Abstract
Human pluripotent stem cells (PSCs), including embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), provide a dynamic tool for revealing early embryonic development, modeling pathological processes, and developing therapeutics through drug discovery and potential cell replacement. The first step toward the utilities of human PSCs is directed differentiation to functionally specialized cell/tissue types. Following developmental principles, human ESCs, and lately iPSCs, have been effectively differentiated to region- and/or transmitter-specific neuronal and glial types, including cerebral glutamatergic, striatal γ-aminobutyric acid (GABA)-ergic, forebrain cholinergic, midbrain dopaminergic, and spinal motor neurons, as well as astrocytes and oligodendrocytes. These studies also reveal unique aspects of human cell biology, including intrinsically programmed developmental course, differential uses of transcription factors for neuroectoderm specification, and distinct responses to extracellular signals in regulating cell fate. Such information will be instrumental in translating biological findings to therapeutic development.
Keywords: Embryonic stem cells, Induced pluripotent stem cells, Neural stem cells, Patterning, Transcriptional regulation, Transplantation, Drug screening
Introduction
Human PSCs, including those derived from preimplantation embryos, known as ESCs, and those reprogrammed from somatic cells, called iPSCs, have the potential to differentiate to hundreds of cell types of the three embryonic germ layers while maintaining the ability of self-renew [1–4]. Differentiation of human PSCs into specialized cell types in vitro as well as in vivo (e.g., teratoma formation) is essentially recapitulation of embryonic development. Hence, human PSCs are potentially instrumental in revealing early human development, which is otherwise experimentally inaccessible. In this regard, iPSCs derived from patients with developmental disorders may provide a platform to identify missteps in early stages that lead to abnormal development. Like mouse ESCs, human ESCs may be genetically altered to express disease phenotypes, thus offering a dynamic model system for following pathogenic processes. Similarly, iPSCs reprogrammed from patients’ somatic cells, especially those with genetic defects, can potentially achieve the same goal while at the naturally occurring human genetic background. Both genetically modified human ESCs and disease iPSCs may be used for drug discovery. Recently, functional neurons, the so-called iN cells, are induced directly from fibroblasts by overexpression of neuronal transcription factors, such as Ascl1, Brn2, and Mytl [5, 6]. Such a process bypasses stem cell and progenitor stages, thus generating mature neurons in a much shorter time, which may be beneficial for disease modeling. They are nevertheless non-proliferative and thus are not expanded. Functionally specialized cells and tissues that are differentiated from human ESCs and iPSCs or directly reprogrammed may also be employed for regenerative therapy, including personalized cell therapy through reprogramming patients’ somatic cells in vitro and/or in vivo. Therefore, the advent of human PSCs marks the turning point for regenerative medicine.
The utility of human ESCs and iPSCs will be dependent upon our ability to guide them towards functionally specialized and/or disease target cell and tissue types in a robust manner. Over the past decade, progress has been made in directed differentiation of human PSCs, especially ESCs, to certain functional cell types. In this review, we will focus on the neuronal and glial cell types in the central nervous system that have been successfully differentiated from human PSCs, with an intention of drawing principles that will be instructive for our future effort in directing other cell/tissue types from human PSCs.
Developmental principles as a foundation of neural subtype specification
Brain development begins when the inner mass cells, from which ESCs are derived, proliferate, migrate, and differentiate to form three germ layers, the endo-, meso-, and ecto-derm. Activation of fibroblast growth factor (FGF) and/or inhibition of bone morphogenetic protein (BMP) and wingless-type MMTV integration site family (WNT) in the ectoderm results in specialization of neuroectoderm, as unveiled in Xenopus and chick embryos [7, 8]. These morphogens appear to converge on Smad in determining the neuroectoderm fate, at least in Xenopus [9]. They also seem to play similar roles in specifying mouse [10–12] and human [13–16] ESCs toward the neural fate albeit with variations [17]. While strategies aimed at modulating these pathways are instrumental in guiding PSCs toward the neural fate, it is presently unknown what the neuroectoderm transcriptional determinants are and how the signaling pathways regulate neuroectoderm transcription factors, partly due to the complexity of mammalian embryo development [18]. The in vitro PSC neural differentiation, giving its simplicity, could potentially reveal the molecular events underlying neuroectoderm specification.
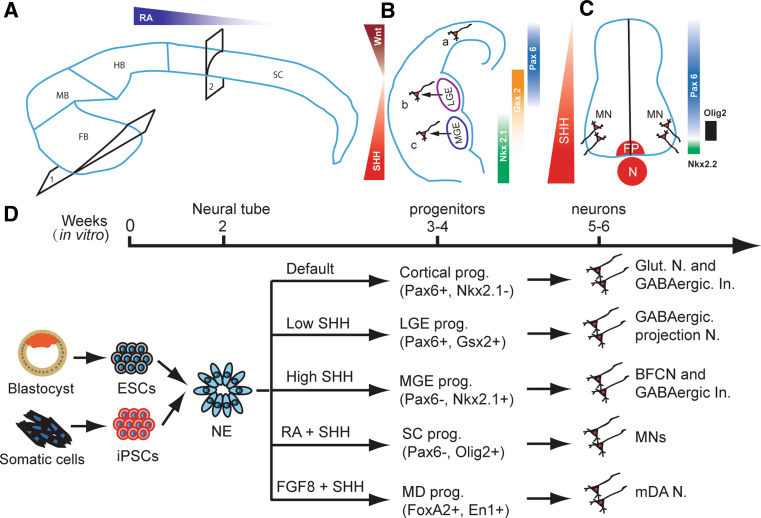
The neuroectoderm is specified first in the head region and gradually extends caudally to form the entire neural plate, which begins to fold and fuse dorsally at the future neck area and forms the neural tube rostrally and caudally. This morphogenesis is dictated by temporarily available gradients of mophogens along the rostro-caudal axis, including FGFs, WNTs, and retinoic acid (RA), as well as along the dorso-verntral axis, including WNTs, BMPs, and sonic hedgehog (SHH). Neural precursors in any given domain of the neural tube are “patterned” by a set of A-P and D-V morphogens at a particular concentration and are fated to functionally specialized neuronal and glial types (Fig. 1). For example, SHH gradients define the transcriptional domains in the developing telencephalon thus patterning the medial ganglionic eminence (MGE) in the ventral and lateral ganglionic eminence (LGE) in the dorsal [19–21] (Fig. 1), whereas in the spinal cord, the ventral to dorsal SHH gradients control the expression of homeodomain transcription factors such as Nkx2.2, Nkx6.1, Olig2, Irx3, and Pax6, thus defining the progenitor domains of five distinct classes of neurons, including motor neurons [22–24]. Although it remains unknown how the regionally patterned progenitors acquire transmitter and/or functional specificity after they become mature neurons and glia, it is the regional patterning principle that guides PSC neural differentiation.
Fig. 1.
Neuronal subtype specification in vivo and vitro. A The developing brain is patterned by morphogens like FGFs and RA along the anterior-posterior axis into forebrain (FB), middle brain (MB), hindbrain (HB), and spinal cord (SC). In each part of the brain and spinal cord, it is further subdivided into multiple domains along the dorsal–ventral axis by morphogens like WNTs, BMPs, and SHH. B In the forebrain, as shown in coronal hemi-section through 1 in a, two opposing morphogens, ventrally derived SHH and dorsally originated WNTs define the cortical, LGE and MGE domains. Progenitors in these domains mainly generate glutamatergic neurons (a), GABAergic projection neurons (b), and BFCNs (c), respectively. GABAergic interneurons are differentiated from all three domains in human. C Similarly, in the spinal cord, as shown in the cross section from site 2 in A, SHH gradients released from notochord (N) and floor plate (FP) define ventral domains in the ventral spinal cord and MNs are generated from the Olig2-expressing ventral progenitors (prog.). D In parallel with in vivo neural development, human ESCs and iPSCs are first differentiated toward Pax6-expressing primitive neuroepithelia (NE) in the absence of morphogens in the first week. In the absence of exogenous morphogens, the NE differentiate to Pax6-expressing cortical progenitors, and subsequently generate glutamatergic neurons (Glut. N.) and GABAergic interneurons (GABAergic In.) by default. In the presence of a low concentration of SHH, the NE become Gsx-expressing LGE progenitors and they later generate DARPP32-expressing GABAergic projection neurons. With higher SHH concentration, the NE are fated to MGE progenitors, which produce BFCNs and GABAergic interneurons. Under RA, the primitive NE are caudalized to hindbrain/spinal cord progenitors, which become Olig2-expressing progenitors in the presence of SHH and then differentiate to motor neurons (MNs). In the presence of FGF8 and SHH, the primitive NE are specified to ventral midbrain progenitors, which then produce mDA neurons
Identification of primitive neuroepithelia for patterning versatile neural progenitors
A neural stem cell (NSC), by definition, can generate neurons, astrocytes and oligodendrocytes while renewing itself. However, NSCs in a particular brain region give rise to neurons and glia characteristic of this but not other parts of the brain. Even in the same brain region, NSCs generate projection neurons during embryonic stages followed by interneurons and glia later in life. Therefore, the potential of NSCs in or isolated from the developing neural tube or developed brain are temporarily and spatially restricted [25, 26]. In contrast, NSCs initially generated during gastrulation may possess a broader spectrum of differentiation potential before they are limited by locally derived morphogens over time. Identification and maintenance of such naive or primitive NSCs from PSCs will be instrumental in directing stem cells to functionally specialized neurons and glia.
Human ESCs, after removal of self-renewing factors (e.g., mouse embryonic fibroblast feeder) and culture in chemically defined medium with or without FGFs and BMP inhibitors, differentiate predominantly to neuroepithelia [14, 27]. For human iPSCs, some of which often do not differentiate to the neural lineage efficiently [28, 29], inhibition of BMPs such as by application of Noggin and SB431542 can significantly increase the neural differentiation efficiency [30]. The neuroepithelia can be readily discerned by characteristic columnar epithelial morphology after 6–8 days of human ESC differentiation, and confirmed by expression of anterior but not posterior neuroectodermal transcription factors, including Pax6 [15, 31]. This expression pattern mirrors that in the developing human neural plate [32]. Initially, these cells organize into rosettes without a lumen and express adhesion molecules like N-cadherin on the surface of individual neuroepithelial cell. Over the following week (days 10–17), the epithelia divide, resulting in compaction and lumen formation in rosettes. At this stage, N-cadherin is gradually concentrated in the lumen side, signaling polarization of the epithelia. They also express additional neuroectoderm transcription factors like Sox1 [31]. These changes in morphology and gene expression patterns suggest that neuroepithelia at these two stages may possess different potentials. Indeed, treatment of early neuroepithelia (days 8–15) with RA efficiently represses expression of anterior genes such as Otx2, Foxg1and Pax6, and induces posterior genes such as Hox genes, whereas the same factor has little effect on regional patterning of neuroepithelia at later stages (>day 15) [15]. Similarly, neuroepithelia generated in the absence of morphogens carry predominantly a dorsal fate by expressing Pax6 and Emx1 but not Nkx2.1. Application of SHH nearly completely represses the dorsal characteristics and induces a ventral forebrain fate by expression of Nkx2.1 [33]. Thus, the early neuroepithelia are referred to as primitive (anterior) neuroepithelia, whereas those at later stages are refractory to morphogen-induced regional patterning, hence regarded as definitive neuroepithelia [31]. Neuroepithelia differentiated from human iPSCs follow the same temporal changes regardless of the cells from which they are reprogrammed [28, 34]. These findings unveil a window of opportunity to direct human PSCs to regionally and functionally specialized neuronal and glial subtypes. They also uncover the unique role of Pax6 in controlling early human brain development [32, 35].
Differentiation of cerebral neurons in the absence of exogenous morphogens
During animal embryogenesis, the anterior neural tube is patterned to the dorsal pallium by WNTs and BMPs [36, 37] and the ventral subpallium by SHH [38], which are fated mainly to glutamatergic and GABAergic neurons, respectively [39–42] (Fig. 1). As discussed above, primitive neuroepithelia differentiated from human PSCs carry a dorsal telencephalic characteristic. Further differentiation of the primitive neuroepithelia in the absence of morphogens results in the generation of neural progenitors that express Pax6, Emx1, Ngn2, and Tbr1, but not ventral transcription factor like Nkx2.1, indicating a predominant dorsal telencephalic fate [33, 43]. This default dorsal fate appears to be determined by high levels of endogenous WNTs relative to lower levels of SHH during human ESC differentiation to telencephalic progenitors [33]. WNTs also up-regulates the truncated form of Gli3, which represses the function of SHH [33]. Consequently, the ventral fate is further suppressed. This contrasts with the largely ventral phenotypes of mouse ESC-derived neural progenitors in the absence of exogenous morphogens. Inhibition of SHH signaling enhances the cerebral fate [44], suggesting that SHH signaling outweighs WNTs during early mouse neural differentiation.
Differentiation of the dorsal human neural progenitors in a chemically defined medium generates mostly glutamatergic neurons but also GABAergic neurons [33]. This is confirmed by whole-cell patch clamping recording, revealing both excitatory and inhibitory synaptic currents [45]. The early presence of GABAergic neurons in differentiation cultures from a nearly pure population of Pax6-positive and Nkx2.1-negative progenitors indicates that some GABAergic neurons may be generated from human cortical progenitors. This in vitro finding is consistent with the fact that a substantial population of GABAergic neurons is developed within cerebral cortex in humans [46], whereas in the mouse cerebral cortex, GABAergic neurons are almost exclusively migrated from the ventral forebrain [47–50]. The finding shows again the maintenance of intrinsic developmental programs of human PSCs.
If the neuroepithelia are differentiated in the absence of exogenous morphogens but as free-floating cell aggregates, they also appear to generate predominantly cortical neuronal progenitors by expressing Foxg1, Emx1, and Pax6. With further culturing in suspension, the progenitors organize into layers, reminiscent of cortical layers. By day 46, neurons expressing Tbr1 and Foxg1 (resembling layer VI neurons) and those expressing Ctip2 and Emx1 appear sequentially [43]. This self-organization is probably driven by patterning factors such as FGF, WNT, and BMP produced within the culture [17], mirroring the birth of neocotex in vivo.
Activation of SHH and/or inhibition of WNTs leads to generation of forebrain GABAergic and cholinergic neurons
Numerous types of neurons reside in the ventral forebrain. The major projection neurons are DARPP32-expressing medium spiny GABAergic neurons in the striatum and cholinergic neurons in the basal forebrain, which originate from LGE and MGE, respectively. The main interneurons are GABAergic neurons, which originate from both LGE and MGE (Fig. 1B). Since the default fate of human PSC-derived neuroepithelia is dorsal because of the overriding effects of WNTs over SHH, it is predicted that inhibition of WNTs and/or activation of SHH would pattern the naive neuroepithelia to a ventral fate. Indeed, a high concentration of SHH, or inhibition of WNTs by dickkopf 1 (DKK1), or together almost completely converts the Pax6-expressing primitive dorsal precursors to ventral progenitors, which now express ventral homeodomain transcription factors, including Gsx2 and Nkx2.1 [33, 51].
Ventral-dorsal SHH gradients, together with dorsal–ventral WNT gradients, determine the progenitor domains in the ventral forebrain. Exposed to low gradients of both SHH and WNTs, progenitors in the dorsal (lateral) subpallium, which develops to LGE, express Pax6 and/or Gsx2 and differentiate to projection GABAergic neurons (Fig. 1). This pattern has been exquisitely replicated in vitro [52]. In the presence of low concentrations of SHH, the progenitor cells exhibit phenotypes of LGE cells, with expression of Gsx2 and low levels of Pax6 but not the more ventral marker Nkx2.1 (Fig. 1D). After removal of SHH, these LGE-like progenitors differentiate to projection GABAergic neurons that express specific markers, including GABA, glutamic acid decarboxylase (GAD) 65/67, and DARPP32, as well as striatal markers like Meis2 and Ctip2 [52]. At an appropriate SHH concentration, the early primitive neuroepithelia are efficiently patterned LGE-like progenitors, which generate an enriched population of DARPP32-expressing GABAergic neurons as high as 87% of the total differentiated cells (Fig. 1D). This is perhaps the highest efficiency by which a neuronal subtype has so far been directed from human PSCs.
In the ventral subpallium, which becomes MGE, progenitors are exposed to high levels of SHH. They express Nkx2.1 and later differentiate to GABAergic interneurons and basal forebrain cholinergic projection neurons (Fig. 1). Indeed, in the presence of high concentrations of SHH, the dorsal transcription factors, Pax6 and Emx1, are completely repressed, whereas the ventral transcription factor Nkx2.1 is highly upregulated. Under this condition, the LGE phenotype, including expression Meis2 and Gsx2, is also repressed. Consequently, a nearly homogeneous population of Nkx2.1-expressing, MGE/POa-like progenitors is generated. Nkx2.1-expressing MGE progenitors normally give rise to GABAergic interneurons and projection cholinergic neurons. Indeed, the majority of the differentiated human cells are GABA-expressing neurons with 14% being cholinergic neurons. The cholinergic population can be increased to 38% if the progenitors are differentiated on top of astrocyte feeder layer [53]. These cholinergic neurons carry forebrain markers such as Foxg1, Otx2, and Nkx2.1, as well as cholinergic transmitter identity markers, including choline acetyltransferase (ChAT) and vesicular acetylcholine transporter (VAChT) (Fig. 1D). Importantly, the in vitro produced human cholinergic neurons, following their transplantation into mice with loss of cholinergic neurons in the medial septum, corrected learning and memory deficit. Furthermore, we found that spinal cord motor neurons that carry the same transmitter as basal forebrain cholinergic neurons (BFCNs) failed to rescue memory deficit [53]. Therefore, both the regional identity and transmitter phenotypes are essential for appropriate function of a neuron.
Generation of ChAT-positive cholinergic neurons from human ESCs has recently been reported [54]. In both cases, nearly a uniform population of human ESC differentiated progenies is ChAT-positive cells. It is mystery how a simple treatment of BMP9 [54] or NGF [55] can persuade human ESCs to uniformly adopt the forebrain cholinergic fate. Furthermore, human ESCs were differentiated in the presence of a very high concentration of RA (10 μM), a strong caudalizing morphogen [54]. It is not clear whether these cells are true cholinergic neurons and whether they are forebrain cholinergic neurons as their regional identity has not been carefully examined. Functional analysis, especially in vivo, will be essential to verify the identity of ChAT-labeled, in vitro-produced cells.
The combination of RA and SHH leads to the generation of spinal cord motor neurons
Spinal cord motor neurons originate from the motoneuron progenitor (pMN) domain in the ventral developing spinal cord. Since human PSC-derived primitive neuroepithelia carry a forebrain identity, it is necessary to caudalize the anterior neuroepithelia. Treatment of neuroepithelia with 0.1 μM of RA in a chemically defined medium from day 10 to 17 completely suppresses the expression of anterior transcription factors such as Otx2 and Foxg1 while activating posterior transcription factors including Hoxb4, Hoxc5, and Hoxc8. Ventralization of the caudal neuroepithelia with SHH from day 14 to 21 effectively limits the cells to a ventral spinal fate, including Olig2-expressing motor neuron progenitors. These progenitors, upon removal of RA and SHH, give rise to Mnx1 (also known as HB9)-expressing, postmitotic motor neurons at the fourth to fifth week [15, 56, 57]. They carry additional motor neuron transcriptional factors such as Lhx3 and Isl ½. This in vitro differentiation process mirrors that in vivo in which postmitotic motor neurons begin to appear in developing human spinal cord at the fifth week. Further culture leads to maturation of these neurons, expressing transmitter-related enzymes, including ChAT, VAChT, and becoming electrically excitable. Importantly, co-culture of the human motor neurons with myocytes causes clustering of acetylcholine receptors on myotubes, as revealed by bungarotoxin staining. Therefore, human ESC-derived motor neurons appear to be functional [15]. Transplantation of human ESCs-derived motor neurons into chick embryonic spinal cord and mouse spinal cord showed survival of grafted neurons, some of which express Nkx6.1 and Mnx1 [58]. However, functionality of these human motor neurons in vivo remains to be demonstrated. The motor neuron differentiation paradigm developed with human ESCs, has also shown efficacy in human iPSCs [28, 29]. In particular, motor neurons are generated from a patient’s iPSCs, including those with amyotrophic lateral sclerosis (ALS) [59] and spinal muscular atrophy (SMA) [60]. Preliminary results indicate that some of the disease phenotypes, such as death of motor neurons, appear to occur in those from SMA iPSCs [60]. Therefore, patient iPSCs could provide a useful model to dissect cellular and molecular mechanisms underlying motor neuron degeneration.
In the above differentiation paradigm, only about 20% of the differentiated progenies are motor neurons [15], leaving the remaining 80% of cells with unknown identities. This is problematic for effective assay development and especially for therapeutic development using the human PSC-derived motor neurons. We have therefore modified the protocol by patterning the primitive neuroepithelia, cultured in suspension, with small molecules, purmorphamine (a SHH agonist) and RA. This modification has led to the generation of over 50% Mnx1-expressing motor neurons [61]. Furthermore, this differentiation system removes the use of recombinant proteins, thus facilitating translation to industrial production and potential clinical applications.
RA often restricts the progenitors to the cervical and brachial spinal cord fate. Hence, motor neurons generated under such a condition are equivalent to those in the cervical and brachial spinal cord. Therefore, alternative strategies need to be developed to direct differentiation of other motoneuron subtypes. Lately, Patani and colleagues revealed that activation of SHH while inhibiting activin/nodal signaling can effectively promote spinal MN specification without exogenous RA. MNs generated through this RA-independent mechanism not only possess caudal, medial motor columnar identities but also with lumbar, lateral motor columns characteristics [62]. Thus, subclasses of spinal cord MNs may be generated from human PSCs.
Alternative routes to dopaminergic neurons
Midbrain dopaminergic neurons (mDA) have been so far the most extensively examined cell type among neural cells that are differentiated from human PSCs. Such enthusiasm stems in part from the expectation that transplantation of stem cell-derived DA neurons is a promising approach for treating Parkinson’s disease (PD) and that transplantation of fetal-derived DA neurons has shown some promises in PD patients [63–65]. Since midbrain DA neurons are localized to the ventral midbrain, a general strategy has been to pattern human ESC-derived neural precursors with FGF8 and SHH in a chemically defined medium [66, 67] or in the presence of stromal cells such as PA6 and MS5 [68–72] or midbrain astrocytes [73]. These neural progenitors differentiate to tyrosine hydroxylase (TH)-expressing DA neurons with variable efficiency, up to 30% among differentiated progenies. The addition of GDNF (25 ng/ml), a known dopaminergic neuroprotectant agent, can significantly increase the yield of dopaminergic-like neurons generated from human ESCs [74]. DA neurons have been similarly differentiated from human iPSCs [75, 76]. Under most conditions, these TH-expressing neurons are DA neurons, as they are generally negative for dopamine β-hydroxylase, an enzyme that turns dopamine to norepinephrine. However, the regional identity of these DA neurons has not been carefully examined. This is in part due to the lack of reliable and robust antibodies for mDA neurons as well as a temporary mismatch of midbrain homeodomain transcription factors and more mature DA neuronal markers. For example, the midbrain homeodomain transcription factor, Engrailed 1 (En1), which often overlaps with TH in mouse DA neurons, is downregulated when the progenitors become post-mitotic TH-expressing DA neurons. Therefore, only a small fraction of TH-expressing DA neurons co-express En1 during a relatively early stage of DA neuron differentiation process from human ESCs [66, 67]. Additionally, more mature markers like dopamine transporter (DAT) are rarely seen in cultured human DA neurons. Therefore, the maturation and function of human ESC-derived DA neurons are generally tested following transplantation into 6-hydroxydopamine (6-OHDA)-lesioned rat stratum. Transplantation of human ESC-derived DA cultures indeed corrects locomotion deficits in those rats, as revealed by apomorphine- or amphetamine-induced rotation tests. Sequential analysis of grafts suggests that the majority of DA neurons are perhaps generated from grafted neural progenitors [66]. It is presently undefined how the grafted neural cells contribute to functional improvement in rodents. It is mostly likely that release of DA from the grafted DA neurons acts like DA supplementation. This is reflected in early symptomatic improvement in grafted rats [73]. However, integration of DA neurons into the striatal circuitry is also possible. So far, the longest survival period post-transplantation is about 5 months [66]. This, however, is not long enough to test if the functional contribution from grafted human ESC derivatives is sustained, potentially through synaptic integration. Animal models under an immune-deficient background would help address this issue. Alternatively, autologous transplantation with DA neurons reprogrammed from the same animals will overcome the need of immune-deficient background. Furthermore, application of existing technologies, such as the use of light-sensitive channelrhodopsin [77, 78] to confirm synaptic and functional connections between grafted DA neurons and their target cells will establish a foundation for stem cell-based therapy for PD.
A recent study found that mDA neurons originate from floor plate cells [79]. Floor plate cells express high levels of SHH and are generally non-neurogenic except in the anterior portion of the midbrain. The anterior midbrain expresses Otx2 and WNT1, which together confer neurogenic potential to floor plate cells, at least in part through the induction of Lmx1a [79]. Hence, an alternative route is to differentiate human PSCs to floor plate cells, which are then activated for neurogenesis for DA neuron generation. Under high concentrations of SHH, large populations of floor plate cells are generated from human ESCs, which express Foxa2 and SHH [80]. Alternatively, overexpression of Gli1 significantly induces the generation of Foxa2-positive precursors [81]. Differentiation of these floor plate cells results in generation of neurons, including some TH-expressing DA neurons [80, 82], suggesting a possibility of generating DA neurons via this alternative route. The bottleneck at this moment is how to activate the neurogenic potential of these floor-plate progenitors. From the principle described above, WNT1 is uniquely expressed in the ventral midbrain and is critical for activating the neurogenic process. WNTs also interact with SHH through Gli3 to regulate dorsal versus ventral fate of neural progenitors that are derived from human ESCs [33]. Thus, it is expected that regulation of WNTs and/or SHH in the Foxa2-expressing progenitors should lead to generation of mDA neurons. Additionally, cells labeled with Foxa2 may not all be floor plate cells, as ventral forebrain progenitors also express Foxa2. Therefore, differentiation of these Foxa2 progenitors to TH-expressing DA neurons may not lead to efficient generation of mDA neurons. It will also be interesting to see if DA neurons generated through the floor plate pathway are functionally distinct from those differentiated using the aforementioned general patterning methods, particularly with regard to their ability to integrate into neural circuitry.
Besides potential application in cell therapy, DA neurons from PD patient’s stem cells may also be useful for revealing pathological processes that underlie DA neuron degeneration in PD and for developing drugs for therapy. IPSCs generated from familial and sporadic PD patients have been differentiated to DA neurons [83]. Transplantation of these PD patients’ DA neurons into the 6-OHDA lesioned rats indicates that these DA neurons behave like regular DA neurons, correcting locomotive deficits [76]. This finding highlights interesting issues. First, human iPSCs can be effectively differentiated to DA neurons using the methods developed with human ESCs. Second, human iPSC-derived DA neurons are functional. Third, despite PD patient origin including genetic defects, DA neural degenerative phenotypes may not manifest in vitro and in a short-term in vivo environment. Experimental interventions and a long-term in vivo environment may be necessary to facilitate phenotypic presentation.
Long march to astroglial generation
Astrocytes are the most abundant cell type in the brain and spinal cord. They participate in almost every aspect of physiology and pathology of the CNS. Hence, generation of enriched populations of functional astrocytes from human ESCs, especially disease iPSCs, would enable better understanding of the roles of astrocytes in pathogenesis of neurological diseases. In particular, cells of the astroglial lineage are generally technically easier for manipulation than neuronal cells. Hence, they may be more readily modified for drug screening or produced for cell therapy.
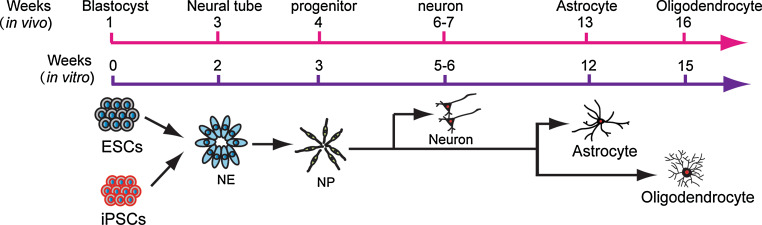
Genesis of astroglial cells follows neurogenesis (Fig. 2). Neural stem/progenitor cells, isolated from brain tissues or derived from mouse ESCs, generate abundant astroglial cells after expansion in vitro, which is almost regarded as a “default” fate. Nevertheless, directed differentiation of enriched populations of functional astroglial cells from human PSCs turns out to be not straightforward. At least two reasons explain the deficiency in this area. First, the specification of astrocytes is poorly understood. In particular, the identity of astroglial progenitors remains enigmatic, partly because of lack of reliable markers [84, 85]. Second, in human, astroglial cells, marked by S100β and glial fibrillary acidic protein (GFAP), begin to appear at 3–4 months of age. This would suggest that astroglia would appear only after long-term culture, which was indeed observed in multiple laboratories [84, 86]. Systematic analysis indicates that astroglial progenitors, marked by a putative glial progenitor marker NF1A [87], are rarely seen at 1 month of human ESC differentiation, but are progressively increased over time. The relatively widely used marker for astroglial progenitors, CD44, is not detected until 2 months of cultures. Instead, the relatively specific astroglial marker, S100β, is detected as early as 2 months in culture. GFAP is found after 3 months of differentiation [88]. This temporal pattern of marker expression corresponds to that of in vivo development. Since human ESC-derived neural precursors generate predominantly neurons in the first 3–4 months [27, 84], it is necessary to suppress neurogenesis and promote gliogenesis. Regular dissociation of neural progenitor aggregates after 1 month of differentiation from human PSCs and then expansion in the presence of EGF significantly reduce the potential of generating β-III tubulin-expressing neurons. Instead, these cells are largely restricted to a glial fate by 2 months of human PSC differentiation, with subsequent sequential expression of NF1A, S100β, CD44, and GFAP. These human astroglial cells also appear to express astrocyte-specific glutamate transporter GLT-1 and can uptake glutamate. Electrophysiological analysis suggests that they possess potassium currents, more closely resembling immature astrocytes. This also corresponds to their ability to promote synaptogenesis of neurons that are growing on astrocytes. These cells are indeed restricted to the astroglial fate as they retain the astrocyte identity following transplantation into the mouse brain. Importantly, the human ESC-derived astroglial progenitors can be expanded continuously for at least 8 months, thus yielding large quantities of uniform population of astroglial cells [88].
Fig. 2.
Temporal course of glial differentiation in vivo and in vitro. In parallel with in vivo development, human PSCs begin to generate neurons in the second month, astrocytes in the third month, and oligodendrocytes in the fourth month
Like neurons, astrocytes in different brain regions exhibit various morphologies and appear to possess unique functions. Astrocytes in the ventral mesencephalon, but not the dorsal mesencephalon or other parts of the brain, express high levels of WNTs and promote the specification of neural progenitors to adopt the midbrain dopaminergic neuronal fate [89, 90]. By patterning primitive neuroepithelial cells with RA or SHH and then differentiating the cells to glial cells, we discovered that now the astroglial progenitors or immature astrocytes also carry homeodomain transcription factors that are appropriate to the positional identity. For example, astroglial progenitors generated in the absence of morphogens carry a dorsal anterior identity by expressing Otx2 but not Hoxb4 or Nkx2.1. In contrast, astroglial progenitors generated in the presence of RA express Hoxb4 but not Otx2. While more studies are needed to determine the functional consequence of regionally specialized astroglial cells, the finding suggests that the positional identity of astroglial cells is determined when primitive neuroepithelia are patterned. The regionally patterned neural progenitors first produce neurons and then generate astrocytes of the same region.
Unexpected twist for oligodendroglial differentiation
Oligodendroglial cells are born after neurons and astroglia (Fig. 2). Most of them are generated in a relatively small domain in the developing ventral brain and spinal cord in an SHH-dependent manner [85, 91]. The progenitors migrate to other parts of the brain and spinal cord and differentiate to myelinating oligodendrocytes. In the spinal cord, most oligodendroglial progenitors are born in the pMN domain. Precursor cells in the pMN domain first generate motor neurons and then switch to oligodendroglial progenitors by co-expressing Olig2 with Nkx2.2 and Sox10. Using the same strategy for spinal motor neuron specification, human ESCs are first induced to Olig2-expressing ventral spinal progenitors with SHH. FGF2 and SHH are then added to inhibit neurogenesis of the Olig2 progenitors, so that the Olig2 progenitors become gliogenic by co-expressing Nkx2.2 in the fifth week. However, these Olig2/Nkx2.2-coexpressing progenitors do not become bipolar Sox10/PDGFR-coexpressing oligodendroglial precursor cells (OPCs) until 2 months later [92, 93]. Therefore, it takes at least 3 months for human ESCs to differentiate to OPCs. This protracted differentiation process coincides with the OPC development in vivo (Fig. 2). All the processes are dependent upon SHH, exogenous or endogenous, as inhibition of SHH signaling by cyclopamine nearly completely abolishes OPC generation. Therefore, SHH-dependent specification of OPCs from human ESCs is essentially the same as what is learned from in vivo analyses of multiple species.
Since the transition from Olig2 progenitors to OPCs takes 8 weeks, the Olig2 progenitors are usually expanded in the presence of morphogens FGF2 and/or EGF. However, few OPCs were generated when the Olig2 progenitors were expanded in the presence of FGF2 whereas the vast majority of the Olig2 progenitors become OPCs in the absence of FGF2. It is surprising because mouse neural progenitors, including Olig2 progenitors, generate robust OPCs after expansion in the presence of FGF2, possibly by promoting SHH production [94–97]. In human cells, FGF2 inhibits SHH expression but facilitates Gli3 expression, thus inhibiting the co-expression of Olig2 with Nkx2.2. The Olig2- or Nkx2.2-expressing progenitors hence generate neurons and astrocytes instead of OPCs [92, 93]. It is not clear why FGF2 in this particular stage of human neural development inhibits oligodendrogliogenesis. It is possible that during that particular neurogenic phase in human development, high levels of FGFs from developing neurons prevent premature oligodendroglial differentiation. Following neurogenesis, FGF levels decrease, which allow oligodendrogliogenesis to take off. This may be one of the mechanisms underlying delayed oligodendrogliogenesis in humans.
The finding that human OPC specification is dependent upon SHH and that FGF2 inhibits SHH signaling explains why human neural progenitors, expanded with FGF2, generate few OPCs [92, 98–101]. It also explains why differentiation of human ESCs without SHH and/or with FGF2 produces only a small fraction of OPCs [102–104]. The near pure population of ‘OPCs’ generated from human ESCs without application of SHH [104] cannot be replicated by us. The downside of the strategy without FGF2 is that the yield of OPCs is low because of the low proliferation rate of Olig2 progenitors. Therefore, there is a need to promote OPC proliferation. Unfortunately, known morphogens for mouse OPCs do not appear to promote human OPC division [92]. Therefore, it seems that human OPCs, at least those of spinal cord characteristics, do not respond to morphogens as predicted from mouse OPCs. This again illustrates the unique aspects of human oligodendroglial development.
As alluded to above, neuronal and glial progenitors are specified when primitive neuroepithelia are patterned. It would be interesting if OPCs of forebrain characteristics may be similarly differentiated from human PSCs and if these OPCs respond to morphogens as predicted from rodent studies.
Generation of retinal cells during human PSC neural differentiation
Retina is part of the CNS. It first differentiates from the rest of the primitive anterior neuroectoderm as an eye field by downregulating neuroectoderm genes and upregulating eye field-specific transcription factors, including Six6, Six3, Rx, Lhx2, and Tll, etc. [105]. Since human ESC/iPSC-derived primitive neuroepithelia exhibit anterior phenotypes with the expression of Pax6, Otx2, and Lhx2 [31], some of them may become retinal stem cells or eye field precursor cells. Coexpression of Pax6 with Rx has been used in animal studies to define eye field precursors [106, 107]. Indeed, co-expression of Pax6 and Rx is observed in our neuroepithelia along neural differentiation, and nearly all neuroepithlia express both Pax6 and Rx by 2 weeks in culture [108]. By day 30, nearly 15% of neuroepithelial spheres express Mitf protein and these cells later become retinal pigment epithelia (RPE). Following RPE differentiation, Mitf gradually decreases, whereas the neural retinal transcription factor Chx10 begins to be expressed by day 40. By day 80 of differentiation, ~20% of all neurospheres contain Crx-expressing photoreceptor precursors, of which ~50% of Crx-positive cells express more mature photoreceptor markers recoverin and the cone photoreceptor-specific protein opsin [108]. This temporal course of retinal cell differentiation from both human ESCs and iPSCs mirrors the course of retinal development in vivo, again highlighting the intrinsic program that is preserved in human PSC differentiation in vitro.
The presence of retinal precursor cells in human PSC neural differentiation cultures would lead to generation of retinal cells in prolonged cultures, including RPE and neural retinal cells. Indeed, RPE are frequently observed spontaneously in neural differentiation cultures [109] (Zhang, unpub. obs.). Since the dorsal part of the optic vesicle is fated into RPE whereas ventral portion give rise to neural retina [110], application of WNTs and Nodal antagonists promotes the differentiation of Rx- and Mitf-positive pigment retinal progenitors, which later generate RPE in the presence of activin and nicotinamide whereas RA and taurine enhance photoreceptor differentiation [107, 111–113]. These results demonstrate that subclasses of retinal cells can be differentiated from human PSCs. RPE cells generated from human iPSCs exhibit similar morphology and gene expression profile as native RPE [114, 115]. These iPS-RPE cells also display functional ion transporters, membrane potential, and polarized vascular endothelial growth factor (VEGF) secretion [115]. Moreover, human iPSC- or ESC-derived RPE cells display survival and migration into retinal layers after transplanting into sodium iodate-injected albino-type adult rabbits [116], and are capable of phagocytosing photoreceptor material in vivo after transplanting into dystrophic rat [114]. These findings suggest that human PSC-derived RPE could be useful for treating patients with visual disabilities, such as Best disease, subtypes of retinitis pigmentosa (RP), and age-related macular degeneration (AMD).
A critical unresolved issue is identification of early retinal stem cells (equivalents of eye field precursors) and pigment versus neural retinal progenitors. As it stands now, the markers used for marking eye field cells in embryonic animal tissues become blurred when applied to neuroepithelia that are differentiated from human PSCs, particularly because many of the retinal markers, like Pax6, Lhx2, and Rx, are uniformly expressed by all human neuroepithelia. One may need to screen through these transcription factors in limited primate embryo tissues to identify sets of both positive and negative transcription factors for eye field (retinal stem) cells, pigment, and neural retinal progenitors. This information will be instrumental in identifying specific precursor populations during neural differentiation and in regulating these precursors for directed differentiation of RPE or photoreceptor cells.
Conclusions and future directions
Directed differentiation of neuronal and glial subtypes from the human ESCs and iPSCs summarized above indicates that this process follows developmental principles. Not only the temporal course of differentiation mirrors that of neuronal and glial birth in embryonic human development but also the transcriptional codes and response to extracellular signals resemble those learned from animal studies. During neural development, regional patterning of naive neuroepithelia is critical to specification of diverse arrays of neuronal and glial types. Likewise, patterning of human PSC-derived primitive neuroepithelia with specific sets of morphogens is essential for specifying region-specific neural progenitors that subsequently generate functionally diversified neuronal and glial classes. Therefore, in vitro differentiation of the human PSCs described above serves as a useful model to reveal early human development.
This evolving human PSC neural differentiation model has also revealed unique aspects of human neural differentiation. The most obvious phenomenon is the temporal course of birth of neural stem cells, neurons, and glial cells, which can be predicted from human embryonic development. This suggests that the intrinsic program of neural cell fates is reserved in cultured human PSCs. The fact that the cerebral transcription factor Pax6 is necessary and sufficient for human but not mouse ESCs to become primitive neuroepithelia indicates that Pax6 is a neuroectoderm determinant in humans. This explains why the “default” fate of human PSC-derived neural progenitors is of cerebral cortical characteristics. This also determines the number of cycles by which these progenitors need to go through in order to produce sufficient numbers of neurons that make up the cerebral cortex in humans. Hence, it should not be alarmed that the human PSC-derived neural precursors “shed” predominantly neurons for many months, nor should it be regarded as maintenance of neural stem cells, and differentiation of glial cells (astrocytes and oligodendrocytes) is a very much “delayed” process. These cellular differentiation programs are not only controlled by transcription determinants but are also coordinated by extracellular signals. In particular, WNT signaling dictates the early phase of neuroectoderm specification. Consequently, differentiation to alternative fates requires regulation of signaling pathways, including WNTs, and this has to happen on primitive neuroepithelia during the second week of human PSC differentiation. It will not be surprising that known regulatory machineries are used by human neural cells in unexpected ways, especially considering that the human brain is an evolutionally most advanced organ. The field should not demand that neural cells from humans behave exactly in the way as they do in animals.
Important for effective differentiation of neuronal and glial subtypes is the patterning of primitive neuroepithelial cells. These cells, appearing at the second week of human PSC differentiation, express a unique set of anterior transcription factors including Pax6. By analogy to the maintenance of PSCs by a transcription network that involves Nanog, Oct4, and Sox2, it is likely that primitive neuroepithelia are specified and maintained by a similar transcription network that includes Pax6. Hence, it will be instrumental in identifying the transcriptional code, especially the targets and partners of Pax6, and their regulatory networks.
The neuronal types successfully differentiated from human PSCs are almost all early born projection neurons. We believe that many other types of projection neurons, including hypothalamic endocrine regulatory neurons, hindbrain serotonergic neurons, cerebellar neurons, and sensory neurons, may well be differentiated from human PSCs following developmental principles and the strategies illustrated above. Developmentally, there are vast arrays of interneurons that are born between projection neurons and glial cells and they are critical for neural function, especially high function in humans. The regulatory mechanisms underlying the birth of interneurons is less well known and relatively fewer tools are available to define them. Similarly, few studies are reported for directed differentiation of sensory neurons from human PSCs. Therefore, directed differentiation of these interneurons will be one of the major future directions.
Directed differentiation of glial cells from human PSCs has not been paid sufficient attention in the past. This is partly because glia are regarded as supporting cells and differentiation of glia as a default consequence of differentiation following neuronal generation. Nevertheless, significant lag in glial differentiation from human PSCs poses a major technical burden to generation of enriched populations of glial cells. As illustrated in astrocyte differentiation, there are many glial subtypes that have yet to be discovered, and human PSC differentiation in fact may facilitate the discovery, given the limited tools available to distinguish glial subtypes in vivo. Additional difficulties in glial differentiation from human PSCs are evaluation of their function in vitro and in vivo. Novel tools will be needed in this regard.
Functional evaluation of human PSC-generated neurons and glia is not straightforward. The main hurdle is the “stretched” time for maturation in vitro and in vivo. Human ESC-derived neurons need several weeks of culture to become electrophysiologically active, especially synaptic transmission. Astrocytes can facilitate the maturation process [45]. Similarly, it takes several weeks or even months for grafted human neurons to integrate into the host neural circuitry [77, 117], thus contributing to behavioral consequences. This simple timing issue demands reconsideration of model systems for testing the function of human neural cells [118]. Because of xenograft nature, a humanized animal or those with immune-deficient background will be essential for assessing the function of human neurons and glia. These model systems will be equally important for evaluating the safety of human stem cell derivatives in the long term.
Neurons and glia derived from transgenic human ESCs or disease iPSCs are proposed for modeling disease processes in a Petri dish. This is perhaps another major use of human stem cells besides developmental studies. One of the major hurdles in disease modeling is the identification and development of disease phenotypes in vitro. Again, timing is everything here. This is particularly difficult for neurodegenerative disorders which evolve over decades in vivo. Reconstruction of cellular components to allow cellular interactions may facilitate phenotypic development, especially under the disease environment. Transplantation of these disease neural cells may also allow development of pathology in the brain and spinal cord environment in the long term.
Acknowledgments
The authors thank members of the Zhang laboratory for reading and commenting on the manuscript. Studies presented from our laboratory have been supported by the National Institute of Neurological Disorders and Stroke (NS045926, NS057778, NS064578), ALS Association, National MS Society (NMSS TR-3761), NYSTEM (C024406), Bleser Family Foundation, Busta Family Foundation, Neuroscience Training Program (T32 GM007507) and partly by a core grant to the Waisman Center from the National Institute of Child Health and Human Development (P30 HD03352).
References
- 1.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 2.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin Ii, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 5.Pang ZP, Yang N, Vierbuchen T, Ostermeier A, Fuentes DR, Yang TQ, Citri A, Sebastiano V, Marro S, Sudhof TC, Wernig M (2011) Induction of human neuronal cells by defined transcription factors. Nature [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 6.Pfisterer U, Kirkeby A, Torper O, Wood J, Nelander J, Dufour A, Bjorklund A, Lindvall O, Jakobsson J, Parmar M. Direct conversion of human fibroblasts to dopaminergic neurons. Proc Natl Acad Sci USA. 2011;108(25):10343–10348. doi: 10.1073/pnas.1105135108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stern CD. Neural induction: old problem, new findings, yet more questions. Development. 2005;132:2007–2021. doi: 10.1242/dev.01794. [DOI] [PubMed] [Google Scholar]
- 8.Wilson SI, Edlund T. Neural induction: toward a unifying mechanism. Nat Neurosci. 2001;4(Suppl):1161–1168. doi: 10.1038/nn747. [DOI] [PubMed] [Google Scholar]
- 9.Fuentealba LC, Eivers E, Ikeda A, Hurtado C, Kuroda H, Pera EM, De Robertis EM. Integrating patterning signals: Wnt/GSK3 regulates the duration of the BMP/Smad1 signal. Cell. 2007;131:980–993. doi: 10.1016/j.cell.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aubert J, Dunstan H, Chambers I, Smith A. Functional gene screening in embryonic stem cells implicates Wnt antagonism in neural differentiation. Nat Biotechnol. 2002;20:1240–1245. doi: 10.1038/nbt763. [DOI] [PubMed] [Google Scholar]
- 11.Tropepe V, Hitoshi S, Sirard C, Mak TW, Rossant J, van der Kooy D. Direct neural fate specification from embryonic stem cells: a primitive mammalian neural stem cell stage acquired through a default mechanism. Neuron. 2001;30:65–78. doi: 10.1016/S0896-6273(01)00263-X. [DOI] [PubMed] [Google Scholar]
- 12.Ying QL, Stavridis M, Griffiths D, Li M, Smith A. Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture. Nat Biotechnol. 2003;21:183–186. doi: 10.1038/nbt780. [DOI] [PubMed] [Google Scholar]
- 13.Itsykson P, Ilouz N, Turetsky T, Goldstein RS, Pera MF, Fishbein I, Segal M, Reubinoff BE. Derivation of neural precursors from human embryonic stem cells in the presence of noggin. Mol Cell Neurosci. 2005;30:24–36. doi: 10.1016/j.mcn.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Zhang SC, Wernig M, Duncan ID, Brustle O, Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol. 2001;19:1129–1133. doi: 10.1038/nbt1201-1129. [DOI] [PubMed] [Google Scholar]
- 15.Li XJ, Du ZW, Zarnowska ED, Pankratz M, Hansen LO, Pearce RA, Zhang SC. Specification of motoneurons from human embryonic stem cells. Nat Biotechnol. 2005;23:215–221. doi: 10.1038/nbt1063. [DOI] [PubMed] [Google Scholar]
- 16.Pera MF, Andrade J, Houssami S, Reubinoff B, Trounson A, Stanley EG, Ward-van Oostwaard D, Mummery C. Regulation of human embryonic stem cell differentiation by BMP-2 and its antagonist noggin. J Cell Sci. 2004;117:1269–1280. doi: 10.1242/jcs.00970. [DOI] [PubMed] [Google Scholar]
- 17.Lavaute TM, Yoo YD, Pankratz MT, Weick JP, Gerstner JR, Zhang SC. Regulation of neural specification from human embryonic stem cells by BMP and FGF. Stem Cells. 2009;27:1741–1749. doi: 10.1002/stem.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pevny L, Placzek M. SOX genes and neural progenitor identity. Curr Opin Neurobiol. 2005;15:7–13. doi: 10.1016/j.conb.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 19.Rallu M, Machold R, Gaiano N, Corbin JG, Mcmahon AP, Fishell G. Dorsoventral patterning is established in the telencephalon of mutants lacking both Gli3 and Hedgehog signaling. Development. 2002;129:4963–4974. doi: 10.1242/dev.129.21.4963. [DOI] [PubMed] [Google Scholar]
- 20.Rash BG, Grove EA. Patterning the dorsal telencephalon: a role for sonic hedgehog? J Neurosci. 2007;27:11595–11603. doi: 10.1523/JNEUROSCI.3204-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aoto K, Nishimura T, Eto K, Motoyama J. Mouse GLI3 regulates Fgf8 expression and apoptosis in the developing neural tube, face, and limb bud. Dev Biol. 2002;251:320–332. doi: 10.1006/dbio.2002.0811. [DOI] [PubMed] [Google Scholar]
- 22.Marti E, Bumcrot DA, Takada R, Mcmahon AP. Requirement of 19 K form of Sonic hedgehog for induction of distinct ventral cell types in CNS explants. Nature. 1995;375:322–325. doi: 10.1038/375322a0. [DOI] [PubMed] [Google Scholar]
- 23.Roelink H, Porter JA, Chiang C, Tanabe Y, Chang DT, Beachy PA, Jessell TM. Floor plate and motor neuron induction by different concentrations of the amino-terminal cleavage product of sonic hedgehog autoproteolysis. Cell. 1995;81:445–455. doi: 10.1016/0092-8674(95)90397-6. [DOI] [PubMed] [Google Scholar]
- 24.Ericson J, Morton S, Kawakami A, Roelink H, Jessell TM. Two critical periods of sonic hedgehog signaling required for the specification of motor neuron identity. Cell. 1996;87:661–673. doi: 10.1016/S0092-8674(00)81386-0. [DOI] [PubMed] [Google Scholar]
- 25.Temple S. The development of neural stem cells. Nature. 2001;414:112–117. doi: 10.1038/35102174. [DOI] [PubMed] [Google Scholar]
- 26.Temple S. Stem cell plasticity–building the brain of our dreams. Nat Rev Neurosci. 2001;2:513–520. doi: 10.1038/35081577. [DOI] [PubMed] [Google Scholar]
- 27.Zhang SC. Neural subtype specification from embryonic stem cells. Brain Pathol. 2006;16:132–142. doi: 10.1111/j.1750-3639.2006.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu BY, Weick JP, Yu J, Ma LX, Zhang XQ, Thomson JA, Zhang SC. Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc Natl Acad Sci USA. 2010;107:4335–4340. doi: 10.1073/pnas.0910012107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boulting GL, Kiskinis E, Croft GF, Amoroso MW, Oakley DH, Wainger BJ, Williams DJ, Kahler DJ, Yamaki M, Davidow L, Rodolfa CT, Dimos JT, Mikkilineni S, Macdermott AB, Woolf CJ, Henderson CE, Wichterle H, Eggan K. A functionally characterized test set of human induced pluripotent stem cells. Nat Biotechnol. 2011;29:279–286. doi: 10.1038/nbt.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27:275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pankratz MT, Li XJ, Lavaute TM, Lyons EA, Chen X, Zhang SC. Directed neural differentiation of human embryonic stem cells via an obligated primitive anterior stage. Stem Cells. 2007;25:1511–1520. doi: 10.1634/stemcells.2006-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang X, Huang CT, Chen J, Pankratz MT, Xi J, Li J, Yang Y, Lavaute TM, Li XJ, Ayala M, Bondarenko GI, Du ZW, Jin Y, Golos TG, Zhang SC. Pax6 is a human neuroectoderm cell fate determinant. Cell Stem Cell. 2010;7:90–100. doi: 10.1016/j.stem.2010.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li XJ, Zhang X, Johnson MA, Wang ZB, Lavaute T, Zhang SC. Coordination of sonic hedgehog and Wnt signaling determines ventral and dorsal telencephalic neuron types from human embryonic stem cells. Development. 2009;136:4055–4063. doi: 10.1242/dev.036624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeng H, Guo M, Martins-Taylor K, Wang X, Zhang Z, Park JW, Zhan S, Kronenberg MS, Lichtler A, Liu HX, Chen FP, Yue L, Li XJ, Xu RH. Specification of region-specific neurons including forebrain glutamatergic neurons from human induced pluripotent stem cells. PLoS One. 2010;5:e11853. doi: 10.1371/journal.pone.0011853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elkabetz Y, Panagiotakos G, Al Shamy G, Socci ND, Tabar V, Studer L. Human ES cell-derived neural rosettes reveal a functionally distinct early neural stem cell stage. Genes Dev. 2008;22:152–165. doi: 10.1101/gad.1616208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Campbell K. Dorsal-ventral patterning in the mammalian telencephalon. Curr Opin Neurobiol. 2003;13:50–56. doi: 10.1016/S0959-4388(03)00009-6. [DOI] [PubMed] [Google Scholar]
- 37.Grove EA, Fukuchi-Shimogori T. Generating the cerebral cortical area map. Annu Rev Neurosci. 2003;26:355–380. doi: 10.1146/annurev.neuro.26.041002.131137. [DOI] [PubMed] [Google Scholar]
- 38.Gunhaga L, Marklund M, Sjodal M, Hsieh JC, Jessell TM, Edlund T. Specification of dorsal telencephalic character by sequential Wnt and FGF signaling. Nat Neurosci. 2003;6:701–707. doi: 10.1038/nn1068. [DOI] [PubMed] [Google Scholar]
- 39.Stuhmer T, Puelles L, Ekker M, Rubenstein JL. Expression from a Dlx gene enhancer marks adult mouse cortical GABAergic neurons. Cereb Cortex. 2002;12:75–85. doi: 10.1093/cercor/12.1.75. [DOI] [PubMed] [Google Scholar]
- 40.Olsson M, Campbell K, Turnbull DH. Specification of mouse telencephalic and mid-hindbrain progenitors following heterotopic ultrasound-guided embryonic transplantation. Neuron. 1997;19:761–772. doi: 10.1016/S0896-6273(00)80959-9. [DOI] [PubMed] [Google Scholar]
- 41.Olsson M, Bjorklund A, Campbell K. Early specification of striatal projection neurons and interneuronal subtypes in the lateral and medial ganglionic eminence. Neuroscience. 1998;84:867–876. doi: 10.1016/S0306-4522(97)00532-0. [DOI] [PubMed] [Google Scholar]
- 42.Marin O, Rubenstein JL. A long, remarkable journey: tangential migration in the telencephalon. Nat Rev Neurosci. 2001;2:780–790. doi: 10.1038/35097509. [DOI] [PubMed] [Google Scholar]
- 43.Eiraku M, Watanabe K, Matsuo-Takasaki M, Kawada M, Yonemura S, Matsumura M, Wataya T, Nishiyama A, Muguruma K, Sasai Y. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell. 2008;3:519–532. doi: 10.1016/j.stem.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 44.Gaspard N, Bouschet T, Hourez R, Dimidschstein J, Naeije G, van den Ameele J, Espuny-Camacho I, Herpoel A, Passante L, Schiffmann SN, Gaillard A, Vanderhaeghen P. An intrinsic mechanism of corticogenesis from embryonic stem cells. Nature. 2008;455:351–357. doi: 10.1038/nature07287. [DOI] [PubMed] [Google Scholar]
- 45.Johnson MA, Weick JP, Pearce RA, Zhang SC. Functional neural development from human embryonic stem cells: accelerated synaptic activity via astrocyte coculture. J Neurosci. 2007;27:3069–3077. doi: 10.1523/JNEUROSCI.4562-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Letinic K, Zoncu R, Rakic P. Origin of GABAergic neurons in the human neocortex. Nature. 2002;417:645–649. doi: 10.1038/nature00779. [DOI] [PubMed] [Google Scholar]
- 47.Tan SS, Kalloniatis M, Sturm K, Tam PP, Reese BE, Faulkner-Jones B. Separate progenitors for radial and tangential cell dispersion during development of the cerebral neocortex. Neuron. 1998;21:295–304. doi: 10.1016/S0896-6273(00)80539-5. [DOI] [PubMed] [Google Scholar]
- 48.Anderson S, Mione M, Yun K, Rubenstein JL. Differential origins of neocortical projection and local circuit neurons: role of Dlx genes in neocortical interneuronogenesis. Cereb Cortex. 1999;9:646–654. doi: 10.1093/cercor/9.6.646. [DOI] [PubMed] [Google Scholar]
- 49.Casarosa S, Fode C, Guillemot F. Mash1 regulates neurogenesis in the ventral telencephalon. Development. 1999;126:525–534. doi: 10.1242/dev.126.3.525. [DOI] [PubMed] [Google Scholar]
- 50.Horton S, Meredith A, Richardson JA, Johnson JE. Correct coordination of neuronal differentiation events in ventral forebrain requires the bHLH factor MASH1. Mol Cell Neurosci. 1999;14:355–369. doi: 10.1006/mcne.1999.0791. [DOI] [PubMed] [Google Scholar]
- 51.Aubry L, Bugi A, Lefort N, Rousseau F, Peschanski M, Perrier AL. Striatal progenitors derived from human ES cells mature into DARPP32 neurons in vitro and in quinolinic acid-lesioned rats. Proc Natl Acad Sci USA. 2008;105:16707–16712. doi: 10.1073/pnas.0808488105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ma LX, Hu B, Liu Y, Liu H, Vermilyea SC, Liu H, Zhang X, Sun Y, Gao L, Li J, Ayala M, Zhang SC. Specification of functional striatal GABAergic projection neurons from human stem cells. Soc Neurosci Abstr. 2010;331:7. [Google Scholar]
- 53.Liu Y, Krencik R, Liu H, Ma LX, Zhang X, Zhang SC. Functional cholinergic neurons from human embryonic stem cells. Soc Neurosci Abstr. 2010;331:5. [Google Scholar]
- 54.Bissonnette CJ, Lyass L, Bhattacharyya BJ, Belmadani A, Miller RJ, Kessler JA. The controlled generation of functional basal forebrain cholinergic neurons from human embryonic stem cells. Stem Cells. 2011;29(5):802–811. doi: 10.1002/stem.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nilbratt M, Porras O, Marutle A, Hovatta O, Nordberg A. Neurotrophic factors promote cholinergic differentiation in human embryonic stem cell-derived neurons. J Cell Mol Med. 2010;14:1476–1484. doi: 10.1111/j.1582-4934.2009.00916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Singh Roy N, Nakano T, Xuing L, Kang J, Nedergaard M, Goldman SA. Enhancer-specified GFP-based FACS purification of human spinal motor neurons from embryonic stem cells. Exp Neurol. 2005;196:224–234. doi: 10.1016/j.expneurol.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 57.Hu BY, Zhang SC. Differentiation of spinal motor neurons from pluripotent human stem cells. Nat Protoc. 2009;4:1295–1304. doi: 10.1038/nprot.2009.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee H, Shamy GA, Elkabetz Y, Schofield CM, Harrsion NL, Panagiotakos G, Socci ND, Tabar V, Studer L. Directed differentiation and transplantation of human embryonic stem cell-derived motoneurons. Stem Cells. 2007;25:1931–1939. doi: 10.1634/stemcells.2007-0097. [DOI] [PubMed] [Google Scholar]
- 59.Dimos JT, Rodolfa KT, Niakan KK, Weisenthal LM, Mitsumoto H, Chung W, Croft GF, Saphier G, Leibel R, Goland R, Wichterle H, Henderson CE, Eggan K. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321:1218–1221. doi: 10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- 60.Ebert AD, Yu J, Rose FF, Jr, Mattis VB, Lorson CL, Thomson JA, Svendsen CN. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature. 2009;457:277–280. doi: 10.1038/nature07677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li XJ, Hu BY, Jones SA, Zhang YS, Lavaute T, Du ZW, Zhang SC. Directed differentiation of ventral spinal progenitors and motor neurons from human embryonic stem cells by small molecules. Stem Cells. 2008;26:886–893. doi: 10.1634/stemcells.2007-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Patani R, Hollins AJ, Wishart TM, Puddifoot CA, Alvarez S, de Lera AR, Wyllie DJ, Compston DA, Pedersen RA, Gillingwater TH, Hardingham GE, Allen ND, Chandran S. Retinoid-independent motor neurogenesis from human embryonic stem cells reveals a medial columnar ground state. Nat Commun. 2011;2:214. doi: 10.1038/ncomms1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bjorklund A, Lindvall O. Cell replacement therapies for central nervous system disorders. Nat Neurosci. 2000;3:537–544. doi: 10.1038/75705. [DOI] [PubMed] [Google Scholar]
- 64.Freed CR, Greene PE, Breeze RE, Tsai WY, Dumouchel W, Kao R, Dillon S, Winfield H, Culver S, Trojanowski JQ, Eidelberg D, Fahn S. Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. N Engl J Med. 2001;344:710–719. doi: 10.1056/NEJM200103083441002. [DOI] [PubMed] [Google Scholar]
- 65.Olanow CW, Goetz CG, Kordower JH, Stoessl AJ, Sossi V, Brin MF, Shannon KM, Nauert GM, Perl DP, Godbold J, Freeman TB. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson’s disease. Ann Neurol. 2003;54:403–414. doi: 10.1002/ana.10720. [DOI] [PubMed] [Google Scholar]
- 66.Yang D, Zhang ZJ, Oldenburg M, Ayala M, Zhang SC. Human embryonic stem cell-derived dopaminergic neurons reverse functional deficit in parkinsonian rats. Stem Cells. 2008;26:55–63. doi: 10.1634/stemcells.2007-0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yan Y, Yang D, Zarnowska ED, Du Z, Werbel B, Valliere C, Pearce RA, Thomson JA, Zhang SC. Directed differentiation of dopaminergic neuronal subtypes from human embryonic stem cells. Stem Cells. 2005;23:781–790. doi: 10.1634/stemcells.2004-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zeng X, Cai J, Chen J, Luo Y, You ZB, Fotter E, Wang Y, Harvey B, Miura T, Backman C, Chen GJ, Rao MS, Freed WJ. Dopaminergic differentiation of human embryonic stem cells. Stem Cells. 2004;22:925–940. doi: 10.1634/stemcells.22-6-925. [DOI] [PubMed] [Google Scholar]
- 69.Schulz TC, Noggle SA, Palmarini GM, Weiler DA, Lyons IG, Pensa KA, Meedeniya AC, Davidson BP, Lambert NA, Condie BG. Differentiation of human embryonic stem cells to dopaminergic neurons in serum-free suspension culture. Stem Cells. 2004;22:1218–1238. doi: 10.1634/stemcells.2004-0114. [DOI] [PubMed] [Google Scholar]
- 70.Perrier AL, Tabar V, Barberi T, Rubio ME, Bruses J, Topf N, Harrison NL, Studer L. Derivation of midbrain dopamine neurons from human embryonic stem cells. Proc Natl Acad Sci USA. 2004;101:12543–12548. doi: 10.1073/pnas.0404700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Park CH, Minn YK, Lee JY, Choi DH, Chang MY, Shim JW, Ko JY, Koh HC, Kang MJ, Kang JS, Rhie DJ, Lee YS, Son H, Moon SY, Kim KS, Lee SH. In vitro and in vivo analyses of human embryonic stem cell-derived dopamine neurons. J Neurochem. 2005;92:1265–1276. doi: 10.1111/j.1471-4159.2004.03006.x. [DOI] [PubMed] [Google Scholar]
- 72.Sonntag KC, Pruszak J, Yoshizaki T, van Arensbergen J, Sanchez-Pernaute R, Isacson O. Enhanced yield of neuroepithelial precursors and midbrain-like dopaminergic neurons from human embryonic stem cells using the bone morphogenic protein antagonist noggin. Stem Cells. 2007;25:411–418. doi: 10.1634/stemcells.2006-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Roy NS, Cleren C, Singh SK, Yang L, Beal MF, Goldman SA. Functional engraftment of human ES cell-derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nat Med. 2006;12:1259–1268. doi: 10.1038/nm1495. [DOI] [PubMed] [Google Scholar]
- 74.Young A, Assey KS, Sturkie CD, West FD, Machacek DW, Stice SL. Glial cell line-derived neurotrophic factor enhances in vitro differentiation of mid-/hindbrain neural progenitor cells to dopaminergic-like neurons. J Neurosci Res. 2010;88:3222–3232. doi: 10.1002/jnr.22499. [DOI] [PubMed] [Google Scholar]
- 75.Swistowski A, Peng J, Liu Q, Mali P, Rao MS, Cheng L, Zeng X. Efficient generation of functional dopaminergic neurons from human induced pluripotent stem cells under defined conditions. Stem Cells. 2010;28:1893–1904. doi: 10.1002/stem.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hargus G, Cooper O, Deleidi M, Levy A, Lee K, Marlow E, Yow A, Soldner F, Hockemeyer D, Hallett PJ, Osborn T, Jaenisch R, Isacson O. Differentiated Parkinson patient-derived induced pluripotent stem cells grow in the adult rodent brain and reduce motor asymmetry in Parkinsonian rats. Proc Natl Acad Sci USA. 2010;107:15921–15926. doi: 10.1073/pnas.1010209107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Weick JP, Johnson MA, Skroch SP, Williams JC, Deisseroth K, Zhang SC. Functional control of transplantable human ESC-derived neurons via optogenetic targeting. Stem Cells. 2010;28:2008–2016. doi: 10.1002/stem.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 79.Ono Y, Nakatani T, Sakamoto Y, Mizuhara E, Minaki Y, Kumai M, Hamaguchi A, Nishimura M, Inoue Y, Hayashi H, Takahashi J, Imai T. Differences in neurogenic potential in floor plate cells along an anteroposterior location: midbrain dopaminergic neurons originate from mesencephalic floor plate cells. Development. 2007;134:3213–3225. doi: 10.1242/dev.02879. [DOI] [PubMed] [Google Scholar]
- 80.Fasano CA, Chambers SM, Lee G, Tomishima MJ, Studer L. Efficient derivation of functional floor plate tissue from human embryonic stem cells. Cell Stem Cell. 2010;6:336–347. doi: 10.1016/j.stem.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 81.Denham M, Thompson LH, Leung J, Pebay A, Bjorklund A, Dottori M. Gli1 is an inducing factor in generating floor plate progenitor cells from human embryonic stem cells. Stem Cells. 2010;28:1805–1815. doi: 10.1002/stem.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cooper O, Hargus G, Deleidi M, Blak A, Osborn T, Marlow E, Lee K, Levy A, Perez-Torres E, Yow A, Isacson O. Differentiation of human ES and Parkinson’s disease iPS cells into ventral midbrain dopaminergic neurons requires a high activity form of SHH, FGF8a and specific regionalization by retinoic acid. Mol Cell Neurosci. 2010;45:258–266. doi: 10.1016/j.mcn.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Soldner F, Hockemeyer D, Beard C, Gao Q, Bell GW, Cook EG, Hargus G, Blak A, Cooper O, Mitalipova M, Isacson O, Jaenisch R. Parkinson’s disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell. 2009;136:964–977. doi: 10.1016/j.cell.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang SC. Defining glial cells during CNS development. Nat Rev Neurosci. 2001;2:840–843. doi: 10.1038/35097593. [DOI] [PubMed] [Google Scholar]
- 85.Rowitch DH. Glial specification in the vertebrate neural tube. Nat Rev Neurosci. 2004;5:409–419. doi: 10.1038/nrn1389. [DOI] [PubMed] [Google Scholar]
- 86.Carpenter MK, Inokuma MS, Denham J, Mujtaba T, Chiu CP, Rao MS. Enrichment of neurons and neural precursors from human embryonic stem cells. Exp Neurol. 2001;172:383–397. doi: 10.1006/exnr.2001.7832. [DOI] [PubMed] [Google Scholar]
- 87.Deneen B, Ho R, Lukaszewicz A, Hochstim CJ, Gronostajski RM, Anderson DJ. The transcription factor NFIA controls the onset of gliogenesis in the developing spinal cord. Neuron. 2006;52:953–968. doi: 10.1016/j.neuron.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 88.Krencik R, Weick JH, Zhang Z, Zhang SC. Specification of transplantable astroglial cells from human pluripotent stem cells. Nat Biotechnol. 2011;29:528–534. doi: 10.1038/nbt.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Castelo-Branco G, Sousa KM, Bryja V, Pinto L, Wagner J, Arenas E. Ventral midbrain glia express region-specific transcription factors and regulate dopaminergic neurogenesis through Wnt-5a secretion. Mol Cell Neurosci. 2006;31:251–262. doi: 10.1016/j.mcn.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 90.Wagner J, Akerud P, Castro DS, Holm PC, Canals JM, Snyder EY, Perlmann T, Arenas E. Induction of a midbrain dopaminergic phenotype in Nurr1-overexpressing neural stem cells by type 1 astrocytes. Nat Biotechnol. 1999;17:653–659. doi: 10.1038/10862. [DOI] [PubMed] [Google Scholar]
- 91.Richardson WD, Kessaris N, Pringle N. Oligodendrocyte wars. Nat Rev Neurosci. 2006;7:11–18. doi: 10.1038/nrn1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hu BY, Du ZW, Li XJ, Ayala M, Zhang SC. Human oligodendrocytes from embryonic stem cells: conserved SHH signaling networks and divergent FGF effects. Development. 2009;136:1443–1452. doi: 10.1242/dev.029447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hu BY, Du ZW, Zhang SC. Differentiation of human oligodendrocytes from pluripotent stem cells. Nat Protoc. 2009;4:1614–1622. doi: 10.1038/nprot.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brustle O, Jones KN, Learish RD, Karram K, Choudhary K, Wiestler OD, Duncan ID, Mckay RD. Embryonic stem cell-derived glial precursors: a source of myelinating transplants. Science. 1999;285:754–756. doi: 10.1126/science.285.5428.754. [DOI] [PubMed] [Google Scholar]
- 95.Billon N, Jolicoeur C, Ying QL, Smith A, Raff M. Normal timing of oligodendrocyte development from genetically engineered, lineage-selectable mouse ES cells. J Cell Sci. 2002;115:3657–3665. doi: 10.1242/jcs.00049. [DOI] [PubMed] [Google Scholar]
- 96.Mcdonald JW, Liu XZ, Qu Y, Liu S, Mickey SK, Turetsky D, Gottlieb DI, Choi DW. Transplanted embryonic stem cells survive, differentiate and promote recovery in injured rat spinal cord. Nat Med. 1999;5:1410–1412. doi: 10.1038/70986. [DOI] [PubMed] [Google Scholar]
- 97.Mcmorris FA, Mckinnon RD. Regulation of oligodendrocyte development and CNS myelination by growth factors: prospects for therapy of demyelinating disease. Brain Pathol. 1996;6:313–329. doi: 10.1111/j.1750-3639.1996.tb00858.x. [DOI] [PubMed] [Google Scholar]
- 98.Zhang SC, Ge B, Duncan ID. Tracing human oligodendroglial development in vitro. J Neurosci Res. 2000;59:421–429. doi: 10.1002/(SICI)1097-4547(20000201)59:3<421::AID-JNR17>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 99.Grever WE, Zhang S, Ge B, Duncan ID. Fractionation and enrichment of oligodendrocytes from developing human brain. J Neurosci Res. 1999;57:304–314. doi: 10.1002/(SICI)1097-4547(19990801)57:3<304::AID-JNR2>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 100.Chandran S, Compston A. Neural stem cells as a potential source of oligodendrocytes for myelin repair. J Neurol Sci. 2005;233:179–181. doi: 10.1016/j.jns.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 101.Chandran S, Compston A, Jauniaux E, Gilson J, Blakemore W, Svendsen C. Differential generation of oligodendrocytes from human and rodent embryonic spinal cord neural precursors. Glia. 2004;47:314–324. doi: 10.1002/glia.20011. [DOI] [PubMed] [Google Scholar]
- 102.Izrael M, Zhang P, Kaufman R, Shinder V, Ella R, Amit M, Itskovitz-Eldor J, Chebath J, Revel M. Human oligodendrocytes derived from embryonic stem cells: effect of noggin on phenotypic differentiation in vitro and on myelination in vivo. Mol Cell Neurosci. 2007;34:310–323. doi: 10.1016/j.mcn.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 103.Kang SM, Cho MS, Seo H, Yoon CJ, Oh SK, Choi YM, Kim DW. Efficient induction of oligodendrocytes from human embryonic stem cells. Stem Cells. 2007;25:419–424. doi: 10.1634/stemcells.2005-0482. [DOI] [PubMed] [Google Scholar]
- 104.Nistor GI, Totoiu MO, Haque N, Carpenter MK, Keirstead HS. Human embryonic stem cells differentiate into oligodendrocytes in high purity and myelinate after spinal cord transplantation. Glia. 2005;49:385–396. doi: 10.1002/glia.20127. [DOI] [PubMed] [Google Scholar]
- 105.Zuber ME, Gestri G, Viczian AS, Barsacchi G, Harris WA. Specification of the vertebrate eye by a network of eye field transcription factors. Development. 2003;130:5155–5167. doi: 10.1242/dev.00723. [DOI] [PubMed] [Google Scholar]
- 106.Mathers PH, Jamrich M. Regulation of eye formation by the Rx and pax6 homeobox genes. Cell Mol Life Sci. 2000;57:186–194. doi: 10.1007/PL00000683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Osakada F, Ikeda H, Mandai M, Wataya T, Watanabe K, Yoshimura N, Akaike A, Sasai Y, Takahashi M. Toward the generation of rod and cone photoreceptors from mouse, monkey and human embryonic stem cells. Nat Biotechnol. 2008;26:215–224. doi: 10.1038/nbt1384. [DOI] [PubMed] [Google Scholar]
- 108.Meyer JS, Shearer RL, Capowski EE, Wright LS, Wallace KA, Mcmillan EL, Zhang SC, Gamm DM. Modeling early retinal development with human embryonic and induced pluripotent stem cells. Proc Natl Acad Sci USA. 2009;106:16698–16703. doi: 10.1073/pnas.0905245106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Klimanskaya I, Hipp J, Rezai KA, West M, Atala A, Lanza R. Derivation and comparative assessment of retinal pigment epithelium from human embryonic stem cells using transcriptomics. Cloning Stem Cells. 2004;6:217–245. doi: 10.1089/clo.2004.6.217. [DOI] [PubMed] [Google Scholar]
- 110.Kagiyama Y, Gotouda N, Sakagami K, Yasuda K, Mochii M, Araki M. Extraocular dorsal signal affects the developmental fate of the optic vesicle and patterns the optic neuroepithelium. Dev Growth Differ. 2005;47:523–536. doi: 10.1111/j.1440-169X.2005.00828.x. [DOI] [PubMed] [Google Scholar]
- 111.Reh TA, Lamba D, Gust J. Directing human embryonic stem cells to a retinal fate. Methods Mol Biol. 2010;636:139–153. doi: 10.1007/978-1-60761-691-7_9. [DOI] [PubMed] [Google Scholar]
- 112.Idelson M, Alper R, Obolensky A, Ben-Shushan E, Hemo I, Yachimovich-Cohen N, Khaner H, Smith Y, Wiser O, Gropp M, Cohen MA, Even-Ram S, Berman-Zaken Y, Matzrafi L, Rechavi G, Banin E, Reubinoff B. Directed differentiation of human embryonic stem cells into functional retinal pigment epithelium cells. Cell Stem Cell. 2009;5:396–408. doi: 10.1016/j.stem.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 113.Osakada F, Jin ZB, Hirami Y, Ikeda H, Danjyo T, Watanabe K, Sasai Y, Takahashi M. In vitro differentiation of retinal cells from human pluripotent stem cells by small-molecule induction. J Cell Sci. 2009;122:3169–3179. doi: 10.1242/jcs.050393. [DOI] [PubMed] [Google Scholar]
- 114.Carr AJ, Vugler AA, Hikita ST, Lawrence JM, Gias C, Chen LL, Buchholz DE, Ahmado A, Semo M, Smart MJ, Hasan S, Da Cruz L, Johnson LV, Clegg DO, Coffey PJ. Protective effects of human iPS-derived retinal pigment epithelium cell transplantation in the retinal dystrophic rat. PLoS One. 2009;4:e8152. doi: 10.1371/journal.pone.0008152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kokkinaki M, Sahibzada N, Golestaneh N. Human iPS-derived retinal pigment epithelium (RPE) cells exhibit ion transport, membrane potential, polarized VEGF secretion and gene expression pattern similar to native RPE. Stem Cells. 2011;29:825–835. doi: 10.1002/stem.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Amirpour N, Karamali F, Rabiee F, Rezaei L, Esfandiari E, Razavi S, Dehghani A, Razmju H, Nasr-Esfahani MH, Baharvand H (2011) Differentiation of human embryonic stem cell-derived retinal progenitors into retinal cells by sonic hedgehog and/or retinal pigmented epithelium and transplantation into the subretinal space of sodium iodate-injected rabbits. Stem Cells Dev Jun 1. (Epub ahead of print) [DOI] [PubMed]
- 117.Muotri AR, Nakashima K, Toni N, Sandler VM, Gage FH. Development of functional human embryonic stem cell-derived neurons in mouse brain. Proc Natl Acad Sci USA. 2005;102:18644–18648. doi: 10.1073/pnas.0509315102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Darsalia V, Allison SJ, Cusulin C, Monni E, Kuzdas D, Kallur T, Lindvall O, Kokaia Z. Cell number and timing of transplantation determine survival of human neural stem cell grafts in stroke-damaged rat brain. J Cereb Blood Flow Metab. 2011;31:235–242. doi: 10.1038/jcbfm.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]