Abstract
There is considerable evidence that sphingosine kinases play a key role in cancer progression, which might involve positive selection of cancer cells that have been provided with a survival and growth advantage as a consequence of over-expression of the enzyme. Therefore, inhibitors of sphingosine kinase represent a novel class of compounds that have potential as anti-cancer agents. Poor inhibitor potency is a major issue that has precluded successful translation of these compounds into the clinic. However, recent discoveries have shown that sphingosine kinase 1 is an allosteric enzyme and that some inhibitors offer improved effectiveness by inducing proteasomal degradation of the enzyme or having nanomolar potency. Herein, we provide a perspective about these recent developments and highlight the importance of translating basic pharmacological and biochemical findings on sphingosine kinase into new drug discovery programmes for treatment of cancer.
Keywords: sphingosine 1-phosphate, sphingosine kinase, cell death, proteasomal degradation, allosterism
The role of sphingosine kinase in cancer
The levels of the bioactive sphingolipid, sphingosine 1-phosphate (S1P) are controlled by its synthesis (conversion of sphingosine to S1P, catalysed by the two isoforms of sphingosine kinase, SK1 and SK2) and removal (by cleavage of S1P catalysed by S1P lyase or dephosphorylation catalysed by S1P phosphatase) (1). S1P binds to sphingosine 1-phosphate (S1P)-specific G-protein coupled receptors termed S1P1–5 (1). S1P also binds to intracellular protein targets (see later).
There is evidence of a major role for sphingosine kinase in human cancers. For instance, there is elevated SK1 mRNA transcript and/or SK1 protein expression in stomach, lung, brain, colon, kidney and breast cancers and non-Hodgkins lymphoma (1). Indeed, we reported that high tumour expression of SK1 is correlated with poor patient survival rates and induction of tamoxifen resistance in ER+ breast cancer patients (n = 304) (2, 3). Moreover, S1P promotes migration of ER+ MCF-7 breast cells via an SK1-dependent mechanism, and this might suggest a role for SK1 in metastasis (2). Ectopic over-expression of SK1 in MCF-7 cells also induces resistance to tamoxifen (see (1) for review). In addition, SK1 expression is higher in ER− compared with ER+ breast tumours and this is correlated with a poorer prognosis (see (1) for review). Similarly, high expression of SK1 in astrocytoma correlates with poor prognosis and knock-down of SK1 reduces glioblastoma cell proliferation (see (1) for review). Therefore, SK1 appears to play a role in two major hallmarks of cancer, namely enhanced proliferation and metastasis/invasion. In addition, the over-expression of SK1 in fibroblasts induces their transformation to fibrosarcoma (see (1) for review). S1P is also involved in regulating angiogenesis and creation of a tumour microenvironment. This is exemplified by the use of the sphingosine analogue, FTY720, which is converted to (S)-FTY720 phosphate in vivo by SK2 and has recently been licensed (FDA/EMA-Gilenya™) for the treatment of relapsing multiple sclerosis (4). (S)-FTY720 phosphate binds to S1P1,3,4,5 and is a functional antagonist of S1P1 (4), while FTY720 is a direct inhibitor of SK1 activity (5). Moreover, FTY720 reduces tumour metastasis in a mouse melanoma model and decreases tumour cell proliferation and increases apoptosis due to inhibition of neovascularisation (see (1) for review). FTY720 also decreases metastasis in a breast cancer mouse model which is associated with deformed and decreased filopodia formation in the cancer cells (see (1) for review). Additional in vivo evidence supports a role for SK1 as a chemotherapeutic ‘sensor’ for promotion of tumourgenesis. Large vascularised resistant tumours are formed when cancer cells over-expressing SK1 are injected or implanted into mice (see (1) for review).
There are multiple mechanisms that regulate the expression of SK1. For instance, the SK1 gene is regulated by AP2, Sp1, SMAD4 (6), and HIF2α (see (1) for review), suggesting that SK1 expression might be controlled by mitogen-activated protein kinase signalling, cytokines, and hypoxia (in solid tumours). Moreover, a number of growth factors and steroid hormones regulate the expression of SK1, such as TGFβ, oestrogen, and progesterone (1, 7, 8). SK1 expression in cells is also regulated by proteolysis. For instance, cathepsin B has been implicated in regulating lysosomal degradation of SK1 in podocytes (9). SK1 expression is also regulated by the ubiquitin-proteasomal pathway in LNCaP prostate cancer and MCF-7 breast cancer cells (5, 10), raising the possibility that this route of degradation might be de-regulated in certain cancers. In summary, altered expression of SK1 underlies the major cancer promoting properties of this enzyme. Cancer cells that over-express SK1 appear to exhibit a non-oncogenic addiction for SK1 (see (1) for review). This is defined by a positive selection of cancer cells because elevated SK1 expression confers a survival and growth advantage to these cells.
SK2 also has a role in cancer. Thus, siRNA knock-down of SK2 in breast or colon cancer cells reduces doxorubicin-induced expression of p21 (a cyclin-dependent kinase inhibitor) and G2/M arrest and enhances doxorubicin-induced apoptosis. Moreover, breast or colon cancer progression is reduced upon knock-down of SK2 (see (1) for review). In addition, EGF stimulates the ERK1-catalysed phosphorylation of SK2 on Ser 351 and Thr578, which is required for the migration of MCF-7 breast cancer cells in response to this growth factor (see (1) for review).
The need for S1P therapeutics
The major objective of drug discovery has focused on new molecules that are capable of agonising/antagonising S1P1–5. A prominent example is FTY720, which via transformation to (S)-FTY720 phosphate has been developed as a functional S1P1 antagonist (4). Another approach is to reduce the bioavailability of S1P at its receptors using S1P neutralising antibodies. Formulation of humanised S1P neutralising antibody is currently being evaluated in Phase 1 clinical trials for solid tumours (11).
However, in addition to acting on cell-surface S1P receptors, S1P also binds to and modulates the activity of intracellular proteins. Novel intracellular targets of S1P include histone deacetylase (HDAC) (12), TRAF2 (13), p21 activated protein kinase 1 (PAK1) (14), and prohibitin 2 (15). S1P formed by SK2 binds to and inhibits HDAC1 and HDAC2 within repressor complexes that are enriched at the promoters of genes encoding p21 (a cyclin-dependent kinase inhibitor) and c-fos (a transcriptional regulator). S1P inhibition of HDACs increases histone acetylation, thereby promoting expression of p21 and c-fos (12). The identification of TRAF2 as a target for S1P formed by SK1 (13) is particularly important given its role in regulating NFκB in inflammation and cancer. S1P activates TRAF2, which is an E3 ligase that catalyses the ubiquitination of RIP1 (Lys63-signalling ubiquitination), required for NFκB activation (13). Indeed, NFκB is linked with the acquisition of resistance of prostate cancer cells to docetaxel (16). S1P formed by SK1 has also been shown to directly activate PAK1 (14). Heregulin-induced migration requires SK1 and is contingent on a heregulin-induced co-localisation of SK1 with filamin A in lamellopodia in A7 melanoma cells. Down-regulation of SK1 markedly reduces activation of PAK1 and phosphorylation of filamin A (14). S1P formed by SK2 also binds directly to prohibitin-2 (PHB2) at the inner mitochondrial membrane and induces the correct assembly of the cytochrome c oxidase complex (respiratory complex IV) required for oxidative phosphorylation (15).
SK inhibitors
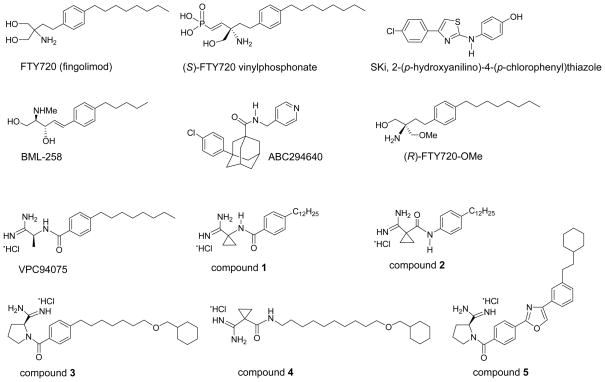
The studies described above highlight the importance of limiting the intracellular action of S1P in cancer by developing inhibitors of SK1 and SK2. Indeed, there has been a concerted effort to produce inhibitors. One of the first to be identified was SKi (or SKI-II, 2-(p-hydroxyanilino)-4-(p-chlorophenyl)thiazole, Fig. 1), which inhibits both SK1 and SK2 activity. SKi reduces intracellular S1P, inhibits proliferation, and induces apoptosis in various cancer cell lines (17). In vivo studies demonstrated good orally bioavailability and inhibition of tumour growth (18). A water-soluble sphingosine analogue, BML-258 (SK1-I; (2R,3S,4E)-N-methyl-5-(4′-pentylphenyl)-2-aminopent-4-ene-1,3-diol, Fig. 1) is an SK1-selective inhibitor (19) that is effective both in vitro and in vivo (20). ABC294640 (3-(4-chlorophenyl)-adamantane-1-carboxylic acid (pyridin-4-ylmethyl)amide, Fig. 1) is a selective competitive (with sphingosine) SK2 inhibitor (21), which is an effective orally bioavailable anti-cancer agent, and inhibits tumour proliferation and migration in vitro (21, 22). ABC294640 induces autophagic cell death in PC-3 prostate, MDA-MB-231 breast, and A-489 kidney tumour cells (22). (R)-FTY720-OMe is a new analogue where one of the prochiral hydroxyl groups of FTY720 has been replaced by a methoxy group (Fig. 1 (23)). The rationale for replacing one of the hydroxyl groups with a methyl ether was to block the site that is phosphorylated by SK2. (R)-FTY720-OMe is a specific, competitive (with sphingosine) inhibitor of SK2 (with no effect on SK1) with a Kic of 16 μM (23). The enantiomer of (R)-FTY720-OMe does not inhibit SK2. (R)-FTY720-OMe also induces growth arrest of MCF-7 breast cancer cells, thereby providing an additional means of reducing proliferation of these cells. (R)-FTY720-OMe also inhibits S1P-induced actin rearrangement in MCF-7 cells, thereby preventing formation of a migratory phenotype, suggesting an additional application to preventing metastasis (23).
Fig. 1.
Structural formulae of FTY720 (fingolimod), (S)-FTY720 vinylphosphonate, SKi, BML-258, (R)-FTY720-OMe, ABC294640 and six amidine-based analogues that have been demonstrated to inhibit SK1 and/or SK2 activity.
Recent enzyme kinetic studies revealed that FTY720 is a novel competitive (with sphingosine) inhibitor of SK1 with a Kic of 2μM (5, 24). The KM of SK2 for FTY720 is 18.2 μM (13.8μM for sphingosine) (25). However, the Vmax for FTY720 is appreciably lower than for sphingosine (FTY720, 5.1 nmol/min/mg protein; sphingosine, 43 nmol/min/mg protein); thus, FTY720 is also an effective inhibitor of SK2 activity where sphingosine is the substrate (unpublished). SKi is a mixed inhibitor of SK1 (with sphingosine) with Kic =17 μM and Kiu = 48.3 μM and thus, at low micromolar concentration, favours competitive inhibition (24). We have synthesised multiple analogues of FTY720 and established that minor modifications in chemical scaffold have profound effects on effectiveness and selectivity (Fig. 1). Inhibitor characterisation studies reveal that (S)-FTY720 vinylphosphonate inhibits SK1 in an uncompetitive manner (with sphingosine) with Kiu = 14.5 μM (24). This mode of inhibition is indicative of allosterism contingent on formation of the sphingosine-SK1 complex. Two new FTY720 analogues, a conjugate of sphingosine with a fluorophore (Bodipy-sphingosine (Bdp-So)) and (S)-FTY720 regioisomer (in which the position of the amino group and a hydroxyl group are interchanged in the molecule), stimulate SK1 activity (24), thereby providing additional evidence for the presence of allosteric site(s). SK1 is an oligomeric protein (minimally a dimer) containing non-cooperative catalytic sites and that the allosteric site(s) exert an auto-inhibition of the catalytic site. We have proposed a model in which the allosteric site exists in an ‘on’ and ‘off’ state in equilibrium (24). Thus, the binding of (S)-FTY720 vinylphosphonate to SK1 stabilises the ‘on’ state, which is predicted to have longer lifetime in terms of auto-inhibiting activity than SK1 with an unbound allosteric site. Conversely, activators of SK1, e.g. Bdp-So and (S)-FTY720 regioisomer, stabilise the ‘off’ state to relieve the inhibition of SK1 activity by the allosteric site and to therefore, stimulate the enzyme activity.
An issue of effectiveness: ability to induce the proteasomal degradation of SK1
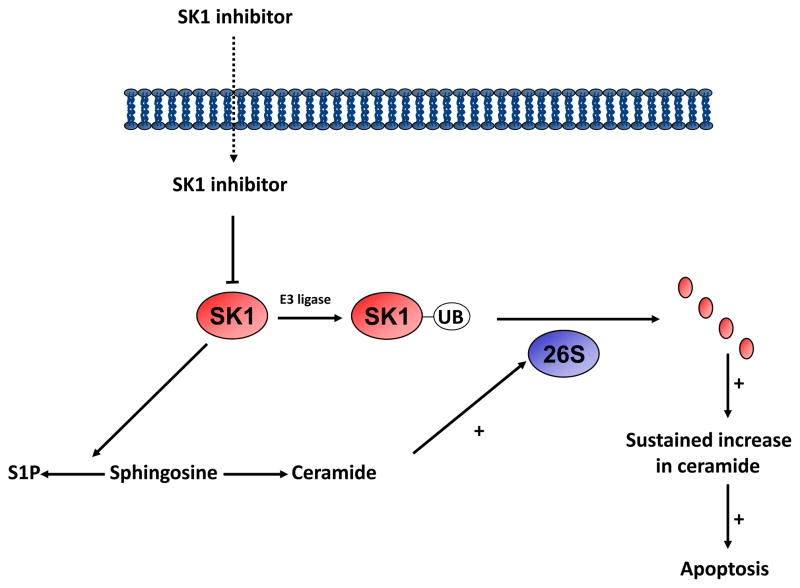
We have proposed an entirely novel mechanism for the interaction of inhibitors with SK1 that offers additional opportunities for drug discovery. SK1 inhibitors activate the ubiquitin-proteasomal degradation pathway to remove SK1 from MCF-7 breast cancer and LNCaP prostate cancer cells (5, 10, 24) (Fig. 2). The SKi-induced proteasomal degradation of SK1 is reduced by the proteasomal inhibitor, MG132. In podocytes, SKi also induces the proteolytic degradation of SK1, but this is mediated by a lysosomal- and cathepsin B (CA074Me-sensitive)-dependent pathway (9). The treatment of prostate cancer cells with the cathepsin B inhibitor CA074Me has no effect on the SKi-induced degradation of SK1 suggesting that this route of degradation does not operate in these cells (10). The proteasomal degradation of SK1 is partly dependent on the inhibition of SK1 activity, the accumulation of ceramide, and the subsequent ceramide-induced activation of the proteasome (10). SK1 is polyubiquitinated, and SK1 inhibitors enhance the rate at which this modified version of the enzyme is degraded by the proteasome (10). Evidence to support a role for ceramide was obtained using the mycotoxin Fuminosin B1, which inhibits the conversion of dihydrosphingosine to dihydroceramide and sphingosine to ceramide catalysed by ceramide synthase. Fuminosin B1 treatment partially reversed the effect of SKi on the proteasomal degradation of SK1 (10). The creation of an SK1 null cancer cell leads to the onset of apoptosis (5, 10). This is associated with a reduction in intracellular S1P levels and an elevation of C22:0-ceramide (10). Moreover, these effects are recapitulated by siRNA knock-down of SK1 in MCF-7 cells (see (1) for review). We also found that FTY720 and (S)-FTY720 vinylphosphonate induce the proteasomal degradation of SK1 in MCF-7 cells (5, 24), suggesting that a wide range of structurally diverse SK1 inhibitors utilise this common pathway to remove SK1 from cancer cells. FTY720 itself also has additional targets (See (1) for review), including activation of PP2A (26) and which might have a significant effect on cell-cycle progression.
Fig. 2.
A schematic representation of the effect of SK1 inhibitors in promoting the ubiquitin-proteasomal degradation of SK1. Acute inhibition of SK1 by the SK1 inhibitor appears to promote a ceramide-dependent proteasomal degradation of SK1 that is polyubiquitinated under basal conditions. 26S represents the proteasome.
An issue of potency: designing new nanomolar inhibitors
To date, most inhibitors of SK1 exhibit Ki or IC50 values in the micromolar range, which largely preclude their use as therapeutic agents. This places constraints on bioavailability and increases the potential for ‘off-target’ effects, even though some of these inhibitors are effective in in vivo cancer models. This has, therefore, hampered progress to the clinic. However, recent advances in this area have generated optimism that new inhibitors may become available that can be translated to the clinic. For example, Macdonald, Lynch, and colleagues have recently synthesised amidine-based SK1 inhibitors with nanomolar potency that reduce endogenous S1P levels in human leukemia U937 cells (27). The lead compound was the amidine analogue VPC94075 ((S)-N-(1-amino-1-iminopropan-2-yl)-4-octylbenzamide hydrochloride; see Fig. 1), which exhibits selectivity for SK (competitive with sphingosine), with no activity against diacylglycerol kinase and protein kinase C. Selectivity for SK1 over SK2 was dependent on the length of the apolar tail. Compounds with a short (C8 and C10) apolar tail inhibited both SK1 and SK2, while those with a longer tail length (C12) such as compounds 1 and 2 (Fig. 1) were selective for SK1. This can be explained by a model in which the catalytic binding pocket of SK1 is larger than for SK2. The SK1/SK2 selectivity of compound 2 was higher than that of compound 1, suggesting that the orientation of the carboxamide moiety (phenyl-NHCO- vs. phenyl-CONH-) affects ligand binding to SK1 and SK2. Analogues containing a pyrrolidine-2-carboximidamide head group were synthesized; for example, compounds 3 and 4 (Fig. 1) that exhibited Ki values of 75 and 110 nM for SK1 with an 80- and 470-fold selectivity for SK1 over SK2, respectively (27). An SK1 homology model based on the crystal structure of diacylglycerol kinase was employed in which linkers were docked into the SK1 model. A class of heteroaromatic compounds with six fewer rotatable bonds was also synthesized. Of these, oxazole 5 (Fig. 1) was identified as an SK1 inhibitor with a Ki = 47 nM and 180-fold selectivity over SK2. Therefore, a major barrier to translation of SK1 inhibitors, with respect to potency, has been removed.
An attack on cancer: the ‘Hydra effect’
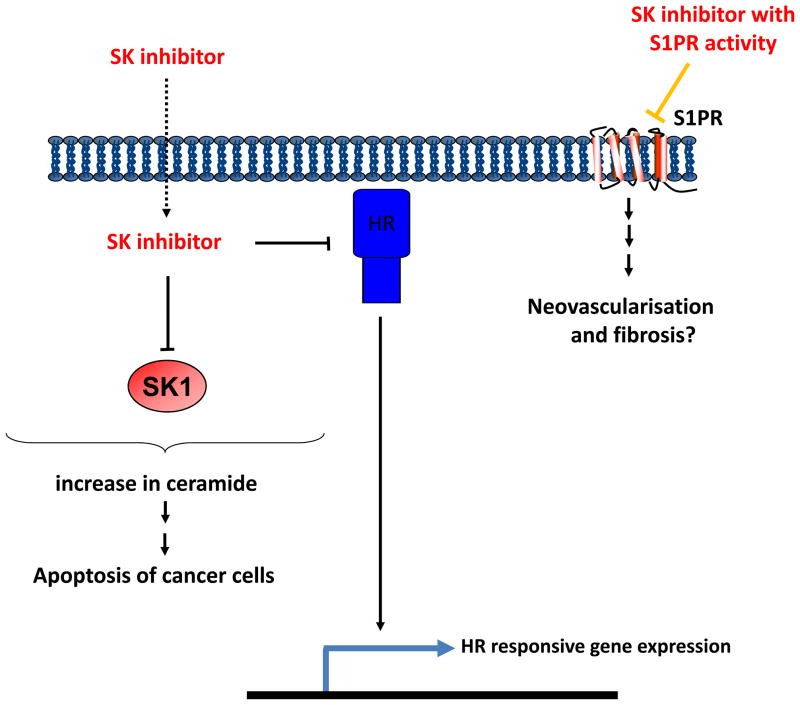
S1P formed by SK1 can be released from cancer cells and has the potential to bind to S1P receptors on the cell surface to inhibit apoptosis. This process is called ‘inside-out’ signalling (see (1) for review). Indeed, high membrane S1P1 expression in tumours of ER+ breast cancer patients (n = 304) is associated with the acquisition of resistance to tamoxifen (3). S1P released from cancer cells also has the potential to bind to S1P receptors on endothelial cells to promote their proliferation, leading to neovascularisation of the tumour. S1P can also act on fibroblasts within tumours, which might also function to sustain cancer progression through growth factors released from the fibrotic cells (28). Thus, a compound that combines properties that block these cellular interactions would be highly advantageous in the treatment of cancer. Antagonism of S1P receptors might be effective at blocking neovascularisation, fibrosis, and cancer cell proliferation, while binding of the compound to SK1 might promote its proteasomal degradation to induce apoptosis of fibroblasts and cancer cells (Fig. 3). Multiple effects may also underlie the action of SKi and ABC294640, which in addition to inhibiting SK1/SK2 and SK2, respectively, can bind to and antagonise the oestrogen receptor in breast cancer cells (29, 30). SKi, FTY720, or (S)-FTY720 vinylphosphonate also reduce the expression of the androgen receptor in androgen-independent LNCaP-AI cells (5), thereby offering an additional therapeutic option, as the constitutively active androgen receptor is responsible for driving androgen-independent growth of these cells.
Fig. 3.
A schematic model of the ‘Hydra effect’ of SK inhibitors. Inhibitors such as (S)-FTY720 vinylphosphonate that exhibit multiple actions on S1P-related proteins, including S1P1, SK1, and SK2 might be usefully exploited to launch a multi-prong attack on cancer. HR represents hormone receptor corresponding to androgen or oestrogen receptors.
(S)-FTY720 vinyl phosphonate also inhibits SK2 (23) and is a full antagonist of S1P1,3,4 (Ki 208 nM, 15 nM, and 1190 nM, respectively) and a partial antagonist of S1P2 and S1P5 (31). The fact that (S)-FTY720 vinylphosphonate exhibits a Ki of 14.5 μM for SK1 inhibition (24) is not incompatible with a dual action at both S1P receptor and SK1, even under conditions where the extracellular concentration of (S)-FTY720 vinylphosphonate is in the nanomolar range. We draw analogy with the action of FTY720. In this regard, nanomolar concentrations of FTY720 are clinically effective in the treatment of multiple sclerosis. FTY720 is phosphorylated by SK2 to provide the clinically effective agent, (S)-FTY720 phosphate, which acts on four of the five S1P receptors. SK2 exhibits a KM of 18 μM for FTY720 (25). Therefore, from an extracellular nanomolar concentration of FTY720, it seems likely that the cells concentrate the inhibitor, such that an intracellular concentration can accumulate into the range where it can be efficiently phosphorylated by SK2. This would also bring the concentration of FTY720 into a range that inhibits and induces proteasomal degradation of SK1 in cancer cells.
(S)-FTY720 vinylphosphonate (which is a putative allosteric inhibitor (24)) induces the proteasomal degradation of an N-terminal variant of SK1 (86 amino-acid N-terminal variant) in androgen-independent LNCaP-AI cells (10). This is significant because the N-terminal SK1 variant is resistant or less sensitive to proteasomal degradation induced by SKi and FTY720 respectively in these cells (10). Therefore, allosteric inhibitors might force SK1 to adopt a conformation that improves susceptibility to proteasomal degradation. These findings are important because resistance to chemotherapeutic agents is correlated with high expression of SK1. This includes prostate cancer cells resistant to camptothecin, pancreatic cancer cells to gemcitabine and chronic myeloid leukaemia (CML) cells to imatinib (see (1) for review). Therefore, targeting SK1 to the proteasome opens avenues for restoring sensitivity of cancer cells to chemotherapeutic agents and provides a rationale for combination therapies with SK1 inhibitors.
Conclusion
The evidence presented here suggests that SK1 inhibitors have the potential to be the ‘golden sword’ of Hercules because they may cut off the many heads of cancer. Thus, inhibitors that can kill cancer cells by this ‘Hydra effect’ (named after the Greek mythological monster with nine heads) offer the potential for translation to the clinic. There are several additional important questions that have been raised by recent advances. For instance, can new sphingosine analogues be developed to generate a wider structurally diverse library of compounds with nanomolar potency? Is the enhanced potency of amidine SK1 inhibitors augmented by the proteasomal degradation of SK1? In addition to these questions, we suggest that SK allosterism offers new drug discovery opportunities. Indeed, the elucidation of the structure of sphingosine kinases by X-ray crystallography may provide the means by which development of high potency novel catalytic and allosteric inhibitors can be accelerated.
Acknowledgments
Financial support: SP/NJP: Cancer Research UK (23158/A7536); RB: National Institutes of Health (HL-083187)
Abbreviations
- AP2
Activating protein 2
- Bcl2
B cell lymphoma 2
- ER
oestrogen receptor
- HDAC
histone deacetylase
- HIF
hypoxic-inducible factor
- PAK1
p21 activated protein kinase 1
- SK
sphingosine kinase
- S1P
sphingosine 1-phosphate
- Sp1
specificity protein 1
- TGF
transforming growth factor
- TRAF2
tumour necrosis factor receptor associated factor 2
- UB
ubiquitin
Footnotes
Disclosure: No conflicts of interest
References
- 1.Pyne NJ, Pyne S. Sphingosine 1-phosphate and cancer. Nature Rev Cancer. 2010;10:489–503. doi: 10.1038/nrc2875. [DOI] [PubMed] [Google Scholar]
- 2.Long JS, Edwards J, Watson C, Tovey S, Mair K, Schiff R, et al. Sphingosine kinase 1 induces tolerance to human epidermal growth factor receptor 2 and prevents formation of a migratory phenotype in response to sphingosine 1-phosphate in estrogen receptor positive breast cancer cells. Mol Cell Biol. 2010;30:3827–3841. doi: 10.1128/MCB.01133-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watson C, Long JS, Orange C, Tannahill CL, Mallon E, McGlynn LM, et al. High expression of sphingosine 1-phosphate receptors, S1P1 and S1P3, sphingosine kinase 1, and extracellular signal-regulated kinase-1/2 is associated with development of tamoxifen resistance in estrogen receptor-positive breast cancer patients. Am J Pathology. 2010;177:2205–2215. doi: 10.2353/ajpath.2010.100220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R, Bruns C, et al. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem. 2002;277:21453–21457. doi: 10.1074/jbc.C200176200. [DOI] [PubMed] [Google Scholar]
- 5.Tonelli F, Lim KG, Loveridge C, Long J, Pitson SM, Tigyi G, et al. FTY720 and (S)-FTY720 vinylphosphonate inhibit sphingosine kinase 1 and promote its proteasomal degradation in human pulmonary artery smooth muscle, breast cancer and androgen-independent prostate cancer cells. Cell Signal. 2010;22:1536–1542. doi: 10.1016/j.cellsig.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ren S, Babelova A, Moreth K, Xin C, Eberhardt W, Doller A, et al. Transforming growth factor-beta2 upregulates sphingosine kinase-1 activity, which in turn attenuates the fibrotic response to TGF-beta2 by impeding CTGF expression. Kidney Int. 2009;76:857–867. doi: 10.1038/ki.2009.297. [DOI] [PubMed] [Google Scholar]
- 7.Kono Y, Nishiuma T, Nishimura Y, Kotani Y, Okada T, Nakamura S, et al. Sphingosine kinase 1 regulates differentiation of human and mouse lung fibroblasts mediated by TGF-beta1. Am J Respir Cell Mol Biol. 2007;37:395–404. doi: 10.1165/rcmb.2007-0065OC. [DOI] [PubMed] [Google Scholar]
- 8.Jeng YJ, Suarez VR, Izban MG, Wang HQ, Soloff MS. Progesterone-induced sphingosine kinase-1 expression in the rat uterus during pregnancy and signaling consequences. Am J Physiol Endocrinol Metab. 2007;292:E1110–1121. doi: 10.1152/ajpendo.00373.2006. [DOI] [PubMed] [Google Scholar]
- 9.Ren S, Xin C, Pfeilschifter J, Huwiler A. A novel mode of action of the putative sphingosine kinase inhibitor 2-(p-hydroxyanilino)-4-(p-chlorophenyl)thiazole (SKI II): induction of lysosomal sphingosine kinase 1 degradation. Cell Physiol Biochem. 2010;26:97–104. doi: 10.1159/000315110. [DOI] [PubMed] [Google Scholar]
- 10.Loveridge C, Tonelli F, Leclecq T, Lim KG, Long S, Berdyshev E, et al. The sphingosine kinase 1 inhibitor 2-(p-hydroxyanilino)-4-(p-chlorophenyl)thiazole induces proteasomal degradation of sphingosine kinase 1 in mammalian cells. J Biol Chem. 2010;285:38841–38852. doi: 10.1074/jbc.M110.127993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Visentin B, Vekich JA, Sibbald BJ, Cavalli AL, Moreno KM, Matteo RG, et al. Validation of an anti-sphingosine 1-phosphate antibody as a potential therapeutic in reducing growth, invasion and angiogenesis in multiple tumour lineages. Cancer Cell. 2006;9:225–238. doi: 10.1016/j.ccr.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 12.Hait NC, Allegood J, Maceyka M, Strub GM, Harikumar KB, Singh SK, et al. Regulation of histone acetylation in the nucleus by sphingosine 1-phosphate. Science. 2009;325:1254–1257. doi: 10.1126/science.1176709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alvarez SE, Harikumar KB, Hait NC, Allegood J, Strub GM, Kim EY, et al. Sphingosine 1-phosphate is a missing co-factor for the E3 ligase TRAF2. Nature. 2010;465:1084–1088. doi: 10.1038/nature09128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maceyka M, Alvarez SE, Milstien S, Spiegel S. Filamin A links sphingosine kinase 1 and sphingosine-1-phosphate receptor 1 at lamellipodia to orchestrate cell migration. Mol Cell Biol. 2008;28:5687–5697. doi: 10.1128/MCB.00465-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strub GM, Paillard M, Liang J, Gomez L, Allegood JC, Hait NC, et al. Sphingosine-1-phosphate produced by sphingosine kinase 2 in mitochondria interacts with prohibitin 2 to regulate complex IV assembly and respiration. FASEB J. 2011;25:600–612. doi: 10.1096/fj.10-167502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Domingo-Domenech J, Oliva C, Rovira A, Codony-Servat J, Bosch M, Filella X, et al. Interleukin 6, a nuclear-κB target predicts resistance to docetaxel in hormone-independent prostate cancer and nuclear factor-κB inhibition by PS-1145 enhances docetaxel anti-tumour activity. Clin Cancer Res. 2006;12:5578–5586. doi: 10.1158/1078-0432.CCR-05-2767. [DOI] [PubMed] [Google Scholar]
- 17.French KJ, Schrecengost RS, Lee BD, Zhuang Y, Smith SN, Eberly JL, et al. Discovery and evaluation of inhibitors of human sphingosine kinase. Cancer Res. 2003;63:5962–5969. [PubMed] [Google Scholar]
- 18.French KJ, Upson JJ, Keller SN, Zhuang Y, Yun JK, Smith CD. Antitumour activity of sphingosine kinase inhibitors. J Pharmacol Exp Ther. 2006;318:596–603. doi: 10.1124/jpet.106.101345. [DOI] [PubMed] [Google Scholar]
- 19.Paugh SW, Paugh BS, Rahmani M, Kapitonov D, Almenara JA, Kordula T, et al. A selective sphingosine kinase 1 inhibitor integrates multiple molecular therapeutic targets in human leukaemia. Blood. 2008;112:1382–1391. doi: 10.1182/blood-2008-02-138958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kapitonov D, Allegood JC, Mitchell C, Hait NC, Almenara JA, Adams JK, et al. Targeting sphingosine kinase 1 inhibits AKT signalling induces apoptosis and suppresses growth of human glioblastoma cells and xenografts. Cancer Res. 2009;69:6915–6923. doi: 10.1158/0008-5472.CAN-09-0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.French KJ, Zhuang Y, Maines LW, Gao P, Wang W, Beljanski V, Upson JJ, et al. Pharmacology and antitumour activity of ABC294640, a selective inhibitor of sphingosine kinase-2. J Pharmacol Exp Ther. 2010;333:129–139. doi: 10.1124/jpet.109.163444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beljanski V, Knaak C, Smith CD. A novel sphingosine kinase inhibitor induces autophagy in tumour cells. J Pharmacol Exp Ther. 2010;333:454–464. doi: 10.1124/jpet.109.163337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim KG, Sun C, Bittman R, Pyne NJ, Pyne S. (R)-FTY720 methyl ether is a specific sphingosine kinase 2 inhibitor: effect on sphingosine kinase 2 expression in HEK 293 cells and actin rearrangement and survival of MCF-7 breast cancer cells. Cell Signal. 2011 doi: 10.1016/j.cellsig.2011.05.010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lim KG, Tonelli F, Li Z, Lu X, Bittman R, Pyne S, et al. FTY720 analogues as sphingosine kinase 1 inhibitors: Enzyme inhibition kinetics, allosterism, proteasomal degradation and actin rearrangement in MCF-7 breast cancer cells. J Biol Chem. 2011;286:18633–18640. doi: 10.1074/jbc.M111.220756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Billich A, Bornacin F, Devay P, Mechtcheriakova D, Urtz N, Baumruker T. Phosphorylation of the immunomodulatory drug, FTY720 by sphingosine kinase. J Biol Chem. 2003;278:47408–47415. doi: 10.1074/jbc.M307687200. [DOI] [PubMed] [Google Scholar]
- 26.Matsuoka Y, Nagahara Y, Ikekita M, Shinomiya T. A novel immunosuppressive agent FTY720 induced Akt dephosphorylation in leukemia cells. Br J Pharmacol. 2003;138:1303–1312. doi: 10.1038/sj.bjp.0705182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kennedy AJ, Mathews TP, Kharel Y, Field SD, Moyer ML, East JE, et al. Development of amidine-based sphingosine kinase 1 nanomolar inhibitors and reduction of sphingosine 1-phosphate in human leukemia cells. J Med Chem. 2011;54:3524–3548. doi: 10.1021/jm2001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hawinkels LJ, Ten Dijke P. Exploring anti-TGF-β therapies in cancer and fibrosis. Growth Factors. 2011 doi: 10.3109/08977194.2011.595411. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 29.Antoon JW, Meacham WD, Bratton MR, Slaughter EM, Rhodes LV, Ashe HB, et al. Pharmacological inhibition of sphingosine kinase isoforms alters estrogen receptor signaling in human breast cancer. J Mol Endocrinol. 2011;46:205–216. doi: 10.1530/JME-10-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Antoon JW, White MD, Meacham WD, Slaughter EM, Muir SE, Elliott S, et al. Antiestrogenic effects of the novel sphingosine kinase-2 inhibitor ABC294640. Endocrinology. 2010;151:5124–5135. doi: 10.1210/en.2010-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valentine WJ, Kiss GN, Liu JES, Gotoh M, Murakami-Murofushi K, Pham TC, et al. (S)-FTY720-vinylphosphonate, an analogue of the immunosuppressive agent FTY720, signals as a pan-antagonist of sphingosine 1-phosphate GPCR and inhibits autotaxin activity. Cell Signal. 2010;22:1543–1553. doi: 10.1016/j.cellsig.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]