Abstract
Background
Schizophrenia is a chronic and devastating brain disorder characterized by hallucinations and delusions, symptoms reflecting impaired reality testing. While animal models have captured negative symptoms and cognitive deficits associated with schizophrenia, none have addressed these defining, positive symptoms.
Methods
Here we tested the performance of adults given neonatal ventral hippocampal lesions (NVHL), a neurodevelopmental model of schizophrenia, in two taste aversion procedures.
Results
Normal and NVHL rats formed aversions to a palatable food when the food was directly paired with nausea, but only NVHL rats formed a food aversion when the cue predicting that food was paired with nausea. The failure of NVHL rats to fully discriminate real from imagined food parallels the failure of people with schizophrenia to differentiate internal thoughts/beliefs from reality.
Conclusions
These results further validate the NVHL model of schizophrenia and provide a means of assessing impaired reality testing in variety of animal models.
Keywords: schizophrenia, psychosis, mediated devaluation, neonatal ventral hippocampal lesion, hallucination, reality testing
Introduction
Schizophrenia is a chronic brain disorder affecting on average 1% of the US adult population. Amongst the more devastating symptoms are hallucinations and delusions. These symptoms are thought to reflect impaired reality testing, where reality testing refers to “the capacity to distinguish internal fantasy from external reality (1).” Although these symptoms form a core and defining feature of schizophrenia, existing animal models used to study the disease typically do not address them. Indeed, these aspects of schizophrenia have been described as difficult or impossible to capture in animal models (2).
As a result, animal research has focused primarily on capturing the so-called negative symptoms and cognitive deficits. This approach has been remarkably successful. For example, in one neurodevelopmental model in which neonatal rats receive ventral hippocampal lesions (NVHL), adult rats later exhibit a number of symptoms paralleling those observed in schizophrenic patients, including impaired in sensory-motor gating (3), hyperactivity in response to stimulants (4), comorbitity with drug use (5) and abnormal social behaviors (6). Despite the success of the NVHL and other models in recreating some aspects of schizophrenia (7, 8), our understanding of the disorder – and our ability to design effective treatments – might progress more rapidly if impaired reality testing could be assessed (9).
Here we utilized a simple taste aversion procedure combined with Pavlovian conditioning to assess the ability of rats to distinguish between a real and internal representation of a palatable food. We found that normal rats were readily able to distinguish the two. As a result, they appropriately reduced consumption of the food (and learned behaviors directed toward obtaining the food) when the food was directly paired with induced-nausea but not when only the representation of the food was paired with induced-nausea. By contrast, NVHL rats reduced food consumption in both settings. We suggest that this approach provides a tool with which to assess rudimentary reality testing in animal models of schizophrenia.
Methods and Materials
Subjects
Timed pregnant Long-Evans females were obtained at embryonic days 15–18 from Charles River (Wilmington, MA, USA) and were individually housed with free access to food and water in a temperature- and humidity-controlled environment with a 12-h:12-h light/dark cycle (lights on at 7:00 A.M.). Neonatal pups were left undisturbed until postnatal day (PD) 6 or 7, when they were weighed and sex was determined. Following surgery (see below) at approximately PD21, animals were weaned and housed in pairs of like lesion status. Upon reaching adulthood (PD56), animals were single-housed. Before training rats were reduced to 85% of their baseline weights. All procedures adhered to guidelines set forth by the University of Maryland School of Medicine Animal Care and Use Committee and the National Institutes of Health.
Surgical procedures
Between postnatal day 6 and 8, male pups (15–20 g) received either an excitotoxic lesion of the ventral hippocampus (NVHL; n=17) or a control procedure (n=15). Pups were anesthetized via hypothermia; NVHL pups received bilateral infusions (0.3 μl per side; 0.15 μl/min) of ibotenic acid (10 μg/μl in artificial CSF [aCSF]) into the ventral hippocampus, at coordinates of 3 mm posterior to bregma, 3.5 mm lateral to bregma, and 5 mm from the surface of the skull. Control rats received bilateral infusions of aCSF into the ventral hippocampus at the same coordinates. Following recovery, pups were returned to their mothers.
Apparatus
Testing was conducted in 16 standard sized behavioral boxes (12 × 10 × 12 inches) using equipment modules purchased from Coulbourn Instruments. A recessed food cup was located in the center of the right wall approximately 2 cm above the floor. The food cup was connected to a feeder mounted outside of the chamber to deliver 45 mg sucrose pellets (plain sugar or cocoa-flavored, Research Diets). For conditioned taste aversion an external speaker approximately 20 cm from the chamber was connected to a white noise (72 db) generator. For representation-mediated taste aversion a cue light was placed directly above the food cup approximately 10 cm from the floor. Data were collected by Graphic State behavioral software from Coulbourn Instruments.
Behavioral Measures
The primary measure of conditioning for both CTA and RMTA was food cup rate, specifically, the number of food cup entries per minute during the white noise or cue light. The primary measure of eating was number of pellets consumed. For CTA raw consumption numbers are shown. For RMTA the change in pellet consumption [(# of pellets consumed pre-nausea) – (# of pellets consumed post-nausea)] was calculated. Positive numbers would indicate a taste aversion was formed.
Conditioned taste aversion
All rats (Con, n=14; NVHL, n=17) received eight days of conditioning. Each day consisted of 12 pairings of 30-s white noise with delivery of three cocoa-flavored pellets (time between trials was 7–10 min). Over the next 6 days, conditioned taste aversion training and probe testing was conducted. Rats in each lesion group were divided into paired (Con, 7; NVHL, 9) and unpaired groups (Con, 7; NVHL, 8). On days 1 and 3 Paired rats were given access to 100 cocoa pellets while unpaired rats placed in the chambers with no access to pellets. Following 10 min all rats were removed and received intraperitoneal (IP) injections of 0.3 M lithium chloride [LiCl - 5 ml/kg]. This dose and delivery of LiCl naturally induces nausea/gastric irritation and when given following food consumption results in a conditioned taste aversion (10, 11). On days 2 and 4, unpaired rats were given 10-min access to 100 cocoa-flavored pellets while paired rats placed in the chambers with no access to pellets. Previous studies have shown that spacing LiCl-induced nausea and food consumption by 24 hrs prevents the acquisition of a conditioned taste aversion (12). Thus, while both Paired and Unpaired rats experienced the food and became nauseas, only Paired rats should form a conditioned taste aversion. On day 5 an extinction probe was given in which all rats received 12, 30-s presentation of white noise in the absence of any pellets (time between trials was 7–10 min). On day 6 a consumption test was given in which all rats had access to 100 pellets.
Representation-mediated taste aversion
Over three days, rats (Con, n=11; NVHL, n=11) received a total of 6 or 12 pairings of 30-s cue light illumination with delivery of 3 plain sugar pellets (time between trials was 7–10 min). Following conditioning to the cue light, rats were given a 10-min consumption test, in which 100 sugar pellets were placed in the recessed food cup. This would serve as a baseline for measuring subsequent taste aversion. Next, in two consecutive 60-min sessions; nausea was induced by IP injection of 0.3 M LiCl (5 ml/kg) and rats were placed in the experimental chamber. Five minutes into each session the cue light was illuminated for 30-s. In this way cue light illumination (and presumably any representation of sugar elicited by the cue light) was paired with nausea. The following day a consumption test identical to the first was given to determine if pairing the cue light and nausea resulted in any aversion to the actual sugar pellets.
Statistical analyses
Data were acquired using Coulbourn GS2 software. Raw data were processed in Matlab to extract food cup rate during cue and baseline periods. In order to be included in consumption analysis for either representation-mediated or conditioned taste aversion rats must have eaten at least 40 of 100 pellets in the baseline consumption test. Data were analyzed with ANOVA using Statistica. Post-hoc comparisons were made with Tukey’s Honestly Significant Difference. In all cases p < 0.05 was considered significant.
Results
Histological results
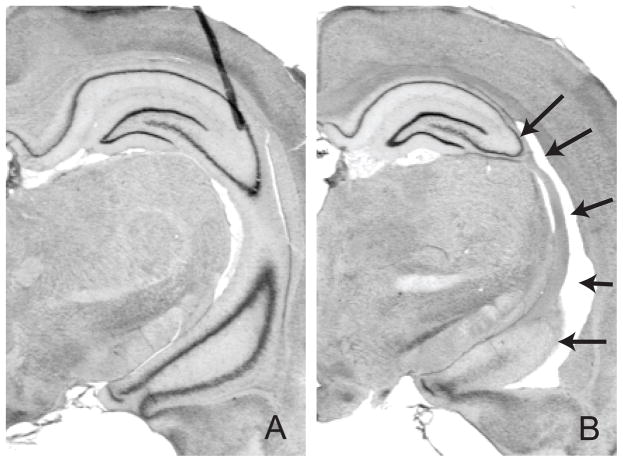
Nissl-stained hippocampal sections from NVHL animals exhibited varying degrees of cell loss, cavitation, enlarged ventricles, and cellular disorganization restricted to the ventral subiculum, ventral CA1, and/or CA3 region. Control-treated animals showed no evidence of damage to either the hippocampus or adjacent areas. A representative photomicrograph from a Control rat (Fig 1A) and NVHL rat (Fig 1B) is shown.
Figure 1.
Representative control and neonatal ventral hippocampal lesions. Photomicrographs from Nissl-stained (A) Control and (B) NVHL are shown. Black arrows point to regions of cellular loss & disorganization and ventricular enlargement.
Conditioned taste aversion
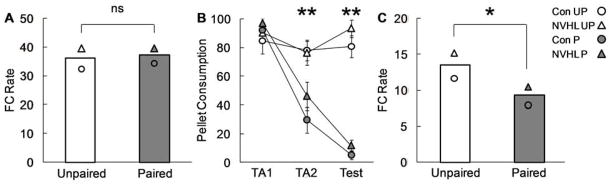
Experimental timeline and training procedures are shown in Figure 2A and 2B. Control and NVHL rats were trained that a white noise predicted cocoa-flavored pellets (Fig 3A). ANOVA [(lesion – Con, NVHL) × (group – paired, unpaired)] for mean food cup rate during the white noise on the final day of conditioning found no significant main effects or any interactions with lesion or group (all ps > 0.1). Control and NVHL rats were then divided into two groups: Paired and Unpaired. Paired rats received the pellets with nausea induced by lithium chloride injection. Unpaired rats received the pellets at the same time as Paired rats but nausea was induced 24 hrs later. As expected, only paired rats formed an aversion to the pellets (Fig 3B). ANOVA for cocoa pellet consumption [(day – TA1, TA2, Test) × (lesion – Con, NVHL) × (group – paired, unpaired)] revealed significant effects of day (F2,54 = 68.10, p < 0.01), group (F1,27 = 33.62, p < 0.01), and a day × group interaction (F2,54 = 62.11, p < 0.01). Post-hoc comparisons found significant differences between Unpaired and Paired consumption on TA2 and Test (ps < 0.01). Importantly, however, there were neither main effects nor interactions with lesion (ps > 0.1).
Figure 2.
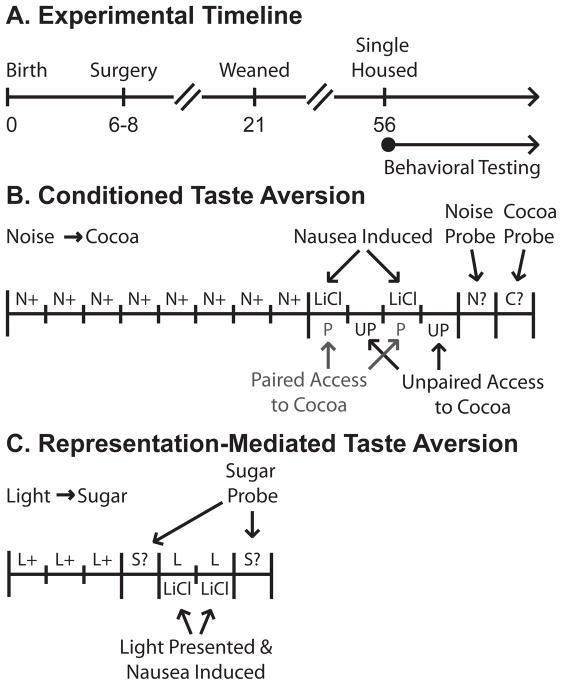
Experimental timeline and outline of behavioral procedures. (A) Postnatal days 0, 6–8, 21 and 56 are marked. These days corresponded to Birth, Surgery, Weaning and Single-Housing. After day 56 (adulthood) rats were food restricted and behavioral testing took place. (B) Conditioned taste aversion took place over 14 days. Noise → Cocoa conditioning was given for 8 consecutive days. Over days 9–12 Paired rats were given cocoa pellets and made nauseas on the same day while Unpaired rats ate cocoa and were made nauseas on separate days. Behavioral responding to the Noise alone was tested on day 13 and a final cocoa consumption test given on day 14. (C) Representation-mediated taste aversion took place over 7 days. Light → Sugar conditioning was given for 3 consecutive days, with half the rats receiving 2 trials/day and half receiving 4 trials/day. On day 4 a baseline sugar consumption test was given. Over days 5–6 rats were made nauseas and the cue light illuminated. A final sugar consumption test was given on day 8.
Figure 3.
Control and NVHL performance in conditioned taste aversion. (A) Mean food cup rate on the final day of noise → cocoa training for Control (circle) and NVHL (triangle) rats in the Unpaired (white) and Paired (grey) conditions. Symbols represent group means; bars represent combined Control and NVHL means for Unpaired and Paired groups. (B) Mean ± SEM pellet consumption over the 2 days of taste aversion training (TA1 and TA2) and the final test. (C) Mean food cup rate in the extinction probe. (ns = not significant, *p < 0.05, **p < 0.01)
Subsequently, a probe test was conducted in which the white noise was presented in the absence of pellets (Fig 3C). As expected, Paired rats reduced food cup responding during the white noise, relative to Unpaired rats, demonstrating that conditioned responding was sensitive to the current value of the pellets. ANOVA [(lesion – Con, NVHL) × (group – paired, unpaired)] for food cup rate to the white noise revealed a significant effect of group (F(1,27) = 4.31, p < 0.05), but no significant main effects or interactions with lesion (ps > 0.1). Note that the overall reduction in responding during the probe, relative to conditioning, was due to extinction resulting from the omission of cocoa-pellets. Importantly, ANOVA which included trial (1–12) as a factor found identical patterns of extinction for Control and NVHLs (ps > 0.1).
Representation-mediated taste aversion
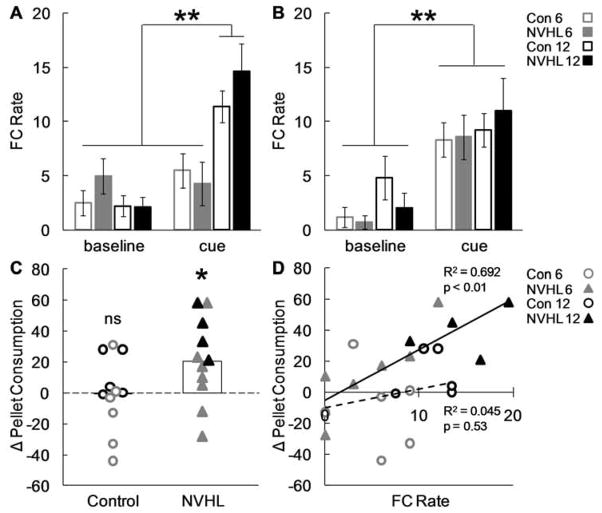
Experimental timeline and training procedures are shown in Figure 2A and 2C. Control and NVHL rats were trained that cue light illumination predicted sugar pellets; rats received a total of either 6 or 12 light-sugar pairings (Fig 4A). Training increased food cup responding during the cue light, compared to baseline, for rats that received 12 but not 6 pairings. Accordingly, ANOVA [(lesion – Con, NVHL) × (period – baseline, cue) × (trials – 6, 12)] for food cup rate on the final day of conditioning found a significant period × trial interaction (F(1,18) = 17.84, p < 0.01). Post-hoc testing confirmed that food cup rates during the cue were elevated over baseline for rats receiving 12 trials of conditioning (p < 0.01) but not for rats receiving 6 trials (p > 0.1). No effects of or interactions with lesion were found (ps > 0.1), indicating that NVHL rats learned at the same rate as Controls.
Figure 4.
Control and NVHL performance in representation-mediated taste aversion. (A) Mean ± SEM food cup rate during baseline and cue periods on the final day of conditioning are shown for Control (open bar) and NVHL rats (filled bar) that received 6 (grey) or 12 (black) light-sugar pairings. (B) Mean ± SEM food cup rate during baseline and cue periods for light-nausea pairings. (C) Change in consumption following light-nausea pairings is plotted for all individuals: Control (open circle), NVHL (filled triangle), 6 (grey), 12 (black). The more positive the number the greater the taste aversion that was formed. When necessary data points were jittered along the x-axis to avoid overlap. White bars indicate group means for Control and NVHL, collapsing 6 and 12 cue-sugar pairings. (D) The relationship between conditioned responding (x-axis) and the change in pellet consumption (y-axis) is plotted for Control and NVHLs. The trendline and the square of the correlation coefficient are shown for each group; Control: dotted line, NVHL: solid line. (ns = not significant, *p < 0.05, **p < 0.01)
Following conditioning rats were given free access to 100 sugar pellets in the experimental chamber in order to assess baseline food consumption. There was no difference between baseline consumption levels in Control and NVHL rats (p > 0.1). Next, on two consecutive days, all rats received pairings of the cue light and LiCl-induced nausea in the training chamber without food present. Control and NVHL rats showed identical patterns of increased responding to the food cup during light presentation on these days (Figure 4B). ANOVA [(lesion – Con, NVHL) × (period – baseline, cue) × (trials – 6, 12)] for food cup rate found a main effect of period (F (1,18) = 53.05, p < 0.01) but no effects nor any interactions with lesion (ps > 0.1).
Finally, rats were again given free access to 100 pellets in the experimental chamber. When consumption was compared to the earlier baseline (Fig. 4C), only NVHL rats had formed a significant aversion to the sugar pellets. ANOVA [(lesion – Con, NVHL) × (test – pre-nausea, post-nausea) × (trials – 6, 12)] comparing baseline consumption to that following cue-nausea pairings found a significant main effect of test (F(1,18) = 6.46, p < 0.05), a significant test × trials interaction (F(1,18) = 6.33, p < 0.05) and critically, a lesion × test interaction (F(1,18) = 5.66, p < 0.05). Post-hoc comparisons confirmed that NVHLs (p < 0.05) but not Controls (p > 0.1) significantly reduced their pellet consumption between the two tests. Additionally when food cup rate was plotted against the change in pellet consumption for each rat (Fig. 4D) there was a significant positive correlation for NVHL rats (R2 = 0.692, p < 0.01) but not for Controls (R2 = 0.045, p = 0.53). Thus, although both NVHL and Control rats appeared to learn similarly from the 6 or 12 trials of conditioning, NVHL rats more strongly formed a representation-mediated taste aversion.
Discussion
Here we have shown that NVHL rats exhibit markedly enhanced RMTA compared to controls. This was despite Control and NVHLs showing identical cue conditioning, taste aversion learning and an ability to utilize the current value of the reward to guide responding. Normal performance in these aspects of testing is particularly noteworthy because it suggests the enhanced RMTA exhibited by NVHL rats cannot be readily explained by facilitated learning about reward or nausea, as might be expected given previous work showing facilitated learning after damage to the hippocampal system (13, 14). However if NVHLs simply formed stronger reward associations, then they might have conditioned more rapidly and certainly should have reduced responding more than controls during the noise probe at the end of CTA. As noted above, they performed like controls in both tests. Similarly, if NVHLs formed stronger associations with nausea, they should have more quickly suppressed food consumption in CTA. This also did not occur. In this regard, it is also worth noting that the pattern of behavioral deficits shown by NVHL rats differs from that shown by adult rats given hippocampal lesions (8).
Instead a more parsimonious account of enhanced RMTA in NVHLs is that while both Controls and NVHLs formed representations of reward, NVHLs were less able to distinguish these representations of reward from actual reward. As a result, in NVHL rats, the imagined reward was more effective as a substitute for actual reward in the RMTA procedure. The difficulty in obtaining RMTA in normal adult rats is consistent with prior reports, indicating that this effect is transient and depends heavily on subjects receiving a precise amount of training (15). In this regard, the robust effect in NVHL rats is particularly striking.
The goal of this study was to apply a potential behavioral assessment of reality testing to the NVHL model of schizophrenia. As noted earlier, impaired reality testing in people with schizophrenia is best evidenced by delusions – internal beliefs persisting despite abundant and contrary real world evidence – and hallucinations – experiencing events not actually present in the real world. At the core of both delusions and hallucinations is a failure to distinguish between internal beliefs or experiences and external reality. A similar failure to distinguish imagined representations from reality may underlie RMTA. That this proposed means of assessing reality testing revealed markedly impaired function in the NVHL model of schizophrenia is intriguing, and extends the deficits this model produces into the realm of positive symptoms. Of course, the NVHL is but a single model of schizophrenia. Finding enhanced RMTA in a variety of schizophrenia models, or schizophrenic patients themselves, would greatly strengthen our claims. Nevertheless, the NVHL model is the most extensively studied neurodevelopmental model of schizophrenia with near 100 publications, and it is in use in a few dozen labs. While behavioral deficits reported in NVHL rats so far involve cognitive and social deficits, the problem NVHL rats have with RMTA may be highly relevant to hallucinations and delusions.
Of course realizing the potential of this procedure will require additional work on a number of fronts. First, treatment with D-2 blocking antipsychotics reduces positive symptoms in people and also reverses the NVHL effect in a number of behavioral paradigms. Demonstrating a similar reversal for NVHLs in RMTA would greatly strengthen claims of modeling positive symptoms of schizophrenia. Second, neurophysiological investigations (single-unit recording, immediate early genes, etc.) would provide much needed evidence on the neural basis of enhanced RMTA in NVHLs. Current, albeit scant, evidence suggests that RMTA is dependent on associative representations encoded in the basolateral amygdala (16). Notably the medial prefrontal cortex is thought to modulate the acquisition and/or expression of these amygdalar representations (17). One attractive hypothesis is that these projections allow prefrontal regions to selectively modulate – sculpt – associative representations in amygdala, so that they can be dissociated from the neural populations encoding actual rewards during learning. Such a sculpting process might occur rapidly with learning. This idea would be consistent with the observation that NVHL rats exhibit altered dopaminergic control of local inhibitory circuits in medial prefrontal cortex (18), resulting in hyper-excitable and ‘noisy’ networks in this region (19). The resultant decline in the signal-to-noise ratio in medial prefrontal cortex might result in dysregulation of the amygdala, thereby prolonging the period of sensitivity to RMTA. Supporting this, both prefrontal hyperactivity (20) and decreased prefrontal-amygdalar communication (21, 22) has been reported in people with schizophrenia.
Future experiments using the behavioral procedures employed here may help identity the neural dysfunction associated with positive symptoms of schizophrenia. A better understanding of these neural circuits may aide our ability to develop novel therapies – potentially decreasing the burden of schizophrenia on individuals and society.
Acknowledgments
This work was supported by grants from the NIDA (R01-DA-015718 to GS), the NIMH (R01-MH-57683 to PO’D) and an Institutional Ruth L. Kirschstein National Research Service Award to MAM (T32-NS-07375).
Footnotes
Financial Disclosures: The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kaplan HI, Sadock BJ, Grebb JA. Kaplan and Sadock’s Synpopsis of Psychiatry: Behavioral Sciences Clinical Psychiatry. 7. Baltimore: Williams & Wilkins; 1994. [Google Scholar]
- 2.Feifel D, Shilling PD. Promise and pitfalls of animal models of schizophrenia. Curr Psychiatry Rep. 2010;12:327–334. doi: 10.1007/s11920-010-0122-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lipska BK, Swerdlow NR, Geyer MA, Jaskiw GE, Braff DL, Weinberger DR. Neonatal excitotoxic hippocampal damage in rats causes post-pubertal changes in prepulse inhibition of startle and its disruption by apomorphine. Psychopharmacology (Berl) 1995;122:35–43. doi: 10.1007/BF02246439. [DOI] [PubMed] [Google Scholar]
- 4.Lipska BK, Jaskiw GE, Weinberger DR. Postpubertal emergence of hyperresponsiveness to stress and to amphetamine after neonatal excitotoxic hippocampal damage: a potential animal model of schizophrenia. Neuropsychopharmacology. 1993;9:67–75. doi: 10.1038/npp.1993.44. [DOI] [PubMed] [Google Scholar]
- 5.Chambers RA, Self DW. Motivational responses to natural and drug rewards in rats with neonatal ventral hippocampal lesions: an animal model of dual diagnosis schizophrenia. Neuropsychopharmacology. 2002;27:889–905. doi: 10.1016/S0893-133X(02)00365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sams-Dodd F, Lipska BK, Weinberger DR. Neonatal lesions of the rat ventral hippocampus result in hyperlocomotion and deficits in social behaviour in adulthood. Psychopharmacology (Berl) 1997;132:303–310. doi: 10.1007/s002130050349. [DOI] [PubMed] [Google Scholar]
- 7.Chen J, Lipska BK, Weinberger DR. Genetic mouse models of schizophrenia: from hypothesis-based to susceptibility gene-based models. Biol Psychiatry. 2006;59:1180–1188. doi: 10.1016/j.biopsych.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 8.Tseng KY, Chambers RA, Lipska BK. The neonatal ventral hippocampal lesion as a heuristic neurodevelopmental model of schizophrenia. Behavioural Brain Research. 2009;204:295–305. doi: 10.1016/j.bbr.2008.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDannald M, Schoenbaum G. Toward a model of impaired reality testing in rats. Schizophrenia Bulletin. 2009;35:664–667. doi: 10.1093/schbul/sbp050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hatfield T, Han JS, Conley M, Gallagher M, Holland P. Neurotoxic lesions of basolateral, but not central, amygdala interfere with pavlovian second-order conditioning and reinforcer devaluation effects. Journal of Neuroscience. 1996;16:5256–5265. doi: 10.1523/JNEUROSCI.16-16-05256.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roitman MF, Wheeler RA, Tiesinga PH, Roitman JD, Carelli RM. Hedonic and nucleus accumbens neural responses to a natural reward are regulated by aversive conditioning. Learn Memory. 2010;17:539–546. doi: 10.1101/lm.1869710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh T, McDannald MA, Haney RZ, Cerri DH, Schoenbaum G. Nucleus Accumbens Core and Shell are Necessary for Reinforcer Devaluation Effects on Pavlovian Conditioned Responding. Frontiers in Integrative Neuroscience. 2010;4:126. doi: 10.3389/fnint.2010.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.White NM, McDonald RJ. Acquisition of a spatial conditioned place preference is impaired by amygdala lesions and improved by fornix lesions. Behavioural Brain Research. 1993;55:269–281. doi: 10.1016/0166-4328(93)90122-7. [DOI] [PubMed] [Google Scholar]
- 14.Bussey TJ, Clea Warburton E, Aggleton JP, Muir JL. Fornix lesions can facilitate acquisition of the transverse patterning task: a challenge for “configural” theories of hippocampal function. Journal of Neuroscience. 1998;18:1622–1631. doi: 10.1523/JNEUROSCI.18-04-01622.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holland PC. Amount of training effects in representation-mediated food aversion learning: no evidence of a role for associability changes. Learn Behav. 2005;33:464–478. doi: 10.3758/bf03193185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dwyer DM, Killcross S. Lesions of the basolateral amygdala disrupt conditioning based on the retrieved representations of motivationally significant events. Journal of Neuroscience. 2006;26:8305–8309. doi: 10.1523/JNEUROSCI.1647-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sotres-Bayon F, Quirk GJ. Prefrontal control of fear: more than just extinction. Current Opinion in Neurobiology. 2010;20:231–235. doi: 10.1016/j.conb.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tseng KY, Lewis BL, Hashimoto T, Sesack SR, Kloc M, Lewis DA, et al. A neonatal ventral hippocampal lesion causes functional deficits in adult prefrontal cortical interneurons. Journal of Neuroscience. 2008;28:12691–12699. doi: 10.1523/JNEUROSCI.4166-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gruber AJ, Calhoon GG, Shusterman I, Schoenbaum G, Roesch MR, O’Donnell P. More is less: a disinhibited prefrontal cortex impairs cognitive flexibility. Journal of Neuroscience. 2010;30:17102–17110. doi: 10.1523/JNEUROSCI.4623-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor SF, Welsh RC, Chen AC, Velander AJ, Liberzon I. Medial frontal hyperactivity in reality distortion. Biol Psychiatry. 2007;61:1171–1178. doi: 10.1016/j.biopsych.2006.11.029. [DOI] [PubMed] [Google Scholar]
- 21.Hoptman MJ, D’Angelo D, Catalano D, Mauro CJ, Shehzad ZE, Kelly AM, et al. Amygdalofrontal Functional Disconnectivity and Aggression in Schizophrenia. Schizophrenia Bulletin. 2009 doi: 10.1093/schbul/sbp012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Radulescu AR, Mujica-Parodi LR. A systems approach to prefrontal-limbic dysregulation in schizophrenia. Neuropsychobiology. 2008;57:206–216. doi: 10.1159/000151731. [DOI] [PubMed] [Google Scholar]