Abstract
Autism is a common neurodevelopmental disorder with genetic and environmental components. Though unproven, genetic susceptibility to high mercury (Hg) body burden has been suggested as an autism risk factor in a subset of children. We hypothesized that exposure to “safe” Hg levels could be implicated in the etiology of autism if genetic susceptibility altered Hg's metabolism or intracellular compartmentalization. Genetic sequences of four genes implicated in the transport and response to Hg were screened for variation and association with autism. LAT1 and DMT1 function in Hg transport, and Hg exposure induces MTF1 and MT1a. We identified and characterized 74 variants in MT1a, DMT1, LAT1 and MTF1. Polymorphisms identified through screening 48 unrelated individuals from the general and autistic populations were evaluated for differences in allele frequencies using Fisher's exact test. Three variants with suggestive p-values <0.1 and four variants with significant p-values <0.05 were followed-up with TaqMan genotyping in a larger cohort of 204 patients and 323 control samples. The pedigree disequilibrium test was used to examine linkage and association. Analysis failed to show association with autism for any variant evaluated in both the initial screening set and the expanded cohort, suggesting that variations in the ability of the four genes studied to process and transport Hg may not play a significant role in the etiology of autism.
Keywords: Mercury, Autism, LAT1, DMT1, MTF1, MT1a
1. Introduction
Autism is a common neurodevelopmental disorder with 0.1 to 0.2% prevalence in the U.S (Network, 2007) and is part of the autism spectrum disorders (ASD, a collective term which also encompasses Asperger's syndrome and children diagnosed with “pervasive developmental disorder, not otherwise specified”)(Pickett and London, 2005). Autism is characterized by repetitive patterns of behavior, impairments in social interaction and disrupted communication which are usually evident by age 3.
Autism is a complex disorder with both genetic and environmental components (Rapin, 1997). Genome-wide linkage screens have identified multiple genetic loci proposed to impact susceptibility and the overall autistic phenotype, but few surpass the significant linkage threshold value and fail to be consistently replicated (Vorstman et al. , 2006). In addition, positive findings from candidate gene analysis (summarized in recent reviews (Abrahams and Geschwind, 2008, Freitag et al. , 2010)) are not consistently replicated across studies (Newschaffer et al. , 2007). Autism is 90% heritable (Freitag, 2007), yet defined gene mutations, copy number variations and genetic syndromes account for only 10-20% of autism ASD cases(Abrahams and Geschwind, 2008) suggesting complex interactions between multiple genes and/or with environmental factors influence the risk and severity of autism (Folstein and Rosen-Sheidley, 2001). Furthermore, it has been hypothesized that variation in the interplay between inherited vulnerabilities and different environmental exposures may account for the observed heterogeneity in the autism phenotype (Landrigan, 2010).
Mercury (Hg) has been implicated as an environmental risk factor for autism (Schultz, 2010). Exposure to this known neurotoxicant can result from a variety of sources, and its metabolism and toxicity depends upon the chemical form. Consumption of predator fish is the most prevalent source of organic methylmercury (MeHg)(Aschner et al. , 2010). In contrast, ethylmercury (EtHg) is found at high concentrations (~50 %) in the preservative thimerosal used in multidose vials of childhood vaccines to prevent fungal and bacterial contamination. Finally, inhalation of elemental Hg vapor occurs in some occupational settings such as industry, dentistry (amalgam tooth fillings) and extraction of gold. Metallic Hg in dental amalgams can release both Hg vapor and divalent mercury (Hg2+) into surrounding tissues. Topically applied skin creams, infant teething powders and contaminated food are also routes of inorganic Hg exposure (Casarett, 2001, Counter and Buchanan, 2004).
Hg levels have been evaluated in autistic children and both increased and decreased levels were reported, leading to inconclusive results. Gene expression studies correlated with low blood Hg levels are significantly different between autistic boys compared to non-autistic boys, suggesting the possibility that autistic children might metabolize Hg differently compared to non-autistic children (Stamova B et al. , 2011). Inherited variations in metal transporters or metal processing genes may render certain individuals more susceptible to Hg toxicity and, in the context of environmental exposure to Hg, could be associated with autism.
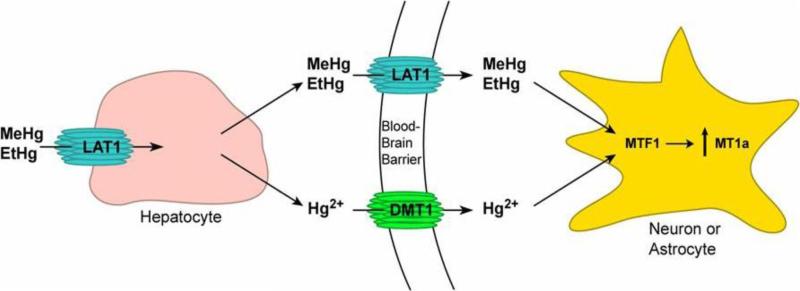
In this study, we screened the genetic sequences of four key genes involved in Hg response and transport for variation and any association with autism. The large neutral amino acid transporter type 1 (LAT1) transports MeHg and likely EtHg, while the divalent metal transporter 1 (DMT1) transports inorganic Hg, a byproduct of cleaved MeHg and EtHg. Metal-regulatory transcription factor 1 (MTF1) induces transcription of metallothionein 1a (MT1a) which binds to both inorganic and organic Hg and works to alleviate metal toxicity. (Figure 1)
Figure 1. Function of LAT1, DMT1, MTF1 and MT1a in Hg metabolism and transport into the brain.
Upon Hg exposure, organic Hg (MeHg or EtHg) is transported into liver hepatocytes by LAT1, where a portion is decomposed into Hg2+. Hg2+ is transported into the brain by DMT1, while remaining MeHg and/or EtHg are taken up by LAT1 into the brain via molecular mimicry of methionine. The presence of MeHg, EtHg and/or Hg2+ in neurons or astrocytes then triggers MTF1 to upregulate MT1a in response to this heavy metal stress.
In contrast to Hg exposure at toxic levels, even at Hg blood levels within Centers for Disease Control and Prevention (CDC) or US Environmental Protection Agency (EPA) accepted values (considered “safe” levels) could be implicated in the etiology of autism due to genetic susceptibility. We hypothesized that even in the absence of elevated Hg levels, inherited variations in metal regulatory genes could potentially increase susceptibility to Hg toxicity by altering the transport, binding, efflux, or tissue distribution of Hg in the body. Prenatal and postnatal exposure to Hg could then act synergistically with these genetic susceptibility factors to manifest the behavioral and developmental deficits present in autism.
2. Materials and methods
2.1 Data Set
All probands were between 4 and 22 years of age with an IQ above 35. As samples were collected prior to 2001, the year thimerosal was revoked from the US market, the probands were likely exposed to thimerosal for their primary vaccinations. All probands were evaluated using both Autism Diagnostic Interview-Revised and Autism Diagnostic Observation Schedule (all modules) to strictly select those with autism and not ASD. Each proband was also evaluated using the Childhood Autism Rating Scale, the Repetitive Behavior Scale, the Aberrant Behavior Checklist, and the Social Responsiveness Scale. Each proband also had a dysmorphology examination to screen for phenocopies.
The initial screening data set consisted of 24 Caucasian autistic individuals and 24 Caucasian normal controls, gender-matched, from the general population.
The larger data set used for TaqMan genotyping of rs2285230, rs1048230, rs33914778 and rs12444670 consisted of a total of 130 families, of which 65 were multiplex families. There were 204 autistic individuals (166 males and 38 females) and 323 control individuals (161 males and 162 females).
However, the TaqManassay for rs12444670 could only successfully genotype 482 individuals, representing 185 autistic individuals and 297 control individuals (294 males and 188 females, with 235 founders and 247 non-founders).
2.2 Primer Design
Primer3 (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi) and PrimerFinder software (J. Smith and K. Bradley, unpublished) were used to design a series of overlapping amplimers spanning the genes of interest (DMT1, MT1a, MTF1 and LAT1) to amplify all exons, exon-intron boundaries, 5‘ and 3‘ untranslated regions, and up to 1000 bp of the promoter region (Table 1). Primer sets were screened on a 2% agarose gel to confirm specific amplification and the correct amplicon size. A total of 100 primer sets were required to amplify the coding and near-coding regions of all four genes combined.
Table 1.
Summary of investigated genes
| Gene Name | Gene Symbol | Location | Gene Size | # of exons |
|---|---|---|---|---|
| Divalent Metal Transporter 1 | DMT1 (aka SLC11a2, NRAMP2) | 12q13 | 43,999 bp | 17* |
| Metallothionein 1a | MT1a | 16q13 | 2941 bp | 3 |
| Metal-regulatory transcription factor 1 | MTF1 | 1p33 | 3302 bp | 11 |
| Neutral amino acid transporter, system L 1 | LAT1 (aka SLC7a5) | 16q24.3 | 5183 bp | 10 |
additional exons possible due to alternate splicing
2.3 Polymorphism Screening and Detection
To detect polymorphisms in DMT1, MT1a, MTF1 and LAT1, we screened an initial data set consisting of 48 Caucasian individuals (24 cases and 24 controls). Fragments were PCR amplified (20 uL reactions containing Platinum SuperMix, 10 ng of human genomic DNA or 20 ng of autism DNA, and 40 ng/uL each of forward and reverse primers) in a thermal cycler (MJ Research, Waltham, MA). Annealing temperatures were adjusted based on GC content.
Single strand conformation polymorphism (SSCP) analysis was employed to screen all of the exons, 5‘ and 3‘ untranslated regions, 1kb upstream of the transcript start, and the exonic bordering regions for genetic variations. DNA fragments were electrophoresed on a 0.5X non-denaturing polyacrylamide gel (15 watts, 4°C for 10-16 hours, depending on product size) with 0.6X TBE running buffer and then visualized by silver stain as previously described (von Deimling et al. , 1993). Polymorphic fragments were sequenced to characterize the specific variant (Vanderbilt University DNA Sequencing Facility or GenHunter Corporation, Nashville, TN).
2.4 TaqMan Genotyping
SNP genotyping was performed using Assays-On-Demand or Assays-by-Design (Applied Biosystems, Foster City, CA) with the ABI PRISM 7900HT Sequence Detection System (Applied Biosystems). Amplification was performed in a 384-well DNA Engine Tetrad 2 Peltier Thermal Cycler (MJ Research, Waltham, MA) with the following conditions: 94°C for 10 min; 92°C for 15 sec, 60°C for 1 min (50 cycles); and held at 4°C. Systematic genotyping errors were minimized through quality control (QC) checks with duplicated samples.
2.5 Statistical Analysis
In the initial screening set of 48 individuals, Hardy-Weinberg Equilibrium (HWE) was calculated in cases and controls with Fisher's exact test. Polymorphisms in both the control and autistic populations were evaluated for differences in allele and genotype frequencies using Fisher's exact test. A p-value <0.05 was considered significant, and a p-value <0.1 was considered suggestive.
Polymorphisms with a suggestive p-value <0.1 or a significant p-value <0.05 were evaluated further in a larger data set of 527 individuals using TaqMan genotyping methods. Allele frequencies and Hardy Weinberg equilibrium were calculated using all genotyped founders in a family, if the family did not contain any genotyped founders, one individual was selected randomly for each family to determine the allele frequencies. The pedigree disequilibrium test (PDT) was performed to examine linkage and association at each marker (Martin et al. , 2001, Martin et al. , 2000). For this analysis 123 trios were used to calculate the sum and average PDT values, that are based on allele counts and the genotype PDT value that is based on genotype counts.
3. Results
Of the 74 polymorphisms currently identified in LAT1, DMT1, MTF1 and MT1a, 17 were novel and 5 were in coding regions, 2 of which were nonsynonymous SNPs (Table 2). Analysis with Fisher's exact test revealed 4 polymorphisms exhibited allele or genotype frequencies that differed significantly (p<0.05) between autistic and control populations (a novel SNP in MTF1, rs12444670 in LAT1, a novel SNP in DMT1 and rs2285230 in DMT1) while 3 polymorphisms (rs33914778 in LAT1, rs11169655 in DMT1, and rs1048230 in DMT1) had suggestive p-values (p<0.1) (Table 2).
Table 2.
Polymorphisms identified in four candidate genes
| Location | Nucleotide Change | Amino Acid Substitution | Case Minor Allele Frequency | Control Minor Allele Frequency | rs number | P-value Allele | P-value Genotype |
|---|---|---|---|---|---|---|---|
| MTF1 | |||||||
| 5′ near gene | NT_032977.9:g.8298029G>A | – | 0.07 (A) | 0.15 (A) | rs61778086 | 0.328 | 0.306 |
| 5′ near gene | NT_032977.9:g.8298155T>A | – | 0.13 (T) | 0.21 (T) | rs9660548 | 0.400 | 0.629 |
| 5′ near gene | NT_032977.9:g.8297694T>G | – | 0.02 (G) | 1.00 (T) | novel | 0.480 | 0.482 |
| 5′ near gene | NT_032977.9:g.8297555T>C | – | 0.02 (C) | 1.00 (T) | novel | 0.475 | 0.475 |
| Intron | NM_005955.2:c.648-109G>A | – | 0.14 (A) | 0.24 (A) | rs112836202 | 0.393 | 0.497 |
| Intron | NM_005955.2:c.648-104T>C | – | 0.02 (C) | 0.00 (C) | rs112943950 | 1.000 | 1.000 |
| Intron | NM_005955.2:c.780-60C>T | – | 0.40 (C) | 0.29 (C) | rs3762359 | 0.368 | 0.599 |
| Intron | NM_005955.2:c.853+93T>C | – | 0.40 (T) | 0.32 (T) | rs3912368 | 0.472 | 0.754 |
| Intron | NM_005955.2:c.780-60C>T | – | 0.04 (T) | 0.02 (T) | rs3762359 | 0.627 | 0.602 |
| Intron | NM_005955.2:c.853+123C>T | – | 0.19 (T) | 0.18 (T) | rs28378413 | 1.00 | 0.750 |
| Exon | NM_005955.2:c.1731T>C | NP_005946.2:p.Asn577Asn | 1.00 (T) | 0.02 (C) | novel | 0.472 | 0.456 |
| Intron | NM_005955.2:c.1767+104C>T | – | 0.02 (T) | 1.00 (C) | novel | 1.000 | 1.000 |
| Intron | NM_005955.2:c.1767+173G>T | – | 0.50 (T) | 0.02 (T) | novel | 0.000 | 0.000 |
| 3′ UTR | NM_005955.2:c.*145C>G | – | 1.00 (G) | 0.02 (C) | novel | 1.000 | 1.000 |
| MT1A | |||||||
| 5′ near gene | NT_010498.15:g.10286040G>A | – | 0.35 (G) | 0.33 (G) | rs1827213 | 1.000 | 1.000 |
| 5′ near gene | NT_010498.15:g.10286056G>A | – | 0.02 (A) | 1.00 (G) | novel | 1.000 | 1.000 |
| 5′ near gene | NT_010498.15:g.10286066A>G | – | 0.13 (G) | 0.09 (G) | rs35346959 | 0.509 | 0.707 |
| 5′ near gene | NT_010498.15:g.10286214T>G | – | 0.09 (G | 0.07 (G) | rs56178387 | 1.000 | 1.000 |
| 5′ near gene | NT_010498.15:g.10286215A>C | – | 0.09 (C) | 0.07 (C) | rs56031221 | 1.000 | 1.000 |
| Intron | NM_005946.2:c.29-28A>G | – | 0.31 (A) | 0.25 (A) | rs11076161 | 0.653 | 0.560 |
| Intron | NM_005946.2:c.29-33A>G | – | 0.31 (A) | 0.25 (A) | rs11076160 | 0.656 | 0.560 |
| Exon | NM_005946.2:c.80C>A | NP_005937.2:p.Thr27Asn | 0.35 (C) | 0.27 (C) | rs111640851 | 0.484 | 0.626 |
| Intron | NM_005946.2:c.94+8T>C | – | 0.35 (T) | 0.25 (T) | rs11647171 | 0.353 | 0.525 |
| Intron | NM_005946.2:c.94+49G>T | – | 0.10 (T) | 0.07 (T) | rs7190725 | 0.756 | 0.701 |
| Intron | NM_005946.2:c.94+169G>A | – | 0.10 (A) | 0.07 (A) | rs8049883 | 0.687 | 0.713 |
| Intron | NM_005946.2:c.94+198_94+199ins2 | – | 0.10 (N) | 0.07 (N) | rs72537170 | 0.729 | 0.708 |
| 3′ near gene | NT 010498.15:g.10288310C>G | – | 0.31 (C) | 0.26 (C) | rs904777 | 0.634 | 0.892 |
| 3′ near gene | NT_010498.15:g.10288350C>T | – | 0.10 (C) | 0.07 (C) | rs904776 | 0.700 | 0.840 |
| LAT1 | |||||||
| 5′ near gene | NT_010498.15:g.41518754A>G | – | 0.18 (C) | 0.23 (C) | rs33923136 | 0.643 | 0.897 |
| 5′ near gene | NT_010498.15:g.41518738G>C | – | 0.18 (G) | 0.23 (G) | rs33942725 | 0.639 | 0.896 |
| 5′ near gene | NT_010498.15:g.41518691G>A | – | 0.37 (T) | 0.40 (T) | rs33914778 | 0.698 | 0.061 |
| 5′ near gene | NT_010498.15:g.41518453C>G | – | 0.18 (C) | 0.23 (C) | rs4429303 | 0.650 | 0.889 |
| 5′ near gene | NT_010498.15:g41518425G>A | – | 0.07 (T) | 0.06 (T) | rs33988046 | 0.711 | 1.000 |
| 5′ near gene | NT_010498.15:g.41518368A>G | – | 0.18 (C) | 0.23 (C) | rs8055157 | 0.638 | 0.880 |
| 5′ near gene | NT_010498.15:g.41518358C>T | – | 0.01 (A) | 0.00 (A) | rs33971919 | 1.000 | 1.000 |
| 5′ near gene | NT_010498.15:g.41518330A>G | – | 0.33 (C) | 0.35 (C) | rs4356486 | 0.727 | 0.342 |
| Intron | NM_003486.5:c.538+231G>A | – | 0.02 (A) | 1.00 (G) | novel | 1.000 | 1.000 |
| Intron | NM_003486.5:c.538+248C>T | – | 0.14 (T) | 0.05 (T) | novel | 0.269 | 0.655 |
| Intron | NM_003486.5:c.771-35 771-31delATGAT | – | 0.20 (N) | 0.13 (N) | novel | 0.579 | 0.782 |
| Intron | NM_003486.5:c.939+55C>T | – | 0.00 (T) | 0.02 (T) | novel | 0.479 | 0.488 |
| Intron | NM_003486.5:c.1044-35C>T | – | 0.26 (T) | 0.13 (T) | rs2287120 | 0.194 | 0.249 |
| Exon | NM_003486.5:c.1131C>T | NP_003477.4:p.Leu377Leu | 0.02 (A) | 0.02 (A) | rs114164039 | 1.000 | 1.000 |
| Intron | NM_003486.5:c.1141-59G>A | – | 0.20 (A) | 0.04 (A) | rs12444670 | 0.031 | 0.146 |
| 3′ UTR | NM_003486.5:c.*458G>A | – | 0.00 (A) | 0.02 (A) | rs16943310 | 0.500 | 0.481 |
| 3′ UTR | NM_003486.5:c.*438C>G | – | 0.25 (G) | 0.03 (G) | rs1060253 | 0.66 | 0.77 |
| 3′ UTR | NM_003486.5:c.*579C>T | – | 0.05 (T) | 0.03 (T) | novel | 1.00 | 1.00 |
| 3′ UTR | NM_003486.5:c.*679C>T | – | 0.02 (T) | 0.00 (T) | novel | 1.00 | 1.00 |
| 3′ UTR | NM_003486.5:c.*1282G>A | – | 0.25 (A) | 0.25 (A) | rs1060257 | 1.00 | 1.00 |
| 3′ UTR | NM_003486.5:c.*1306 *1307ins3 | – | 0.48 (-) | 0.48 (GGG) | rs10692037 | 0.827 | 0.100 |
| 3′ UTR | NM_003486.5:c.*1727G>A | – | 0.02 (A) | 0.05 (A) | rs78174881 | 0.585 | 0.601 |
| 3′ UTR | NM_003486.5:c.*2093C>T | – | 0.14 (T) | 0.26 (T) | rs74039959 | 0.169 | 0.138 |
| 3′ UTR | NM_003486.5:c.*2101C>G | – | 0.25 (G) | 0.34 (G) | rs1060266 | 0.487 | 0.597 |
| 3′ UTR | NM_003486.5:c.*2105C>T | – | 0.09 (A) | 0.05 (A) | novel | 0.690 | 0.681 |
| 3′ UTR | NM_003486.5:c.*2307C>T | – | 0.09 (T) | 0.00 (T) | rs16943300 | 0.109 | 0.112 |
| 3′ UTR | NM_003486.5:c.*2748insT | – | 0.02 (N) | 0.00 (N) | novel | 1.000 | 1.000 |
| 3′ UTR | NM_003486.5:c.*2746insT | – | 0.00 (N) | 0.03 (N) | novel | 0.382 | 0.382 |
| DMT1 | |||||||
| 5′ near gene | NT_029419.12:g.13547773C>T | – | 0.48 (G) | 0.43 (A) | rs427020 | 0.408 | 0.744 |
| Intron | NM_000617.2:c.309+44A>C | – | 0.30 (A) | 0.25 (A) | rs224589 | 0.642 | 0.823 |
| Intron | NM_000617.2:c.607+12G>A | – | 0.19 (A) | 0.16 (A) | rs224454 | 0.698 | 0.722 |
| Intron | NM_000617.2:c.1197+45T>C | – | 0.14 (C) | 0.17 (C) | rs224568 | 0.792 | 0.860 |
| Intron | NM_000617.2:c.1197+48CTT(5 7) | – | 0.74 (n=5) | 0.83 (n=5) | novel | 0.023 | 0.033 |
| Intron | NT_029419.12:g.13529836G>-/A | – | 0.41 (C) | 0.31 (C) | rs2018673 | 0.366 | 0.169 |
| Intron | NM_000617.2:c.1198-209A>T | – | 0.09 (T) | 0.24 (T) | rs11169655 | 0.075 | 0.208 |
| Exon | NM_000617.2:c.1254T>C | NP_000608.1:p.Ile418Ile | 0.09 (C) | 0.24 (C) | rs1048230 | 0.084 | 0.225 |
| Exon | NM_000617.2:c.1498C>T | NP_000608.1:p.Arg500Trp | 0.02 (T) | 1.00 (C) | rs17216086 | 1.000 | 1.000 |
| Intron | NM_000617.2:c.1576-14A>G | – | 0.11 (A) | 0.03 (A) | rs161044 | 0.219 | 0.207 |
| 3′ UTR | NM_000617.2:c.*358A>G | – | 0.16 (G) | 0.30 (G) | rs2285230 | 0.033 | 0.010 |
| 3′ UTR | NM_000617.2:c.*390G> A | – | 0.20 (A) | 0.18 (A) | rs224446 | 0.718 | 0.836 |
| 3′ UTR | NM_000617.2:c.*1031G>A | – | 0.05 (G) | 0.02 (G) | rs150909 | 0.583 | 0.594 |
| 3′ UTR | NM_000617.2:c.*1273G>A | – | 0.15 (A) | 0.22 (A) | rs11169654 | 0.198 | 0.352 |
| 3′ UTR | NM_000617.2:c.*1876T>C | – | 0.44 (T) | 0.50 (C) | rs149411 | 0.673 | 0.793 |
| 3′ near gene | NT_029419.12:g.13518372G>A | – | 0.41 (C) | 0.48 (C) | rs706804 | 0.525 | 0.207 |
| 3′ near gene | NT_029419.12:g.13518229A>G | – | 0.28 (C) | 0.26 (C) | rs15897 | 1.000 | 1.000 |
| 3′ near gene | NT_029419.12:g.13518054G>T | – | 0.30 (C) | 0.26 (C) | rs829022 | 0.825 | 0.897 |
Four of the polymorphisms with suggestive or significant p-values were successfully evaluated further in a larger data set of 527 individuals (204 affected individuals and 323 unaffected individuals) using TaqMan genotyping methods. The pedigree disequilibrium test (PDT) was applied to examine linkage and association at each marker, and they were not statistically significant for association with autism (Table 3). However, the remaining three markers (a novel SNP in MTF1, a novel SNP in DMT1 and rs11169655 in DMT1) with suggestive or significant p-values could not be properly quantified in the larger cohort due to the lack of reliable genotyping assays.
Table 3.
Pedigree Disequilibrium Test (PDT) analysis
| Gene | Variant | MAF1 | HWE2 | AVE PDT3 |
|---|---|---|---|---|
| DMT1 | rs2285230 | 0.1746 | 0.2671 | 0.1066 |
| DMT1 | rs1048230 | 0.1752 | 0.2671 | 0.1663 |
| LAT1 | rs33914778 | 0.4223 | 0.5166 | 0.1051 |
| LAT1 | rs12444670 | 0.266 | 0.6453 | 0.3704 |
MAF indicates the Minor Allele Frequency
HWE indicates the Hardy-Weinberg Equilibrium
AVE PDT indicates the average pedigree disequilibrium test value
4. Discussion
In the U.S. ~8% of the women of childbearing age have Hg levels exceeding EPA limits (Schober et al. , 2003). At high concentrations, MeHg is highly toxic, especially during central nervous system (CNS) development. Inhaled Hg vapor deposits in the CNS, and organic Hg (e.g. MeHg, EtHg) is readily transported across the blood-brain barrier (BBB).
After a link between autism and EtHg toxicity was suggested in 1998 (Wakefield et al. , 1998) for children who had received measles-mumps-rubella (MMR) vaccines containing thimerosal, it was removed from childhood vaccines in the United States in 2001, but is still widely used in developing countries. A critical review of the 12 original studies examining this link concluded that an association could not be established because of methodological flaws (Parker et al. , 2004). Prenatal and infant exposure to EtHg in vaccines containing thimerosal was not associated with an increased risk of autism or ASD (Price et al. , 2010).
However, the possibility that the risk for autism might be altered due to an individual's gene profile that modifies their susceptibility cannot be excluded (Aschner and Ceccatelli, 2010). Even in the absence of toxic Hg levels, “safe” Hg levels could be implicated in the etiology of autism due to genetic susceptibility. Variants in metal regulatory genes could alter Hg's metabolism or intracellular compartmentalization An autistic individual may not have a higher Hg level, but rather they might handle what is condered a “safe” Hg level differently than a non-autistic individual depending on genetic susceptibility (for example, a genetic profile that lowers the threshold of what Hg level could be tolerated) While there are many genes involved in metal regulation, the four candidate genes exhaustively screened in this study are strong candidates as they are directly related to Hg transport and adaptation.
DMT1 is ubiquitously expressed in tissues including the brain and liver (Gunshin et al. , 1997). It is non-specific and transports numerous divalent metals, including Hg2+ and Fe2+ (Forbes and Gros, 2003, Gunshin, Mackenzie, 1997, Picard et al. , 2000). Elemental, metallic or inhaled Hg is oxidized to Hg2+ after absorption by various tissues. Organic mercury (EtHg and MeHg) can be biotransformed to Hg2+ (Casarett, 2001).
While much is known about MeHg pharmacokinetics, data on EtHg is scarce. EtHg passes through the BBB more slowly, but it decomposes into inorganic Hg faster (Magos, 2003). Many of the theories concerning organic Hg toxicity suggest that it is the inorganic form that causes the damage. We reasoned that polymorphisms in this gene are likely to govern brain uptake mechanisms for decomposed EtHg.
LAT1 is restricted to specific tissues such as brain, placenta, fetal liver, testis, bone marrow and tumor cells (Kanai and Endou, 2003, Kanai et al., 1998). Importantly, LAT1 is highly expressed on both sides of the BBB membrane (Boado et al. , 1999, Matsuo et al. , 2000, Pardridge, 1998) and is the major carrier of neutral amino acids from blood into the brain.
MeHg has a high affinity for reduced sulfhydryl groups and binds to the thiol groups of L-cysteine (Cys). Because the MeHg-Cys complex structurally mimics the amino acid methionine, uptake across the BBB is facilitated by LAT1 (Aschner and Clarkson, 1988, Simmons-Willis et al. , 2002, Yin et al. , 2008). Prenatal exposure to MeHg is especially damaging, as it rapidly crosses both the placenta and the BBB. Since LAT1 is also expressed at the placental barrier, the transport of toxic compounds such as EtHg through this barrier is one of the important determinants in fetal toxicity (Kanai and Endou, 2003).
Human metallothioneins (MTs) are part of a gene cluster on chromosome 16q13 that contains four isoforms (MT1, MT2, MT3 and MT4) and 17 subtypes of MT genes. MT1a, a subtype of MT1, is a small metal-binding protein with important functions in metal metabolism and protection (Lichtlen and Schaffner, 2001). MT1 has a high affinity for toxic heavy metals and is induced following Hg exposure (Aschner, 1996). As a potent antioxidant, it is upregulated in response to Hg and MeHg, as well as a variety of other metals including Cd2+, Cu2+, Ag2+ and Zn2+ (Schultz, 2010) thus attenuating metal-induced reactive oxygen species (ROS) damage(Maret, 2000).
MTF1 is a transcription factor which plays a role in cell stress conditions such as heavy metal overload. One major target of MTF1 is the MT genes. In response to Hg and other types of heavy metals, MTF1 binds to the metal response element (MRE) in the promoter region of MT genes to induce transcription. Polymorphisms in MTF1 could decrease an individual's ability to initiate transcription of genes critical for cell stress response. It is speculated that MTF1 could also bind to MREs in DMT1(Lee et al. , 1998), providing another means of Hg regulation. Upon the presence of Hg, DMT1 would be transcriptionally upregulated to increase the available amount of this protein for metal transport.
One family-based association study revealed a polymorphism in MTF1 was linked autism spectrum disorder (p=0.02)(Serajee et al. , 2004). However, after performing Bonferroni correction, the linkage result was no longer statistically significant. We examined this SNP in our study, but it was not present in the Caucasian individuals in our data set.
Our initial sample set of 48 individuals was adequate for SNP discovery, as we were interested in genetic effects that are common risk factors in the population. Power calculations indicate this was sufficient to detect a polymorphism occurring at a 5% level in the autistic population with 91% confidence (and 99.2% confidence of detection in the total population of 48 individuals screened).
While seven polymorphisms initially exhibited suggestive or significant p-values when evaluated in the small set of 48 individuals, further analysis performed on a much larger cohort of 527 individuals confirmed that four of these variants were not significantly associated with autism. The remaining three variants could not be properly quantified in the larger cohort due to lack of reliable genotyping assays available at the time the study was performed. At present their association with autism in a larger population is inconclusive and will need to be examined further in future studies.
The levels of Hg exposure in the studied cohort may have acted as a confounder for this study. We would expect that for each individual in which genetic variants were evaluated for association to autism, that exposure to Hg (even at “safe” levels) could further contribute to the development of autism following the hypothesis that these genetic variants alter the way that Hg is then processed in the body. For this reason, we specifically selected our autistic cohort to include probands who were likely exposed to thimerosal for their primary vaccinations, as these samples were collected prior to 2001 (the year that thimerosal was revoked from the US market). According to the CDC's National Immunization Survey, the estimated vaccination coverage for the proportion of the US population that received vaccines before thimerosal was removed from the US market is 90.5% (CDC, 2001).
This study is valuable as it is an exhaustive screen for genetic variants in MTF1, MT1a, LAT1 and DMT1. The advantage of screening all exons, exonintron boundaries, 5‘ and 3‘ untranslated regions, and up to 1000 bp of the promoter region was that it encompassed polymorphisms located in coding regions which might affect protein structure or folding, as well as those non-coding regions possibly involved in transcript splicing or stability . In contrast with studies which examined known candidate SNPs, we performed a thorough screen throughout the gene, and identified seventeen polymorphisms in this study that have not been previously reported.
The frequency data for all validated genetic variants identified in the four critical metal metabolism genes evaluated in this study will be useful for future studies regarding other disorders with altered metal content. Increased aluminum, copper and zinc are present in Alzheimer's disease (Liu et al. , 2005). Menkes and Wilson's diseases result in copper insufficiency and copper accumulation, respectively (Daniel et al. , 2004). Copper, manganese and iron levels are altered in prion disease (Wong et al. , 2001). Iron overload has been observed in the brains of patients with Alzheimer's disease (increased iron in Lewy bodies), Parkinson's disease and Huntington's disease (Moos and Morgan, 2004). Since DMT1, MT1 and MTF1 function in regulating levels of the metals mentioned above, it is possible that polymorphisms identified in this study may also explain the altered metal content in these other disorders.
Our analysis failed to show association with autism for any of the variants identified in LAT1, DMT1, MTF1 and MT1a which were evaluated in both the initial screening set and the expanded cohort, suggesting that variations in the ability of these four genes to process and transport heavy metals may not play a significant role in the etiology of autism.
The same genes we evaluated likely play an important role in the etiology of other neurodegenerative diseases. For example, because of the multispecificity of LAT1, it has been proposed to transport compounds other than MeHg and EtHg, which mimic amino acid structures, including toxins implicated in the pathogenesis of amyotrophic lateral sclerosis (ALS), Parkinsonian dementia (Weiss and Choi, 1988), trigeminal neuropathy (Patel et al. , 1993), Huntington's disease, and HIV/AIDS (Eastman and Guilarte, 1991, Stone, 2001). Polymorphisms identified in LAT1 may have broad relevance to understanding the genetic factors that influence individual susceptibility to toxins in a broad range of neurodegenerative diseases.
In addition to metal regulation, MTF1 is critical for liver development and has a variety of other targets related to cell stress defense (glutathione), development (C/EBPalpha), oxidative stress (Sepw1)(Wimmer et al. , 2005), hypoxia (Nrdg1), xenobiotic components, and reactive oxygen intermediates (α-foetoprotein, AFP). Furthermore, MTF1 is implicated in cytoskeletal organization (Csrp1)(Wimmer, Wang, 2005) and cancer (Ndrg1)(Wimmer, Wang, 2005) and PlGF (Green et al. , 2001)). Consequently, polymorphisms identified in MTF1 may have broad significance to each condition listed above.
MTs are associated with a number of diseases (Simpkins, 2000) and are implicated in ageing and neurodegenerative brain disorders. Interestingly, MT1 and MT2 have been shown to be neuroprotective in animal models of familial ALS (Nagano et al. , 2001) and multiple sclerosis (Espejo et al. , 2001). In addition, MT levels are also elevated in cancer (Schmid et al. , 1993, Zelger et al. , 1993). It has been proposed that MT is the “danger signal” that indicates cellular damage has occurred in order to mount an active immune response (Yin et al. , 2005). MT in the extracellular environment facilitates the movement of white blood cells to the site of inflammation (Yin, Knecht, 2005). The discovery that MTs mediate leukocyte chemotaxis implies that these proteins could be associated with autoimmune disease and toxicant exposure.
The knowledge gained from this study expands the current understanding of environmental influences on the susceptibility to Hg neurotoxicity and how it relates to autism. Identifying markers of susceptibility is of great clinical concern for they can be incorporated into epidemiological studies to better predict health risks and used to help improve health protection policies.
Highlights.
Polymorphisms identified through screening 48 unrelated individuals from the general and autistic populations were evaluated for differences in allele frequencies.
Overall, we identified and characterized 75 variants in MT1a, DMT1, LAT1 and MTF1.
Despite the fact that the analysis failed to show association with autism for any variant, we believe that given the debate (and unfounded theories on the role of thimerosal in the etiology of autism and related disorders, such as autism spectrum disorder) the results presented herein are highly important and will dispel some of the myths on Hg and these disorders.
Acknowledgements
This study was supported by NIH grants T32 ES007028 and T32 MH065782 to SEO and NIEHS grant R01 07331 to MA.
Abbreviations
- Hg
Mercury
- LAT1
L-type amino acid transporter 1
- DMT1
Divalent Metal Transporter 1
- MTF1
Metal-regulatory Transcription Factor 1
- MT1a
Metallothionein 1a
- CDC
Centers for Disease Control and Prevention
- EPA
US Environmental Protection Agency
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
REFERENCES
- Abrahams BS, Geschwind DH. Advances in autism genetics: on the threshold of a new neurobiology. Nature reviews Genetics. 2008;9:341–55. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner M. The functional significance of brain metallothioneins. Faseb J. 1996;10:1129–36. doi: 10.1096/fasebj.10.10.8751715. [DOI] [PubMed] [Google Scholar]
- Aschner M, Ceccatelli S. Are neuropathological conditions relevant to ethylmercury exposure? Neurotoxicity research. 2010;18:59–68. doi: 10.1007/s12640-009-9113-2. [DOI] [PubMed] [Google Scholar]
- Aschner M, Clarkson TW. Uptake of methylmercury in the rat brain: effects of amino acids. Brain Res. 1988;462:31–9. doi: 10.1016/0006-8993(88)90581-1. [DOI] [PubMed] [Google Scholar]
- Aschner M, Onishchenko N, Ceccatelli S. Toxicology of alkylmercury compounds. Met Ions Life Sci. 2010;7:403–34. doi: 10.1039/BK9781847551771-00403. [DOI] [PubMed] [Google Scholar]
- Boado RJ, Li JY, Nagaya M, Zhang C, Pardridge WM. Selective expression of the large neutral amino acid transporter at the blood-brain barrier. Proc Natl Acad Sci U S A. 1999;96:12079–84. doi: 10.1073/pnas.96.21.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casarett D. Toxicology: The Basic Science of Poisons. 6th ed. McGraw-Hill; New York: 2001. [Google Scholar]
- CDC National, State, and Urban Area Vaccination Coverage Levels Among Children Aged 19--35 Months --- United States, 2001. 2001.
- Counter SA, Buchanan LH. Mercury exposure in children: a review. Toxicol Appl Pharmacol. 2004;198:209–30. doi: 10.1016/j.taap.2003.11.032. [DOI] [PubMed] [Google Scholar]
- Daniel KG, Harbach RH, Guida WC, Dou QP. Copper storage diseases: Menkes, Wilsons, and cancer. Front Biosci. 2004;9:2652–62. doi: 10.2741/1424. [DOI] [PubMed] [Google Scholar]
- Eastman CL, Guilarte TR. Cytotoxicity of 3-hydroxykynurenine: implications for CNS damage in neonatal vitamin B-6 deficiency. Adv Exp Med Biol. 1991;294:625–9. doi: 10.1007/978-1-4684-5952-4_81. [DOI] [PubMed] [Google Scholar]
- Espejo C, Carrasco J, Hidalgo J, Penkowa M, Garcia A, Saez-Torres I, et al. Differential expression of metallothioneins in the CNS of mice with experimental autoimmune encephalomyelitis. Neuroscience. 2001;105:1055–65. doi: 10.1016/s0306-4522(01)00252-4. [DOI] [PubMed] [Google Scholar]
- Folstein SE, Rosen-Sheidley B. Genetics of autism: complex aetiology for a heterogeneous disorder. Nat Rev Genet. 2001;2:943–55. doi: 10.1038/35103559. [DOI] [PubMed] [Google Scholar]
- Forbes JR, Gros P. Iron, manganese, and cobalt transport by Nramp1 (Slc11a1) and Nramp2 (Slc11a2) expressed at the plasma membrane. Blood. 2003;102:1884–92. doi: 10.1182/blood-2003-02-0425. [DOI] [PubMed] [Google Scholar]
- Freitag CM. The genetics of autistic disorders and its clinical relevance: a review of the literature. Molecular psychiatry. 2007;12:2–22. doi: 10.1038/sj.mp.4001896. [DOI] [PubMed] [Google Scholar]
- Freitag CM, Staal W, Klauck SM, Duketis E, Waltes R. Genetics of autistic disorders: review and clinical implications. Eur Child Adolesc Psychiatry. 2010;19:169–78. doi: 10.1007/s00787-009-0076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CJ, Lichtlen P, Huynh NT, Yanovsky M, Laderoute KR, Schaffner W, et al. Placenta growth factor gene expression is induced by hypoxia in fibroblasts: a central role for metal transcription factor-1. Cancer Res. 2001;61:2696–703. [PubMed] [Google Scholar]
- Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, et al. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388:482–8. doi: 10.1038/41343. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Endou H. Functional properties of multispecific amino acid transporters and their implications to transporter-mediated toxicity. J Toxicol Sci. 2003;28:1–17. doi: 10.2131/jts.28.1. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Segawa H, Miyamoto K, Uchino H, Takeda E, Endou H. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98). J Biol Chem. 1998;273:23629–32. doi: 10.1074/jbc.273.37.23629. [DOI] [PubMed] [Google Scholar]
- Landrigan PJ. What causes autism? Exploring the environmental contribution. Curr Opin Pediatr. 2010;22:219–25. doi: 10.1097/MOP.0b013e328336eb9a. [DOI] [PubMed] [Google Scholar]
- Lee PL, Gelbart T, West C, Halloran C, Beutler E. The human Nramp2 gene: characterization of the gene structure, alternative splicing, promoter region and polymorphisms. Blood Cells Mol Dis. 1998;24:199–215. doi: 10.1006/bcmd.1998.0186. [DOI] [PubMed] [Google Scholar]
- Lichtlen P, Schaffner W. The “metal transcription factor” MTF-1: biological facts and medical implications. Swiss Med Wkly. 2001;131:647–52. doi: 10.4414/smw.2001.09672. [DOI] [PubMed] [Google Scholar]
- Liu G, Garrett MR, Men P, Zhu X, Perry G, Smith MA. Nanoparticle and other metal chelation therapeutics in Alzheimer disease. Biochim Biophys Acta. 2005;1741:246–52. doi: 10.1016/j.bbadis.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Magos L. Neurotoxic character of thimerosal and the allometric extrapolation of adult clearance half-time to infants. J Appl Toxicol. 2003;23:263–9. doi: 10.1002/jat.918. [DOI] [PubMed] [Google Scholar]
- Maret W. The function of zinc metallothionein: a link between cellular zinc and redox state. J Nutr. 2000;130:1455S–8S. doi: 10.1093/jn/130.5.1455S. [DOI] [PubMed] [Google Scholar]
- Martin ER, Bass MP, Kaplan NL. Correcting for a potential bias in the pedigree disequilibrium test. Am J Hum Genet. 2001;68:1065–7. doi: 10.1086/319525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin ER, Monks SA, Warren LL, Kaplan NL. A test for linkage and association in general pedigrees: the pedigree disequilibrium test. Am J Hum Genet. 2000;67:146–54. doi: 10.1086/302957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo H, Tsukada S, Nakata T, Chairoungdua A, Kim DK, Cha SH, et al. Expression of a system L neutral amino acid transporter at the blood-brain barrier. Neuroreport. 2000;11:3507–11. doi: 10.1097/00001756-200011090-00021. [DOI] [PubMed] [Google Scholar]
- Moos T, Morgan EH. The metabolism of neuronal iron and its pathogenic role in neurological disease: review. Ann N Y Acad Sci. 2004;1012:14–26. doi: 10.1196/annals.1306.002. [DOI] [PubMed] [Google Scholar]
- Nagano S, Satoh M, Sumi H, Fujimura H, Tohyama C, Yanagihara T, et al. Reduction of metallothioneins promotes the disease expression of familial amyotrophic lateral sclerosis mice in a dose-dependent manner. Eur J Neurosci. 2001;13:1363–70. doi: 10.1046/j.0953-816x.2001.01512.x. [DOI] [PubMed] [Google Scholar]
- Network AaDDM. 2007.
- Newschaffer CJ, Croen LA, Daniels J, Giarelli E, Grether JK, Levy SE, et al. The epidemiology of autism spectrum disorders. Annu Rev Public Health. 2007;28:235–58. doi: 10.1146/annurev.publhealth.28.021406.144007. [DOI] [PubMed] [Google Scholar]
- Pardridge WM. Blood-brain barrier carrier-mediated transport and brain metabolism of amino acids. Neurochem Res. 1998;23:635–44. doi: 10.1023/a:1022482604276. [DOI] [PubMed] [Google Scholar]
- Parker SK, Schwartz B, Todd J, Pickering LK. Thimerosal-containing vaccines and autistic spectrum disorder: a critical review of published original data. Pediatrics. 2004;114:793–804. doi: 10.1542/peds.2004-0434. [DOI] [PubMed] [Google Scholar]
- Patel NJ, Fullone JS, Anders MW. Brain uptake of S-(1,2-dichlorovinyl)glutathione and S-(1,2-dichlorovinyl)-L-cysteine, the glutathione and cysteine S-conjugates of the neurotoxin dichloroacetylene. Brain Res Mol Brain Res. 1993;17:53–8. doi: 10.1016/0169-328x(93)90072-w. [DOI] [PubMed] [Google Scholar]
- Picard V, Govoni G, Jabado N, Gros P. Nramp 2 (DCT1/DMT1) expressed at the plasma membrane transports iron and other divalent cations into a calcein-accessible cytoplasmic pool. J Biol Chem. 2000;275:35738–45. doi: 10.1074/jbc.M005387200. [DOI] [PubMed] [Google Scholar]
- Pickett J, London E. The neuropathology of autism: a review. J Neuropathol Exp Neurol. 2005;64:925–35. doi: 10.1097/01.jnen.0000186921.42592.6c. [DOI] [PubMed] [Google Scholar]
- Price CS, Thompson WW, Goodson B, Weintraub ES, Croen LA, Hinrichsen VL, et al. Prenatal and infant exposure to thimerosal from vaccines and immunoglobulins and risk of autism. Pediatrics. 2010;126:656–64. doi: 10.1542/peds.2010-0309. [DOI] [PubMed] [Google Scholar]
- Rapin I. Autism. N Engl J Med. 1997;337:97–104. doi: 10.1056/NEJM199707103370206. [DOI] [PubMed] [Google Scholar]
- Schmid KW, Ellis IO, Gee JM, Darke BM, Lees WE, Kay J, et al. Presence and possible significance of immunocytochemically demonstrable metallothionein over-expression in primary invasive ductal carcinoma of the breast. Virchows Arch A Pathol Anat Histopathol. 1993;422:153–9. doi: 10.1007/BF01607167. [DOI] [PubMed] [Google Scholar]
- Schober SE, Sinks TH, Jones RL, Bolger PM, McDowell M, Osterloh J, et al. Blood mercury levels in US children and women of childbearing age, 1999-2000. Jama. 2003;289:1667–74. doi: 10.1001/jama.289.13.1667. [DOI] [PubMed] [Google Scholar]
- Schultz S. Does thimerosal or other mercury exposure increase the risk for autism? A review of current literature. Acta Neurobiol Exp. 2010;70:187–95. doi: 10.55782/ane-2010-1790. [DOI] [PubMed] [Google Scholar]
- Serajee FJ, Nabi R, Zhong H, Huq M. Polymorphisms in xenobiotic metabolism genes and autism. J Child Neurol. 2004;19:413–7. doi: 10.1177/088307380401900603. [DOI] [PubMed] [Google Scholar]
- Simmons-Willis TA, Koh AS, Clarkson TW, Ballatori N. Transport of a neurotoxicant by molecular mimicry: the methylmercury-L-cysteine complex is a substrate for human L-type large neutral amino acid transporter (LAT) 1 and LAT2. Biochem J. 2002;367:239–46. doi: 10.1042/BJ20020841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpkins CO. Metallothionein in human disease. Cell Mol Biol (Noisy-le-grand) 2000;46:465–88. [PubMed] [Google Scholar]
- Stamova B, Green PG, Tian Y, Hertz-Picciotto I, Pessah IN, Hansen R, et al. Correlations Between Gene Expression and Mercury Levels in Blood of Boys With and Without Autism. Neurotox Res. 2011;19:31–48. doi: 10.1007/s12640-009-9137-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone TW. Endogenous neurotoxins from tryptophan. Toxicon. 2001;39:61–73. doi: 10.1016/s0041-0101(00)00156-2. [DOI] [PubMed] [Google Scholar]
- von Deimling A, Bender B, Louis DN, Wiestler OD. A rapid and non-radioactive PCR based assay for the detection of allelic loss in human gliomas. Neuropathol Appl Neurobiol. 1993;19:524–9. doi: 10.1111/j.1365-2990.1993.tb00481.x. [DOI] [PubMed] [Google Scholar]
- Vorstman JA, Staal WG, van Daalen E, van Engeland H, Hochstenbach PF, Franke L. Identification of novel autism candidate regions through analysis of reported cytogenetic abnormalities associated with autism. Mol Psychiatry. 2006 doi: 10.1038/sj.mp.4001781. [DOI] [PubMed] [Google Scholar]
- Wakefield AJ, Murch SH, Anthony A, Linnell J, Casson DM, Malik M, et al. Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet. 1998;351:637–41. doi: 10.1016/s0140-6736(97)11096-0. [DOI] [PubMed] [Google Scholar]
- Weiss JH, Choi DW. Beta-N-methylamino-L-alanine neurotoxicity: requirement for bicarbonate as a cofactor. Science. 1988;241:973–5. doi: 10.1126/science.3136549. [DOI] [PubMed] [Google Scholar]
- Wimmer U, Wang Y, Georgiev O, Schaffner W. Two major branches of anti-cadmium defense in the mouse: MTF-1/metallothioneins and glutathione. Nucleic Acids Res. 2005;33:5715–27. doi: 10.1093/nar/gki881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong BS, Chen SG, Colucci M, Xie Z, Pan T, Liu T, et al. Aberrant metal binding by prion protein in human prion disease. J Neurochem. 2001;78:1400–8. doi: 10.1046/j.1471-4159.2001.00522.x. [DOI] [PubMed] [Google Scholar]
- Yin X, Knecht DA, Lynes MA. Metallothionein mediates leukocyte chemotaxis. BMC Immunol. 2005;6:21. doi: 10.1186/1471-2172-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z, Jiang H, Syversen T, Rocha JB, Farina M, Aschner M. The methylmercury-L-cysteine conjugate is a substrate for the L-type large neutral amino acid transporter. Journal of neurochemistry. 2008;107:1083–90. doi: 10.1111/j.1471-4159.2008.05683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelger B, Hittmair A, Schir M, Ofner C, Ofner D, Fritsch PO, et al. Immunohistochemically demonstrated metallothionein expression in malignant melanoma. Histopathology. 1993;23:257–63. doi: 10.1111/j.1365-2559.1993.tb01198.x. [DOI] [PubMed] [Google Scholar]