Abstract
Introduction
In men, idiopathic osteoporosis (IOP) is often associated with low serum insulin-like growth factor (IGF-1) and reduced bone formation. The characteristics of premenopausal women with IOP are not well defined. We aimed to define the clinical, reproductive, and biochemical characteristics of premenopausal women with unexplained osteoporosis.
Methods
This is a cross-sectional study of 64 women with unexplained osteoporosis, 45 with fragility fractures, 19 with low bone mineral density (BMD; Z-score less than or equal to −2.0) and 40 normal controls. The following are the main outcome measures: clinical and anthropometric characteristics, reproductive history, BMD, gonadal and calciotropic hormones, IGF-1, and bone turnover markers (BTMs).
Results
Subjects had lower BMI and BMD than controls, but serum and urinary calcium, serum estradiol, vitamin D metabolites, IGF-1, and most BTMs were similar. Serum parathyroid hormone (PTH) and the resorption marker, tartrate-resistant acid phosphatase (TRAP5b), were significantly higher in both groups of subjects than controls and directly associated in all groups. Serum IGF-1 and all BTMs were directly associated in controls, but the association was not significant after controlling for age. There was no relationship between serum IGF-1 and BTMs in subjects. There were few differences between women with fractures and low BMD.
Conclusions
Higher serum TRAP5b and PTH suggest that increased bone turnover, possibly related to subclinical secondary hyperparathyroidism could contribute to the pathogenesis of IOP. The absence of differences between women with fractures and those with very low BMD indicates that this distinction may not be clinically useful to categorize young women with osteoporosis.
Keywords: Bone turnover markers, IGF-1, Premenopausal osteoporosis
Introduction
Most young men and premenopausal women with osteoporosis have an underlying disorder or have been exposed to medications that interfered with acquisition of peak bone mass or caused excessive bone loss thereafter [1–13]. Osteoporosis, which affects young, otherwise healthy individuals with intact gonadal function and no secondary cause of bone loss is termed “idiopathic” osteoporosis (IOP) [14].
Although IOP affects both genders equally, most research on its etiology has focused on men. In male IOP, calciotropic hormones are usually normal and hypercalciuria is common [4, 7, 11, 12, 15, 16]. High sex hormone-binding globulin (SHBG) levels, low free estradiol (E2) and testosterone [17–22], and low serum insulin-like growth factor-1 (IGF-1) levels [20, 23–25] have been reported, as have direct correlations between both serum IGF-1 and E2 and histomorphometric parameters of bone formation [15, 20, 25]. Although increased turnover has been observed [26, 27], most studies report heterogeneous remodeling [7] or reduced bone formation [15, 16, 20, 25, 28], consistent with osteoblast dysfunction [29] or a defect in their recruitment to remodeling sites [6, 30].
The few small studies that have addressed IOP in premenopausal women have reported smaller stature, lower body mass index (BMI), and a family history of osteoporosis [9, 10, 13, 31]. In a small retrospective study of transiliac crest biopsies from women with IOP [32], we found histomorphometric evidence of increased bone resorption and decreased bone formation, suggesting osteoblast dysfunction similar to men with IOP. We also found lower follicular phase E2 and higher serum resorption markers in 13 women with IOP [31], similar to a recent report by Peris et al. [13]. However, in contrast to our findings in men with IOP [15], serum IGF-1 did not differ from controls and did not correlate with bone mineral density (BMD) [31]. Thus, the etiology and pathogenesis of IOP in premenopausal women remain unclear.
In prior studies of IOP in premenopausal women, the definition of osteoporosis varies, some requiring a history of low-trauma fracture [7, 32], others only low BMD [13, 31]. However, fracture risk in premenopausal women is very low compared to postmenopausal women [33, 34] and the relationship between BMD and fracture incidence in premenopausal women has not been clearly established. Thus, International Society for Clinical Densitometry guidelines recommend that the diagnosis of osteoporosis in young women should not be made solely on the basis of isolated low BMD measurements [35] without a history of fragility fracture or secondary cause of bone loss. Using high-resolution peripheral quantitative computed tomography (HR-pQCT), however, we recently reported that premenopausal women with idiopathic low BMD measurements have microarchitectural deterioration of the peripheral skeleton (distal radius and tibia) comparable to young women with unexplained fragility fractures [36, 37].
To address these issues, we conducted a cross-sectional, case–control study to define the clinical, reproductive, densitometric, and biochemical characteristics of women with a history of unexplained fragility fractures (IOP) and those with idiopathic low BMD (ILBMD). Based upon our previous findings, we hypothesized that affected women would have lower estrogen levels, higher bone resorption markers, and lower bone formation markers than normal controls, but that serum IGF-1 levels would not differ. We also hypothesized that women with fractures would differ clinically and biochemically from those with ILBMD.
Materials and methods
Patient population
Premenopausal women, aged 18–48, were recruited at Columbia University, New York, NY and Creighton University, Omaha, NE by advertisement, self- or physician referral. Subjects were included in the ILBMD group if they had low areal BMD (aBMD) by dual energy X-ray absorptiometry (DXA; T-score less than or equal to −2.5 or Z-score less than or equal to −2.0) at the spine or proximal femur. Subjects were included in the IOP group if they had a documented low-trauma fracture after age 18, whether or not they had low aBMD. Fractures were ascertained by review of radiographs or radiograph report and categorized as low trauma (equivalent to a fall from a standing height or less) after a case review by a physician panel (ES, AC, RRR, EMS). Skull and digit fractures were excluded. IOP subjects could not participate in the study until more than 3 months had elapsed after their most recent fracture. To qualify as normal controls, women were required to have normal aBMD by DXA (T-score greater than or equal to −1.0 or Z-score greater than or equal to −1.0) and no history of adult low-trauma fractures.
We defined premenopausal status as regular menses off hormonal contraception and early follicular phase follicle stimulating hormone (FSH) levels <20 mIU/mL. Exclusion criteria were amenorrhea lasting 6 months or longer after menarche (other than pregnancy or lactation), fewer than eight menses per year, and a delivery or lactation in the last year. All participants had a detailed history, physical and biochemical evaluation to exclude secondary causes of osteoporosis, including disorders causing premenopausal estrogen deficiency, eating disorders associated with amenorrhea, endocrinopathies (e.g., hyperthyroidism, Cushing’s syndrome, prolactinoma), celiac or other gastrointestinal diseases, abnormal mineral metabolism (e.g., osteomalacia, hyperparathyroidism), marked hypercalciuria (>300 mg/gCr), and drug exposures (e.g., glucocorticoids, anticonvulsants, anticoagulants, methotrexate). Women with serum 25-hydroxyvitamin D (25-OHD) levels below 10 ng/ml were excluded. As vitamin D deficiency and insufficiency are common in otherwise normal adults and commercial assays variable, women with screening 25-OHD levels between 10 and 20 ng/ml were eligible if their serum parathyroid hormone (PTH) was normal (10–65 pg/ml). All subjects provided written informed consent. The Institutional Review Boards of both institutions approved these studies.
Historical characteristics and questionnaires
Study physicians conducted comprehensive history and physical examinations and completed questionnaires on medical, surgical, family and fracture history, medication usage, menstrual and reproductive history, weight, dieting and exercise history, caffeine, tobacco and alcohol usage. Height was measured by Harpenden stadiometer and weight by balance beam scale. Dietary intake of calories, various nutrients, calcium, and vitamin D was quantified using the Block food frequency questionnaire [38]. Physical activity was assessed using the modified Baecke questionnaire [39]. Eating disorders were assessed by interview and the Eating Attitudes Questionnaire [40].
Laboratory assessments
To assess for secondary causes of osteoporosis, fasting morning blood was drawn and a 24-h urine collected during the early follicular phase of the menstrual cycle on the participant’s usual diet and supplement regimen and analyzed in a clinical laboratory (Quest Diagnostics, Madison, NJ) for complete blood count, erythrocyte sedimentation rate, serum electrolytes, renal and hepatic function, FSH, celiac antibodies (tissue transglutaminase, endomysial), protein electrophoresis, thyroid function, PTH, and 25-OHD; 24-h urine was analyzed for free cortisol, calcium, and creatinine. Additional serum was archived and stored at −80°C for batch analyses in the Bone Marker Laboratory of the Metabolic Bone Diseases Program and the CTSA Biomarkers Core Laboratory at CUMC. Some subjects who met the laboratory inclusion criteria based upon screening assays had results outside of the eligibility range on research analyses of archived samples. We retained these subjects in the study and report here results of the archived samples measured in research laboratories.
The following assays were used: E2, double antibody 125I radioimmunoassay (Siemens Medical Solutions Diagnostics, New York, NY); SHBG, ELISA (Alpco Diagnostics, Salem, NH); 25-OHD, radioimmunoassay (Diasorin, Stillwater, MN); 1,25 dihydroxyvitamin D [1, 25(OH)2D], radioimmunoassay (Diasorin, Stillwater, MN); intact PTH, radioimmunoassay (Scantibodies, Santee, CA); N-terminal propeptides of procollagen type 1 (P1NP) (IDS, Scottsdale, AZ); osteocalcin (N-mid OC), ELISA (IDS, Scottsdale, AZ) bone specific alkaline phosphatase (BAP), ELISA (Quidel, Santa Clara, CA); C-telopeptide (CTx), ELISA (IDS, Scottsdale, AZ); N-telopeptides (NTx) in urine, ELISA (Inverness Medical, Princeton, NJ); creatinine in urine by colorimetric assay (Integra 400 Plus, Roche Diagnostics, Indianapolis, IN); Tartrate-resistant acid phosphatase isoform 5b (TRAP5b), ELISA (IDS, Scottsdale, AZ); insulin-like growth factor 1 (IGF-1) was analyzed by radioimmunoassay (Alpco Diagnostics, Salem, NH) in the MECORE Laboratory, St. Josephs Hospital, Bangor, ME. Free estradiol was calculated based on previously described methods [41].
Calcium absorption efficiency was measured in a subset utilizing a timed blood sample obtained 5 h after ingestion of a small tracer dose of 45Ca (10 μCi) as part of a standardized meal according to the method of Heaney and Recker [42].
Areal bone mineral density and body composition by DXA
Areal BMD was measured by DXA (QDR-4500, Hologic Inc., Walton, MA) at Columbia and Creighton University Medical Centers. T- and Z-scores compared subjects and controls with young–normal and age-matched populations of the same race and sex, as provided by the manufacturer. Body composition was measured by DXA: whole body (excluding head) fat and lean mass, and truncal fat and lean mass. Scanners at both sites were cross-calibrated with a reference phantom to read BMD within 1% at baseline and at 6-month intervals throughout the study.
Spine radiographs
All subjects and controls had anterior–posterior and lateral spine radiographs to screen for prevalent vertebral fractures; fractures were defined by the semi-quantitative method used in the Study of Osteoporotic fractures [43]. The study radiologist (R.B.S.) was blinded to the study group.
Statistical analysis
Statistical analyses were performed using SAS software (SAS Institute, Cary NC, USA). To limit type I error caused by multiple comparisons, we first used ANOVA models as an omnibus test to examine overall differences among the three groups (controls, IOP, ILBMD) for each variable. Between-groups comparisons are presented only for variables that initially showed significant overall differences among the three groups based on ANOVA models. Between-groups comparisons were conducted using Student’s t tests. Pearson correlation coefficients were calculated to test associations between variables. Multivariate linear and logistic regression analyses were used to control for covariates. All data are expressed as mean ± standard deviation. Results were considered significant with p<0.05.
Results
Clinical characteristics of the study groups
Of 64 affected subjects, 45 had a history of low-trauma adult fractures (IOP group); 19 had low aBMD but no history of low-trauma adult fractures (idiopathic low BMD or ILBMD group). However, 16% reported high-trauma adult fractures and 26% reported childhood fractures. The number of fractures per IOP subject ranged from 1 to 12; 25 subjects had multiple fractures, ranging from 2 to 12. Eleven subjects had vertebral, 11 had rib, seven had hip, five had pelvic, 12 had forearm, six had humerus, six had lower leg, six had ankle, and nine had metatarsal fractures. The mean age at first adult fracture was 30±9 years, and the mean time between the evaluation and the most recent fracture was 4±4 years.
Subjects and controls were of similar age (Table 1). By ANOVA, only weight, BMI, BMD, family history of osteoporosis, and menarchal age differed significantly. IOP and ILBMD subjects weighed significantly less and had lower BMI than controls. Although controls had higher whole body and truncal fat than subjects, these differences did not reach statistical significance. Women in the ILBMD group were significantly more likely than controls and IOPs to report a family history of osteoporosis.
Table 1.
Characteristics of the study groups
| Control, n=40 | IOP, n=45 | ILBMD, n=19 | |
|---|---|---|---|
| Anthropometric characteristics | |||
| Age (years) | 37.3±8.2 | 37.0±7.7 | 39.6±6.3 |
| Height (cm) | 165.4±7.3 | 163.8±7.2 | 162.2±5.7 |
| Weight (kg) | 70.7±14.6 | 63.0±14.9* | 57.1±10.2*** |
| BMI (kg/m2) | 25.8±4.7 | 23.4±4.9* | 21.6±3.5** |
| Body composition (%) | |||
| Whole body fat | 35.5±7.2 | 33.9±7.8 | 33.3±6.0 |
| Trunk fat | 32.5±8.9 | 30.5±9.3 | 28.9±7.4 |
| Bone mineral density (g/cm2) | |||
| Lumbar spine (L1–4) | 1.099±0.093 | 0.875±0.139*** | 0.793±0.078***, ## |
| Total hip | 0.984±0.075 | 0.792±0.138*** | 0.736±0.090*** |
| Femoral neck | 0.878±0.075 | 0.677±0.129*** | 0.615±0.089*** |
| Distal radius (1/3) | 0.721±0.051 | 0.692±0.047** | 0.679±0.045** |
| Fracture, family, and reproductive history | |||
| Adult high-trauma fractures | 5% | 7% | 16% |
| Childhood/adolescent fractures | 18% | 29% | 26% |
| Family history of osteoporosis | 50% | 58% | 84%*, # |
| Menarchal age (years) | 12.6±1.2 | 12.9±1.0 | 13.7±1.5**, # |
| Menses/year (n) | 12.0±0.9 | 12.1±0.7 | 12.0±0.7 |
| Nulliparous | 25% | 27% | 21% |
| Age at first pregnancy | 26.1±5.7 | 25.5±6.0 | 24.8±7.9 |
| Pregnancies (n) | 1.9±1.7 | 1.9±1.5 | 2.5±2.9 |
| Live births (n) | 1.4±1.2 | 1.5±1.2 | 1.8±2.3 |
| Lactation (%) | 60% | 47% | 63% |
| Lactation (months) | 12.6±11.4 | 14.6±9.7 | 36.8±58.0 |
| Oral contraception >1 year (%) | 78% | 75% | 70% |
| Block Food Frequency Questionnaire results (N=40 controls, 37 IOP, 15 ILBMD) | |||
| Calories/day | 1,736±685 | 1,695±673 | 1,870±604 |
| Protein (gm/day) | 68.4±28.9 | 61.4±18.2 | 75.1±24.2 |
| % Protein in diet | 15.9±3.1 | 15.0±2.9 | 16.3±2.8 |
| % Fat in diet | 36.7±6.2 | 38.6±5.6 | 38.6±9.6 |
| Phosphate (mg/day) | 1,204±488 | 1,038±329 | 1,346±437## |
| Sodium (mg/day) | 2,527±1,151 | 2,287±903 | 2,392±745 |
| Dietary calcium (mg/day) | 861±398 | 683±269* | 952±425# |
| Calcium from supplements | 231±360 | 564±460*** | 563±423** |
| Total calcium (mg/day) | 1,091±475 | 1,247±592 | 1,515±495** |
| Dietary vitamin D (IU/day) | 145±94 | 103±56 | 138±77 |
| Vitamin D from supplements (IU/day) | 130±156 | 170±174 | 91±127 |
| Total vitamin D (IU/day) | 275±184 | 272±183 | 230±151 |
| General questionnaire data | |||
| Calcium supplements (current, %) | 35% | 63%* | 83%***, # |
| Multivitamin (current, %) | 56% | 53% | 30% |
| Thyroid hormone (ever, %) | 5% | 5% | 5% |
| Selective seratonin reuptake inhibitors (SSRI, %) | 3% | 16% | 15% |
| Caffeinated beverages/day | 2.8±1.9 | 2.7±2.2 | 1.8±1.7 |
| ETOH (current drinks/week) | 0.5±0.3 | 0.6±0.4 | 0.9±0.6*, # |
| Smoking (ever, %) | 33% | 21% | 42% |
| Smoking (current, %) | 5% | 5% | 11% |
| Exercise (current, min/week) | 143±54 | 141±81 | 136±56 |
| Exercise (past, min/week) | 165±72 | 150±86 | 124±65 |
p<0.05 vs controls;
p<0.01 vs controls;
p<0.001 vs controls;
p<0.05 IOP vs ILBMD;
p<0.01 IOP vs ILBMD
Bone mineral density
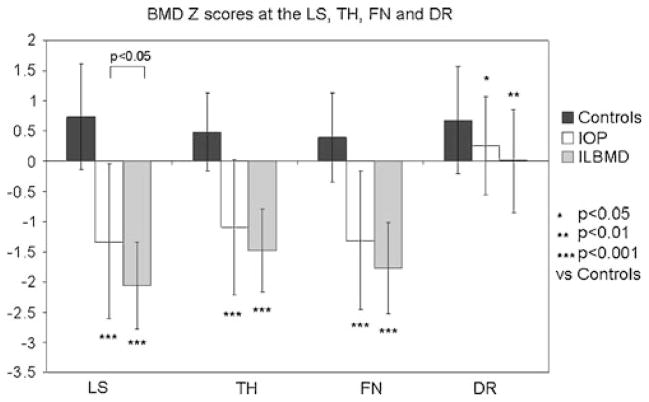
Absolute BMD (Table 1) and Z-scores (Fig. 1) differed significantly between-groups at all sites. BMD was lowest in the ILBMD group, in whom inclusion was based on BMD criteria. Of the ILBMD subjects, 53% had low Z-scores at the spine only, 26% at the hip only, and 21% at both sites. Although included on the basis of fractures rather than BMD criteria, the IOP group had significantly lower aBMD at all sites than controls. Among IOP subjects, only 53% had BMD Z-scores that were below the expected range for age (less than or equal to −2.0) while 47% had BMD Z-scores that were within the expected range for age (greater than −2.0). In 12 (27%) IOP subjects, BMD Z-scores were greater than or equal to −1.0 at all sites. BMD differences remained significant (p<0.03) after controlling for BMI.
Fig. 1.
BMD Z-scores at the LS, total hip (TH), femoral neck (FN), and distal radius (DR)
Reproductive history
Except for menarchal age, reproductive history did not differ between subjects and controls (Table 1). Menarche was slightly but significantly later in the ILBMD group than both IOP and control subjects; the difference remained significant (p<0.05) after controlling for current BMI. Menarchal age did not correlate with BMI, body fat, or BMD at any site or in any group. Variability in reported number of months of lactation was high, particularly in the ILBMD group, who tended to have more months of lactation compared to the controls (p=0.05). Two outliers in the ILBMD group reporting 180 and 132 months of lactation are likely to explain the differences seen. Neither parity nor months of lactation correlated with BMD at any site in any group.
Diet, medications, exercise, tobacco, and alcohol
As weight and BMI were lower in both groups of subjects, we investigated whether dietary composition, supplement, and lifestyle habits differed between subjects and controls (Table 1). By ANOVA, there were significant between-groups differences in dietary phosphate, dietary and supplemental calcium, and total calcium intake. Although the ILBMD group weighed less, caloric and protein intake were similar to the IOP and control groups; however, dietary calcium and phosphate intake in the ILBMD group were significantly higher than the IOP group. The proportion of IOP and ILBMD subjects using calcium supplements, and calcium intake from supplements, were significantly higher than controls; in addition, more ILBMD than IOP subjects used supplements. Total calcium intake was significantly higher in the ILBMD than the control group.
Selective serotonin reuptake inhibitor use appeared higher in subjects than controls, but the difference was not significant (p=0.11 by ANOVA). There were no significant overall between-groups differences for other medications, tobacco exposure, caffeine, or time spent exercising. Although the ILBMD group reported significantly higher alcohol consumption than the other groups, alcohol intake was low in all groups (approximately one drink per week on average).
Biochemistries
FSH and estradiol
Early follicular phase FSH, SHBG, total and calculated free estradiol did not differ significantly between groups (Table 2). Neither total nor free estradiol correlated with bone turnover markers or with BMD at any site in any group.
Table 2.
Biochemical characteristics of the study groups
| Control, n=40 | IOP, n=45 | ILBMD, n=19 | |
|---|---|---|---|
| FSH (mIU/ml) | 7.2±3.5 | 7.5±3.7 | 7.5±2.5 |
| Total E2 (pg/ml) | 38.6±33.9 | 37.7±29.1 | 35.3±23.6 |
| SHBG (nmol/L) | 70.3±31.8 | 85.9±39.8 | 83.4±41.2 |
| Free E2 (pmol/L) | 1.74±1.39 | 1.90±3.29 | 1.40±0.88 |
| Free E2 (%) | 1.3±0.4 | 1.2±0.3 | 1.2±0.3 |
| Calcium (albumin-adjusted; mg/dL) | 9.0±0.3 | 9.1±0.6 | 8.9±0.3 |
| Phosphorus (mg/dL) | 3.5±0.4 | 3.4±0.5 | 3.6±0.6 |
| 25-OHD (ng/mL) | 30.3±13.4 | 36.9±16.4 | 37.7±12.3 |
| 1,25(OH)2D (pg/ml) | 48.7±10.8 | 54.6±18.5 | 56.3±12.2 |
| PTH (pg/mL) | 22.4±9.1 | 29.2±10.6** | 29.5±15.6* |
| 24-h urine calcium (mg/g creatinine) | 161.6±67.5 | 183.9±88.5 | 210.5±69.8 |
| 24-h urine calcium (mg/kg) | 3.0±1.4 | 3.3±2.1 | 4.2±2.0 |
| Calcium absorption (%; F) | 31.8±7.2 | 32.7±7.5 | 30.0±8.2 |
| P1NP (3g/L) | 46.6±17.9 | 46.5±18.0 | 51.7±17.5 |
| BAP (U/L) | 20.3±6.4 | 22.0±6.6 | 21.5±5.0 |
| Osteocalcin (ng/ml) | 15.4±7.9 | 15.7±8.1 | 15.5±6.0 |
| CTx (ng/ml) | 0.287±0.186 | 0.354±0.194 | 0.333±0.127 |
| TRAP5b (U/L) | 1.57±0.95 | 2.24±1.22** | 2.31±1.01** |
| Urine NTx nMBCE/mMCr | 35.6±15.5 | 43.7±22.5 | 36.4±18.8 |
| IGF-1 (ng/ml) | 184.2±54.7 | 182.0±53.8 | 171.9±56.4 |
| Urine cortisol (mcg/24 h) | 58.8±30.8 | 67.6±84.3 | 67.4±53.4 |
No significant differences seen between IOP and ILBMD. ΦPerformed in a subset (n=33 in Controls, n=26 in IOP, n=9 in ILBMD)
p<0.05 vs controls;
p<0.01 vs controls
Abbreviations: FSH, follicle stimulating hormone; E2, estradiol; SHBG, sex hormone-binding globulin; 25-OHD, 25-hydroxyvitamin D; 1,25 (OH)2D, 1,25 dihydroxyvitamin D; P1NP, N-terminal propeptides of procollagen type 1; BAP, bone specific alkaline phosphatase; CTx, C-telopeptide; TRAP5b, tartrate resistant acid phosphatase isoform 5b, NTx, N-telopeptide; IGF-1, insulin-like growth factor-1
Serum minerals and calciotropic hormones
Serum calcium and phosphate levels did not differ between subjects and controls (Table 2). Mean serum 25-OHD was slightly but not significantly higher in both groups of Subjects (p=0.07). Serum 25-OHD levels below 20 ng/mL were found in nine subjects in the IOP group (22%), two in the ILBMD group (11%) and 11 controls (28%; p = NS). Among subjects and controls with serum 25-OHD levels below 20 ng/mL, the mean PTH level was normal (24.3± 11.3 pg/mL; normal, 14–66 pg/ml). Serum albumin corrected calcium levels did not differ between those with 25-OHD below 20 ng/mL compared to the others in any study group; total calcium intake was significantly lower in those with 25-OHD below 20 ng/mL in the IOP group only (916±554 mg/day vs 1,390±579 mg/day; p=0.049).
Serum PTH was significantly higher in both ILBMD and IOP groups than controls, although all means were within the normal range. None of the controls had serum PTH levels >38 pg/ml. In contrast, 12 subjects had PTH levels =40 pg/ml (mean, 46.4±8.8 pg/ml); they did not differ significantly from those with levels <40 pg/ml with regard to age, anthropometric features, BMD, vitamin D metabolites, or bone turnover markers (BTMs; data not shown), except for serum P1NP which was lower in the women with higher PTH (41.6±9.2 vs 50.0±19.4 pg/ml; P=0.03). Calcium absorption, measured in a subset, did not differ between the groups.
Urinary calcium excretion, assessed on the subjects’ typical diet and supplement regimen and expressed as milligrams per gram creatinine or milligrams per kilogram, was slightly but not significantly higher in both groups of subjects (Table 2). Urinary calcium above 250 mg/24 h was present in 30% of controls, 22% of IOP, and 39% of ILBMD subjects. Urinary calcium above 4 mg/kg was present in similar proportions: 23% of controls, 24% of IOP, and 39% of ILBMD subjects; these differences were not significant. Those with urine calcium excretion above or below 4 mg/kg did not differ significantly in terms of PTH, 1,25(OH)2D, 25-OHD, or total calcium intake.
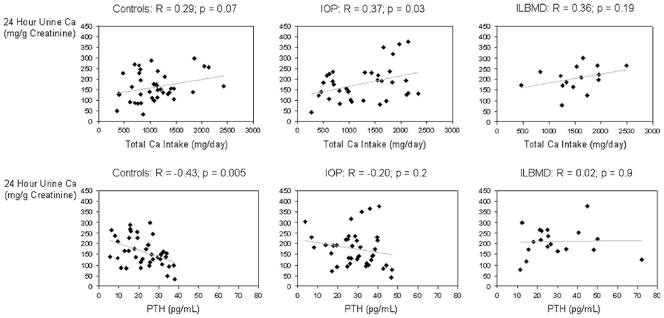
Urinary calcium excretion was directly, but not significantly, associated with total calcium intake in controls (R=0.29; p=0.07) and ILBMD subjects (R=0.36; p=0.19); in contrast, the association was significant in IOP subjects (R=0.37; p=0.03; Fig. 2). Urinary calcium excretion was inversely associated with serum PTH in controls (R=−0.43; p=0.005), but there was no significant association with PTH in either the IOP or ILBMD groups (Fig. 2). Serum PTH did not correlate significantly with serum calcium, phosphate, 25-OHD, 1,25(OH)2D, calcium absorption or calcium intake in any group. Urinary calcium excretion did not correlate with phosphate, sodium, or protein intake in any group.
Fig. 2.
Twenty-four-hour urine Ca vs total Ca intake and PTH: scatter plots and correlation coefficients (R) showing associations between a 24-h urinary calcium excretion and total dietary calcium intake in control, IOP, and ILBMD groups and between b 24-h urinary calcium excretion and serum PTH in control, IOP, and ILBMD groups
Serum 25-OHD was inversely associated with lumbar spine (LS), TH, and FN BMD in the IOP group (R=−0.38 to −0.43; p=0.005–0.02). PTH, 1,25(OH)2D, urinary calcium excretion, and calcium absorption did not correlate significantly with BMD at any site in any group.
Bone turnover markers
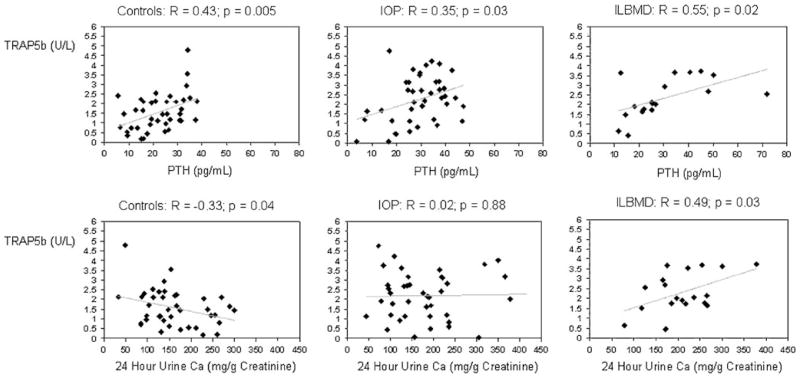
Serum BAP, osteocalcin, P1NP and CTx, and urinary NTx did not differ significantly among the groups. In contrast, TRAP5b was significantly higher in both groups of subjects (by 43% in IOPs; p=0.007 and 47% in ILBMDs; p=0.008) than controls. TRAP5b was directly associated with serum PTH (Fig. 3) in all three groups. PTH also correlated directly with serum BAP in controls (R=0.41; p=0.008), serum osteocalcin in IOP (R=0.39; p=0.01), and serum CTx in ILBMD (R=0.48; p=0.04). TRAP5b was inversely associated with urinary calcium excretion (milligrams per gram of creatinine) in controls (R=−0.33; p=0.04); in contrast, it was directly associated with urinary calcium in the ILBMD group (R=+0.49; p=0.03; Fig. 3). There was no association between TRAP5b and urinary calcium in the IOP group (R=0.02; p=0.9).
Fig. 3.
TRAP5b vs PTH and 24-h urine Ca: scatter plots and correlation coefficients (R) showing associations between a TRAP5b and serum PTH in control, IOP, and ILBMD groups and between b TRAP5b and 24-h urinary calcium excretion in control, IOP, and ILBMD groups
Four IOP subjects fractured between 3 and 6 months before enrollment. Mean TRAP5b in these four subjects was 2.47±1.16 U/L, not significantly different from the other IOP subjects. In addition, no other BTM differed significantly between these four subjects and the remainder of the IOP group.
No consistent relationships were detected between BTMs and BMD. All BTMs correlated inversely and significantly with age in the controls (R=−0.34 to −0.66; p=<0.0001–0.04). Similar relationships were seen between age and CTx, osteocalcin, and P1NP in the IOP subjects (R=−0.32 to −0.54; p=0.0002–0.04), while no significant relationships were seen between age and BTMs in the ILBMD group.
IGF−1 and cortisol
Mean serum IGF-1 levels did not differ between IOP subjects and controls (Table 2). Similarly, in a subset, IGFBP3 and the IGF-1/IGFBP3 ratio did not differ among the groups (data not shown). As expected, IGF-1 correlated inversely with age in each group (R=−0.77 to −0.55; p=0.001 to <0.0001). Serum IGF-1 was directly and significantly associated with all bone turnover markers in the controls (R=0.41–0.62; p=0.009 to <0.0001) (Table 3). Except for BAP, these relationships did not remain significant after controlling for age. In contrast, IGF-1 did not correlate with any bone turnover marker in either group of subjects or in the IOP and ILBMD groups combined (R=−0.09–0.09; p=0.5–0.9), and the results did not change with the addition of age as a covariate. IGF-1 did not correlate significantly with BMD at any site in any group.
Table 3.
Serum IGF-1 and bone turnover markers
| Correlation with IGF-1 (R; p)
|
Correlation with IGF-1 in analyses controlling for age (R; p)
|
|||||
|---|---|---|---|---|---|---|
| Control | IOP | ILBMD | Control | IOP | ILBMD | |
| BAP | 0.62; <0.0001 | 0.04; 0.8 | −0.03; 0.9 | 0.32; 0.04 | 0.06; 0.7 | 0.09; 0.7 |
| Osteocalcin | 0.61; <0.0001 | −0.04; 0.8 | −0.01; 1.0 | 0.21; 0.2 | 0.27; 0.09 | 0.15; 0.5 |
| P1NP | 0.47; 0.002 | 0.16; 0.3 | −0.02; 0.9 | 0.03; 0.8 | 0.02; 0.9 | 0.17; 0.5 |
| TRAP5b | 0.41; 0.008 | 0.01; 0.9 | −0.22; 0.4 | 0.03; 0.8 | 0.15; 0.4 | 0.17; 0.5 |
| CTx | 0.47; 0.002 | 0.16; 0.3 | −2.0; 0.4 | 0.11; 0.5 | 0.20; 0.2 | 0.31; 0.2 |
| NTx | 0.41; 0.009 | −0.11 0.5 | −0.11; 0.7 | 0.25; 0.13 | 0.23; 0.16 | 0.07; 0.8 |
Subgroup analyses
The IOP subjects with normal BMD at all sites (n=12) had higher BMI (28.5±5.7 vs 21.5±2.9 kg/m2; p=0.002), lower serum 25-OHD levels (27±21 vs 41±13 ng/mL; p=0.02) and lower urine calcium excretion (140±52 vs 201±94 mg/gCr; p=0.01) compared to IOP subjects with low BMD, but did not differ in any other respect. The ILBMD subjects with low LS BMD only did not differ from the other ILBMD subjects in any respect.
Discussion
This is the largest study to characterize clinical and biochemical features of premenopausal women with unexplained osteoporosis, and the only one to investigate whether women with a history of low-trauma adult fractures differ clinically or biochemically from those with low BMD but no low-trauma adult fractures. Compared to controls, women in the IOP and ILBMD groups weighed less, had lower BMD, and were more likely to take calcium supplements. A family history of osteoporosis was most common in the women with ILBMD, who also had the latest menarchal age and highest calcium intake of the three groups. Serum reproductive hormones, calcium, vitamin D metabolites, IGF1, urinary calcium excretion, bone formation markers, and certain resorption markers were comparable between the affected groups and also controls. In contrast, serum PTH and TRAP5b were comparably and significantly higher in the IOP and ILBMD groups than controls, although mean PTH was well within the normal range; there were direct correlations between PTH and TRAP5b in all three groups.
The lower BMI in our subjects is consistent with our previous studies of premenopausal osteoporosis [9, 10, 13, 31] and those of Peris et al. [9, 10, 13, 31].
In contrast, reproductive hormones, indices of mineral metabolism, and BTMs differed from other studies of women with IOP. In a small study of 13 women with IOP, we found lower follicular phase serum estradiol levels and higher bone resorption markers (urinary NTx) in women with IOP [31]. The discrepancies between our previous and new results may be because our current sample size is larger and none of the women were receiving hormonal contraception. Neither study measured estrogen levels accross the menstrual cycle, which is likely to be a more accurate assessment of estrogen exposure than a single measurement in the follicular phase.
Also, in contrast to prior studies [13, 31], urinary NTx did not differ between subjects and controls. Similarly, serum CTx did not differ. In contrast, TRAP5b was higher in both the IOP and ILBMD groups than controls and correlated directly with serum PTH in all three groups. To our knowledge, TRAP5b has not previously been evaluated in IOP. TRAP5b is thought by some [44] to be a specific marker for the activity of differentiated osteoclasts, while others [45] believe that it reflects the number of differentiated osteoclasts rather than their activity. Our finding that TRAP5b was elevated in IOP and ILBMD in the face of no differences in CTx or NTx supports the view that TRAP5b provides different information. Although, the significance of the elevated TRAP5b levels in IOP and ILBMD is presently unclear, ongoing histomorphometric analysis of iliac crest biopsies from our subjects should help elucidate this.
In men with IOP, we and others have linked reduced serum levels of IGF-1 to reduced BMD [15, 23], and some have observed significant correlations between reduced IGF-1 levels and histomorphometric parameters of bone formation [15, 20]. In contrast to studies in men, but similar to our previous small study of women with IOP [31], serum IGF-1 did not differ between osteoporotic and normal women and did not correlate with BMD. There were the expected inverse associations between IGF-1 and age in all three groups and IGF-1 correlated inversely with all bone turnover markers in controls. However, most of the associations were no longer significant after controlling for age, and thus likely reflect co-linearity between age, IGF-1, and BTMs. In contrast, although IGF-1 was inversely related to age in both IOP and ILBMD groups, and BTMs were also inversely related to age in the IOP group, serum IGF-1 did not correlate significantly with any bone turnover marker in either the IOP or ILBMD group. It is unclear why the significant unadjusted associations between IGF-1 and BTMs in the controls were not detected in the affected subjects; it may be because the relationship between IGF-1 and bone turnover may indeed be altered in the subjects. In addition, as IGF-1 is predominantly bound to IGFBP3 and restricted to the intravascular space, measurement of total circulating serum IGF-1 levels may not reflect the levels available in the bone microenvironment of a particular tissue [46].
Several studies of men [4, 7, 11, 12, 15, 16] and one of women [13] with IOP have found a substantial proportion with hypercalciuria. While our study design excluded subjects and controls with marked hypercalciuria (>300 mg/g creatinine), we included women with less markedly elevated urinary calcium excretion to examine whether this feature distinguished affected subjects from controls. Similar to Peris et al. [13], we too found that urinary calcium excretion was higher in affected subjects than controls, although results did not reach statistical signficance. However, our results may not be directly comparable to the Peris study, as the authors did not provide information regarding dietary calcium and supplement use in their subjects [13].
While some subjects and controls had serum 25-OHD concentrations below 20 ng/ml, a level currently considered to represent vitamin D deficiency, the prevalence of D deficiency did not differ between the groups, and PTH was not higher in those with lower serum 25-OHD levels. However, although mean PTH levels were normal in subjects and controls, PTH levels were significantly higher in the subjects. Of note, PTH levels correlated significantly and positively with TRAP5b levels in all three groups suggesting that elevated PTH could be contributing to higher osteoclast number and/or bone resorption. However, PTH did not correlate with vitamin D levels in these groups, and the pattern of differences in indices of mineral metabolism between affected subjects and controls did not support either primary or secondary hyperparathyroidism related to calcium or vitamin D deficiency.
We considered whether higher PTH levels and higher TRAP5b levels could reflect some degree of secondary hyperparathyroidism related to renal calcium losses in the affected subjects. Control subjects had expected physiologic relationships between calcium intake, urinary calcium excretion, and PTH; higher calcium intake was associated with a trend towards higher calcium excretion, which was associated with lower PTH and lower TRAP5b. In contrast, in both IOPs and ILBMDs, urinary calcium varied widely with both low and high values present among those with higher PTH and TRAP5b. This suggests heterogeneity in the pathogenesis of both IOP and ILBMD. Various causes of secondary hyperparathyroidism, including both calcium deficiency and hypercalciuria, may have limited our interpretation of these data. Studies of calcium balance on standardized intakes would be a useful avenue to pursue in future attempts to understand the pathogenesis of IOP as it relates to renal calcium losses and intestinal calcium malabsorption.
One of the original goals of this study was to determine whether subjects included on the basis of fracture (IOP) and those included on the basis of low BMD (ILBMD) differed in any significant way. Overall, we found rather few differences between the IOP and ILBMD groups. Both affected groups differed from controls in terms of weight, BMI, BMD, and calcium intake. In addition, both affected groups had elevated PTH and TRAP5b compared to controls. ILBMD patients differed from IOP patients in only a few ways: later menarche, family history of osteoporosis, phosphate intake, and alcohol intake. Because of the limited sample sizes here and the potential for ascertainment bias, it is difficult to determine which, if any, of these differential features are pathogenetically related to low BMD. Women in this group may have had BMD measured or responded to study advertisement because of a family history of osteoporosis or because of knowledge of the relationship between osteoporosis and later menarchal age [47, 48]. Phosphate and alcohol intake were not unusually elevated in any group and the statistically significant differences between the groups are unlikely to be clinically significant. It is interesting that although the ILBMD group included no subjects with adult low-trauma fractures, a substantial proportion had a history of childhood or adolescent fractures and also of high-trauma fractures. This observation suggests that defects in bone quality may develop early, that a history of childhood fractures may presage lower adult BMD and/or abnormal bone quality, as recently reported by Ferrari and colleagues [49], and also that the absence of a fracture history may simply be due to the lack of an appropriately severe injury. This concept is also supported by our HR-pQCT results that showed microarchitectural deterioration and reduced estimated bone stiffness at the radius and tibia, regardless of whether subjects had sustained a fracture or had low BMD [36]. Thus, this distinction does not appear to be clinically useful, at least in terms of understanding the pathogenesis of IOP.
This study has several limitations. Our younger subjects may not have reached peak bone mass. We may have misclassified fractures as low trauma when in fact they did not reflect abnormal fragility. Our sample size was too small to permit subgroup analyses by fracture type. Premenopausal women who have BMD measured or volunteered for this study may have done so because of a family history of osteoporosis, thus biasing our findings related to the presence of family history. Likewise, those diagnosed with osteoporosis or low BMD were already taking supplements prior to study participation, which limits interpretation of our findings regarding vitamin D levels and urinary calcium excretion in these subjects. As only cross-sectional data are available, we cannot determine whether observed abnormalities result from ongoing bone loss or past insults, which have now stabilized or resolved. In particular, we may have failed to discern differences in most BTMs because structural defects seen resulted from past rather than ongoing insults to bone.
Our study also has important strengths. To our knowledge, this is the largest case–control study of women with IOP, and the first to compare those with low-trauma fractures (IOP) to those with ILBMD. Both affected subjects and controls were carefully characterized, and common causes of secondary osteoporosis were excluded by detailed clinical and biochemical evaluation.
In conclusion, as yet, no clear-cut unifying etiology has emerged in our study of IOP in premenopausal women. The consistent finding of elevated TRAP5b that was directly related to PTH secretion in both groups of subjects suggests that increased bone turnover, possibly related to some degree of secondary hyperparathyroidism could contribute to their condition. However, this disorder may also be the result of a heterogeneous array of underlying causes. Overall, the similarities between premenopausal women with fractures and those with low BMD suggest that they represent a clinical continuum and support a mandate for careful individualized evaluations and tailored treatment plans.
Acknowledgments
These studies are supported by the following NIH funding sources: RO1AR49896 (E.S.), K24 AR052661 (E.S.), K23 AR054127 (A.C.), and UL1 RR024156 (S.C.).
Footnotes
Conflicts of interest None.
Contributor Information
A. Cohen, Email: ac1044@columbia.edu, Columbia University, New York, NY, USA, Department of Medicine, College of Physicians and Surgeons, Columbia University, PH8-864, 630 West 168th Street, New York 10032 NY, USA
R. R. Recker, Creighton University, Omaha, NE, USA
J. Lappe, Creighton University, Omaha, NE, USA
D. W. Dempster, Columbia University, New York, NY, USA, Helen Hayes Hospital, West Haverstraw, NY, USA
S. Cremers, Columbia University, New York, NY, USA
D. J. McMahon, Columbia University, New York, NY, USA
E. M. Stein, Columbia University, New York, NY, USA
J. Fleischer, Columbia University, New York, NY, USA
C. J. Rosen, Maine Medical Center, Portland, ME, USA
H. Rogers, Columbia University, New York, NY, USA
R. B. Staron, Columbia University, New York, NY, USA
J. LeMaster, Creighton University, Omaha, NE, USA
E. Shane, Columbia University, New York, NY, USA
References
- 1.Ebeling PR. Osteoporosis in men. New insights into aetiology, pathogenesis, prevention and management. Drugs Aging. 1998;13:421–434. doi: 10.2165/00002512-199813060-00002. [DOI] [PubMed] [Google Scholar]
- 2.Gennari L, Bilezikian JP. Osteoporosis in men. Endocrinol Metab Clin North Am. 2007;36:399–419. doi: 10.1016/j.ecl.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 3.Gourlay ML, Brown SA. Clinical considerations in premenopausal osteoporosis. Arch Intern Med. 2004;164:603–614. doi: 10.1001/archinte.164.6.603. [DOI] [PubMed] [Google Scholar]
- 4.Kelepouris N, Harper KD, Gannon F, Kaplan FS, Haddad JG. Severe osteoporosis in men. Ann Intern Med. 1995;123:452–460. doi: 10.7326/0003-4819-123-6-199509150-00010. [DOI] [PubMed] [Google Scholar]
- 5.Khan A. Premenopausal women and low bone density. Can Fam Physician. 2006;52:743–747. [PMC free article] [PubMed] [Google Scholar]
- 6.Khosla S. Idiopathic osteoporosis: is the osteoblast to blame?—Author’s response. J Clin Endocrinol Metab. 1998;83:716. doi: 10.1210/jcem.83.2.4587-5. [DOI] [PubMed] [Google Scholar]
- 7.Khosla S, Lufkin EG, Hodgson SF, Fitzpatrick LA, Melton LJ., 3rd Epidemiology and clinical features of osteoporosis in young individuals. Bone. 1994;15:551–555. doi: 10.1016/8756-3282(94)90280-1. [DOI] [PubMed] [Google Scholar]
- 8.Lewiecki EM. Low bone mineral density in premenopausal women. South Med J. 2004;97:544–550. doi: 10.1097/00007611-200406000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Moreira Kulak CA, Schussheim DH, McMahon DJ, Kurland E, Silverberg SJ, Siris ES, Bilezikian JP, Shane E. Osteoporosis and low bone mass in premenopausal and perimenopausal women. Endocr Pract. 2000;6:296–304. doi: 10.4158/EP.6.4.296. [DOI] [PubMed] [Google Scholar]
- 10.Peris P, Guanabens N, Martinez de Osaba MJ, Monegal A, Alvarez L, Pons F, Ros I, Cerda D, Munoz-Gomez J. Clinical characteristics and etiologic factors of premenopausal osteoporosis in a group of Spanish women. Semin Arthritis Rheum. 2002;32:64–70. doi: 10.1053/sarh.2002.33725. [DOI] [PubMed] [Google Scholar]
- 11.Peris P, Guanabens N, Monegal A, Suris X, Alvarez L, Martinez de Osaba MJ, Hernandez MV, Munoz-Gomez J. Aetiology and presenting symptoms in male osteoporosis. Br J Rheumatol. 1995;34:936–941. doi: 10.1093/rheumatology/34.10.936. [DOI] [PubMed] [Google Scholar]
- 12.Peris P, Martinez-Ferrer A, Monegal A, Martinez de Osaba MJ, Alvarez L, Ros I, Muxi A, Reyes R, Guanabens N. Aetiology and clinical characteristics of male osteoporosis. Have they changed in the last few years? Clin Exp Rheumatol. 2008;26:582–588. [PubMed] [Google Scholar]
- 13.Peris P, Ruiz-Esquide V, Monegal A, Alvarez L, Martinez de Osaba MJ, Martinez-Ferrer A, Reyes R, Guanabens N. Idiopathic osteoporosis in premenopausal women. Clinical characteristics and bone remodelling abnormalities. Clin Exp Rheumatol. 2008;26:986–991. [PubMed] [Google Scholar]
- 14.Heshmati HM, Khosla S. Idiopathic osteoporosis: a heterogeneous entity. Ann Med Interne (Paris) 1998;149:77–81. [PubMed] [Google Scholar]
- 15.Kurland ES, Rosen CJ, Cosman F, McMahon D, Chan F, Shane E, Lindsay R, Dempster D, Bilezikian JP. Insulin-like growth factor-I in men with idiopathic osteoporosis. J Clin Endocrinol Metab. 1997;82:2799–2805. doi: 10.1210/jcem.82.9.4253. [DOI] [PubMed] [Google Scholar]
- 16.Zerwekh JE, Sakhaee K, Breslau NA, Gottschalk F, Pak CY. Impaired bone formation in male idiopathic osteoporosis: further reduction in the presence of concomitant hypercalciuria. Osteoporos Int. 1992;2:128–134. doi: 10.1007/BF01623819. [DOI] [PubMed] [Google Scholar]
- 17.Evans SF, Davie MW. Low body size and elevated sex-hormone binding globulin distinguish men with idiopathic vertebral fracture. Calcif Tissue Int. 2002;70:9–15. doi: 10.1007/s00223-001-2018-6. [DOI] [PubMed] [Google Scholar]
- 18.Gillberg P, Johansson AG, Ljunghall S. Decreased estradiol levels and free androgen index and elevated sex hormone-binding globulin levels in male idiopathic osteoporosis. Calcif Tissue Int. 1999;64:209–213. doi: 10.1007/s002239900604. [DOI] [PubMed] [Google Scholar]
- 19.Legrand E, Hedde C, Gallois Y, Degasne I, Boux de Casson F, Mathieu E, Basle MF, Chappard D, Audran M. Osteoporosis in men: a potential role for the sex hormone binding globulin. Bone. 2001;29:90–95. doi: 10.1016/s8756-3282(01)00478-1. [DOI] [PubMed] [Google Scholar]
- 20.Pernow Y, Hauge EM, Linder K, Dahl E, Saaf M. Bone histomorphometry in male idiopathic osteoporosis. Calcif Tissue Int. 2009;84:430–438. doi: 10.1007/s00223-009-9239-5. [DOI] [PubMed] [Google Scholar]
- 21.Pietschmann P, Kudlacek S, Grisar J, Spitzauer S, Woloszczuk W, Willvonseder R, Peterlik M. Bone turnover markers and sex hormones in men with idiopathic osteoporosis. Eur J Clin Investig. 2001;31:444–451. doi: 10.1046/j.1365-2362.2001.00836.x. [DOI] [PubMed] [Google Scholar]
- 22.Van Pottelbergh I, Goemaere S, Zmierczak H, Kaufman JM. Perturbed sex steroid status in men with idiopathic osteoporosis and their sons. J Clin Endocrinol Metab. 2004;89:4949–4953. doi: 10.1210/jc.2003-032081. [DOI] [PubMed] [Google Scholar]
- 23.Ljunghall S, Johansson AG, Burman P, Kampe O, Lindh E, Karlsson FA. Low plasma levels of insulin-like growth factor 1 (IGF-1) in male patients with idiopathic osteoporosis. J Intern Med. 1992;232:59–64. doi: 10.1111/j.1365-2796.1992.tb00550.x. [DOI] [PubMed] [Google Scholar]
- 24.Pernow Y, Granberg B, Saaf M, Weidenhielm L. Osteoblast dysfunction in male idiopathic osteoporosis. Calcif Tissue Int. 2006;78:90–97. doi: 10.1007/s00223-005-0158-9. [DOI] [PubMed] [Google Scholar]
- 25.Reed BY, Zerwekh JE, Sakhaee K, Breslau NA, Gottschalk F, Pak CY. Serum IGF 1 is low and correlated with osteoblastic surface in idiopathic osteoporosis. J Bone Miner Res. 1995;10:1218–1224. doi: 10.1002/jbmr.5650100812. [DOI] [PubMed] [Google Scholar]
- 26.Perry HM, 3rd, Fallon MD, Bergfeld M, Teitelbaum SL, Avioli LV. Osteoporosis in young men: a syndrome of hyper-calciuria and accelerated bone turnover. Arch Intern Med. 1982;142:1295–1298. doi: 10.1001/archinte.142.7.1295. [DOI] [PubMed] [Google Scholar]
- 27.Pacifici R, Rifas L, Teitelbaum S, Slatopolsky E, McCracken R, Bergfeld M, Lee W, Avioli LV, Peck WA. Spontaneous release of interleukin 1 from human blood monocytes reflects bone formation in idiopathic osteoporosis. Proc Natl Acad Sci USA. 1987;84:4616–4620. doi: 10.1073/pnas.84.13.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johansson AG, Eriksen EF, Lindh E, Langdahl B, Blum WF, Lindahl A, Ljunggren O, Ljunghall S. Reduced serum levels of the growth hormone-dependent insulin-like growth factor binding protein and a negative bone balance at the level of individual remodeling units in idiopathic osteoporosis in men. J Clin Endocrinol Metab. 1997;82:2795–2798. doi: 10.1210/jcem.82.9.4148. [DOI] [PubMed] [Google Scholar]
- 29.Marie PJ, de Vernejoul MC, Connes D, Hott M. Decreased DNA synthesis by cultured osteoblastic cells in eugonadal osteoporotic men with defective bone formation. J Clin Invest. 1991;88:1167–1172. doi: 10.1172/JCI115418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parfitt A, Weinstein R. Idiopathic osteoporosis: is the osteoblast to blame? J Clin Endocrinol Metab. 1998;83:716. doi: 10.1210/jcem.83.2.4587-5. [DOI] [PubMed] [Google Scholar]
- 31.Rubin MR, Schussheim DH, Kulak CA, Kurland ES, Rosen CJ, Bilezikian JP, Shane E. Idiopathic osteoporosis in premenopausal women. Osteoporos Int. 2005;16:526–533. doi: 10.1007/s00198-004-1716-0. [DOI] [PubMed] [Google Scholar]
- 32.Donovan MA, Dempster D, Zhou H, McMahon DJ, Fleischer J, Shane E. Low bone formation in premenopausal women with idiopathic osteoporosis. J Clin Endocrinol Metab. 2005;90:3331–3336. doi: 10.1210/jc.2004-2042. [DOI] [PubMed] [Google Scholar]
- 33.Thompson PW, Taylor J, Dawson A. The annual incidence and seasonal variation of fractures of the distal radius in men and women over 25 years in Dorset, UK. Injury. 2004;35:462–466. doi: 10.1016/S0020-1383(03)00117-7. [DOI] [PubMed] [Google Scholar]
- 34.Wu F, Mason B, Horne A, Ames R, Clearwater J, Liu M, Evans MC, Gamble GD, Reid IR. Fractures between the ages of 20 and 50 increase women’s risk of subsequent fractures. Arch Int Med. 2002;162:33–36. doi: 10.1001/archinte.162.1.33. [DOI] [PubMed] [Google Scholar]
- 35.Lewiecki EM. Premenopausal bone health assessment. Curr Rheumatol Rep. 2005;7:46–52. doi: 10.1007/s11926-005-0008-9. [DOI] [PubMed] [Google Scholar]
- 36.Cohen A, Liu XS, Stein EM, McMahon DJ, Rogers HF, Lemaster J, Recker RR, Lappe JM, Guo XE, Shane E. Bone microarchitecture and stiffness in premenopausal women with idiopathic osteoporosis. J Clin Endocrinol Metab. 2009;94:4351–4360. doi: 10.1210/jc.2009-0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu XS, Cohen A, Shane E, et al. Individual trabeculae segmentation (ITS)-based morphological analysis of high-resolution peripheral quantitative computed tomography images detects abnormal trabecular plate and rod microarchitecture in premenopausal women with idiopathic osteoporosis. J Bone Miner Res. 2010;25:1496–1505. doi: 10.1002/jbmr.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Subar AF, Thompson FE, Kipnis V, Midthune D, Hurwitz P, McNutt S, McIntosh A, Rosenfeld S. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: the Eating at America’s Table Study. Am J Epidemiol. 2001;154:1089–1099. doi: 10.1093/aje/154.12.1089. [DOI] [PubMed] [Google Scholar]
- 39.Pols MA, Peeters PH, Bueno-De-Mesquita HB, Ocke MC, Wentink CA, Kemper HC, Collette HJ. Validity and repeatability of a modified Baecke questionnaire on physical activity. Int J Epidemiol. 1995;24:381–388. doi: 10.1093/ije/24.2.381. [DOI] [PubMed] [Google Scholar]
- 40.Mintz LB, O’Halloran MS. The eating attitudes test: validation with DSM-IV eating disorder criteria. J Pers Assess. 2000;74:489–503. doi: 10.1207/S15327752JPA7403_11. [DOI] [PubMed] [Google Scholar]
- 41.Sodergard R, Backstrom T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem. 1982;16:801–810. doi: 10.1016/0022-4731(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 42.Heaney R, Recker R. Estimation of true calcium absorption. Ann Intern Med. 1985;103:516–521. doi: 10.7326/0003-4819-103-4-516. [DOI] [PubMed] [Google Scholar]
- 43.Black DM, Palermo L, Nevitt MC, Genant HK, Epstein R, San Valentin R, Cummings SR. Comparison of methods for defining prevalent vertebral deformities: the study of osteoporotic fractures. J Bone Miner Res. 1995;10:890–902. doi: 10.1002/jbmr.5650100610. [DOI] [PubMed] [Google Scholar]
- 44.Alatalo SL, Halleen JM, Hentunen TA, Monkkonen J, Vaananen HK. Rapid screening method for osteoclast differentiation in vitro that measures tartrate-resistant acid phosphatase 5b activity secreted into the culture medium. Clin Chem. 2000;46:1751–1754. [PubMed] [Google Scholar]
- 45.Henriksen K, Tanko LB, Qvist P, Delmas PD, Christiansen C, Karsdal MA. Assessment of osteoclast number and function: application in the development of new and improved treatment modalities for bone diseases. Osteoporos Int. 2007;18:681–685. doi: 10.1007/s00198-006-0286-8. [DOI] [PubMed] [Google Scholar]
- 46.Khosla S. Editorial: Idiopathic osteoporosis—Is the osteoblast to blame? J Clin Endocrinol Metab. 1997;82:2792–2793. doi: 10.1210/jcem.82.9.4264. [DOI] [PubMed] [Google Scholar]
- 47.Fox KM, Magaziner J, Sherwin R, Scott JC, Plato CC, Nevitt M, Cummings S. Reproductive correlates of bone mass in elderly women. Study of Osteoporotic Fractures Research Group. J Bone Miner Res. 1993;8:901–908. doi: 10.1002/jbmr.5650080802. [DOI] [PubMed] [Google Scholar]
- 48.Ito M, Yamada M, Hayashi K, Ohki M, Uetani M, Nakamura T. Relation of early menarche to high bone mineral density. Calcif Tissue Int. 1995;57:11–14. doi: 10.1007/BF00298989. [DOI] [PubMed] [Google Scholar]
- 49.Ferrari SL, Chevalley T, Van Rietbergen B, Bonjour JP, Rizzoli R. Reduced bone strength in young adult women with clinical fractures during childhood or adolescence in American Society for Bone and Mineral Research Annual Meeting. J Bone Miner Res. 2010;25(Suppl 1) Presentation No: 1127. [Google Scholar]