Abstract
STAT3 has important functions in both tumor cells and the tumor microenvironment to facilitate cancer progression. The STAT regulatory kinase JAK has been strongly implicated in promoting oncogenesis of various solid tumors, including through the use of JAK kinase inhibitors such as AZD1480. However, direct evidence that JAK drives STAT3 function and cancer pathogenesis at the level of the tumor microenvironment has yet to be established clearly. In this study, we show that AZD1480 inhibits STAT3 in tumor-associated myeloid cells, reducing their number and inhibiting tumor metastasis. Myeloid cell-mediated angiogenesis was also diminished by AZD1480, with additional direct inhibition of endothelial cell function in vitro and in vivo. AZD1480 blocked lung infiltration of myeloid cells and formation of pulmonary metastases in both mouse syngeneic experimental and spontaneous metastatic models. Furthermore, AZD1480 reduced angiogenesis and metastasis in a human xenograft tumor model. Although the effects of AZD1480 on the tumor microenvironment were important for the observed anti-angiogenic activity, constitutive activation of STAT3 in tumor cells themselves could block these anti-angiogenic effects demonstrating the complexity of the JAK/STAT signaling network in tumor progression. Together, our results indicated that AZD1480 can effectively inhibit tumor angiogenesis and metastasis mediated by STAT3 in stromal cells as well as tumor cells.
Introduction
Tumor development is affected by signaling within the cancer cells and their interactions with surrounding tissue composed of extracellular matrix components and stromal cells, including endothelial cells (ECs) and immune cells (1). The local tumor microenvironment responds to signaling through inflammatory cells, which release cytokines, chemokines and growth factors to stimulate tumor growth via increased invasion potential of tumor cells. These signals also create immunosuppressive networks that enhance tumor survival (2). Signal transducer and activator of transcription 3 (STAT3) is a point of convergence for multiple oncogenic signaling pathways. Constitutive activation of STAT3 within tumor cells as well as stromal cells promotes cancer cell proliferation, invasion, angiogenesis and immune evasion (3). Activated STAT3 downregulates Th1 cytokines and other mediators critical for potent anti-tumor immune responses. STAT3 driven tumor-derived factors, including interleukin (IL)-6, IL-10 and vascular endothelial growth factor (VEGF), establish a crosstalk between tumor cells and tumor-associated immune cells to ensure persistent STAT3 activation in the tumor microenvironment, thereby creating a ‘feed-forward loop’ (4-7). Activated STAT3 in tumor-associated immune cells leads to expression of a large number of growth factors, angiogenic factors and other molecules crucial for invasion and metastasis (8-10).
The importance of IL-6 in cancer development and progression has been widely documented (11-13). A critical role of JAK in mediating IL-6-induced STAT3 activation has also been established. Although JAK has been viewed as a critical target for treating malignancies of hematopoietic origins, recent studies demonstrate its importance in various solid tumors (14). Recently JAK has also been shown to facilitate sphingosine-1-phosphate receptor-1 (S1PR1)-induced persistent STAT3 activation in both tumor cells and tumor stromal cells (9). We recently showed that AZD1480 is a potent, competitive small-molecule inhibitor of JAK1/2 kinase, and that it is capable of inhibiting STAT3 phosphorylation and tumor growth in a STAT3-dependent manner (14). Although tumor growth was inhibited directly in vivo in each tumor model tested, in some tumor cell lines AZD1480 did not block tumor cell growth in vitro at levels that produced maximal inhibition of STAT3 phosphorylation (14). This suggests the potential important effects of AZD1480 on the tumor microenvironment by inhibiting JAK/STAT signaling. A ZD1480 is currently in early clinical trials for solid and hematologic malignancies (15). Our current study shows that AZD1480 inhibits tumor angiogenesis and metastasis in part by affecting the tumor microenvironment.
Materials and Methods
Reagents
AZD1480 was provided by AstraZeneca (Waltham, MA) and dissolved in DMSO for in vitro studies. For in vivo experiments, AZD1480 was suspended in water supplemented with 0.5% Hypromellose and 0.1% Tween 80. All solvents are from Sigma (St. Louis, MO). Mouse IL-6 was purchased from R&D Systems (Minneapolis, MN). Antibodies against p-STAT3 (Tyr705), p-JAK2 (Tyr1007/1008), JAK2, cleaved caspase 3 (Asp175) (5A1E) and matrix metalloproteinase 9 (MMP9) (G657) were purchased from Cell Signaling Technology (Danvers, MA). Antibodies against STAT3 (C-20) and VEGF (A-20) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA).
Cell lines
Renca murine cell line was a gift from Dr. Alfred Chang (University of Michigan Medical Center, Ann Arbor, MI). Human renal cell carcinoma cell line, 786-O, was generously provided by Dr. William G. Kaelin (Harvard University, MA). The 4T1 mouse mammary tumor cell line and the Calu-6 lung carcinoma cell line were from ATCC. Mouse EC line derived from prostate and colon was kindly provided by S. Huang and J. Fidler (M.D. Anderson Cancer Center, Houston, Texas). All the cell lines above were grown in DMEM or RPMI 1640 with 10% fetal bovine serum (FBS, Sigma, St. Louis, MO). Human umbilical vein endothelial cells (HUVECs) were obtained from Clonetics (San Diego, CA) and cultured on collagen I coated plates in their complete medium (Clonetics, San Diego, CA). 786-O STAT3C and vector-expressing control cell lines were generated and maintained as described previously (16).
Animal models and drug administration
Female BALB/c and athymic nude (NCR-nu/nu) mice (7-8 weeks old) were obtained from National Cancer Institute (Bethesda, MD) and Taconic Laboratories (Germantown, NY). Animal use procedures were approved by the institutional animal care and use committees of Beckman Research Institute at City of Hope and AstraZeneca. For subcutaneous (s.c.) tumor model, 2.5×106 Renca or 786-O cells suspended in 100 μl PBS were injected into the flank of BALB/c or nude mice, respectively. When average tumor volume reached approximately 100-150 mm3, AZD1480 or vehicle was administered by oral gavage either once a day at the dose of 50 mg/kg, or twice daily at 30 mg/kg, as indicated. Tumor size was measured by caliper every other day. For experimental lung metastasis model, 0.1×106 Renca or 1×106 786-O cells suspended in 500 μl PBS were injected via tail vein to BALB/c or nude mice, respectively. Three days later, mice were orally treated with AZD1480 (50mg/kg/d) or vehicle for 21 days for Renca tumors and 60 days for 786-O tumors respectively. For the Calu-6 model, 3 × 106 tumor cells in matrigel were implanted s.c. into the flanks of nude mice, randomized into vehicle (twice daily, BID) and drug treatment (AZD1480, 30 mg/kg BID) groups, and dosed orally daily for 19 days. For spontaneous lung metastasis model, 2×105 4T1 cells suspended in 100 μl PBS were injected in the mammary gland of female BALB/c mice by gently penetrating the skin. AZD1480 (50mg/kg/d) or vehicle was given orally for 21 days.
Flow Cytometry
Cell suspensions from spleen, tumor or lung were prepared as described previously (7) and stained with fluorochrome-conjugated CD11b and Gr1 antibodies (BD Biosciences, San Diego, CA). Data were collected by CyAn ADP Violet Cytometer (Dako Cytomation, Fort Collins, CO), and analyzed with Flowjo (Tree Star).
In vivo Matrigel plug assay
Growth factor-reduced Matrigel (BD Biosciences, San Diego, CA) containing Renca tumor cells and splenic CD11b+/CD11c- myeloid cells enriched from Renca tumor-bearing mice (ratio, 1:10) were implanted s.c. into BALB/c mice. Five days after implantation, AZD1480 (50mg/kg/d) or vehicle was given orally for 4 days. For the Calu-6 matrigel plug assay, 5 × 106 tumor cells in matrigel were implanted into nude mice which were then treated twice daily, beginning on day 2, with vehicle, 30 mg/kg AZD1480, or 6 mg/kg VEGFR inhibitor, orally for 7 days. The plugs were harvested for hemoglobin content measurement by colorimetry using Drabkin reagent (Sigma-Aldrich, St. Louis, MO) and frozen sections of the Renca tumor plugs were stained for CD31.
In vitro tube formation assay
Mouse ECs or HUVECs (5×104 cells/well in 1% FBS RPMI 1640 medium) were seeded on 48-well plates coated with 100 μl of growth factor-reduced Matrigel. Five percent of Renca tumor conditioned medium (collected from cultured Renca tumor cells) with varying doses of AZD1480 or DMSO was added. After 16 h, capillary-like tube formation was quantified by manually counting the cord junctions with at least three branches formed by ECs.
Wound healing migration assay
Mouse ECs were grown on 6-well plates, “wounds” were made by scratching on the confluent cells with a pipette tip. The number of cells migrated into the wound area was counted after incubation with DMSO or AZD1480 (1μM) for 24 h.
Cell viability assay
Renca or 786-O cells suspended in DMEM medium with 5% FBS were seeded in 96-well plates (5,000 per well) to allow adhesion and then treated with DMSO or AZD1480 for 48 h. Cell viability was determined by MTS assay (Promega, Madison, WI) according to instructions. Absorbance at 490 nm was measured with Mikrotek Laborsysteme (Overath, Germany). Mouse ECs and splenic CD11b+/c− myeloid cells enriched from tumor bearing mice were cultured in 5% FBS 1640 RPMI medium. HUVECs were cultured on collagen 1-coated plates in complete medium (Clonetics). All cells are treated with DMSO and AZD1480 at various doses for 24 h. Cell viability was determined by counting cell number manually. All the experiments were repeated 3 times.
Immunofluorescence
Immunofluorescent staining of tumor or lung frozen tissue sections was described previously (10). To prepare lung sections, mouse lungs were perfused with PBS to eliminate circulatory blood. All the representative images were obtained under 200x magnification. CD31+ blood vessels or CD11b+ myeloid cells were counted in 6 random fields (200x).
Real-time quantitative PCR
RNA extraction, cDNA synthesis and real-time PCR were described previously (16). The primers for mouse VEGF, IL-1β, MMP9, FGF-2, S100A8, S100A9 and GAPDH were purchased from SuperArray Bioscience (Frederick, MD).
Western blot
Harvested cells were lysed and tissue samples were homogenized with a homogenizer (Power Gen, Fisher Scientific, Pittsburgh, PA) with modified RIPA buffer. Equal amounts of proteins were subjected to SDS-PAGE and immunoblotted with indicated antibodies.
Evaluation of lung metastasis
Mouse lungs were perfused by intra-tracheal injection of India ink (15% India ink, 85% water, and 3 drops of NH4OH/100 ml) and fixed in Fekete’s solution (300 ml of 70% ethanol, 30 ml of 37% formaldehyde, 5 ml of glacial acetic acid) for 10 min. Metastatic nodules were counted. For histological evaluation of lung micro-metastases, five sections of lung from each mouse were stained with H&E and examined under light microscope. The number of micro-metastatic foci per field (40x) was counted in all five sections.
Intravital multiphoton microscopy
Implantation of 786-O-pRC-vector or 786-O-pRC-STAT3C tumor cells into nude mice and treatment with AZD1480 or vehicle were described previously (14). Tumor vasculature, apoptosis and extracellular matrix were visualized by dextran-rhodamine (red) (Invitrogen, Carlsbad, CA), Annexin V (green) (BioVision, Golden, CO) and Hoechst 33342 (blue) (Sigma,,St. Louis, MO).
Statistics
Two-tailed student’s t test was used for statistical analysis. Differences were considered statistically significant when p <0.05. ***, p < 0.001; **, p < 0.01 and *, p < 0.05.
Results
AZD1480 inhibits Renca tumor growth in vivo with a reduction in tumor myeloid cell infiltration
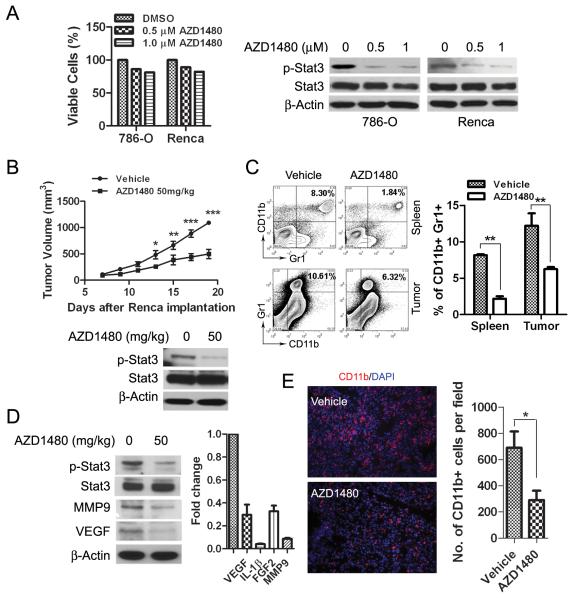
Our previous studies indicated that although AZD1480 could induce tumor growth inhibition and tumor cell apoptosis in vivo, in certain tumor cell lines it did not effectively inhibit tumor cell proliferation and induce apoptosis in vitro (14). Consistent with this observation, we found that AZD1480 treatment of 786-O human renal cancer cells and mouse Renca cells in vitro had only limited reduction in cell viability (Fig. 1A, left panel and Supplementary Fig. S1A), although phosphorylated (p)-JAK2 and p-STAT3 were inhibited (Supplementary Fig. S1B and Fig. 1A, right panel). These findings prompted us to investigate the in vivo antitumor effects of AZD1480 on Renca, a syngeneic murine renal carcinoma model. Renca tumor cells were subcutaneously injected into BALB/c mice and treated with AZD1480 (50mg/kg/d) or vehicle for 21 days. We observed a significant (p<0.001) inhibition of tumor growth in AZD1480-treated group compared with vehicle-treated group (Fig.1B, top). Western blot analyses of the whole tumor lysates revealed a dramatic inhibition of p-STAT3 by AZD1480 treatment (Fig.1B, bottom). These results suggest that AZD1480 has significant antitumor effects in vivo, with inhibition of STAT3 signaling.
Figure 1. AZD1480 inhibits tumor growth in vivo by inhibition of STAT3 and reduction of tumor-associated myeloid cells.
A, Analysis of tumor cell viability after treatment with AZD1480 in vitro. MTS assay showing viability of 786-O and Renca tumor cells 48 h after AZD1480 treatment at indicated doses (left panel) (Bars, SD; n=3). Western blotting evaluating p-STAT3 level in the tumor cells 2 hours after treatment with AZD1480 (right panel). B, Top, AZD1480 inhibits Renca tumor growth. Volumes of Renca tumors in mice treated with AZD1480 or vehicle for 21 continuous days (Bars, SEM; n=10). Bottom, western blot using whole tumor lysates showing total and p-STAT3 levels 2 hours after dosing. C, AZD1480 reduces tumor-associated MDSCs in Renca tumor-bearing mice. Flow cytometry analysis quantifying CD11b+/Gr1+ myeloid cells in spleens and tumors collected 14 days of treatment with AZD1480 or vehicle. Bars show mean ± SEM; n=3 (3 independent experiments with 4 mice per group). D, AZD1480 inhibits p-STAT3 and expression of STAT3-regulated genes in myeloid cells. Western blot and real time PCR detecting p-STAT3 and expression levels of the indicated genes in tumor-infiltrating CD11b+/CD11c- myeloid cells isolated from 4 pooled tumors after 14 days of treatment. All gene expression levels in vehicle treated group were set as 1.0. The experiments were repeated twice with similar results. E, AZD1480 treatment reduces tumor myeloid cell infiltration. Representative images of immunofluorescent staining showing CD11b+ cells in tumor tissues harvested after 10 days of treatment. The numbers of CD11b+ cells per field based on 6 fields/slide, 3 slides per tumor with total of 4 tumor/mice per group were shown; Bars, SEM.
The tumor microenvironment is a complex system composed of many types of cells, many of which play crucial roles in tumor progression (17). In particular, tumor-associated myeloid cells are an important component of the tumor microenvironment that regulates tumor growth and responses to anticancer therapies (18-19). We investigated the effect of targeting the JAK/STAT3 signaling pathway with AZD1480 on tumor-associated myeloid cells. CD11b+/Gr1+ myeloid cells (myeloid-derived suppressor cells [MDSCs]) in spleens and tumors were quantified by flow cytometry analyses in Renca tumor-bearing mice after 21 days of treatment. We observed a 2 to 3-fold reduction of MDSCs in AZD1480-treated groups compared with vehicle groups (p<0.01), as shown in Fig. 1C. It has been demonstrated that constitutively activated STAT3 not only plays a critical role in tumor cell signaling, but also stimulates the accumulation of tumor-associated myeloid cells (7). Therefore, we evaluated whether STAT3 signaling could be regulated by AZD1480 in myeloid cells. Tumor-infiltrating CD11b+/CD11c- myeloid cells isolated from tumor-bearing mice after 14 days of treatment were analyzed. STAT3 phosphorylation was potently inhibited in AZD1480-treated group, and STAT3-dependent, angiogenic and metastasis-promoting factors, VEGF, IL-1β, F G F 2 and MMP9, were downregulated in tumor-infiltrating CD11b+/CD11c- myeloid cells (Fig. 1D). Furthermore, immunostaining of Renca tumor sections for CD11b also indicated a dramatic reduction of CD11b+ myeloid cell infiltration after AZD1480 administration (Fig. 1E). In order to identify whether AZD1480 directly affects myeloid cell tumor-promoting functions, we performed an ex vivo migration assay to examine the effect of AZD1480 on myeloid cell motility. Splenic CD11b+/CD11c- myeloid cells isolated from Renca tumor-bearing mice were subjected to a transwell migration assay. The percentage of migrated myeloid cells was significantly (p<0.05) inhibited by AZD1480 treatment in a dose dependent manner (Supplementary Fig. S2B, left panel), and a reduction of p-STAT3 by AZD1480 treatment in CD11b+/CD11c- myeloid cells was also observed (Supplementary Fig. S2B, right panel).
AZD1480 inhibits tumor angiogenesis in Renca tumor model
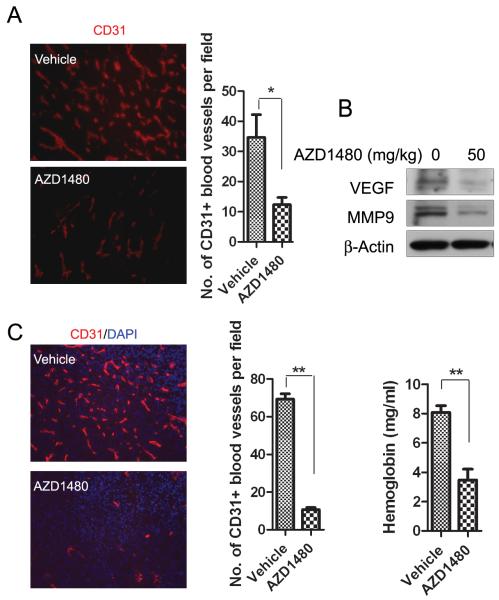
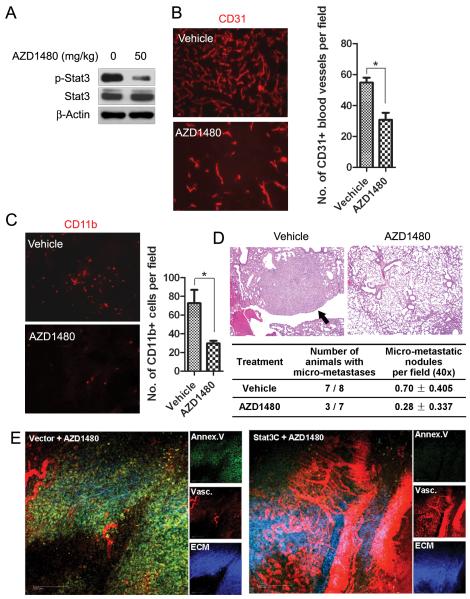
We next investigated the anti-angiogenic effect of AZD1480 on Renca tumors. Following 10 days of treatment, tumors were collected and immunostained for endothelial cell marker, CD31. We observed a more than 3-fold reduction of CD31+ tumor blood vessels in AZD1480-treated mice compared with vehicle-treated (Fig. 2A), along with downregulation of VEGF and MMP9 in whole tumor lysates (Fig. 2B). Emerging evidence has indicated that tumor-associated myeloid cells are important sources of pro-angiogenic factors in the tumor microenvironment (20-21), and our group has previously demonstrated that constitutively activated STAT3 in tumor-associated myeloid cells plays a crucial role in promoting tumor angiogenesis (10). We therefore analyzed the effect of AZD1480 on myeloid cell-induced angiogenesis in a modified matrigel angiogenesis assay. Matrigel plugs containing a mixture of Renca tumor cells and CD11b+/CD11c- myeloid cells (a ratio of 1:10) enriched from spleens of tumor-bearing mice were implanted into BALB/c mice and analyzed by immunostaining for CD31. We found a potent reduction of neovasculature in AZD1480 treatment group (Fig. 2C, left panel). Quantified results indicated a more than 7-fold reduction in CD31+ vasculature comparing AZD1480 with vehicle-treated group (Fig. 2C, middle panel). Measurement of hemoglobin content of matrigel plug also demonstrated that AZD1480 significantly reduced neovascularization (p<0.01) (Fig. 2C, right panel). Taken together, the data suggest that AZD1480 inhibits STAT3 signaling and tumor angiogenesis, at least in part by targeting tumor-associated myeloid cells, in the Renca tumor model. Furthermore, inhibition of vascularization of matrigel plugs and tumor growth has also been observed in the Calu-6 lung carcinoma xenograft model, and in association with inhibition of p-STAT3 and induction of apoptosis (Supplementary Fig. S3). The extent of antiangiogenic effect is comparable to that observed with VEGFR inhibitors (22).
Figure 2. AZD1480 inhibits myeloid cell-mediated angiogenesis in a mouse syngeneic tumor model.
A, AZD1480 suppresses vessel density in Renca tumors. Left, immunostaining with CD31 antibody (red) detecting tumor vasculature in Renca tumor sections obtained after 10 days of treatment with AZD1480 (50mg/kg/d) or vehicle. Right, numbers of blood vessels per field were shown (Bars, SEM; n=5). B, Western blotting of whole tumor lysates showing effects of AZD1480 on VEGF and MMP9 expression 2 hours after dosing. C, AZD1480 inhibits myeloid cell-mediated tumor angiogenesis in vivo. Immunofluorescent staining for CD31+ blood vessels (red) in s.c implanted matrigel plugs containing a mixture of Renca tumor cells and CD11b+/CD11c- myeloid cells after treatment with AZD1480 or vehicle for 9 days. (Left panel; Blue, DAPI). Quantification of CD31+ blood vessels per field (middle panel) and hemoglobin content in the matrigel plugs (right panel) were shown (Bars, SEM; n=4).
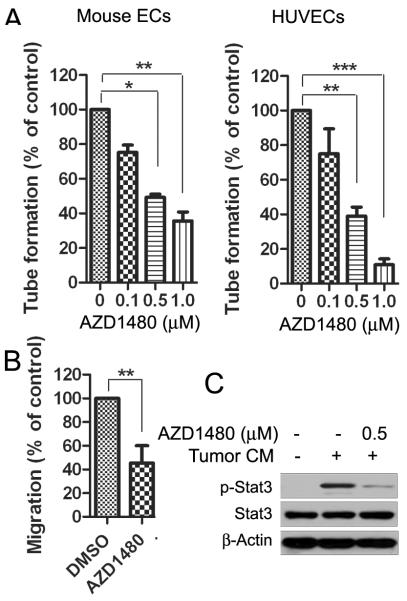
To examine whether targeting STAT3 by AZD1480 directly inhibits the function of endothelial cells (ECs), we analyzed tube formation activity of both mouse ECs and HUVECs in the presence or absence of AZD1480. AZD1480 inhibited both mouse and human EC tube formation induced by Renca tumor conditioned medium in a dose-dependent manner (Fig. 3A). In addition, the effect of AZD1480 on mouse EC migration was measured by a wound healing assay. We observed a significant reduction in the number of cells that migrated into the wound area (p<0.01) (Fig. 3B). The doses required to inhibit EC tube formation and migration were noticeably less than those that affect the viability of mouse and human ECs (Fig. 3A, B and Supplementary Fig. S2A). Moreover, p-STAT3 was evaluated in mouse ECs after treatment of AZD1480 for 2 h followed by 30 min stimulation of Renca tumor conditioned medium. We observed that 0.5 μM of AZD1480 potently inhibited STAT3 phosphorylation induced by Renca tumor conditioned medium (Fig. 3C).
Figure 3. AZD1480 inhibits endothelial cell function in vitro.
A, AZD inhibits tube formation of endothelial cell in vitro. Numbers of tube-like structure formed by AZD1480-treated mouse (left) or human (right) endothelial cells per well were shown (Bars, SD; n=3). B, AZD1480 inhibits endothelial cell migration. Wound healing migration assay to detect the migration of mouse endothelial cells in vitro (Bars, SD; n=3). C, AZD1480 inhibits p-STAT3 in mouse endothelial cells induced by Renca tumor cell conditioned medium. Western blotting showing the effect of AZD1480 on p-STAT3 levels in endothelial cells exposed to the tumor cell-conditioned medium.
AZD1480 inhibits lung metastasis and factors important for pre-metastatic niche formation
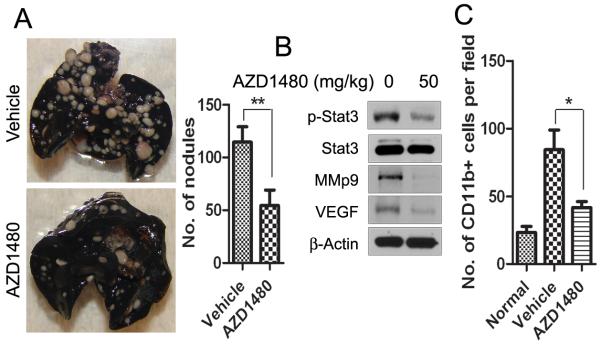
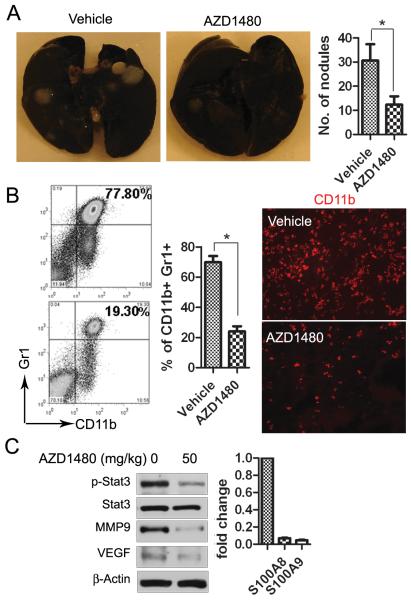
STAT3 has been implicated in tumor migration and metastasis (9, 23-24). Therefore, we tested the effect of AZD1480 on an experimental lung metastasis model. Renca cells were injected into BALB/c mice and AZD1480 or vehicle was given orally 3 days after implantation. As shown in Fig. 4A, the number of metastatic lung nodules was significantly reduced on day 21 by AZD1480 treatment compared with vehicle treatment (p<0.01). Western blot analysis of whole lung lysates revealed reduced p-STAT3, VEGF, and MMP9 (Fig. 4B). It has been shown that the primary tumor influences the lung environment before metastasis occurs (25), and infiltration and accumulation of tumor-associated myeloid cells into the lung play a crucial role in the development of metastasis (26). Thus, we examined whether subcutaneous primary tumor influenced myeloid cell infiltration into the lung and whether AZD1480 treatment blocked this process. We analyzed lung myeloid cell infiltration by immunofluorescent staining in subcutaneous Renca tumor model and found a significant (p<0.05) reduction of CD11b+ myeloid cells in the lungs after 14 days of treatment with AZD1480 (Fig. 4C). These results indicate that AZD1480 can inhibit Renca tumor metastasis.
Figure 4. AZD1480 inhibits experimental lung metastasis of mouse syngeneic tumors.
A, Metastatic nodules were visualized to show the inhibitory effect of AZD1480 on Renca tumor metastasis 21 days after treatment in an experimental lung metastasis model. The numbers of metastatic nodules were shown as mean ± SEM (n=10). B, Western blotting showing p-STAT3 and STAT3-regulated genes in whole lung lysates at day 21, 2 hours after the last dosing. C, AZD1480 treatment reduces lung myeloid cell infiltration in subcutaneous Renca tumor model. Immunofluorescent staining for CD11b+ cells quantifying numbers of myeloid cells per field in the lungs of s.c. Renca tumor-bearing mice after 14 days of treatment with AZD1480 or vehicle. (Bars, SEM; n=4).
AZD1480 inhibits spontaneous lung metastasis and modulates the metastatic environment
We also investigated the effect of AZD1480 on 4T1, a syngeneic mouse mammary carcinoma model that spontaneously develops lung metastasis. 4T1 tumor cells were orthotopically implanted into the mammary glands of mice, and AZD1480 or vehicle was orally administered 3 days after tumor challenge. The number of lung metastatic nodules was significantly (p<0.05) reduced after 21 days of AZD1480 treatment compared with vehicle treatment (Fig. 5A). Meanwhile, we examined lung myeloid cell infiltration in 4T1 tumor-bearing mice by flow cytometry. We observed a 2 to 4-fold reduction of CD11b+/Gr1+ myeloid cells in the lungs as early as 4 days after initial AZD1480 treatment (p<0.05) (Fig. 5B, left panel). Lung tissue sections were subjected to immunofluorescence staining for CD11b antibody. A reduction of lung myeloid cell infiltration after 8 days of AZD1480 treatment was shown (Fig. 5B, right panel). Furthermore, we examined STAT3 signaling in pulmonary CD11b+/CD11c- myeloid cells by either western blot or real-time PCR. As shown in Fig. 5C, p-STAT3 along with VEGF and MMP9, as well as S100A8 and S100A9, all of which have been shown to be important in myeloid cell-mediated distant site metastasis, were inhibited after treatment with AZD1480 compared with vehicle group. To further address the effects of AZD1480 on myeloid cells’ ability to attract 4T1 tumor cells, we performed an ex vivo migration assay. CD11b+/CD11c- myeloid cell conditioned medium was used to induce 4T1 tumor cell migration. The number of migrated tumor cells was significantly decreased in AZD1480 treatment group (p<0.05) (Supplementary Fig. S4). Taken together, these results suggest that AZD1480, by targeting STAT3 signaling, potently reduced the infiltration of myeloid cells into the lung, which could inhibit tumor cell distant colonization.
Figure 5. AZD1480 inhibits spontaneous lung metastasis of mouse syngeneic tumors.
A, Metastatic nodules were visualized and counted to show the inhibitory effect on AZD1480 on 4T1 tumor 21 days after treatment in a spontaneous lung metastasis model. (Bars, SEM; n=12). B, Treatment with AZD1480 reduces lung myeloid cell infiltration. Flow cytometry evaluating pulmonary CD11b+/Gr1+ myeloid cells isolated from 4T1 tumor bearing mice after 8 days of treatment with AZD1480 or vehicle (Left and middle panels), Bars show mean ± SEM; n=3 (3 independent experiments with 4 mice each group). Representative images of immunofluorescent staining showing CD11b+ cells in lung sections after treatment (right panel). C, AZD1480 inhibits p-STAT3 and reduces the expression of STAT3-regulated genes in lung myeloid cells. Western blotting and real time PCR detecting p-STAT3 and expression levels of the indicated genes in lung CD11b+/CD11c- cells after 8 days of treatment. Samples were collected 2 hours after last dosing.
Anti-angiogenic and anti-metastatic effects of AZD1480 on a human renal cell carcinoma xenograft
Previous study indicated the ability of AZD1480 to inhibit growth of various human tumors, including 786-O human renal cell carcinoma, in xenograft models (14). We determined here whether AZD1480 could also inhibit tumor growth through anti-angiogenesis or anti-metastasis in 786-O human renal cell carcinoma xenografts. Western blot analyses of the whole tumor lysates showed a dramatic inhibition of p-STAT3 by AZD1480 treatment (Fig. 6A). Tumor sections were immunostained with CD31 antibody to detect tumor vessels after AZD1480 or vehicle treatment for 35 days. As shown in Fig. 6B, AZD1480 treatment led to a 2 to 2.5 fold reduction in CD31+ blood vessels in 786-O xenografts (p<0.05). We also examined infiltrating myeloid cells in tumors by immunostaining for CD11b. The number of tumor CD11b+ myeloid cells was significantly decreased after AZD1480 treatment (p<0.05) (Fig. 6C). To determine whether the reduction in myeloid cells correlated with inhibition of lung metastasis, we investigated the effect of AZD1480 on an experimental pulmonary metastasis model induced by 786-O tumor cells. Lung tissue was collected and analyzed for metastasis after 2 months of treatment. Seven of 8 mice in vehicle group developed metastasis on histological examination, while only 3 of 7 mice in AZD1480 group developed metastases. The number of micro-metastatic nodules per field in the vehicle group was also significant higher (p<0.05) than that of AZD1480 treated mice (Fig. 6D). These results further indicate that AZD1480 inhibits angiogenesis and metastasis in 786-O xenografts, which is associated with inhibition of myeloid cells by AZD1480 treatment.
Figure 6. AZD1480 inhibits angiogenesis and metastasis in 786-O xenografts and over-expression of constitutively active STAT3 rescues 786-O tumor from angiogenic inhibition.
A, AZD1480 inhibits STAT3 activity in 786-O tumors. Western blotting detecting p-STAT3 level in s.c. 786-O tumor lysate after 35 days of AZD1480 treatment. B, AZD1480 inhibits angiogenesis in 786-O tumors. Immunofluorescent staining showing CD31+ blood vessels in 786-O xenografts after treatment. Numbers of blood vessels per field were shown (Bars, SEM; n=4). C, AZD1480 inhibits myeloid cell infiltration in 786-O xenografts. Immunofluorescent staining for CD11b in tumor tissues quantifies the numbers of myeloid cells (Bars, SEM; n=4). D, AZD1480 inhibits experimental lung metastasis of 786-O tumors. HE staining of lung tissues harvested from athymic nude mice i.v. injected with 786-O cells and treated with AZD1480 or vehicle for 2 months. Arrow in the representative photos indicates metastatic tumor. E, Overexpression of a constitutively active STAT3 (STAT3C) rescues 786-O tumor from angiogenesis inhibition induced by AZD1480. Intravital multiphoton laser microscopy was used to visualize vasculature, apoptosis and extracellular matrix in tumors transfected with STAT3C or vector (Bar, 200 μm).
Since AZD1480 also inhibits JAK2/STAT3 in tumor cells, we investigated the effect of constitutive STAT3 within tumor cells signaling on the tumor stromal angiogenic environment. We stably transfected 786-O cells with either constitutively active STAT3 mutant, STAT3C (27), or control vector, challenged the tumor cells into athymic nude mice and observed the effects of AZD1480 on angiogenesis. Intravital multiphoton laser microscopy was used to visualize tumor vasculature in living mice. As shown in Fig. 6E, 786-O xenografts expressing STAT3C demonstrated resistance to AZD1480-induced angiogenesis inhibition compared with vector control. These data indicate that despite the anti-angiogenic activity of AZD1480 within the tumor microenvironment, tumor autonomous STAT3 signaling can interact with stroma to promote tumor angiogenesis.
Discussion
Previous work has established the importance of JAK1/2 in STAT3-dependent tumorigenesis, and inhibition by AZD1480 resulted in blockage of tumor growth, although direct inhibitory effects on tumor cells were not evident in vitro in some cell lines (14). Moreover, AZD1480 treatment of myeloma cells resulted in decreased tumor proliferation and the induction of apoptosis, which could be seen in the presence of bone marrow stromal cells (28). Our current work demonstrates the effects of AZD1480 on modulating JAK/STAT3 signaling in the tumor microenvironment and reducing tumor angiogenesis and metastasis.
A complex multidirectional interaction exists between tumor cells, surrounding stroma and the microenvironment at metastatic sites (19, 29). The accumulation of myeloid cells has been shown to create a permissive environment at distant organs for metastasis to occur (25, 29-31). In the pre-metastatic niche, recruited myeloid cells in concert with ECs and stromal cells produce a milieu of chemokines, growth factors, extracellular matrix (ECM) proteases and proteins essential for tumor cell invasion to facilitate metastasis (25). It has been shown that STAT3 promotes crosstalk within the tumor stroma allowing tumor cells to interact with myeloid and ECs, and STAT3 within myeloid cells then stimulates endothelial cells resulting in tumor growth, migration and angiogenesis (10), thereby playing an important role in metastatic potential. Our study provides evidence that JAK/STAT3 signaling within the primary tumor microenvironment is critical for myeloid cell infiltration and the formation of tumor vasculature. Furthermore, inhibition of STAT3-mediated myeloid infiltration and angiogenesis with AZD1480 dramatically decreased the formation of metastases. In addition, when a constitutively-activated mutant form of STAT3 was introduced into the tumor cells, treating mice with AZD1480 was not able to inhibit tumor angiogenesis. These results support the importance of factors produced by tumor cells in promoting tumor angiogenesis, and suggest that the antiangiogenic effects of AZD1480 are partly mediated by blocking JAK/STAT3 in tumor cells, highlighting a tumor autonomous mode of antiangiogenic activity distinct from that of VEGFR inhibitors. Taken together, blocking JAK/STAT3 activity with AZD1480 may have promise in the treatment of solid malignancies by inhibiting tumor growth at the primary site and preventing invasion and metastasis.
Supplementary Material
Acknowledgements
We thank the members of the Analytical Cytometry Core, the Light Microscopy Core and Animal Facility at City of Hope, as well as Gregory O’Connor, Maria Pinzon-Ortiz, Troy Patterson and Shenghua Wen at Astrazeneca for their contributions.
Grant Support
This study was supported by the National Institutes of Health (grants R01 CA115815, R01 CA115674-01 and K12CA001727) and by Kure it! Kidney Cancer Funds at City of Hope.
Footnotes
Disclosure of Potential Conflicts of Interest
No Potential conflicts of interest were disclosed.
References
- 1.Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–74. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 3.Yu H, Jove R. The STATs of cancer--new molecular targets come of age. Nat Rev Cancer. 2004;4:97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- 4.Wang T, Niu G, Kortylewski M, Burdelya L, Shain K, Zhang S, et al. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat Med. 2004;10:48–54. doi: 10.1038/nm976. [DOI] [PubMed] [Google Scholar]
- 5.Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol. 2007;7:41–51. doi: 10.1038/nri1995. [DOI] [PubMed] [Google Scholar]
- 6.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kortylewski M, Kujawski M, Wang T, Wei S, Zhang S, Pilon-Thomas S, et al. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat Med. 2005;11:1314–21. doi: 10.1038/nm1325. [DOI] [PubMed] [Google Scholar]
- 8.Kortylewski M, Xin H, Kujawski M, Lee H, Liu Y, Harris T, et al. Regulation of the IL-23 and IL-12 balance by Stat3 signaling in the tumor microenvironment. Cancer Cell. 2009;15:114–23. doi: 10.1016/j.ccr.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee H, Deng J, Kujawski M, Yang C, Liu Y, Herrmann A, et al. STAT3-induced S1PR1 expression is crucial for persistent STAT3 activation in tumors. Nat Med. 2010;16:1421–28. doi: 10.1038/nm.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kujawski M, Kortylewski M, Lee H, Herrmann A, Kay H, Yu H. Stat3 mediates myeloid cell-dependent tumor angiogenesis in mice. J Clin Invest. 2008;118:3367–77. doi: 10.1172/JCI35213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Catlett-Falcone R, Landowski TH, Oshiro MM, Turkson J, Levitzki A, Savino R, et al. Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity. 1999;10:105–15. doi: 10.1016/s1074-7613(00)80011-4. [DOI] [PubMed] [Google Scholar]
- 12.Gao SP, Mark KG, Leslie K, Pao W, Motoi N, Gerald WL, et al. Mutations in the EGFR kinase domain mediate STAT3 activation via IL-6 production in human lung adenocarcinomas. J Clin Invest. 2007;117:3846–56. doi: 10.1172/JCI31871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–13. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hedvat M, Huszar D, Herrmann A, Gozgit JM, Schroeder A, Sheehy A, et al. The JAK2 inhibitor AZD1480 potently blocks Stat3 signaling and oncogenesis in solid tumors. Cancer Cell. 2009;16:487–97. doi: 10.1016/j.ccr.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ioannidis S, Lamb ML, Wang T, Almeida L, Block MH, Davies AM, et al. Discovery of 5-Chloro-N(2)-[(1S)-1-(5-fluoropyrimidin-2-yl)ethyl]-N(4)-(5-methyl-1H-pyr azol-3-yl)pyrimidine-2,4-diamine (AZD1480) as a Novel Inhibitor of the Jak/Stat Pathway. J Med Chem. 2011;54:262–76. doi: 10.1021/jm1011319. [DOI] [PubMed] [Google Scholar]
- 16.Xin H, Zhang C, Herrmann A, Du Y, Figlin R, Yu H. Sunitinib inhibition of Stat3 induces renal cell carcinoma tumor cell apoptosis and reduces immunosuppressive cells. Cancer Res. 2009;69:2506–13. doi: 10.1158/0008-5472.CAN-08-4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Albini A, Sporn MB. The tumour microenvironment as a target for chemoprevention. Nat Rev Cancer. 2007;7:139–47. doi: 10.1038/nrc2067. [DOI] [PubMed] [Google Scholar]
- 18.Schmid MC, Varner JA. Myeloid cells in the tumor microenvironment: modulation of tumor angiogenesis and tumor inflammation. J Oncol. 2010:201026. doi: 10.1155/2010/201026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–52. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618–31. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- 21.Wei D, Le X, Zheng L, Wang L, Frey JA, Gao AC, et al. Stat3 activation regulates the expression of vascular endothelial growth factor and human pancreatic cancer angiogenesis and metastasis. Oncogene. 2003;22:319–29. doi: 10.1038/sj.onc.1206122. [DOI] [PubMed] [Google Scholar]
- 22.Smith NR, James NH, Oakley I, Wainright A, Copley C, Kendrew J, et al. Acute pharmacodynamic and antivascular effects of the vascular endothelial growth factor signaling inhibitor AZD2171 in Calu-6 human lung tumor xenografts. Mol Cancer Ther. 2007;6:2209–19. doi: 10.1158/1535-7163.MCT-07-0142. [DOI] [PubMed] [Google Scholar]
- 23.Barbieri I, Pensa S, Pannellini T, Quaglino E, Maritano D, Demaria M, et al. Constitutively active Stat3 enhances neu-mediated migration and metastasis in mammary tumors via upregulation of Cten. Cancer Res. 2010;70:2558–67. doi: 10.1158/0008-5472.CAN-09-2840. [DOI] [PubMed] [Google Scholar]
- 24.Cheng GZ, Zhang WZ, Sun M, Wang Q, Coppola D, Mansour M, et al. Twist is transcriptionally induced by activation of STAT3 and mediates STAT3 oncogenic function. J Biol Chem. 2008;283:14665–73. doi: 10.1074/jbc.M707429200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hiratsuka S, Watanabe A, Aburatani H, Maru Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol. 2006;8:1369–75. doi: 10.1038/ncb1507. [DOI] [PubMed] [Google Scholar]
- 26.Couzin J. Medicine. Tracing the steps of metastasis, cancer’s menacing ballet. Science. 2003;299:1002–6. doi: 10.1126/science.299.5609.1002. [DOI] [PubMed] [Google Scholar]
- 27.Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, et al. Stat3 as an oncogene. Cell. 1999;98:295–303. doi: 10.1016/s0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- 28.Scuto A, Krejci P, Popplewell L, Wu J, Wang Y, Kujawski M, et al. The novel JAK inhibitor AZD1480 blocks STAT3 and FGFR3 signaling, resulting in suppression of human myeloma cell growth and survival. Leukemia. 2010:1–13. doi: 10.1038/leu.2010.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Psaila B, Lyden D. The metastatic niche: adapting the foreign soil. Nat Rev Cancer. 2009;9:285–93. doi: 10.1038/nrc2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–7. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim S, Takahashi H, Lin WW, Descargues P, Grivennikov S, Kim Y, et al. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457:102–6. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.