Abstract
ARID1A (BAF250A) promotes the formation of SWI/SNF chromatin remodeling complexes containing BRG1 or BRM. ARID1A has emerged as a candidate tumor suppressor based on its frequent mutations in ovarian clear cell and endometrioid cancers and in uterine endometrioid carcinomas. Here we report that restoring wild-type ARID1A expression in ovarian cancer cells that harbor ARID1A mutations is sufficient to suppress cell proliferation and tumor growth in mice, whereas RNAi-mediated silencing of ARID1A in non-transformed epithelial cells is sufficient to enhance cellular proliferation and tumorigenicity. Gene expression analysis identified several downstream targets of ARID1A including CDKN1A and SMAD3, which are well known p53 target genes. In support of the likelihood that p53 mediates the effects of ARID1A on these genes, we demonstrated that p53 was required and sufficient for their regulation by ARID1A. Further, we showed that CDKN1A (encoding p21) acted in part to mediate growth suppression by ARID1A. Lastly, we obtained evidence that the ARID1A/BRG1 complex interacts directly with p53 and that mutations in the ARID1A and TP53 genes were mutually exclusive in tumor specimens. Our results provide functional evidence in support of the hypothesis that ARID1A is a bona fide tumor suppressor that collaborates with p53 to regulate CDKN1A and SMAD3 transcription and tumor growth in gynecological cancers.
Introduction
It has been well established that chromatin remodeling is critical for essentially all aspects of nuclear activities including transcription, DNA replication and DNA damage repair (1–3) and molecular genetic changes in chromatin remodeling genes have emerged as a new mechanism in cancer pathogenesis. Amplification of Rsf-1, a gene participating in ISWI chromatin remodeling, has been demonstrated to promote chromosomal instability, propel tumor progression, and contribute to disease aggressiveness in ovarian and oral cancer (4–8). As well, somatic inactivating mutations have been detected in several SWI/SNF chromatin remodeling genes including PBRM1 (BAF180) (9) in renal cell carcinoma, BRG1 (SMARCA4) in lung carcinoma (10, 11), and ARID1A (BAF250a) in endometrium-related carcinomas including uterine endometrioid carcinoma, ovarian clear cell carcinoma, and ovarian endometrioid carcinoma (12–14). In previous studies (12–14), we have found that 46%–57% of ovarian clear cell carcinomas, 40% of uterine endometrioid carcinomas, and 30% of ovarian endometrioid carcinomas harbor somatic sequence mutations in ARID1A. ARID1A, a homolog of yeast SWI1, encodes a large nuclear protein, p270 (also known as BAF250a), which participates in forming a SWI/SNF chromatin remodeling complex (15). Coordinate activity of the proteins of the SWI/SNF complex is responsible for altering chromatin structure which is required to facilitate several cellular functions including transcription, DNA synthesis and DNA damage repair (16–21). ARID1A binds to DNA non-selectively In vitro and recruits other components to the complex, a process that may confer specificity of the SWI/SNF (22, 23). The core protein and the ATPases, BRG1 or BRM, are responsible for moving or dispersing nucleosomes surrounding specific chromosomal regions such as transcription initiation sites (17, 24). It has been proposed that SWI/SNF gene members play an important role in embryonic development, tissue regeneration, cell senescence, apoptosis and oncogenesis.
Because the majority of somatic mutations involving ARID1A in human cancers are insertions/deletions causing frameshift or nonsense mutations, ARID1A was thought to behave as a tumor suppressor gene, but direct evidence of its tumor suppressor activity has not been demonstrated. In the present study, we examined the effects of ARID1A expression or loss of expression on carcinoma cells and non-transformed cells, respectively. Additionally, we identified ARID1A-regulated genes as a primary step toward unveiling the mechanisms by which ARID1A inhibits tumor growth. Demonstration of tumor suppressor functions of ARID1A should be fundamental for future studies aimed at elucidating the biological roles of ARID1A and SWI/SNF complexes in endometrium-related tumorigenesis and should help clarify how aberrant chromatin remodeling activity participates in cancer development.
Materials and Methods
Plasmid construction and lentivirus production
The full-length cDNA of ARID1A (coding sequence of NM_006015) was sub-cloned from plasmids CMV-T7-hOsa1 (Addgene plasmid 17986) (25) and pCI-neo-BAF250 (2) into pCDNA6-V5/His.b (Invitrogen) and pLenti-puro, with V5/His tags at the C-terminus. The tetracycline-inducible lentiviral vector pLenti-puro was constructed using pLenti4/TO/V5-DEST vector (Invitrogen) as a backbone by removing the Gateway elements between the two EcoRV sites, and by replacing the Zeocin resistance gene with a puromycin resistance gene. The tetracycline repressor was introduced into cells using virus produced from pLenti6/TR (Invitrogen). The control plasmid, pLenti-puro-LacZ, was constructed by cloning lacZ from pLenti6/V5-GW/lacZ (Invitrogen) into the pLenti-puro vector. The shRNALentiviral plasmids were obtained from the RNAi consortium. The short hairpin RNA (shRNA) sequences for ARID1A were: sh1 (TRCN0000059090), CCTCTCTTATACACAGCAGAT, and sh2 (TRCN0000059091), CCGTTGATGAACTCATTGGTT. The shRNA sequences for p21 were: sh1 (TRCN0000040123), CGCTCTACATCTTCTGCCTTA, and sh2 (TRCN0000040126), GACAGATTTCTACCACTCCAA. The shRNA sequence for p53 was (TRCN0000003754) TCAGACCTATGGAAACTACTT. Lentivirus was produced using HEK293FT cells (Invitrogen) with the second generation packaging system pSPAX2 (Addgene plasmid 12260) and pMD2.G (Addgene plasmid 12259). Lentiviral titer was determined using a real-time qPCR method by measuring viral RNA content in viral supernatant as described (26). The firefly luciferase plasmids for the p21 promoter, WWP-Luc (27), and for SMAD3 (28) have been reported previously. The primers used in this study are listed in Table S3.
Tumorigenicity in immunocompromised mice
OSE4 cells were transduced by lentivirus expressing shRNA targeting ARID1A (sh1) or GFP, and two days later the transduced cells were injected into the subcutaneous tissue on the right and left flanks, respectively, of 4–6 week-old nu/nu mice. For each injection site, 4×106 cells mixed 1:1 (v:v) with Matrigel (BD Biosciences) were injected. The tumors were excised and weighted 6 weeks after inoculation. All tumors were confirmed by histopathologic analysis. To restore ARID1A expression in the xenograft model, HEC-1-AARID1A or LacZ inducible cells (2×106 cells/site mixed 1:1 (v:v) with Matrigel) were injected onto the left and right flanks, respectively, of 4–6 week-old nu/nu mice. Starting ten days after inoculation, 125 μg doxycycline per mouse was administered every other day by intraperitoneal injection. Tumors were excised and weighted 17 days after inoculation. Student’s t test was used for statistical analysis using Prism software (GraphPad). Care of experimental animals was in accordance with institutional guidelines (Johns Hopkins Medical Institutions).
Chromatin Immunoprecipitation
Cells were cross-linked with 1% formaldehyde (Sigma) diluted in culture media at room temperature for 10 minutes followed by quenching with 125 mM Glycine for 5 minutes. The chromatin was extracted with ChIP lysis buffer (1% SDS, 10 mM EDTA, and 50 mM Tris, pH 7.5). After one freeze-thaw cycle, the DNA was fragmented twice for 10 minutes using a Bioruptor (Diagenode) sonicator at 30 second on/off cycles set at the highest intensity. The chromatin was precipitated using 2 μg antibody pre-absorbed onto 20 μl Protein A/G (1:1) DYNAL magnetic beads (Invitrogen). After reverse cross-linking, the DNA was purified using a PCR purification kit (Qiagen). Binding to promoter regions was analyzed by qPCR and reported as percent of input. In ChIP-reChIP, immunocomplex from the 1st ChIP was eluted with 10mM DTT in TE at 37 °C. The eluates were then diluted with ten volumes of ChIP lysis buffer and used for the 2nd round ChIP.
Cell Lines
The nature of ovarian surface epithelial cells OSE4 (29) and IOSE-80PC (30), as well as the ovarian clear cell carcinoma cell line, OVISE, (31) were previously described. All the cell lines were cultured in RPMI-1640 supplemented with 10% fetal bovine serum. HEC-1-A cells were obtained from ATCC (Rockville, Maryland). These four cell lines exhibited distinct morphological features, and were also authenticated by the Fragment Analysis Facility at the Johns Hopkins University using the short tandem repeat (STR) DNA profiling (PowerPlex 1.2 System, Promega). The STR profiles of HEC-1-A and OVISE were matched to their original profiles in the cell line database at the Japanese Collection of Research Bioresources (JCRB, http://cellbank.nibio.go.jp/cellbank_e.html). The STR profiles of OSE4 and IOSE-80PC cell lines have not yet been deposited but they were distinct from any cell line reported in the current STR Profile Databases maintained by JCRB and ATCC (http://www.atcc.org). Mycoplasma was tested negative in all cell lines (PlasmoTest, InvivoGen).
Results
Restoring ARID1A expression suppresses tumor growth
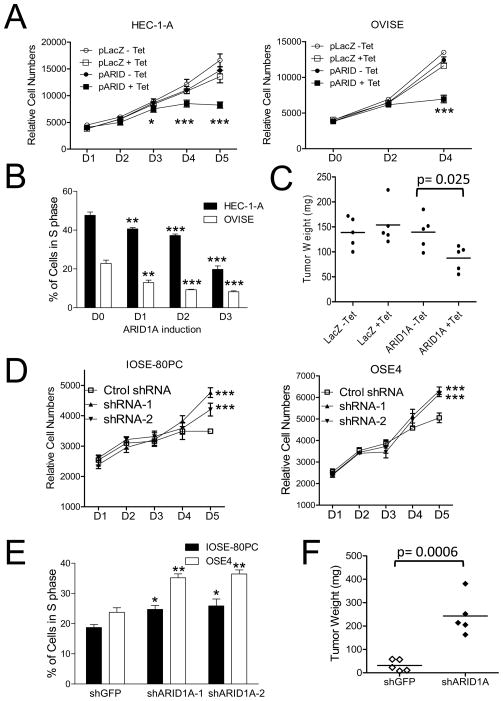
To determine the tumor suppressive function of ARID1A, we restored the expression of wild-type ARID1A in cancer cells harboring ARID1A mutations. Based on mutation information and Western blot analysis (Fig. S1), we selected the HEC-1-A uterine endometrioid carcinoma cell line and the OVISE ovarian clear cell carcinoma cell line as the models. Both cell lines contained homozygous ARID1A mutations and did not express detectable ARID1A protein. A Tet-On tetracycline inducible system was established to control ARID1A expression in both cell lines. Expression of ARID1A in those cells was induced by treating cells with doxycycline, a derivative of tetracycline (Fig. S2). As a control, we also established Tet-On inducible LacZ expression in both cell lines (Fig. S2). Cellular proliferation and the percentage of cells in the S phase of cell cycle were markedly decreased in ARID1A-induced cells as compared to the control groups of cells lacking ARID1A expression (Fig. 1A and 1B). In contrast, induced expression of ARID1A in the ovarian clear cell line, JHOC5, which has wild-type ARID1A and abundantly expressed the protein, had minimal effects on cellular proliferation and cell cycle progression (Fig. S3).
Fig. 1.
Effects of ARID1A on cellular proliferation and tumor growth. (A) A Tet-on inducible system was established in HEC-1-Aand OVISE cancer cell lines which harbor ARID1A inactivating mutations. Growth curve analysis shows that induction of ARID1A expression significantly decreases cellular proliferation as compared with the control groups without ARID1A expression. (B) Ectopic expression of wild-type ARID1A in HEC-1-A and OVISE leads to a decreased population of cells in S-phase. (C) ARID1A expression (ARID1A + Tet) significantly reduces the tumor weight of HEC-1-Atumor xenografts as compared to control tumors without ARID1A induction. (D) As compared to IOSE-80PC and OSE4 epithelial cells transduced with control shRNA lentivirus, cells transduced with virus expressing two different ARID1A shRNAs demonstrate significantly greater proliferation by day 5. (E) The percentage of cells in S phase is increased in IOSE-80PC and OSE4 cells transduced by ARID1A shRNAs (shARID1A-1 and shARID1A-2). (F) OSE4 cells transduced with ARID1A shRNA virus (shRNA 1) were injected into the subcutaneous tissue on the right flank of nu/nu mice, and control (Cont) shRNA virus transduced cells were injected into the left flank. Six weeks later, tumors arising from cells transduced with ARID1A shRNA are larger than tumors arising from cells transduced with control shRNA (p< 0.0006). For panelsA and B, statistical analysis was performed between ARID1A-induced and un-induced cells using t test. For panels D and E, statistical analysis was performed between ARID1A knockdown samples and control samples. *, p<0.05; **, p<0.01; ***, p<0.001 (t test).
To extrapolate the above in vitro findings to an in vivo setting, we established Tet-On inducible HEC-1-A tumor xenografts in athymic nu/nu mice. In the experimental group, ARID1A expression was induced 10 days after tumor cell inoculation, when small subcutaneous tumors were palpable. We found that expression of wild-type ARID1A significantly reduced tumor weights as compared to the control group without restoration of wild-type ARID1A (p= 0.025) (Fig. 1C). As expected, tumor growth in mice was not affected by induction of the control gene, LacZ (Fig. 1C). Immunohistochemistry performed on the excised tumors showed ARID1A nuclear immunoreactivity in ARID1A induced tumors (+Tet) but not in mock induced tumors (−Tet), confirming tetracycline-regulation of ARID1A expression in the tumor xenografts (Fig. S4). Based on immunostaining, the level of ARID1A induction in tumor cells, although varying in intensity, was comparable to mouse stromal cells and endometrium, indicating a physiological level of ARID1A expression in our Tet-On inducible system (Fig. S4).
Silencing of ARID1A expression enhances cellular proliferation and tumorigenicity
Our previous studies (14, 32) together with the Western blot analysis (Fig. S1) demonstrates that ARID1A mutations correlate with loss of protein expression in human endometrium-related cancers. Thus, to simulate the effect of ARID1A mutations, we knocked down ARID1A in two epithelial cell lines, IOSE-80PC and OSE4, which were derived from normal ovarian surface epithelium. It has been demonstrated in an engineered mouse model that ovarian surface epithelium may undergo Mullerian metaplasia to become endometrium tissue and develop ovarian endometrioid carcinoma after genetic inactivation of Wnt/β-catenin and PIK3/Pten pathways (33). Furthermore, ovarian clear cell carcinoma has long been thought to derive from endometriosis. Since normal epithelial cell lines established from uterine endometrium have not been available, ovarian surface epithelial cell lines may represent the relevant models currently available to prove the principle if loss of ARID1A expression promotes oncogenesis.
As compared to cells transduced with control virus carrying GFP-targeting shRNA, cells transduced with ARID1A shRNA virus demonstrated an elevated proliferation rate and an increased cell population in S phase (Fig. 1D, 1E). To determine if ARID1A knockdown affected tumorigenicity in vivo, OSE4 cells, in which ARID1A expression had been knocked down by shRNA, were injected into athymic nu/nu mice. Although OSE4 cells are poorly tumorigenic, they became highly tumorigenic when ARID1A expression was reduced by shRNA (Fig. 1F).
ARID1A regulates p53-controlled genes
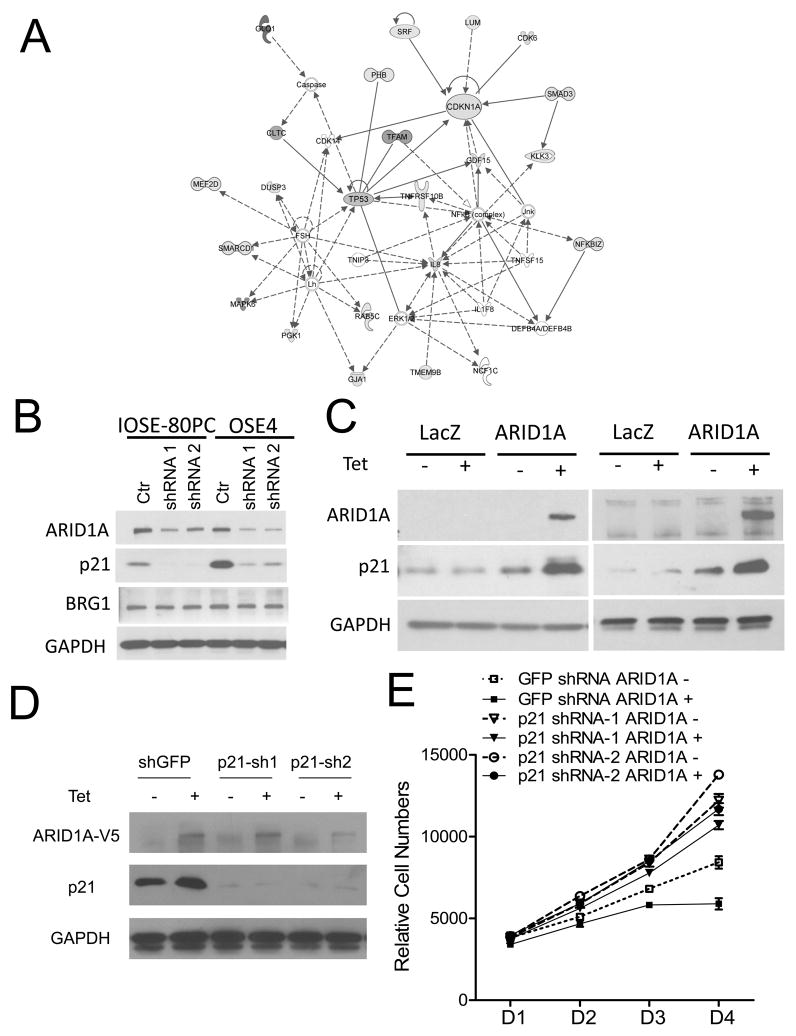
The above results indicate that ARID1A controls cell cycle progression, and downregulation of ARID1A expression leads to increased cellular proliferation. To determine the possible mechanisms, we compared the transcriptomes of IOSE-80PC and OSE4 cells between ARID1A knockdown and control groups using Illumina BeadChip arrays. We identified a total of 104 genes in which transcript levels were up- or down-regulated by at least two folds in both cell lines (Table S1 and Fig. 2A). Those genes downregulated by ARID1A shRNAs included CDKN1A, SMAD3, SMAD5, Nag1 (of TGF-βsuperfamily), TRIM8 and SMARCD1 (BAF60a). Pathway analysis of these AIRD1A-regulated genes demonstrated an enrichment of pathways in cell cycle regulation (Table S2), and, in fact, the top two pathways involved cell cycle regulation. Interestingly, even though the expression levels of p53 remained stable, several p53-related genes were down-regulated by ARID1A knockdown (Fig. 2A). Among them, two prominent molecular hubs, CDKN1A, encoding p21 (Cip1 or WAF1), and SMAD3, were identified. Because of the known biological roles of CDKN1A and SMAD3 in pathways related to cell cycle and p53, we selected both for further characterization in this study. Down-regulation of expression of CDKN1A and SMAD3 by ARID1A knockdown was validated using quantitative RT-PCR or Western blot analyses (Fig. S5A, Fig. 2B). In addition, induction of ARID1A expression led to upregulation of p21 protein in HEC-1-A and OVISE Tet-on cells (Fig. 2C) and to an increase of SMAD3 mRNA levels in OVISE Tet-on cells (Fig. S6A).
Fig. 2.
Identification of ARID1A regulated genes. (A) Ingenuity analysis of networks contributed by 104 differentially expressed ARID1A-regulated candidate genes identified two prominent molecular hubs, CDKN1A encoding p21 (Cip1 or WAF1) and SMAD3. A color copy is shown in Supplementary Fig. S7. (B) Western blot demonstrates that ARID1A knockdown results in reduced expression of ARID1A and p21. (C) Induction of ARID1A expression leads to upregulation of p21 protein in both HEC-1-A (left panel) and OVISE (right panel) cell lines. (D) Western blot shows significant reduction in p21 levels following knockdown with shRNA targeting p21. (E) Two different p21 shRNAs significantly increased cellular proliferation in ARID1A-induced cells as compared to control (GFP) shRNA treated ARID1A-induced HEC-1-A cells (p< 0.01).
To further determine if p21 mediated the growth suppressive effects of ARID1A, we knocked down CDKN1A in the OVISE cell line carrying tet-inducible ARID1A. As expected, ARID1A induction resulted in growth suppression and p21 overexpression, and more importantly, two different p21 shRNAs significantly increased cellular proliferation in cells with ARID1A induction as compared to control (GFP) shRNA treated cells (Fig. 2E), indicating that p21 was responsible, at least in part, for growth suppression following ARID1A induction.
Binding of ARID1A/BRG1/p53 complex to the promoters of CDKN1A and SMAD3
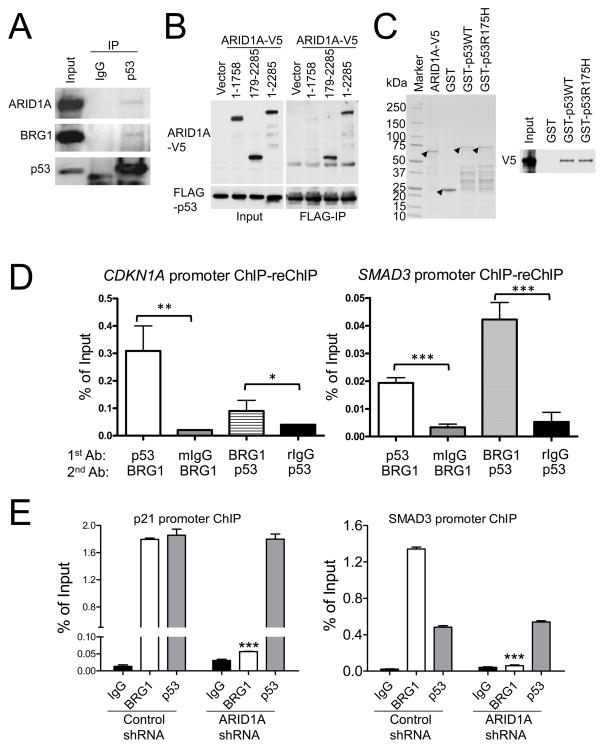
It has been established that ARID1A interacts with BRG1 ATPase to form a SWI/SNF chromatin remodeling protein complex (2, 22). It has also been reported that p53 interacts with SWI/SNF subunits BAF60a, BRG1 and BAF47 (34–36). To determine if p53 bound to ARID1A/BRG1 complex in our experimental system, we performed co-immunoprecipitation in OSE4 cells by pulling down endogenous p53 then blotted with either ARID1A or BRG1 antibodies. As shown in Fig. 3A, both ARID1A and BRG1 co-immunoprecipitated with p53. We further showed that the C-terminus (amino acid 1759–2285) but not the N-terminus (amino acid 1–1758) of ARID1A mediates the interaction with p53 (Fig. 3B). Next, we addressed whether ARID1A and p53 could directly interact with each other. Recombinant GST-p53 (both wild-type p53 and R175H mutant) and the C-terminus of ARID1A (amino acid 1759–2285 with C-terminal V5-tag) were purified from Escherichia coli and used in immunoprecipitation assay (Fig. 3C). Recombinant p53 but not GST pulled down the C-terminus of ARID1A. Moreover, the ARID1A/BRG1 SWI/SNF complex and p53 occupied the same promoter regions of CDKN1A and SMAD3, as demonstrated by sequential and reciprocal chromatin immunoprecipitation (ChIP)-reChIP using p53 and BRG1 antibodies, respectively (Fig. 3D). The above results provide new evidence that p53 is recruited to the ARID1A/BRG1 complex that binds to CDKN1A and SMAD3 promoters.
Fig. 3.
Interaction of p53 with ARID1A/BRG1 SWI/SNF chromatin remodeling complex. (A) Endogenous p53 co-immunoprecipitates with ARID1A and BRG1 in OSE4 cells. As a control, p53 was immunoprecipitated with a mouse anti-p53 antibody and was detected by a rabbit anti-p53 antibody in immunoblotting. (B) C-terminus of ARID1A interacts with p53. V5-tagged full-length (amino acid 1–2285) or fragments of ARID1A were co-stransfected into 293FT cells with FLAG-p53 construct and were applied for immunoprecipitation using FLAG-Ab beads. (C) Coomassie stain of recombinant ARID1A-V5 (amino acid 1759–2285), GST, GST-p53 wild-type (WT) and p53 R175H mutant (left); Western blot of ARID1A-V5 after pull-down with Glutathione beads (right). Arrow heads indicate the bands corresponding to respective proteins. (D) ChIP-reChIP experiments with anti-p53 and BRG1 antibodies. Mouse IgG (mIgG) and rabbit IgG (rIgG) were used as negative controls. (E) Chromatin immunoprecipitation demonstrates that both BRG1 and p53 bind to CDKN1A and SMAD3 promoters, and that knockdown of ARID1A significantly reduces the binding of BRG1 but not p53 to these two promoters. *, p<0.05; **, p<0.01; ***, p<0.001 (t test).
To further delineate the role of ARID1A/BRG1/p53 complex in regulating transcription of CDKN1A and SMAD3, we performed ChIP using antibodies reacting to BRG1. Antibody against p53 was used in the ChIP assay as a control. As shown in Fig. 3E, both BRG1 and p53 bound to the promoter regions of CDKN1A and SMAD3, and, more importantly, knockdown of ARID1A significantly reduced BRG1 binding to both promoter sequences. In contrast, interaction of p53 with CDKN1A and SMAD3 promoter regions was unaffected by reduced expression of ARID1A. Besides, promoter reporter (luciferase) analysis demonstrated that the transcriptional activity of p21 and SMAD3 was decreased by ARID1A knockdown (Fig. S5B).
ARID1A regulates p21 in a p53-dependent fashion
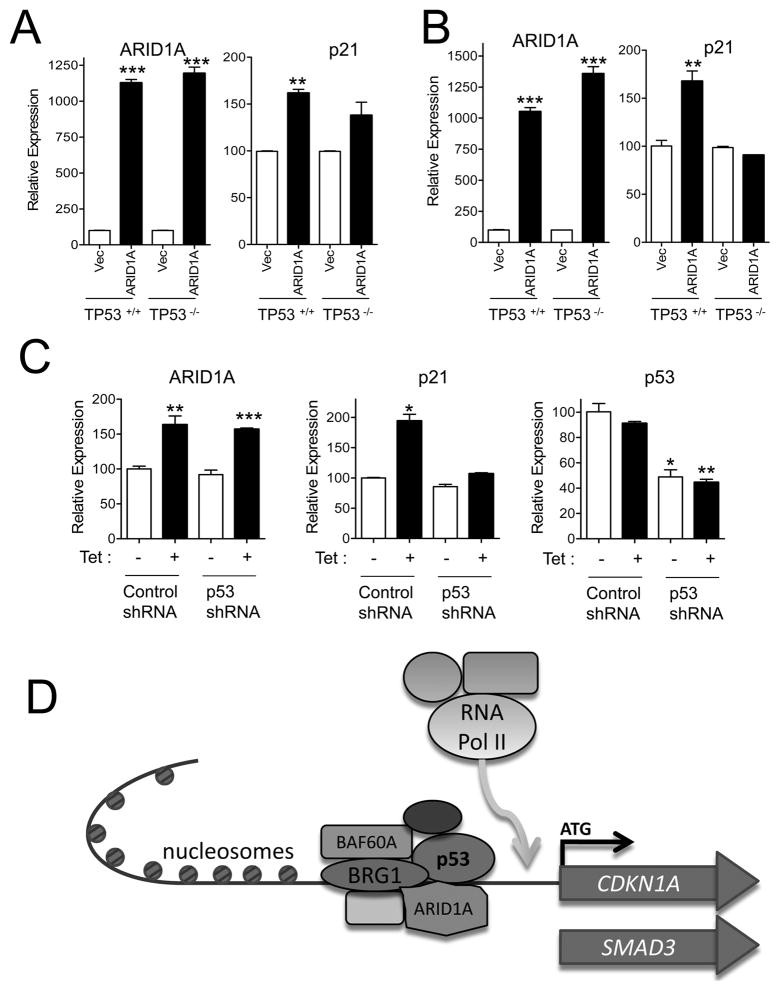
Because p21 is a well established downstream target of p53 and ARID1A interacts with p53, we asked whether ARID1A-regulated p21 transcription depended on the p53 pathway in three cell models. First, two different TP53 knockout cell lines, HCT116TP53−/− and MCF10ATP53−/−, were used. These cells, as well as their parental wild-type counterparts, were transfected with a plasmid expressing ARID1A-V5. We observed that ectopic expression of ARID1A-V5 was associated with increased mRNA levels of p21 in p53 wild-type cells and to a lesser extent in p53 null cells (Fig. 4A and B). Next, we knocked down p53 levels with shRNA in HEC-1-A ARID1A-inducible cells and measured its effect on the transcription level of CDKN1A. The results demonstrated that induction of p21 by ARID1A was significantly reduced by p53-specific shRNA as compared to control shRNA (Fig. 4C). Similarly, SMAD3 upregulation by ectopic expression of ARID1A was compromised in MCF10ATP53−/− cells as compared to MCF10ATP53+/+ cells (Fig. S6B).
Fig. 4.
Regulation of p21 by ARID1A and p53. (A) HCT116TP53−/− and HCT116TP53+/+ cells were transfected with ARID1A-V5. Ectopic expression of ARID1A-V5 is associated with increased mRNA levels of p21 in HCT116TP53+/+ but to a lesser extent in HCT116TP53−/− cells. (B) MCF10ATP53−/− and MCF10ATP53+/+ cells were transfected with ARID1A-V5. Ectopic expression of ARID1A-V5 upregulate p21mRNA levels in MCF10ATP53+/+ cells but not in MCF10ATP53−/− cells. (C) ARID1A inducible HEC-1-A cells were treated with p53 shRNA. ARID1A induction by doxycycline (Tet) increases p21 transcript expression in cells treated with control shRNA but not in cells treated with p53 shRNA. (D) Schematic presentation of SWI/SNF complex containing ARID1A in regulating target gene expression. ARID1A can directly or indirectly recruit p53 to the BAF complex for transcriptional regulation of its downstream targets, e.g., CDKN1Aand SMAD3. p53 may target the SWI/SNF complex to promoters containing a p53 binding site. Upon binding, SWI/SNF alters chromatin structure by displacing nucleosomes to create naked DNA sites to be occupied by transcriptional machinery including RNA polymerase II for transcriptional initiation. *, p<0.05; **, p<0.01; ***, p<0.001(t test).
Correlation of ARID1A mutation and TP53 mutation in tumor tissues
The above results suggest that inactivating mutations in either ARID1A or TP53 result in loss of transcriptional regulation of CDKN1A and SMAD3. To this end, we analyzed the mutational status of ARID1A and TP53 in 77 ovarian clear cell carcinomas and uterine endometrioid carcinomas which were known to have ARID1A and TP53 mutations. Our data showed a mutually exclusive pattern of ARID1A and TP53 mutations (p= 0.031, Fisher’s exact test) (Table 1). Specifically, all 34 tumors with ARID1A mutations were TP53 wild-type, and all 6 carcinomas harboring TP53 mutations contained wild-type ARID1A.
Table 1.
Mutation status of ARID1A and TP53 in 77 ovarian clear cell and uterine endometrioid carcinomas
| TP53 MUT | TP53 WT | Total | |
|---|---|---|---|
| ARID1A MUT | 0 | 34 | 34 |
| ARID1A WT | 6 | 37 | 43 |
| Total | 6 | 71 | 77 |
MUT, mutation; WT, wild-type p=0.031
Discussion
In this study, we demonstrated the tumor suppressive role of ARID1A and proposed the possible mechanisms involved. The evidence for tumor suppressor function of ARID1A comes from its ability to inhibit cellular proliferation and tumor growth when expressed in cancer cells harboring mutated ARID1A and to enhance cellular proliferation and tumorigenecity when its expression is silenced in epithelial cells with wild-type ARID1A. Although ARID1A mutations appear to predominate in endometrium-related carcinomas, inactivation of the ARID1A-containing BAF complex, as a consequence of genetic or epigenetic alterations, may be common in development of other human neoplastic diseases including carcinomas arising from kidney, breast, lung, and stomach (14, 37, 38). For example, deletion of the chromosome 1p35 region harboring ARID1A has been found in more than half of pancreatic carcinomas (39).
It has been established that SWI/SNF chromatin remodeling genes are required for several nuclear activities including transcription. It remains unclear, however, whether they act globally to facilitate transcriptional activity in transcriptionally active domains, or if they locally target a set of selected genes that work in concert for specific cellular functions. The data presented in this study, together with a recent report (40), favor the latter view, as evidenced by downregulation of specific genes upon ARID1A silencing. Analysis of ARID1A downstream genes reveals that the tumor suppressor role of ARID1A mainly involves negative regulation of cell cycle progression and identifies two p53-regulated genes, CDKN1A and SMAD3, which may serve as the major target genes to mediate its tumor suppressor functions. The induction of p21 by ARID1A, and negative regulation of ARID1A on cell-cycle progression in our cell models are in agreement with previous reports which showed that ARID1A is required for cell-cycle arrest after induced differentiation in mouse osteoblastic cells (41, 42).
The regulation of p53-related genes by ARID1A raises the possibility if ARID1A molecularly cooperates with p53 to inhibit tumor growth. In fact, this view is supported by several pieces of evidence reported in this study. Most importantly, both protein co-immunoprecipitation and ChIP-reChIP analyses demonstrate that p53 interacts with ARID1A/BRG1 and the ARID1A/BRG1/p53 complex binds to the promoter regions of CDKN1A and SMAD3. By employing a TP53 knockout cell system and RNA interference strategy, we observed that ARID1A-induced p21 and SMAD3 expression required the presence of p53. Therefore, it is possible that in non-transformed cells, ARID1A and p53 collaborate as a pair of gatekeepers that prevent tumorigenesis by transcriptional activation of tumor-inhibiting downstream genes such as CDKN1A and SMAD3, since the tumor suppressor role of CDKN1A that encodes for p21 has been well established (43) and deficiency of SMAD3 has been reported to propel tumor progression in a mouse model (44). Besides, we found that all tumors with mutated ARID1A contained wild-type TP53, and tumors with mutated TP53 harbored wild-type ARID1A. Because both ARID1A and TP53 appear to be essential for tumor suppression of endometrium-related cancer, concurrent mutations in both genes are thus not required for tumorigenesis. In other words, mutation in either ARID1A or TP53 is sufficient to inactivate the ARID1A/BRG1/p53 complex and silence transcription of CDKN1A and SMAD3.
Our co-immunoprecipitation study using recombinant p53 and ARID1A indicates that ARID1A directly binds to p53 proteins and thus may recruit p53 to the BAF complex for transcriptional regulation of its downstream targets. In this way, p53 may target the SWI/SNF complex to specific promoters with p53 binding sites, while the SWI/SNF complex alters the local chromatin structure by displacing nucleosomes and creating naked DNA sites to be occupied by transcriptional machinery including RNA polymerase II during transcription (Fig. 4C). The mutually exclusive pattern of ARID1A and TP53 mutations supports our view that these tumors do not require concurrent mutations in both genes because as shown in this study mutation of either gene is sufficient to turn off tumor suppressor activity by transcriptionally inactivating the set of genes co-regulated by ARID1A and TP53. It has been established that p53 mutant such as R175H failed to induce p21 expression and cell-cycle arrest (27, 45). One of the functions of mutant p53 is to decrease wild-type p53 binding to its target genes including p21 in a dominant-negative manner (46). Recently it has been reported that mutant p53 protein may bind to a set of unique genes that are not the conventional p53-regulated genes such as GRO1 (47). Thus, the observation that mutant p53 can bind to ARID1A raises the possibility that p53/ARID1A/BRG1 complex might regulate a different set of genes depending on the mutation status of TP53.
It should be noted that OSE4 and IOSE-80PC were immortalized by SV40 large T antigen (29, 48), and one of the functions of T antigen (Tag) is to interact with p53 and inactivate its transcriptional activity (49). Interestingly, it has also been demonstrated that the p53-Tag complex purified from human cells but not from mouse cells still retains p53 transcriptional activity (50), and p53 is required for the transcription of p53-responsive genes including the insulin-like growth factor I and p21 in SV40 Tag immortalized cells (51). Therefore, it warrants further study to determine whether ARID1A binds to p53 alone and/or p53-Tag complex in OSE4 and IOSE-80PC cells. Nevertheless, our data show that ARID1A is required for the expression of several p53-responsive genes, and the interaction between ARID1A and p53 can be further supported by our experiments performed in four cell lines (OVISE, HEC-1-A, HCT116, and MCF10A) that do not express Tag.
Similar to any newly identified cancer genes, there are several important questions remaining to be addressed in order to fully understand their molecular functions and before translational applications in cancer can be proposed. For example, future studies are required to determine the roles of ARID1A mutations in the context of pathway networks, including PI3K/Pten and canonical Wnt pathways, in which alterations have been identified in endometrium-related cancers (52). It will also be important to determine differences in response to therapy, risk for disease recurrence, and overall prognosis between tumors with and without ARID1A mutations. Such information should be valuable in assisting clinical management of patients who suffer from endometrium-related cancer.
In summary, the above data suggest that ARID1A is a negative cell cycle regulator and a tumor suppressor gene. One of the mechanisms involving tumor suppressor function of ARID1A is through the molecular collaboration between ARID1A and p53, as the complex formation is required and sufficient to transcriptionally regulate several p53-related genes, including those with known tumor suppressor functions. We demonstrate that in the presence of wild-type TP53, tumor cells may take advantage of the disruption of the ARID1A/BRG1/p53 complex caused by ARID1A mutations to downregulate p53-regulated genes including p21, SMAD3, and perhaps other genes with tumor suppressor functions. The findings presented in this study suggest a close collaboration between genetic and epigenetic alterations in cancer pathogenesis and provide a new molecular mechanism that contributes to tumor suppression.
Supplementary Material
Acknowledgments
The authors are grateful for reagents that were originally generated by Dr. Fred Bunz for HCT116 TP53 knockout cells, Dr. David J. Weber for MCF10A TP53 knockout cells, Dr. Bert Vogelstein for p21 promoter reporter plasmid, Dr. Thomas J. Kelley for SMAD3 promoter reporter plasmid, and Dr. Weidong Wang for pCI-neo-BAF250 plasmid. The authors also appreciate the technical support from Drs. Chen Xu, Min Gao, pathway analysis from Dr. Jason Xuan and Tian Ye, and tumor samples from Drs. Kuan-Ting Kuo and Tsui-Lien Mao. This study was supported by grants CA129080 and CA103937 from NIH/NCI and an OSB1 grant from HERA Women’s Cancer Foundation.
References
- 1.Liu H, Mulholland N, Fu H, Zhao K. Cooperative activity of BRG1 and Z-DNA formation in chromatin remodeling. Mol Cell Biol. 2006;26:2550–9. doi: 10.1128/MCB.26.7.2550-2559.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nie Z, Xue Y, Yang D, et al. A specificity and targeting subunit of a human SWI/SNF family-related chromatin-remodeling complex. Mol Cell Biol. 2000;20:8879–88. doi: 10.1128/mcb.20.23.8879-8888.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang W, Cote J, Xue Y, et al. Purification and biochemical heterogeneity of the mammalian SWI-SNF complex. EMBO J. 1996;15:5370–82. [PMC free article] [PubMed] [Google Scholar]
- 4.Shih Ie M, Sheu JJ, Santillan A, et al. Amplification of a chromatin remodeling gene, Rsf-1/HBXAP, in ovarian carcinoma. Proc Natl Acad Sci U S A. 2005;102:14004–9. doi: 10.1073/pnas.0504195102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi JH, Sheu JJ, Guan B, et al. Functional analysis of 11q13. 5 amplicon identifies Rsf-1 (HBXAP) as a gene involved in paclitaxel resistance in ovarian cancer. Cancer Res. 2009;69:1407–15. doi: 10.1158/0008-5472.CAN-08-3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheu JJ, Choi JH, Yildiz I, et al. The Roles of Human Sucrose Nonfermenting Protein 2 Homologue in the Tumor-Promoting Functions of Rsf-1. Cancer Res. 2008;68:4050–7. doi: 10.1158/0008-5472.CAN-07-3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sheu JJ, Guan B, Choi JH, et al. Rsf-1, a chromatin remodeling protein, induces DNA damage and promotes genomic instability. J Biol Chem. 2010;285:38260–9. doi: 10.1074/jbc.M110.138735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fang FM, Li CF, Huang HY, et al. Overexpression of a chromatin remodeling factor, Rsf-1/HBXAP, correlates with aggressive oral squanous cell carcinoma. Am J Pathol. 2011 doi: 10.1016/j.ajpath.2011.01.043. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varela I, Tarpey P, Raine K, et al. Exome sequencing identifies frequent mutation of the SWI/SNF complex gene PBRM1 in renal carcinoma. Nature. 2011;469:539–42. doi: 10.1038/nature09639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Medina PP, Romero OA, Kohno T, et al. Frequent BRG1/SMARCA4-inactivating mutations in human lung cancer cell lines. Hum Mutat. 2008;29:617–22. doi: 10.1002/humu.20730. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez-Nieto S, Canada A, Pros E, et al. Massive parallel DNA pyrosequencing analysis of the tumor suppressor BRG1/SMARCA4 in lung primary tumors. Hum Mutat. 2010 doi: 10.1002/humu.21415. [DOI] [PubMed] [Google Scholar]
- 12.Jones S, Wang TL, Shih IM, et al. Frequent mutations of chromatin remodeling gene ARID1A in ovarian clear cell carcinoma. Science. 2010;330:228–31. doi: 10.1126/science.1196333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wiegand KC, Shah SP, Al-Agha OM, et al. ARID1A mutations in endometriosis- associated ovarian carcinomas. N Engl J Med. 2010;363:1532–43. doi: 10.1056/NEJMoa1008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guan B, Mao TL, Panuganti PK, et al. Mutation and loss of expression of ARID1A in uterine low-grade endometrioid carcinoma. Am J Surg Pathol. 2011;35:625–32. doi: 10.1097/PAS.0b013e318212782a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hurlstone AF, Olave IA, Barker N, van Noort M, Clevers H. Cloning and characterization of hELD/OSA1, a novel BRG1 interacting protein. Biochem J. 2002;364:255–64. doi: 10.1042/bj3640255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Rechem C, Boulay G, Leprince D. HIC1 interacts with a specific subunit of SWI/SNF complexes, ARID1A/BAF250A. Biochem Biophys Res Commun. 2009;385:586–90. doi: 10.1016/j.bbrc.2009.05.115. [DOI] [PubMed] [Google Scholar]
- 17.Ho L, Crabtree GR. Chromatin remodelling during development. Nature. 2010;463:474–84. doi: 10.1038/nature08911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reisman D, Glaros S, Thompson EA. The SWI/SNF complex and cancer. Oncogene. 2009;28:1653–68. doi: 10.1038/onc.2009.4. [DOI] [PubMed] [Google Scholar]
- 19.Gao X, Tate P, Hu P, Tjian R, Skarnes WC, Wang Z. ES cell pluripotency and germ-layer formation require the SWI/SNF chromatin remodeling component BAF250a. Proc Natl Acad Sci U S A. 2008;105:6656–61. doi: 10.1073/pnas.0801802105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krosl J, Mamo A, Chagraoui J, et al. A mutant allele of the Swi/Snf member BAF250a determines the pool size of fetal liver hemopoietic stem cell populations. Blood. 2010;116:1678–84. doi: 10.1182/blood-2010-03-273862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alessio N, Squillaro T, Cipollaro M, Bagella L, Giordano A, Galderisi U. The BRG1 ATPase of chromatin remodeling complexes is involved in modulation of mesenchymal stem cell senescence through RB-P53 pathways. Oncogene. 2010;29:5452–63. doi: 10.1038/onc.2010.285. [DOI] [PubMed] [Google Scholar]
- 22.Dallas PB, Pacchione S, Wilsker D, Bowrin V, Kobayashi R, Moran E. The human SWI-SNF complex protein p270 is an ARID family member with non-sequence-specific DNA binding activity. Mol Cell Biol. 2000;20:3137–46. doi: 10.1128/mcb.20.9.3137-3146.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilsker D, Patsialou A, Zumbrun SD, et al. The DNA-binding properties of the ARID-containing subunits of yeast and mammalian SWI/SNF complexes. Nucleic Acids Res. 2004;32:1345–53. doi: 10.1093/nar/gkh277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X, Nagl NG, Wilsker D, et al. Two related ARID family proteins are alternative subunits of human SWI/SNF complexes. Biochem J. 2004;383:319–25. doi: 10.1042/BJ20040524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inoue H, Furukawa T, Giannakopoulos S, Zhou S, King DS, Tanese N. Largest subunits of the human SWI/SNF chromatin-remodeling complex promote transcriptional activation by steroid hormone receptors. J Biol Chem. 2002;277:41674–85. doi: 10.1074/jbc.M205961200. [DOI] [PubMed] [Google Scholar]
- 26.Sastry L, Johnson T, Hobson MJ, Smucker B, Cornetta K. Titering lentiviral vectors: comparison of DNA, RNA and marker expression methods. Gene Ther. 2002;9:1155–62. doi: 10.1038/sj.gt.3301731. [DOI] [PubMed] [Google Scholar]
- 27.El-Deiry WS, Tokino T, Velculescu VE, et al. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–25. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 28.Lee HJ, Lee JK, Miyake S, Kim SJ. A novel E1A-like inhibitor of differentiation (EID) family member, EID-2, suppresses transforming growth factor (TGF)-beta signaling by blocking TGF-beta-induced formation of Smad3-Smad4 complexes. J Biol Chem. 2004;279:2666–72. doi: 10.1074/jbc.M310591200. [DOI] [PubMed] [Google Scholar]
- 29.Nitta M, Katabuchi H, Ohtake H, Tashiro H, Yamaizumi M, Okamura H. Characterization and tumorigenicity of human ovarian surface epithelial cells immortalized by SV40 large T antigen. Gynecol Oncol. 2001;81:10–7. doi: 10.1006/gyno.2000.6084. [DOI] [PubMed] [Google Scholar]
- 30.Choi JH, Choi KC, Auersperg N, Leung PC. Overexpression of follicle-stimulating hormone receptor activates oncogenic pathways in preneoplastic ovarian surface epithelial cells. J Clin Endocrinol Metab. 2004;89:5508–16. doi: 10.1210/jc.2004-0044. [DOI] [PubMed] [Google Scholar]
- 31.Gorai I, Nakazawa T, Miyagi E, Hirahara F, Nagashima Y, Minaguchi H. Establishment and characterization of two human ovarian clear cell adenocarcinoma lines from metastatic lesions with different properties. Gynecol Oncol. 1995;57:33–46. doi: 10.1006/gyno.1995.1097. [DOI] [PubMed] [Google Scholar]
- 32.Maeda D, Mao T-L, Fukayama M, et al. Clinicopathological significance of loss of ARID1A Immunoreactivity in ovarian clear cell carcinoma. Int J Mol Sci. 2010;11:5120–8. doi: 10.3390/ijms11125120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu R, Hendrix-Lucas N, Kuick R, et al. Mouse model of human ovarian endometrioid adenocarcinoma based on somatic defects in the Wnt/beta-catenin and PI3K/Pten signaling pathways. Cancer Cell. 2007;11:321–33. doi: 10.1016/j.ccr.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 34.Oh J, Sohn DH, Ko M, Chung H, Jeon SH, Seong RH. BAF60a interacts with p53 to recruit the SWI/SNF complex. J Biol Chem. 2008;283:11924–34. doi: 10.1074/jbc.M705401200. [DOI] [PubMed] [Google Scholar]
- 35.Lee D, Kim JW, Seo T, Hwang SG, Choi EJ, Choe J. SWI/SNF complex interacts with tumor suppressor p53 and is necessary for the activation of p53-mediated transcription. J Biol Chem. 2002;277:22330–7. doi: 10.1074/jbc.M111987200. [DOI] [PubMed] [Google Scholar]
- 36.Naidu SR, Love IM, Imbalzano AN, Grossman SR, Androphy EJ. The SWI/SNF chromatin remodeling subunit BRG1 is a critical regulator of p53 necessary for proliferation of malignant cells. Oncogene. 2009;28:2492–501. doi: 10.1038/onc.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang X, Nagl NG, Jr, Flowers S, Zweitzig D, Dallas PB, Moran E. Expression of p270 (ARID1A), a component of human SWI/SNF complexes, in human tumors. Int J Cancer. 2004;112:636. doi: 10.1002/ijc.20450. [DOI] [PubMed] [Google Scholar]
- 38.Huang J, Zhao YL, Li Y, Fletcher JA, Xiao S. Genomic and functional evidence for an ARID1A tumor suppressor role. Genes Chromosomes Cancer. 2007;46:745–50. doi: 10.1002/gcc.20459. [DOI] [PubMed] [Google Scholar]
- 39.Birnbaum DJ, Adelaide J, Mamessier E, et al. Genome profiling of pancreatic adenocarcinoma. Genes Chromosomes Cancer. 2011 doi: 10.1002/gcc.20870. [DOI] [PubMed] [Google Scholar]
- 40.Inoue H, Giannakopoulos S, Parkhurst CN, et al. Target genes of the largest human SWI/SNF complex subunit control cell growth. Biochem J. 2011;434:83–92. doi: 10.1042/BJ20101358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagl NG, Jr, Patsialou A, Haines DS, Dallas PB, Beck GR, Jr, Moran E. The p270 (ARID1A/SMARCF1) subunit of mammalian SWI/SNF-related complexes is essential for normal cell cycle arrest. Cancer Res. 2005;65:9236–44. doi: 10.1158/0008-5472.CAN-05-1225. [DOI] [PubMed] [Google Scholar]
- 42.Nagl NG, Jr, Zweitzig DR, Thimmapaya B, Beck GR, Jr, Moran E. The c-myc gene is a direct target of mammalian SWI/SNF-related complexes during differentiation-associated cell cycle arrest. Cancer Res. 2006;66:1289–93. doi: 10.1158/0008-5472.CAN-05-3427. [DOI] [PubMed] [Google Scholar]
- 43.Abbas T, Dutta A. p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer. 2009;9:400–14. doi: 10.1038/nrc2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sodir NM, Chen X, Park R, et al. Smad3 deficiency promotes tumorigenesis in the distal colon of ApcMin/+ mice. Cancer Res. 2006;66:8430–8. doi: 10.1158/0008-5472.CAN-06-1437. [DOI] [PubMed] [Google Scholar]
- 45.Baker SJ, Markowitz S, Fearon ER, Willson JK, Vogelstein B. Suppression of human colorectal carcinoma cell growth by wild-type p53. Science. 1990;249:912–5. doi: 10.1126/science.2144057. [DOI] [PubMed] [Google Scholar]
- 46.Willis A, Jung EJ, Wakefield T, Chen X. Mutant p53 exerts a dominant negative effect by preventing wild-type p53 from binding to the promoter of its target genes. Oncogene. 2004;23:2330–8. doi: 10.1038/sj.onc.1207396. [DOI] [PubMed] [Google Scholar]
- 47.Yan W, Chen X. Identification of GRO1 as a critical determinant for mutant p53 gain of function. J Biol Chem. 2009;284:12178–87. doi: 10.1074/jbc.M900994200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choi JH, Lee KT, Leung PC. Estrogen receptor alpha pathway is involved in leptin- induced ovarian cancer cell growth. Carcinogenesis. 2011;32:589–96. doi: 10.1093/carcin/bgq276. [DOI] [PubMed] [Google Scholar]
- 49.Ali SH, DeCaprio JA. Cellular transformation by SV40 large T antigen: interaction with host proteins. Semin Cancer Biol. 2001;11:15–23. doi: 10.1006/scbi.2000.0342. [DOI] [PubMed] [Google Scholar]
- 50.Sheppard HM, Corneillie SI, Espiritu C, Gatti A, Liu X. New insights into the mechanism of inhibition of p53 by simian virus 40 large T antigen. Mol Cell Biol. 1999;19:2746–53. doi: 10.1128/mcb.19.4.2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bocchetta M, Eliasz S, De Marco MA, Rudzinski J, Zhang L, Carbone M. The SV40 large T antigen-p53 complexes bind and activate the insulin-like growth factor-I promoter stimulating cell growth. Cancer Res. 2008;68:1022–9. doi: 10.1158/0008-5472.CAN-07-5203. [DOI] [PubMed] [Google Scholar]
- 52.Di Cristofano A, Ellenson LH. Endometrial carcinoma. Annu Rev Pathol. 2007;2:57–85. doi: 10.1146/annurev.pathol.2.010506.091905. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.