamber system to the bottom compartment in response to chemoattractant factors. Previously CIM-plates have been used for real-time analysis of cell migration. (Daouti et al., 2008; Greiner et al., 2011; Ungefroren et al., 2011). In detail, the membrane of the top chamber of a CIM-plate was coated with fibronectin by adding 40 μL of 20 μg/mL fibronectin in PBS solution and incubating at 37°C for 30 min. 180 μL of EGM-2 (complete media for HUVEC) or EGM-2MV (complete media for MEC and LEC) or EBM (serum free media) was added to the bottom chambers. For MDA-MB-231 migration, 180 μL of RPMI-1640 media (Gibco) supplemented with 10% FBS and antibiotics (1% penicillin/streptomycin) was added to the bottom chamber well. The two chambers were assembled together, 30 μL of serum free media was added to the top chamber and the assembled plate was incubated in a tissue culture incubator at 37°C for at least 1 h. The equilibrated plate was removed from the incubator and 100 μL of the trypsinized cells (45,000 HUVEC/well; 30,000 MEC/well; 120,000 LEC/well; 200,000 MDA-MB-231 cells/well) were added to the top chamber. After 30 min incubation at room temperature, the stabilized chamber was loaded in the RTCA machine and the cell index was measured continuously for 20 h. Cell indices at 20 h were selected for analysis.
Inhibition of migration by one peptide (SP5031) was also investigated in a wound healing type assay to validate the migration data from the RTCA system. This assay was performed using the Oris™ Pro Cell Migration assay (Platypus Technologies, WI). Briefly, 25,000 cells/well in complete media were added to the 96-well plate containing stoppers to prevent the cells from settling in the center region of the wells. Cells were allowed to adhere for 4 h, after which the stoppers were removed. Fully supplemented media with or without peptide was added to the wells to allow migration to the previously blocked area in the center of the wells. After 18 h cells were stained with Calcein AM (0.5 μg/ml) (Invitrogen, CA) in PBS supplemented with Ca2+ and Mg2+ and the cells that migrated to the center of the well were quantified by reading fluorescence at 485/530 nm on a Victor V plate reader (Perkin Elmer, MA) and also imaged using a Nikon microscope (Eclipse T-100); images were acquired with the CCD Sensicam mounted on a Nikon microscope (Cooke Company, MI). The detection of only the cells that migrated into the previously restricted region is made possible by using a detection mask at the bottom of the plate which obstructs the other parts of the well.
2.7. LEC adhesion assay
ACEA E-plates (Roche Diagnostics) were used to measure the extent of LEC adhesion in the RTCA system. Cell impedance is expressed as cell index which indicates the degree of cell adhesion to the electrodes. Recently the ACEA E-plates have been used for real-time analysis of cell adhesion (Salo et al., 2009; Foldynova-Trantirkova et al., 2010; Ohkawa et al., 2010). In detail, 100 μL of 2X concentrated peptide solutions were added to the appropriate wells of an E-plate. LEC (25,000 cells/well) in 100 μL of EGM-2MV media were added next to each well diluting the peptides to the appropriate final concentrations. After equilibrating at room temperature for 30 min, the E-plate was loaded into the RTCA personal system. Cell indices at 1 h were analyzed.
For the validation of adhesion data from the RTCA system we performed a conventional cell adhesion assay. LEC were labeled with Cell Tracker Green (Invitrogen, CA) according to the manufacturer’s protocol. Briefly, cells were incubated with complete media containing 10μM Cell Tracker Green for 30 min under normal growth conditions. Labeling media was replaced with complete growth media and cells were allowed to incubate at 37°C with 5% CO2 for an additional 30 minutes to complete the labeling process. Following labeling, cells were plated at a density of 10,000 cells/well in a black 96-well plate with a clear bottom. While cells were labeled the well surface was incubated with peptide at concentrations matching the future cell incubation to keep the peptide on the well surface from depletion after PBS washing. Cells were added to wells containing peptide in media or media with matching concentration of DMSO (< 0.2%) and incubated for 2 h. Cells were washed three times with PBS after which the fluorescence intensity was measured using the Victor V plate reader. Images of one randomly selected view per well were acquired using CCD Sensicam mounted on the Nikon microscope.
2.8. LEC capillary-like tube formation assay
A LEC capillary-like tube formation assay was performed to determine the effect of the somatotropin peptides on lymphangiogenesis. Basement membrane extracellular matrix (Matrigel, BME, BD Bioscience) was thawed at 4°C overnight. A 96-well plate and 200 μL pipette tips were also kept at 4°C overnight and both the plate and tips were placed on ice during the entire experiment. 50 μL of Matrigel was loaded in each well of the 96-well plate and the plate was incubated at 37°C in a tissue culture incubator for 30 min to allow the matrix to polymerize. Trypsinized LEC were mixed with the peptide making the appropriate cell density (15,000 cells/well) and peptide concentrations (100, 50, 25μM). 100 μL of cell and peptide mixture was added on top of the gel in the 96-well plate. The plate was then incubated at 37°C in a tissue culture incubator and the formation of the capillary-like tubes was observed after 0, 6, 12 and 24 h. At 24 h, the wells were imaged using a Nikon microscope. Quantification of tube formation was assisted by S.CORE, a web based image analysis system (S.CO BioLifescience). Tube formation indices represent the degree of tube formation. The indices were calculated using the equation below. The values of the variables used in the equation were obtained automatically by S.CORE.
2.9. Statistical analysis
Error bars correspond to SEM, unless otherwise stated. Differences between a control and a peptide treated group are regarded as significant when P is less than 0.05 using the Student’s t test.
3. Results
3.1. D2-40, the podoplanin antibody and the LYVE-1 antibody positively identify LEC
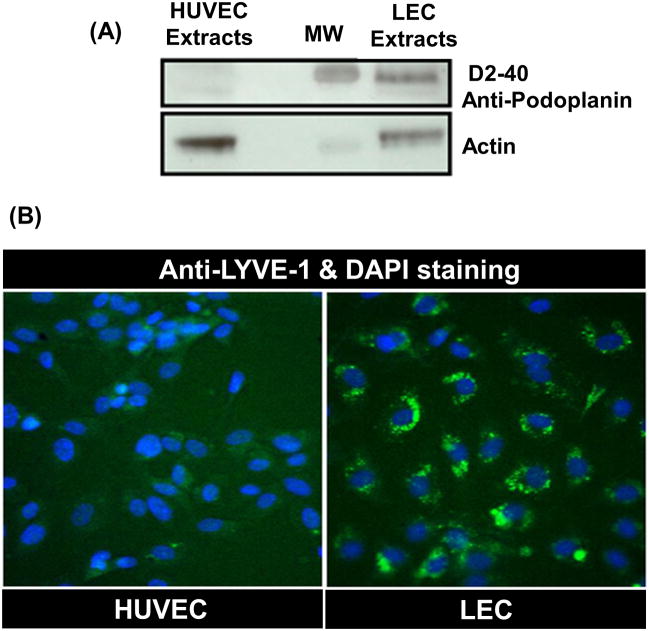
Western blotting and an immunohistochemistry assay were carried out on LEC and HUVEC. The western blotting result showed the absence of the specific band for podoplanin, the LEC marker in HUVEC extracts. However, the specific band was present in high amounts in the LEC extracts (Fig. 1A). An immunohistochemistry assay showed that the LYVE-1 was only expressed in LEC and not in HUVEC (Fig. 1B).
Fig. 1. Specific staining for LEC markers.
(A) Western blotting with D2-40, podoplanin antibody on HUVEC and LEC. Lane 1 demonstrates the absence of the specific band for D2-40 LEC marker in HUVEC extracts while the band is strongly visible in the LEC extracts (Lane 4).
(B) An immunohistochemistry assay with LYVE-1 antibody on HUVEC and LEC. The immunohistochemistry assay shows that LEC is positive for anti-LYVE-1 LEC marker (green colored), however HUVEC is not. A merged image with DAPI staining (blue colored) shows that LYVE-1 receptor proteins (green colored) are expressed in LEC membranes specifically not in HUVEC membranes.
3.2. Somatotropin peptides have anti-proliferative effects on both lymphatic and blood endothelial cells
To investigate the anti-proliferative activity of somatotropin peptides a WST-1 based proliferation assay was performed on HUVEC, MEC and LEC. Neuropeptide FF receptor 2 (SP5029), chorionic somatomammotropin (SP5030), chorionic somatomammotropin-like 1 (SP5032) and placental lactogen (SP5033) were potent inhibitors of HUVEC proliferation exhibiting IC50 values below 50μM. A peptide from brush border myosin-1 (SP5028) inhibited the proliferation of MEC with IC50 below 30μM. Finally in the LEC proliferation assay two peptides, one derived from the IL-17 receptor C (SP5001) and the other derived from the placental lactogen (SP5033) were potent with IC50 values below 60μM (Table 2).
Table 2. IC50 values in proliferation assay with somatotropin peptides.
Somatotropin peptides were tested against HUVEC, MEC and LEC using WST-1 cell proliferation reagent.
| Peptide Name | IC50 (μM ± 95% Cl)
|
||
|---|---|---|---|
| HUVEC | MEC | LEC | |
| SP5001 | 50.7 ± 31.8 | 52.4 ± 27.8 | 27.3 ± 19.6 |
| SP5028 | 58.2 ± 35.2 | 27.7 ± 17.4 | more than 200 |
| SP5029 | 34.9 ± 15.4 | more than 200 | more than 200 |
| SP5030 | 17.7 ± 11.2 | 93.2 ± 42.3 | more than 200 |
| SP5031 | 96.9 ± 34.3 | 83.4 ± 21.9 | 157.6 ± 122.2 |
| SP5032 | 36.5 ± 25.1 | 80.1 ± 47.9 | Not converged |
| SP5033 | 14.7 ± 8.1 | 81.1 ± 37.2 | 59.8 ± 27.0 |
3.3. Somatotropin peptides exhibit cytotoxic and apoptotic activities
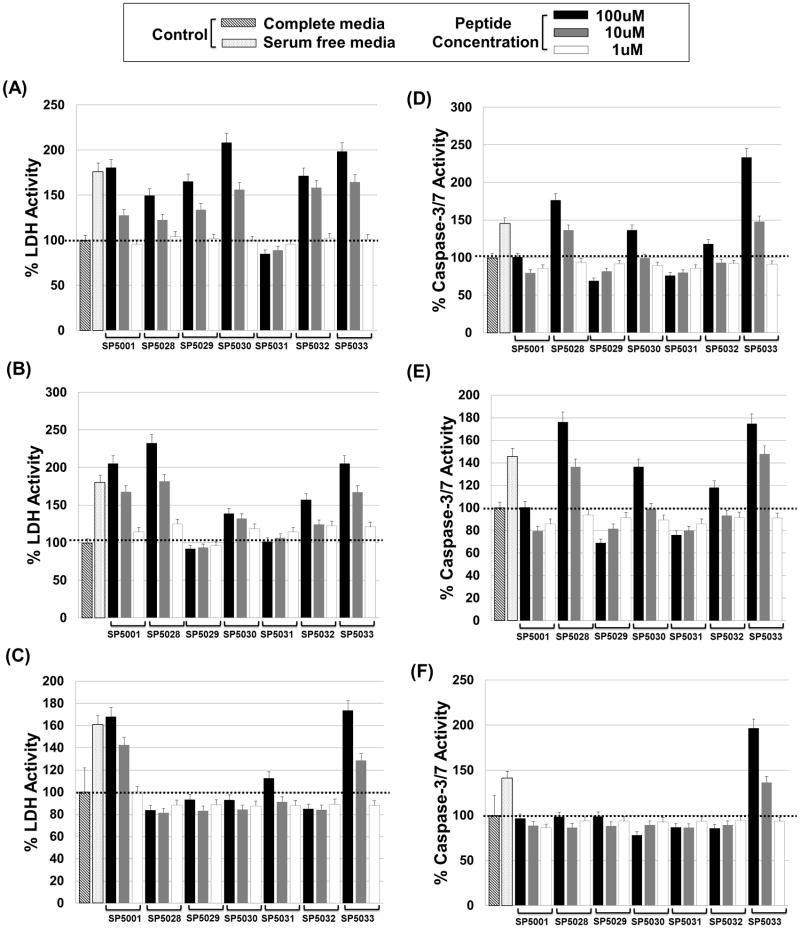
Cytotoxic effects of the peptides on HUVEC, MEC and LEC were determined by measuring the lactate dehydrogenase (LDH) released from damaged cell membranes with CytoTox-ONE Homogeneous Membrane Integrity assay kit (Promega, WI). The normal level of LDH released from cells incubated in complete media for 72 h was set as 100%. The amount of LDH released from cells incubated in serum free media after the same time was more than 150% of the amount seen when cells were grown in complete media presumably because of the cytotoxic stress from starvation. A concentration of 100μM of all the peptides tested in cells grown in complete media except SP5031 showed more than 150% LDH activity compared to the control when HUVEC were tested. Among them SP5030, SP5032 and SP5033 had significant cytotoxic activity at 10μM (Fig. 2A). 100μM of SP5001, SP5028 and SP5033 exhibited more than 200% LDH activity compared to the control on MEC. Even at 10μM these three peptides showed more than 150% LDH level (Fig. 2B). However only SP5001 and SP5033 showed significant LDH activity compared to the control on LEC (Fig. 2C).
Fig. 2. The cytotoxic and apoptotic activity of the peptides.
The cytotoxic effect of the peptides was determined by using CytoTox-ONE Homogeneous Membrane Integrity assay kit detecting lactate dehydrogenase (LDH) released from damaged cells. Peptide induced apoptotic activity was detected by using a Caspase-Glo 3/7 apoptosis detection assay kit. HUVEC, MEC and LEC were incubated at 37°C in complete media with or without peptides for 72 h before these two assays. The LDH or caspase-3/7 signals in the normal condition with only complete media were defined as negative control with 100% LDH or caspase-3/7 activity (black dotted lines). Starved condition with serum free media or peptide treatment induced more LDH or caspase-3/7 activity. (A) Percent LDH activity after treating HUVEC with somatotropin peptides. (B) Percent LDH activity on MEC. (C) Percent LDH activity on LEC. (D) Percent caspase-3/7 activity after treating HUVEC with somatotropin peptides. (E) Percent caspase-3/7 activity on MEC. (F) Percent caspase-3/7 activity on LEC.
Peptide mediated apoptosis of HUVEC, MEC and LEC were tested with a Caspase-3/7 Glo apoptosis detection reagent (Promega, WI). Apoptosis assays are in general more specific than the LDH cytotoxicity assay. 100μM of SP5028, SP5030 and SP5033 induced significant caspase-3/7 activity compared to the control on HUVEC (Fig. 2D). SP5028, SP5030, SP5032 and SP5033 were active against MEC in the apoptosis assay (Fig. 2E). Finally only SP5033 induced significant apoptosis on LEC (Fig. 2F).
3.4. Somatotropin peptides inhibit migration of lymphatic and blood endothelial cells
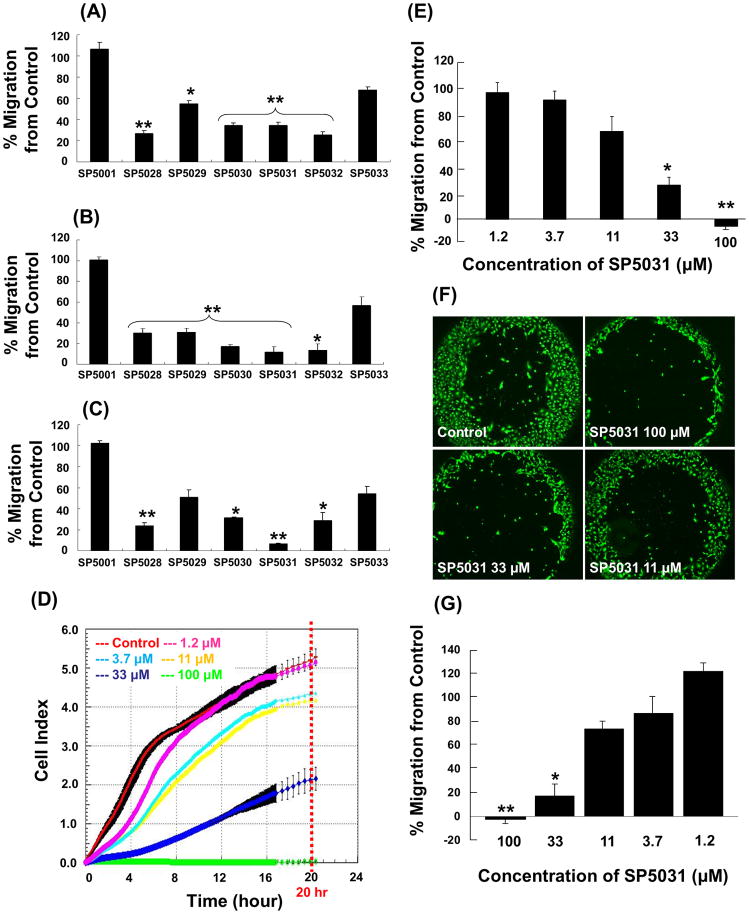
Real time cell analysis (RTCA) system and ACEA CIM-plates (Roche diagnostics) were used to measure the potency of inhibition of migration of HUVEC, MEC and LEC by the various peptides. 50μM of almost all the peptides showed statistically significant migration inhibitory effects on HUVEC, MEC and LEC (Fig. 3). Four peptides: SP5028, SP5030, SP5031 and SP5032 inhibited the migration of HUVEC by more than 65% from control (% inhibition of 73.8%, 65.3%, 65.5% and 74.6%, respectively) (Fig. 3A). All peptides tested except SP5001 and SP5033, dramatically inhibited the migration of MEC (Fig. 3B). Three peptides: SP5028, SP5030 and SP5032 strongly inhibited the migration of LEC (76.1%, 68.7% and 71.3%, respectively). The 14-mer peptide from the transmembrane protein 45A human (SP5031) was the most potent inhibitor of LEC migration (more than 93.6%) (Fig. 3C). Real-time LEC migration was determined with RTCA system for 20 h with different concentrations of SP5031. The IC50 for inhibition of LEC migration by SP5031 was 25.1μM at 20 h after migration (Fig. 3D, E). The wound healing cell migration assay was performed on LEC with SP5031 to validate the data from RTCA system. The results showed a dose response similar to the impedance based migration data from the RTCA system (Fig. 3F, G). SP5031 also showed statistically significant migration inhibition of MDA-MB-231, a breast cancer cell line. Specifically 25, 50 and 100μM SP5031 inhibited MDA-MB-231 cell migration by 46.9%, 83.2% and 97.7% respectively (Fig. 6).
Fig. 3. Migration inhibitory activity of the somatotropin peptides.
Somatotropin peptides were tested against HUVEC, MEC and LEC for their anti-migratory activity. The peptide concentration was 50μM and cell indices at 20 h were used for data analysis. (A) Percent HUVEC migration. * P < 0.05 versus control and ** P < 0.03 versus control. (B) Percent MEC migration. * P < 0.05 versus control, ** P < 0.03 versus control. (C) Percent LEC migration. * P < 0.05 versus control and ** P < 0.02 versus control. (D) Real-time migration data with SP5031 (0, 1.2, 3.7, 11, 33 and 100μM) on LEC. (E) Percent LEC migration with SP5031 after 20 h of migration using RTCA migration assay. * P < 0.05 versus control and ** P < 0.01 versus control. (F) The image of the wound healing LEC migration assay with SP5031. (G) The quantified percent LEC migration with SP5031 using the wound healing cell migration assay. * P < 0.05 versus control and ** P < 0.02 versus control.
Fig. 6. MDA-MB-231 migration with SP5031.
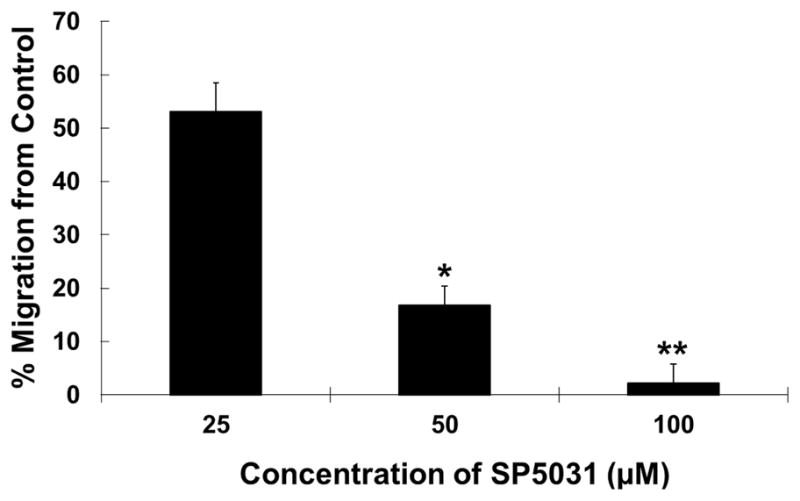
Migration of MDA-MB-231 in the presence of the SP5031 peptide. The peptide concentrations were 25, 50, 100μM and cell indices at 20 h were used for data analysis. * P < 0.03 versus control and ** P < 0.01 versus control.
3.5. Somatotropin peptides block LEC adhesion
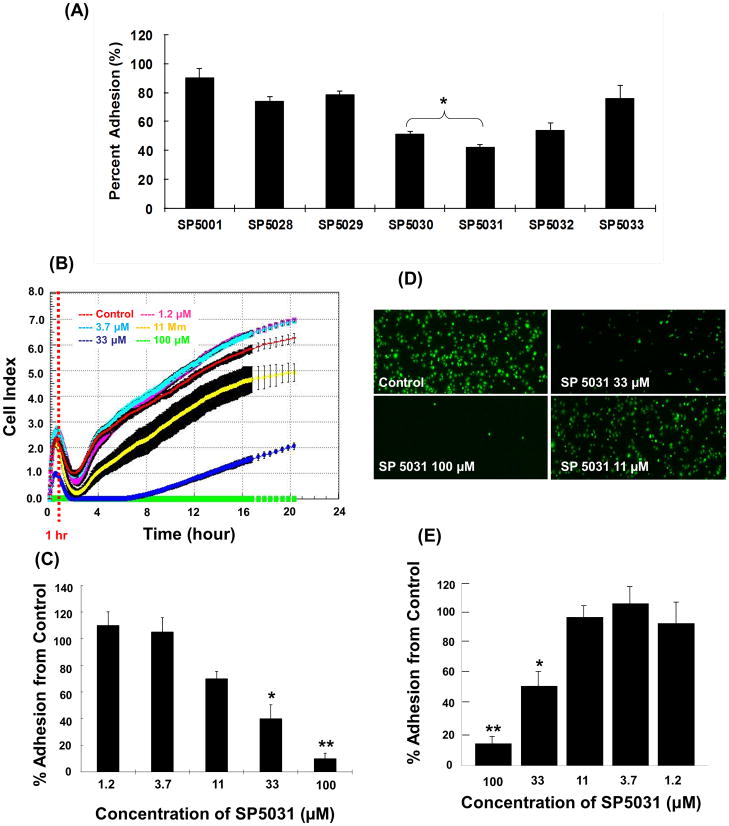
The ACEA E-plates (Roche) were used to evaluate LEC adhesion with or without peptides. All peptides were screened at a concentration of 50μM and the most potent peptide was subsequently tested at concentrations of 100, 33, 11, 3.7, 1.2μM to obtain an IC50 value. SP5001, SP5028 and SP5029 inhibited the LEC adhesion only slightly (19.5%, 18.4% and 21.8%, respectively). However, SP5030, SP5031 and SP5032 showed strong inhibition of LEC adhesion inhibition (49%, 58% and 46%, respectively) (Fig. 4A). Among them the peptide derived from the transmembrane protein 45A human (SP5031) inhibited LEC adhesion most potently with an IC50 value of 24.2μM (Fig. 4B, C). The conventional cell adhesion assay was carried out on LEC with SP5031 peptide to validate the LEC adhesion data from RTCA system. The result showed that SP5031 blocked LEC adhesion in a dose-dependent manner (Fig. 4D, E). However the conventional cell adhesion method was less sensitive than the RTCA system at the low concentrations (below 33μM) of SP5031. These experiments demonstrate that the results from the E-plate based RTCA adhesion assay are reliable and that it may be a more sensitive methodology to measure cell adhesion than the conventional adhesion assay.
Fig. 4. Adhesion inhibitory activity of the somatotropin peptides on LEC.
(A) LEC adhesion in the presence of 50μM of somatotropin peptides at 1 h using the RTCA method. * P < 0.05 versus control. (B) Real-time LEC adhesion data in the presence of different concentrations of SP5031 (0, 1.2, 3.7, 11, 33, 100μM). (C) LEC adhesion with SP5031 after 1 h of adhesion using RTCA. * P < 0.05 versus control and ** P < 0.02 versus control. (D) The image of LEC adhesion with different concentrations of SP5031. (E) The quantified percent LEC adhesion. * P < 0.05 versus control and ** P < 0.02 versus control.
3.6. Somatotropin peptides inhibit LEC tube formation
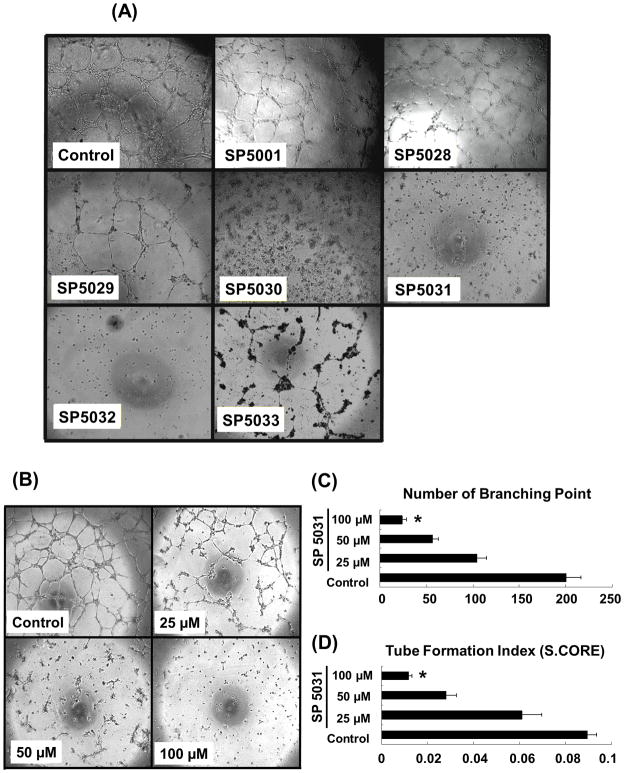
Non-reduced matrigel with EGM-2MV complete media was used in the LEC capillary-like tube formation assay because it promotes robust tube formation allowing us to test the inhibitory effects of the peptides. All peptides were tested at 100μM to show their anti-tube formation activities. SP5030, SP5031 and SP5032 showed pronounced activities so that tube formation was completely blocked (Fig. 5A). Three selected candidates (SP5030, 5031, 5032) were tested at lower concentrations of 50μM and 25μM to obtain the minimum concentration for complete tube inhibition. The peptide from transmembrane protein 45A (SP5031) blocked LEC tube formation most effectively showing a dose response (Fig. 5B). The tube formation index was decreased by 89%, 67% and 22% by 100, 50 and 25μM of the SP5031 respectively.
Fig. 5. Capillary-like tube formation assay with somatotropin peptides on LEC.
(A) LEC tube formation in the presence of 100μM of the peptides at 24 h. (B) LEC tube formation in the presence of different concentrations (0, 25, 50, 100μM) of SP5031 at 24 h. (C) Number of branching point. * P < 0.01 versus control. (D) Tube formation index (S.CORE). * P < 0.01 versus control. Quantification of tube formation was assisted by S.CORE web based image analysis system.
4. Discussion
Small peptides are emerging and promising agents in developing new therapeutics for different diseases (Saladin et al., 2009; Rosca et al., 2011). Peptide agents have significant merits compared to conventional proteins and large synthetic molecules. The advantages of peptides as drugs over proteins are high specificity, low immunogenicity and toxicity, better solubility in water and stable product quality between batches (Sulochana and Ge, 2007). One disadvantage is short half-life in vivo, which could be overcome by peptide modification, conjugation to a macromolecule, or combination with a delivery vehicle (Rosca et al., 2011; Bhise et al., 2011; Grdisa, 2011).
Previously several anti-angiogenic peptides were identified, derived from endogenous proteins such as thrombospondin-1, collagen, laminin, decorin, platelet factor-4, kininostatin, and VEGFR. Among them a number of anti-angiogenic peptides are in clinical trials (Sulochana and Ge, 2007; Rosca et al., 2011). Although many trials have been conducted for anti-angiogenic peptides, peptide agents for inhibiting lymphangiogenesis have not been reported. In this study we investigated the anti-lymphangiogenic effects of small somatotropin peptides. The peptides from IL-17 receptor C (SP5001) and placental lactogen (SP5033) potently inhibited LEC proliferation (Table 2). Four peptides derived from brush border myosin-1 (SP5028), chorionic somatomammotropin (SP5030), transmembrane protein 45A (SP5031) and chorionic somatomammotropin-like 1 (SP5032) strongly inhibited LEC migration and adhesion (Fig. 3, 4). SP5030, SP5031 and SP5032 were strong inhibitors of the LEC capillary-like tube formation. Among them SP5031 was the most active LEC tube formation inhibitor (Fig. 5).
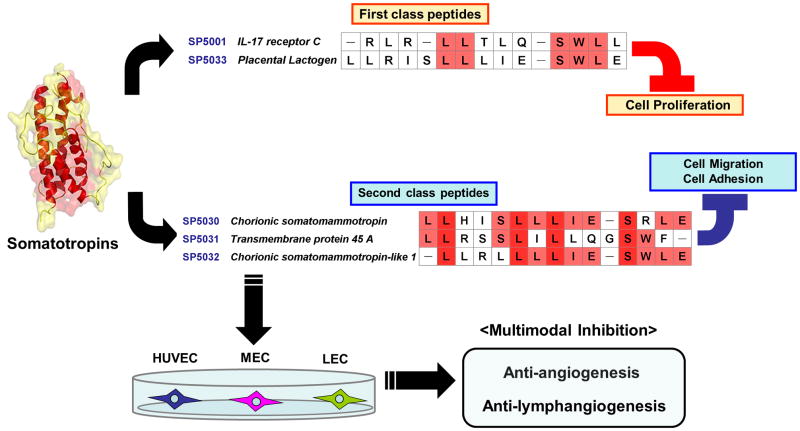
In the present study we could identify two classes of lymphangiogenesis inhibitors. The first class which includes SP5001 and SP5033 inhibit lymphangiogenesis by inhibiting proliferation of lymphatic endothelial cells and the second class which includes SP5030, SP5031 and SP5032 inhibit lymphangiogenesis by mainly inhibiting lymphatic endothelial cell migration and adhesion (Fig. 7). SP5033 showed significant apoptotic activity on HUVEC, LEC and MEC exhibiting caspase-3/7 activity (Fig. 2D, E and F). Interestingly the amino acid sequence of SP5033 (LLRISLLLIESWLE) was very similar to the previously studied 14-mer tilted peptide (LLRISLLLIQSWLE) from 16-kDa fragments of prolactin (PRL) (Nguyen et al., 2006) with a difference only in the 10th amino acid. The glutamic acid (Glu, E) in SP5033 is replaced with the glutamine (Gln, Q) in the tilted PRL peptide. It has been reported that the tilted PRL peptide led to cell apoptosis (Nguyen et al., 2006). Also it was shown that PRL protein induced apoptosis in a prostate cancer model (Giuffrida et al., 2010). These results with prolactin (PRL) peptide and protein suggest that the PRL protein has active domains which may include the sequence of SP5033 exhibiting apoptotic activity. Also the apoptotic property of SP5033 may be associated with its structure which may be conserved regardless of the 10th amino acid replacement. However SP5001, the other peptide in this class, was not associated with caspase-3/7 activity even though it showed potent proliferation inhibitory effect as seen by increased LDH levels (Fig. 2). This suggests that SP5001 has anti-proliferative activity through other pathways such as arresting the cell cycle or other cytotoxic pathways that are different from the caspase-3/7 dependent apoptosis pathway. The second class peptides comprising SP5030, SP5031 and SP5032 inhibited LEC migration and adhesion and were potent lymphatic tube formation inhibitors compared to the first class of peptides (Fig. 3, 4 and 5). These two classes of peptides allow one to manipulate endothelial cell proliferation and migration separately via independent pathways. The receptors and signaling pathways targeted by the two classes of peptides resulting in the control of cell proliferation and migration remain to be identified.
Fig. 7. Classification of somatotropin peptides.
Two classes of somatotropin peptides were determined. The first class of peptides, SP5001 and SP5033, mainly inhibit the proliferation of both lymphatic and blood endothelial cells. The second class of peptides including SP5030, SP5031 and SP5032, potently inhibit migration and adhesion of lymphatic and blood endothelial cells. Their sequences are shown and the conserved amino acids within each class are highlighted. Dark red colored amino acids represent common residues of all these peptides in the two classes.
We have identified several anti-lymphangiogenic sequences derived from various proteins including IL-17 receptor C, brush border myosin-1, neuropeptide FF receptor 2, transmembrane protein 45A, which are not associated with angiogenesis or lymphangiogenesis. These finding may contribute to the understanding of the poorly understood physiological roles of these proteins. For example our present study of SP5001, the peptide derived from IL-17 receptor C protein, could lead us to hypothesize that there might be lymphangiogenesis-related physiological roles of the IL-17 receptor C protein which is known as a receptor for IL-17A and F. Recently it was reported that a ligand IL-17 promotes the expression of VEGFC in non-small-cell lung carcinomas inducing lymphangiogenesis and inflammation (Chen et al., 2010). We had not expected this correlation between IL-17 and lymphangiogenesis before we identified SP5001. We hypothesize that IL-17 receptor C might work as an endogenous scavenger of IL-17 and the active site for anti-lymphangiogenic and anti-inflammatory activity could be closely related to our peptide sequence; further studies are required to validate this hypothesis.
In this study we identified that SP5031 is the best inhibitor of lymphangiogenesis by blocking LEC migration, adhesion and tube formation potently. Surprisingly we found that SP5031 significantly inhibited the migration of the highly metastatic MDA-MB-231 triple-negative breast cancer cells as well. SP5031 blocked the migration of MDA-MB-231 induced by a full complement of media which includes 10% FBS by 83% at a concentration of 50μM (Fig. 6). These dual inhibition results suggest that SP5031 may have both anti-tumorigenic and anti-lymphangiogenic activity.
There are three important consequences of inhibiting migration of MDA-MB-231 cells in addition to blocking lymphangiogenesis. The first is that SP5031 could delay the transport of tumor cells into the lymph nodes by decreasing motility of the cancer cells. Previous data suggest that the lymph node metastasis is greatly facilitated by migration of tumor cells into the lymph nodes via chemotactic agents (Shields et al., 2007). Secondly, inhibition of MDA-MB-231 migration by SP5031 suggests that SP5031 may have direct anti-tumorigenic activity. Cancer cell migration is cooperative with tumor microenvironment for further tumorigenesis (Hanahan and Weinberg, 2011). Thus the inhibition of cancer cell migration can be a phenotype of anti-tumorigenesis. Finally anti-lymphangiogenic activity of SP5031 will decrease the density of lymphatic vessels around the tumor by inhibiting new lymphatic vessel formation, which could help to limit tumor metastasis. Additional studies are needed to characterize anti-tumor and anti-metastatic activity of SP5031 in vivo in different cancer models.
When it comes to anti-angiogenic efficacy of the peptides, we have identified three leading peptides: peptides derived from brush border myosin-1 (SP5028), chorionic somatomammotropin (SP5030) and chorionic somatomammotropin-like 1 (SP5032). Interestingly both SP5030 and SP5032 are derived from chorionic somatomammotropin related proteins. Their amino acid sequences are very similar: 10 of 13–14 amino acids are identical between the two sequences. These anti-angiogenic peptides could be applied to the treatment of different angiogenesis dependent diseases such as cancer, age-related macular degeneration (AMD), or rheumatoid arthritis.
Angiogenesis and lymphangiogenesis are both important processes in health and diseases. Somatotropin peptides are active in both inhibiting lymphangiogenesis and angiogenesis (Fig. 7). These properties are expected be useful for modulating those phenomena in diseases. In particular these peptides would be applied to prevent the deadly spread of cancer cells by inhibiting not only tumor growth but also peritumoral lymphatics. Alternatively, a single compound that targets both tumor-associated blood vessels and tumor-associated lymphatic vessels may decrease tumor size and decrease the incidence of local and distant metastases. Thus these peptides should be further tested in vivo for their simultaneous inhibition of tumor growth and metastasis and other angiogenesis-dependent diseases.
Highlights.
Novel somatotropin peptides were identified using a bioinformatics-based tool.
Somatotropin peptides inhibit both angiogenesis and lymphangiogenesis.
A peptide, transmembrane protein 45A, is a potent cell migration and adhesion inhibitor.
A peptide derived from interleukin 17 receptor C is a potent cell proliferation inhibitor.
The peptide derived from, transmembrane protein 45A, is the most potent lymphangiogenesis inhibitor.
Acknowledgments
This work was supported by the National Institutes of Health grants R21 CA131931 and R01 CA138264.
Abbreviations
- LEC
lymphatic endothelial cell
- MEC
microvascular endothelial cell
- HUVEC
human umbilical vein endothelial cell
- CCR7
chemokine receptor 7
- VEGF
vascular endothelial growth factor
- NRP-2
neuropilin-2
- LYVE-1
lymphatic vessel endothelial hyaluronan receptor 1
- Prox-1
prospero homeobox protein 1
- COX-2
cyclooxygenase 2
- MMP-2/9
matrix metalloproteinase 2/9
- m-TOR
mammalian target of rapamycin
- TSP1
thrombospondin 1
- IL-17
interleukin 17
- PRL/GH
human prolactin/growth hormone
- AMD
age-related macular degeneration
- DAPI
4′,6-diamidino-2-phenylindole
- LDH
lactate dehydrogenase
- RTCA
real time cell analysis
- CIM-plates
cell invasion and migration plates
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achen MG, Jeltsch M, Kukk E, Makinen T, Vitali A, Wilks AF, et al. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4) Proc Natl Acad Sci U S A. 1998;95:548–53. doi: 10.1073/pnas.95.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerji S, Ni J, Wang SX, Clasper S, Su J, Tammi R, et al. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol. 1999;144:789–801. doi: 10.1083/jcb.144.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhise NS, Shmueli RB, Sunshine JC, Tzeng SY, Green JJ. Drug delivery strategies for therapeutic angiogenesis and antiangiogenesis. Expert Opin Drug Deliv. 2011;8:485–504. doi: 10.1517/17425247.2011.558082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caunt M, Mak J, Liang WC, Stawicki S, Pan Q, Tong RK, et al. Blocking neuropilin-2 function inhibits tumor cell metastasis. Cancer Cell. 2008;13:331–42. doi: 10.1016/j.ccr.2008.01.029. [DOI] [PubMed] [Google Scholar]
- Chen X, Xie Q, Cheng X, Diao X, Cheng Y, Liu J, et al. Role of interleukin-17 in lymphangiogenesis in non-small-cell lung cancer: Enhanced production of vascular endothelial growth factor C in non-small-cell lung carcinoma cells. Cancer Sci. 2010;101:2384–90. doi: 10.1111/j.1349-7006.2010.01684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniele A, Zito AF, Giannelli G, Divella R, Asselti M, Mazzocca A, et al. Expression of metalloproteinases MMP-2 and MMP-9 in sentinel lymph node and serum of patients with metastatic and non-metastatic breast cancer. Anticancer Res. 2010;30:3521–7. [PubMed] [Google Scholar]
- Daouti S, Li WH, Qian H, Huang KS, Holmgren J, Levin W, et al. A selective phosphatase of regenerating liver phosphatase inhibitor suppresses tumor cell anchorage-independent growth by a novel mechanism involving p130Cas cleavage. Cancer Res. 2008;68:1162–9. doi: 10.1158/0008-5472.CAN-07-2349. [DOI] [PubMed] [Google Scholar]
- Foldynova-Trantirkova S, Sekyrova P, Tmejova K, Brumovska E, Bernatik O, Blankenfeldt W, et al. Breast cancer-specific mutations in CK1epsilon inhibit Wnt/beta-catenin and activate the Wnt/Rac1/JNK and NFAT pathways to decrease cell adhesion and promote cell migration. Breast Cancer Res. 2010;12:R30. doi: 10.1186/bcr2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J, Klagsbrun M. Angiogenic factors. Science. 1987;235:442–7. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- Forster R, Schubel A, Breitfeld D, Kremmer E, Renner-Muller I, Wolf E, et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- Giuffrida D, Perdichizzi A, Giuffrida MC, La Vignera S, D’Agata R, Vicari E, et al. Does prolactin induce apoptosis? Evidences in a prostate cancer in vitro model. J Endocrinol Invest. 2010;33:313–7. doi: 10.1007/BF03346592. [DOI] [PubMed] [Google Scholar]
- Grdisa M. The delivery of biologically active (therapeutic) peptides and proteins into cells. Curr Med Chem. 2011;18:1373–9. doi: 10.2174/092986711795029591. [DOI] [PubMed] [Google Scholar]
- Greiner M, Kreutzer B, Jung V, Grobholz R, Hasenfus A, Stohr RF, et al. Silencing of the SEC62 gene inhibits migratory and invasive potential of various tumor cells. Int J Cancer. 2011;128:2284–95. doi: 10.1002/ijc.25580. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- He Y, Rajantie I, Pajusola K, Jeltsch M, Holopainen T, Yla-Herttuala S, et al. Vascular endothelial cell growth factor receptor 3-mediated activation of lymphatic endothelium is crucial for tumor cell entry and spread via lymphatic vessels. Cancer Res. 2005;65:4739–46. doi: 10.1158/0008-5472.CAN-04-4576. [DOI] [PubMed] [Google Scholar]
- Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E, et al. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J. 1996;15:290–8. [PMC free article] [PubMed] [Google Scholar]
- Kaipainen A, Korhonen J, Mustonen T, van Hinsbergh VW, Fang GH, Dumont D, et al. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci U S A. 1995;92:3566–70. doi: 10.1073/pnas.92.8.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagiannis ED, Popel AS. A systematic methodology for proteome-wide identification of peptides inhibiting the proliferation and migration of endothelial cells. Proc Natl Acad Sci U S A. 2008;105:13775–80. doi: 10.1073/pnas.0803241105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WW, Hutnik M, Gehr G. Antiangiogenesis in haematological malignancies. Br J Haematol. 2008;143:622–31. doi: 10.1111/j.1365-2141.2008.07372.x. [DOI] [PubMed] [Google Scholar]
- Li WW, Talcott KE, Zhai AW, Kruger EA, Li VW. The role of therapeutic angiogenesis in tissue repair and regeneration. Adv Skin Wound Care. 2005;18:491–500. doi: 10.1097/00129334-200511000-00013. [DOI] [PubMed] [Google Scholar]
- Lin J, Lalani AS, Harding TC, Gonzalez M, Wu WW, Luan B, et al. Inhibition of lymphogenous metastasis using adeno-associated virus-mediated gene transfer of a soluble VEGFR-3 decoy receptor. Cancer Res. 2005;65:6901–9. doi: 10.1158/0008-5472.CAN-05-0408. [DOI] [PubMed] [Google Scholar]
- Nguyen NQ, Tabruyn SP, Lins L, Lion M, Cornet AM, Lair F, et al. Prolactin/growth hormone-derived antiangiogenic peptides highlight a potential role of tilted peptides in angiogenesis. Proc Natl Acad Sci U S A. 2006;103:14319–24. doi: 10.1073/pnas.0606638103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrmén C, Tammela T, Petrova TV, Alitalo K. Biological basis of therapeutic lymphangiogenesis. Circulation. 2011;123:1335–51. doi: 10.1161/CIRCULATIONAHA.107.704098. [DOI] [PubMed] [Google Scholar]
- Ohkawa Y, Miyazaki S, Hamamura K, Kambe M, Miyata M, Tajima O, et al. Ganglioside GD3 enhances adhesion signals and augments malignant properties of melanoma cells by recruiting integrins to glycolipid-enriched microdomains. J Biol Chem. 2010;285:27213–23. doi: 10.1074/jbc.M109.087791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts N, Kloos B, Cassella M, Podgrabinska S, Persaud K, Wu Y, et al. Inhibition of VEGFR-3 activation with the antagonistic antibody more potently suppresses lymph node and distant metastases than inactivation of VEGFR-2. Cancer Res. 2006;66:2650–7. doi: 10.1158/0008-5472.CAN-05-1843. [DOI] [PubMed] [Google Scholar]
- Rosca EV, Koskimaki JE, Rivera CG, Pandey NB, Tamiz AP, Popel AS. Anti-angiogenic peptides for cancer therapeutics. Curr Pharm Biotechnol. 2011;12:1101–16. doi: 10.2174/138920111796117300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saladin PM, Zhang BD, Reichert JM. Current trends in the clinical development of peptide therapeutics. IDrugs. 2009;12:779–84. [PubMed] [Google Scholar]
- Salo S, Boutaud A, Hansen AJ, He L, Sun Y, Morales S, et al. Antibodies blocking adhesion and matrix binding domains of laminin-332 inhibit tumor growth and metastasis in vivo. Int J Cancer. 2009;125:1814–25. doi: 10.1002/ijc.24532. [DOI] [PubMed] [Google Scholar]
- Schacht V, Ramirez MI, Hong YK, Hirakawa S, Feng D, Harvey N. T1alpha/podoplanin deficiency disrupts normal lymphatic vasculature formation and causes lymphedema. EMBO J. 2003;22:3546–56. doi: 10.1093/emboj/cdg342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields JD, Fleury ME, Yong C, Tomei AA, Randolph GJ, Swartz MA. Autologous chemotaxis as a mechanism of tumor cell homing to lymphatics via interstitial flow and autocrine CCR7 signaling. Cancer Cell. 2007;11:526–38. doi: 10.1016/j.ccr.2007.04.020. [DOI] [PubMed] [Google Scholar]
- Stacker SA, Achen MG, Jussila L, Baldwin ME, Alitalo K. Lymphangiogenesis and cancer metastasis. Nat Rev Cancer. 2002;2:573–83. doi: 10.1038/nrc863. [DOI] [PubMed] [Google Scholar]
- Sulochana KN, Ge R. Developing antiangiogenic peptide drugs for angiogenesis-related diseases. Curr Pharm Des. 2007;13:2074–86. doi: 10.2174/138161207781039715. [DOI] [PubMed] [Google Scholar]
- Tammela T, Alitalo K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell. 2010;140:460–76. doi: 10.1016/j.cell.2010.01.045. [DOI] [PubMed] [Google Scholar]
- Timoshenko AV, Chakraborty C, Wagner GF, Lala PK. COX-2-mediated stimulation of the lymphangiogenic factor VEGF-C in human breast cancer. Br J Cancer. 2006;94:1154–63. doi: 10.1038/sj.bjc.6603067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungefroren H, Sebens S, Groth S, Gieseler F, Fandrich F. Differential roles of Src in transforming growth factor-ss regulation of growth arrest, epithelial-to-mesenchymal transition and cell migration in pancreatic ductal adenocarcinoma cells. Int J Oncol. 2011;38:797–805. doi: 10.3892/ijo.2011.897. [DOI] [PubMed] [Google Scholar]
- Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98:769–78. doi: 10.1016/s0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- Yuan L, Moyon D, Pardanaud L, Breant C, Karkkainen MJ, Alitalo K, et al. Abnormal lymphatic vessel development in neuropilin 2 mutant mice. Development. 2002;129:4797–806. doi: 10.1242/dev.129.20.4797. [DOI] [PubMed] [Google Scholar]