Abstract
HtrA1, a member of serine protease family, has been previously found to be involved in resistance to chemotherapy in ovarian cancer although the underlying mechanism is not clear. Using mixture-based oriented peptide library approach, we previously identified X-linked inhibitor of apoptosis protein (XIAP), a member of the inhibitor of apoptosis proteins (IAPs) family as a potential substrate of HtrA1. The aim of this work is to investigate the link between HtrA1 and XIAP proteins and their relationships with chemoresistance in ovarian cancer. Our results showed that recombinant XIAP was degraded by purified wild type HtrA1 but not mutant HtrA1 in vitro. Consistent with the in vitro data, co-immunoprecipitation assays showed that HtrA1 and XIAP formed a protein complex in vivo. Ectopic expression of HtrA1 led to decreased level of XIAP in OV167 and OV202 ovarian cancer cells, while knockdown of HtrA1 resulted in increased level of XIAP in SKOV3 ovarian cancer cells. Furthermore, over-expression of HtrA1 in OV202 cells promoted cell sensitivity to cisplatin-induced apoptosis which could be reversed by increased expression of XIAP. The cleavage of XIAP induced by HtrA1 was enhanced by cisplatin treatment. Taken together, our experiments have identified XIAP as a novel substrate of HtrA1 and the degradation of XIAP by HtrA1 contributes to cell response to chemotherapy, suggesting that restoring the expression of HtrA1 may be a promising treatment strategy for ovarian cancer.
Keywords: HtrA1, XIAP, apoptosis, chemoresistance, ovarian cancer
INTRODUCTION
Epithelial cancer of the ovary is the most lethal gynecologic malignancy in the United States, with approximately 22,000 new cases and 16,000 deaths occurring annually [1]. Despite initial responses to surgery and chemotherapy in up to 80% of cases, more than 75% of affected women ultimately die from recurrence and the development of chemoresistant sites [2]. To improve patient survival, it is crucial to develop novel therapeutic strategies that overcome chemoresistance. However, much remains to be discovered regarding the molecular mechanism that underlies the development of chemoresistance.
HtrA1 belongs to a widely conserved family of serine proteases initially identified in prokaryotes as essential chaperones/proteases required for survival at elevated temperatures (hence named high temperature requirement A HtrA) [3]. Later, these proteases were also identified in mammalian systems as regulators of diverse signaling pathways [4, 5]. We previously identified that the loss of HtrA1 in ovarian cancers may contribute to in vivo chemoresistance [6, 7]. HtrA1 expression was upregulated by cisplatin treatment in ovarian cancer cell lines. Forced expression of HtrA1 enhanced cisplatin-induced cytotoxicity. Clinical data also revealed that patients with ovarian cancers expressing higher levels of HtrA1 showed a higher response rate than those with lower levels of HtrA1 expression [7]. However, the mechanism by which HtrA1 regulates the programmed cell death remains largely unknown yet.
HtrA1-induced apoptosis depends on its serine protease activity [7], suggesting an unknown substrate is involved in conferring chemoresistance. Using mixture-based oriented peptide library screening, we determined HtrA1 consensus cleavage site motifs [4], through which X-linked inhibitor of apoptosis protein (XIAP) is matched as one potential candidate of the substrates of HtrA1. As a member of the inhibitor of apoptosis proteins (IAPs) family, XIAP plays a critical role in apoptosis regulation. By directly inhibiting the initiation and execution phases of the caspase cascade, XIAP protects cell from death in response to various cellular assaults [8]. In ovarian cancer, expression of XIAP has been recognized as an important cell survivor factor that endows cell resistance to cisplatin-induced apoptosis [9–12]. Based on these facts, we hypothesized that HtrA1 may sensitize cell to chemotherapy through degrading XIAP. In this paper, we investigated the link between HtrA1 and XIAP proteins and their relationships with chemoresistance in ovarian cancer.
MATERIALS AND METHODS
Cell culture, transfection and drug treatment
Human ovarian cancer cell lines SKOV3 and TOV21G were obtained from the American Type Culture Collection (ATCC) (Manassas, VA, U S) and cultured according to the provider’s recommendation. OV167 and OV202 cell lines were established and cultured as described previously [7]. Cells were transfected with plasmids using Lipofectamine Plus (Invitrogen) according to the manufacturer’s recommendation. Cisplatin was purchased from Calbiochem (La Jolla, CA, US).
Plasmids
Protease active wide type (WT) HtrA1 proteins were generated as previously described [7]. HtrA1 targeting shRNA (sh1 and sh2) and non-targeting shRNA (NT) were purchased from Sigma-Aldrich. pcDNA3-Xiap-Myc was purchased from Addgene (Cambridge, MA, US).
In vitro synthesis of XIAP protein
XIAP protein was expressed in the TNT Coupled Reticulocyte Lysate System (Promega) according to the manufacturer’s instruction. Specifically, 1μg of pcDNA-XIAP plasmids was added into 25 μl reaction system and incubated at 30°C for 90 mins.
Purification of recombinant HtrA1 protein
Human recombinant HtrA1 proteins were purified from bacteria as previously described [7].
In vitro degradation
Each reaction contained 2.5μl of XIAP product from TNT synthesis system and 0.5μg of HtrA1 protein, which was incubated in a reaction buffer (50 mM HEPES, pH 7.4, 200 mM NaCl, 10 mM CaCl2) in a total volume of 20 μl at 37°C. Reaction products were collected at different time points and resolved on SDS-PAGE and either determined by the radioactivity of 35S or immunoblotted with specific XIAP antibodies.
Western blot analysis
Western blot analysis was performed as described previously [7]. Whole cell lysates were analyzed with the following antibodies: anti-HtrA1, XIAP and β-actin. Anti-HtrA1 antibody was rabbit polyclonal antibody and mouse monoclonal antibody raised as previously described [7]. Monoclonal antibody against β-actin was purchased from Sigma-Aldrich. Polyclonal antibody against XIAP was purchased from Cell Signaling Technology (Danver, MA, US).
Real-time RT-PCR
Total RNA was extracted from cells using Trizol reagent (Invitrogen) and reverse transcribed. Primers of XIAP were purchased from SABiosciences (Frederick, MD, US) and real-time PCR was performed according to the provider’s recommendation.
Clonogenic survival assays
Stable clones expressing non-targeting and HtrA1 shRNA (sh1 and sh2) were seeded at 1000 cells/well in 6-well plates, cultured overnight, treated with various concentrations of cisplatin for 24 hours, washed, and then incubated in drug-free medium for 2 weeks. Colonies were stained with Coomassie blue and counted.
Flow cytometry
1×106 of cells were incubated with PI solution with final concentration of 2 μg/ml at room temperature for 15 mins in the dark. Apoptotic cells were quantified by flow cytometry (FACS Calibur; Becton Dickinson, San Jose, CA, US) using a single laser, emitting excitation light at 488 nm.
Immunoprecipitation
SKOV3 and TOV21G cells were lysed on ice for half an hour. Immunoprecipitates were obtained using rabbit anti-XIAP antibodies conjugated to protein A/G–agarose (Santa Cruz, CA, US).
Statistical analysis
All experiments were performed at least three times in triplicate. Results were expressed as mean ±s.d. Two-tailed Student’s t test and ANOVA followed by Newman-Keuls test were performed using Prism 3.0 (GraphPad Software, La Jolla, CA). P < 0.05 was considered statistically significant.
RESULTS
HtrA1 cleaves XIAP in vitro
To determine if XIAP is a substrate of HtrA1, we purified recombinant WT HtrA1 to examine if HtrA1 can degrade XIAP in vitro. Purified serine protease mutant HtrA1 (SA HtrA1) served as controls to show the requirement of protease activity during the process of XIAP degradation. XIAP protein was expressed in the TNT Coupled Reticulocyte Lysate System (Promega). WT and SA HtrA1 proteins were separately added into XIAP synthesis system and incubated together at 30°C for 90 mins. The expression of XIAP protein was determined by the radioactivity of 35S as well as western blot with specific anti-XIAP antibodies. As shown in Figure 1A and 1B, XIAP protein was successfully expressed in this in vitro system (lane 2). When WT HtrA1 protein was added in the in vitro system, XIAP was degraded (lane 3) while no effect on XIAP protein was manifested when SA HtrA1 protein was added (lane 4).
Figure 1. Degradation of XIAP by HtrA1 in vitro.
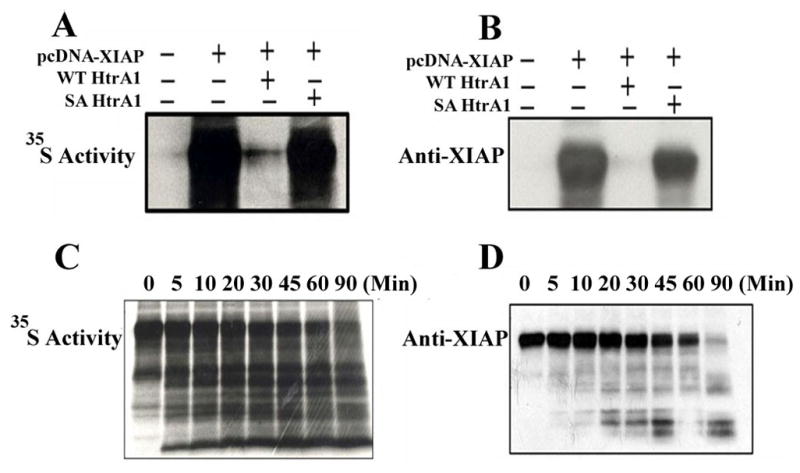
XIAP protein was expressed in the TNT Coupled Reticulocyte Lysate System (Promega). Purified WT or SA HtrA1 proteins were added into XIAP protein synthesis system with a final concentration of 10ng/μl and incubate at 30 °C for 90 mins in a total volume of 25μl reaction. The reaction system without adding XIAP plasmids served as negative control. Reaction products were resolved on SDS-PAGE and the expression of XIAP was either determined by the radioactivity of 35S (Figure 1A) or immunoblotted with specific XIAP antibodies (Figure 1B). WT HtrA1 protein was incubated with recombinant XIAP in a cell-free reaction system (50 mM HEPES, pH 7.4, 200 mM NaCl, 10 mM CaCl2) at 37 °C in a 20 μl volume. Lysates were collected at indicated time points and separated on SDS-PAGE gel and determined by the radioactivity of 35S (Figure 1C) or immunoblotted with specific XIAP antibodies (Figure 1D).
Our results showed that XIAP was almost completely degraded by WT HtrA1 protein at 90 min after incubation (lane 3 in Figure 1A and 1B). To further determine the kenetics of HtrA1-mediated degradation of XIAP, a time course experiment was set up. Reaction products of the in vitro digest assay were collected at indicated time points and separated on SDS-PAGE gel. As shown in Figure 1C and D, the degradation of XIAP resulted in numerous smaller bands, supporting the cleavage of XIAP by HtrA1 is through multiple sites. As HtrA1 successfully cleaved XIAP in a cell free system, it suggests this cleavage process does not require the participation of any other mediator. Taken together, our results demonstrated that HtrA1 can degrade XIAP in vitro, and XIAP is a direct substrate for HtrA1.
HtrA1 cleaves XIAP in vivo
To test whether HtrA1 can also cleave XIAP in vivo, OV202 ovarian cancer cells which express low levels of HtrA1 protein were transfected with plasmids encoding WT HtrA1 cDNA. As shown in Figure 2A, increased expression of WT HtrA1 correlates with decreased level of XIAP, suggesting a dose-dependent degradation of XIAP by WT HtrA1. Similar results were also obtained in OV167 cells (Figure 2B). Analysis of XIAP mRNA level using reverse transcription (RT)–PCR showed that HtrA1 did not alter XIAP mRNA expression (Figure 2C and D), suggesting that the degradation of XIAP by HtrA1 occurred at protein level in OV202 and OV167 cells.
Figure 2. HtrA1 degrades XIAP at protein level.
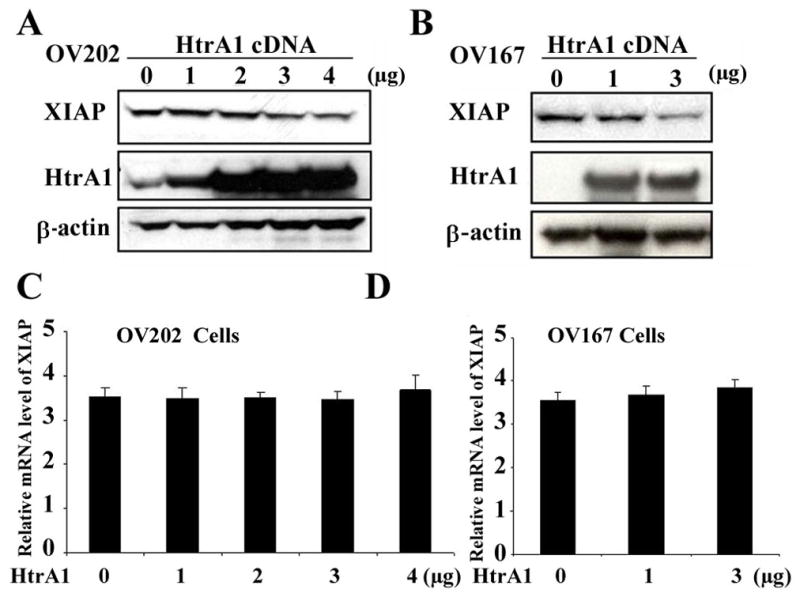
A–B, cleavage of XIAP by HtrA1 in ovarian cancer cells. OV202 (A) or OV167 (B) ovarian cancer cells were transfected with indicated amounts of plasmids encoding WT HtrA1cDNA. 24 h post transfection, cell lysates were collected and XIAP levels were determined by western blot analysis. C–D, expression of XIAP mRNA remains unchanged after transfection with plasmids encoding HtrA1 cDNA. OV202 (C) and OV167 (D) cells were transfected with indicated amount of HtrA1 cDNA. RNA samples were collected at 24 hours after transfection and mRNA level of XIAP was determined by real-time RT-PCR. Bars represent mean ± s.d. of three independent experiments performed in triplicate.
HtrA1 and XIAP associate with each other in vitro and in vivo
In order to determine if HtrA1 associates with XIAP in vitro, immunoprecipitation with anti-HtrA1 antibody was performed in the products from XIAP in vitro synthesis system in which SA mutant HtrA1 proteins were added. The results showed that XIAP was co-immunoprecipitated with SA mutant HtrA1 protein (Figure 3A). To determine if such protein complex also existed in vivo, we also examined the association between endogenous HtrA1 and XIAP in SKOV3 and TOV-21G cell lysates using reverse immunoprecipitation with anti-XIAP antibody. As shown in Figure 3B, the endogenous HtrA1 was also co-precipitated with endogenous XIAP in lysates from both cell lines, demonstrating that these two proteins form a complex both in vitro and in vivo.
Figure 3. HtrA1 interacts with XIAP in vitro and in vivo.
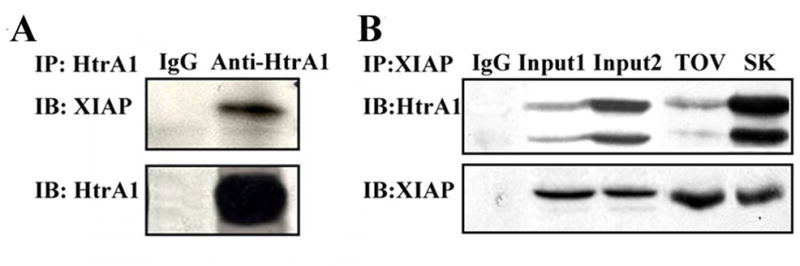
A, exogenous SA HtrA1 associates with XIAP. Immuno-precipitation assay was performed using mouse antibodies specific to HtrA1 in products from XIAP synthesis system in which SA HtrA1 protein was added. Mouse IgG served as control. B, endogenous XIAP associates HtrA1. Immunoprecipitation assay was performed using rabbit antibodies specific to XIAP in cell lysates from TOV-21G (TOV) and SKOV3 (SK) ovarian cancer cells. Mouse IgG served as negative control. Input 1 and 2 from TOV-21G cells and SKOV3 cells respectively, served as positive controls.
Cisplatin enhances the effect of HtrA1-mediated XIAP degradation
Previous studies reported that cisplatin can up-regulate the expression of HtrA1 [7] while down-regulate XIAP expression [11]. However, whether the downregulated XIAP level is attributed in part to the activation of HtrA1 induced by cisplatin hasn’t been studied. To explore the role of HtrA1 and XIAP during chemotherapy, OV167 cells which do not express HtrA1 protein were transfected with plasmids encoding WT HtrA1 and then treated with 10 μM cisplatin for 24h. The results showed that, cisplatin treatment caused a decrease of the level of XIAP in both control cells and HtrA1 expressing cells, compared to untreated cells. However, over-expression of HtrA1 led to a bigger decrease in XIAP after cisplatin treatment (Figure 4A) compared to control cells. Similar results were observed in OV202 cells (Figure 4B).
Figure 4. CDDP induces the cleavage of XIAP by HtrA1.
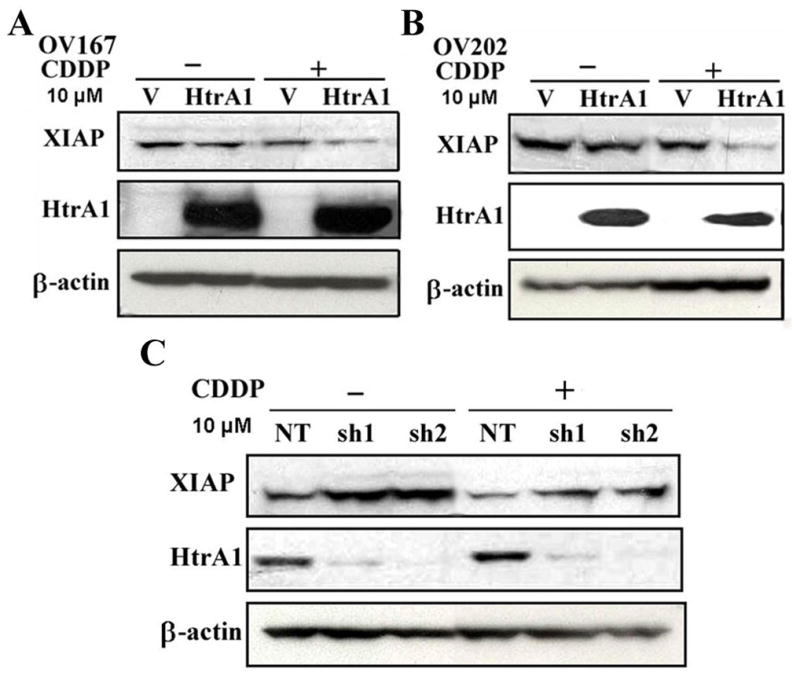
OV167 (A) or OV202 (B) cells were transiently transfected with plasmids encoding WT HtrA1cDNA, followed by the treatment of cisplatin (CDDP) (10 μM) for 24h. HtrA1 and XI0AP levels were determined by Western-blot analysis. C down-regulation of HtrA1 leads to higher level of XIAP under CDDP treatment. SKOV3 clonal cells with NT or HtrA1 shRNA (sh1 and sh2) were treated with 10 μM CDDP for 24h and cell lysates were collected followed by Western-blot analysis.
Next, we further examined the effects of downregulation of HtrA1 on XIAP level under cisplatin treatment using previously described SKOV3 cells transfected with non-targeting (NT) or HtrA1 shRNA (sh1 and sh2) [13]. After these cells were treated with cisplatin for 24 h, cell lysates were collected and followed by western blot analysis. As shown in Figure 4C, treatment with cisplatin decreased the level of XIAP. However, the level of XIAP was higher in cells with down-regulated HtrA1 expression than in the control cells expressing NT shRNA. These results demonstrated that HtrA1 is required for cisplatin-induced degradation of XIAP in vivo.
HtrA1 contributes to cell sensitivity to cisplatin-induced apoptosis which can be reversed by XIAP expression
The involvement of XIAP in chemoresistance is well documented [9, 12, 14]. To determine whether HtrA1-induced inactivation of XIAP will sensitize cells to drug induced apoptosis, we performed colony formation assay using isogenic ovarian cancer cells stably expressing HtrA1 shRNA (sh1 and sh2) compared with non-targeting shRNA control. Consistent with previous reports[7], stable suppression of HtrA1 in SKOV3 and TOV-21G cells showed a significant increase in clonogenic survival under cisplatin treatment compared with cells expressing non-targeting shRNA (Figure 5A and B). Enhanced expression of HtrA1 led to significantly higher rate of cisplatin induced cytotoxicity compared with vector-transfected cells as determined by trypan blue assay (Figure 5C). Additionally, overexpression of XIAP before the addition of cisplatin reversed the pro-apoptosis effects of HtrA1 under cisplatin treatment (Figure 5D). Taken together, these results demonstrated that XIAP is a substrate for HtrA1-induced sensitivity to cisplatin treatment
Figure 5. HtrA1 contributes to cell sensitivity to chemotherapy which can be reversed by XIAP expression.
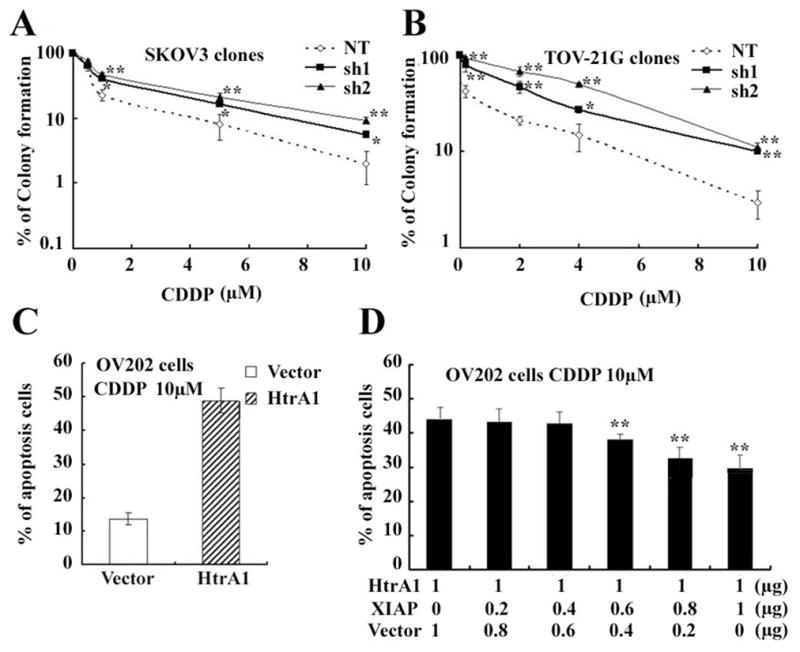
A–B downregulation of HtrA1 confers cell resistance to cisplatin induced apoptosis. Stable clones in SKOV3 and TOV-21G cells expressing non-targeting and HtrA1 shRNA (sh1 and sh2) were seeded at 1000 cells/well in 6-well plates, treated with various concentrations of cisplatin for 24 hours, washed, and then incubated in drug-free medium for 2 weeks. Colonies were stained with Coomassie blue and counted. C over-expression of HtrA1 promotes cisplatin toxicity in ovarian cancer cells. OV202 cells were transfected with plasmids encoding WT HtrA1 and treated with Cisplatin for 24h. Apoptotic cells were counted by typan blue staining. D XIAP expression reverses the cell sensitivity to chemotherapy endowed by HtrA1. OV202 cells were transfected with 1μg plasmids encoding HtrA1 cDNA plus various amounts of plasmids encoding XIAP cDNA. After 24h of transfection, cells were treated with 10 μM cisplatin. Cell apoptosis were evaluated by flow cytometry after PI staining 24 h later. Data are expressed as mean ± s.d. from 3 independent trials performed at least in triplicate. *P < 0.05, **P < 0.01
DISCUSSION
Although cisplatin derivatives are first-line chemotherapeutic agents for the treatment of epithelial ovarian cancer, chemoresistance remains a major hurdle to successful therapy and the molecular mechanisms involved are poorly understood [15]. We previously demonstrated HtrA1 promotes apoptosis and renders cell sensitive to chemotherapy in ovarian cancer [7]. In the present study, we further investigated the mechanisms underlying HtrA1-induced apoptosis. Specifically, we have identified XIAP as a novel substrate of HtrA1 in mediating its pro-apoptotic action.
XIAP, a 57KD protein, is a member of IAPs family. IAPs were first identified in baculoviruses, where they function to keep the host cell alive while the viruses replicate. Eight IAPs have recently been identified in human cells including neuronal apoptosis inhibitory protein (NAIP), XIAP, cellular inhibitor of apoptosis protein-1 (C-IAP-1), C-IAP-2, IAP-like protein 2, (ILP2), melanoma IAP (ML-IAP), BRUCE and survivin[16,17]. All IAPs contain one or more baculovirus inhibitor of apoptosis repeat (BIR) domains. XIAP is the most potent and broadest inhibitor of cell death of all IAPs family members [16]. Accumulating evidence indicates XIAP is a major contributor to chemoresistance in ovarian cancer [15, 18–20]. Cisplatin consistently decreases XIAP content and induces apoptosis in cisplatin-sensitive, but not cisplatin-resistant cells [11, 15]. Downregulation of XIAP in chemoresistant cells renders the cells sensitive to the cytotoxic actions of cisplatin, while over-expression of XIAP in chemosensitive cells causes a reversion to the chemoresistant phenotype [11, 20]. The inability of cisplatin to downregulate XIAP is considered as an important contributing factor for chemoresistance in human ovarian cancer.
Here, we have identified a new upstream regulator of XIAP, HtrA1. Depending on its serine protease activity, HtrA1 can cleave XIAP both in vitro and in vivo. We previously found that HtrA1’s proapoptotic function is correlated with activation of caspase-3 and/or -7 depending on its serine protease activity in ovarian cancer [7]. The results presented here demonstrate that the cleavage of XIAP mediated by HtrA1 plays a key role in HtrA1-mediated apoptosis. It has been shown that XIAP contains three amino-terminal BIR domains. To prevent cell death, it directly binds to and inhibits the upstream initiator caspase-9 by its BIR3 region, and the downstream effector caspases-3 and -7 by the BIR2 domain, which enables itself to attenuate both mitochondria/cytochrome c- and death receptor-mediated apoptosis [8]. The identification of XIAP as a substrate of HtrA1 brings a key mechanistic link for the increased activity of caspase-3 and/or -7 observed after cisplatin treatment. Specifically, cisplatin induces the upregulation of HtrA1, leading to the degradation of XIAP, which results in increased activation of caspase-3 and/or -7.
Besides HtrA1, two mitochondrial proteins, Smac/DIABLO and Omi/HtrA2, have been reported to be involved in the inactivation of XIAP during apoptosis [5, 8, 21]. Smac/Diablo has been shown to inhibit XIAP by directly binding to XIAP at the same domains that mediate the interactions of XIAP with the caspases, and thereby facilitate propagation of the apoptotic cascade via the executioner caspases [22,23]. HtrA2, similar to HtrA1, also belongs to HtrA family in which all members share a highly conserved chymotrypsin like serine protease domain and one PDZ domain at the C-terminus. Unlike Smac/DIABLO, HtrA2 can not only bind with XIAP, but also through its protease activity, cleave XIAP [24–26]. HtrA2 is reported to mediate cisplatin-induced cell death in renal cells and colon cancer cells [27, 28]. The cleavage action of XIAP by HtrA2 may contribute to decreased XIAP level observed in OV167 control cells which do not express HtrA1 after cisplatin treatment (Figure 4A). However, the results that expression of HtrA1 enhanced the decrease of XIAP under cisplatin treatment, clearly shows an additional layer of regulation of XIAP by HtrA1. Our findings discovered a cytosol inhibitor of XIAP, adding new knowledge to the mechanism of the inactivation of XIAP induced by chemotherapy.
Our findings may provide a novel target for therapeutic intervention of overcoming chemoresistance to cisplatin. Therapeutic approaches to enhance HtrA1 expression which results in downregulation of XIAP may increase the sensitivity of ovarian cancer cells to cisplatin treatment. In fact, several approaches to downregulate expression of XIAP have been employed to block tumor growth. For example, XIAP antisense oligonucleotide significantly increases cell death of cancer cells in vitro, impedes tumor growth or causes tumor regression and prolongs animal survival in various xenograft models, including ovarian cancer [9, 29–31]. In a clinical phase I/II trial, XIAP antisense oligonucleotide AEG35156 has shown improved efficacy in combination with chemotherapy in patients with AML refractory to a single induction regimen [32]. Accordingly, a number of IAP-binding pro-apoptotic compounds that mimic the sequence corresponding to the N-terminal tetrapeptide of Smac/DIABLO, have been developed [17] and shown to effectively synergize with TRAIL and Bortezomib in inducing cell death [33]. Our results described here suggest that therapeutic approaches to increase HtrA1 expression may synergize with other approaches to degrade the XIAP on conquering chemoresistance in ovarian cancer.
In summary, our findings bring a new link between the expression of serine protease HtrA1 and the stability of anti-apoptosis factor XIAP, providing new insights into the mechanism of HtrA1-induced cell apoptosis. The identification of new enzyme that can inactivate XIAP leads to a better understanding of the development of resistant phenotype in ovarian cancer.
Acknowledgments
Grant support: This work was funded by grants from the National Cancer Institute CA12340 (to V.S. and J.C.), the Mayo Clinic Bernard and Edith Waterman Center for Cancer Genetics (to V.S.) and the Ovarian Cancer Research Fund PEO/MC/01.08 (to X. H.)
We thank members of the Shridhar lab for stimulating discussions.
Abbreviations
- HtrA
high temperature requirement A
- WT
wide type
- SA
serine-to-alanine mutant at catalytic site, position 328
- XIAP
X-linked inhibitor of apoptosis
Footnotes
The authors declare no conflict of interest.
References
- 1.Chien JR, Aletti G, Bell DA, Keeney GL, Shridhar V, Hartmann LC. Molecular pathogenesis and therapeutic targets in epithelial ovarian cancer. J Cell Biochem. 2007;102:1117–29. doi: 10.1002/jcb.21552. [DOI] [PubMed] [Google Scholar]
- 2.Chobanian N, Dietrich CS., 3rd Ovarian cancer. Surg Clin North Am. 2008;88:285–99. vi. doi: 10.1016/j.suc.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Clausen T, Southan C, Ehrmann M. The HtrA family of proteases: implications for protein composition and cell fate. Molecular cell. 2002;10:443–455. doi: 10.1016/s1097-2765(02)00658-5. [DOI] [PubMed] [Google Scholar]
- 4.Chien J, He X, Shridhar V. Identification of tubulins as substrates of serine protease HtrA1 by mixture-based oriented peptide library screening. J Cell Biochem. 2009;107:253–63. doi: 10.1002/jcb.22121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chien J, Campioni M, Shridhar V, Baldi A. HtrA serine proteases as potential therapeutic targets in cancer. Curr Cancer Drug Targets. 2009;9:451–68. doi: 10.2174/156800909788486704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chien J, Staub J, Hu SI, Erickson-Johnson MR, Couch FJ, Smith DI, Crowl RM, Kaufmann SH, Shridhar V. A candidate tumor suppressor HtrA1 is downregulated in ovarian cancer. Oncogene. 2004;23:1636–44. doi: 10.1038/sj.onc.1207271. [DOI] [PubMed] [Google Scholar]
- 7.Chien J, Aletti G, Baldi A, Catalano V, Muretto P, Keeney GL, Kalli KR, Staub J, Ehrmann M, Cliby WA, Lee YK, Bible KC, Hartmann LC, Kaufmann SH, Shridhar V. Serine protease HtrA1 modulates chemotherapy-induced cytotoxicity. J Clin Invest. 2006;116:1994–2004. doi: 10.1172/JCI27698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schimmer AD, Dalili S, Batey RA, Riedl SJ. Targeting XIAP for the treatment of malignancy. Cell Death Differ. 2006;13:179–88. doi: 10.1038/sj.cdd.4401826. [DOI] [PubMed] [Google Scholar]
- 9.Shaw TJ, Lacasse EC, Durkin JP, Vanderhyden BC. Downregulation of XIAP expression in ovarian cancer cells induces cell death in vitro and in vivo. Int J Cancer. 2008;122:1430–4. doi: 10.1002/ijc.23278. [DOI] [PubMed] [Google Scholar]
- 10.Ding X, Mohd AB, Huang Z, Baba T, Bernardini MQ, Lyerly HK, Berchuck A, Murphy SK, Buermeyer AB, Devi GR. MLH1 expression sensitises ovarian cancer cells to cell death mediated by XIAP inhibition. Br J Cancer. 2009;101:269–77. doi: 10.1038/sj.bjc.6605180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J, Feng Q, Kim JM, Schneiderman D, Liston P, Li M, Vanderhyden B, Faught W, Fung MF, Senterman M, Korneluk RG, Tsang BK. Human ovarian cancer and cisplatin resistance: possible role of inhibitor of apoptosis proteins. Endocrinology. 2001;142:370–80. doi: 10.1210/endo.142.1.7897. [DOI] [PubMed] [Google Scholar]
- 12.Ma JJ, Chen BL, Xin XY. XIAP gene downregulation by small interfering RNA inhibits proliferation, induces apoptosis, and reverses the cisplatin resistance of ovarian carcinoma. Eur J Obstet Gynecol Reprod Biol. 2009;146:222–6. doi: 10.1016/j.ejogrb.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 13.He X, Ota T, Liu P, Su C, Chien J, Shridhar V. Downregulation of HtrA1 promotes resistance to anoikis and peritoneal dissemination of ovarian cancer cells. Cancer Res. 2010;70:3109–18. doi: 10.1158/0008-5472.CAN-09-3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ndozangue-Touriguine O, Sebbagh M, Mérino D, Micheau O, Bertoglio J, Bréard J. A mitochondrial block and expression of XIAP lead to resistance to TRAIL-induced apoptosis during progression to metastasis of a colon carcinoma. Oncogene. 2008;27:6012–22. doi: 10.1038/onc.2008.197. [DOI] [PubMed] [Google Scholar]
- 15.Fraser M, Leung B, Jahani-Asl A, Yan X, Thompson WE, Tsang BK. Chemoresistance in human ovarian cancer: the role of apoptotic regulators. Reprod Biol Endocrinol. 2003;1:66–79. doi: 10.1186/1477-7827-1-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eckelman BP, Salvesen GS, Scott FL. Human inhibitor of apoptosis proteins: why XIAP is the black sheep of the family. EMBO Rep. 2006;7:988–94. doi: 10.1038/sj.embor.7400795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Srinivasula SM, Ashwell JD. IAPs: what’s in a name? Mol Cell. 2008;30:123–35. doi: 10.1016/j.molcel.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sapi E, Alvero AB, Chen W, O’Malley D, Hao XY, Dwipoyono B, Garg M, Kamsteeg M, Rutherford T, Mor G. Resistance of ovarian carcinoma cells to docetaxel is XIAP dependent and reversible by phenoxodiol. Oncol Res. 2004;14:567–78. doi: 10.3727/0965040042707943. [DOI] [PubMed] [Google Scholar]
- 19.Mansouri A, Zhang Q, Ridgway LD, Tian L, Claret FX. Cisplatin resistance in an ovarian carcinoma is associated with a defect in programmed cell death control through XIAP regulation. Oncol Res. 2003;13:399–404. doi: 10.3727/096504003108748410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sasaki H, Sheng Y, Kotsuji F, Tsang BK. Down-regulation of X-linked inhibitor of apoptosis protein induces apoptosis in chemoresistant human ovarian cancer cells. Cancer Res. 2000;60:5659–66. [PubMed] [Google Scholar]
- 21.Vaux DL, Silke J. Mammalian mitochondrial IAP binding proteins. Biochem Biophys Res Commun. 2003;304:499–504. doi: 10.1016/s0006-291x(03)00622-3. [DOI] [PubMed] [Google Scholar]
- 22.Wu G, Chai J, Suber TL, Wu JW, Du C, Wang X, Shi Y. Structural basis of IAP recognition by Smac/DIABLO. Nature. 2000;408:1008–12. doi: 10.1038/35050012. [DOI] [PubMed] [Google Scholar]
- 23.Verhagen AM, Ekert PG, Pakusch M, Silke J, Connolly LM, Reid GE, Moritz RL, Simpson RJ, Vaux DL. Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell. 2000;102:43–53. doi: 10.1016/s0092-8674(00)00009-x. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki Y, Takahashi-Niki K, Akagi T, Hashikawa T, Takahashi R. Mitochondrial protease Omi/HtrA2 enhances caspase activation through multiple pathways. Cell Death Differ. 2004;11:208–16. doi: 10.1038/sj.cdd.4401343. [DOI] [PubMed] [Google Scholar]
- 25.Yang QH, Church-Hajduk R, Ren J, Newton ML, Du C. Omi/HtrA2 catalytic cleavage of inhibitor of apoptosis (IAP) irreversibly inactivates IAPs and facilitates caspase activity in apoptosis. Genes Dev. 2003;17:1487–96. doi: 10.1101/gad.1097903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Srinivasula SM, Gupta S, Datta P, Zhang Z, Hegde R, Cheong N, Fernandes-Alnemri T, Alnemri ES. Inhibitor of apoptosis proteins are substrates for the mitochondrial serine protease Omi/HtrA2. J Biol Chem. 2003;278:31469–72. doi: 10.1074/jbc.C300240200. [DOI] [PubMed] [Google Scholar]
- 27.Cilenti L, Kyriazis GA, Soundarapandian MM, Stratico V, Yerkes A, Park KM, Sheridan AM, Alnemri ES, Bonventre JV, Zervos AS. Omi/HtrA2 protease mediates cisplatin-induced cell death in renal cells. Am J Physiol Renal Physiol. 2005;288:F371–9. doi: 10.1152/ajprenal.00154.2004. [DOI] [PubMed] [Google Scholar]
- 28.Pruefer FG, Lizarraga F, Maldonado V, Melendez-Zajgla J. Participation of Omi Htra2 serine-protease activity in the apoptosis induced by cisplatin on SW480 colon cancer cells. J Chemother. 2008;20:348–54. doi: 10.1179/joc.2008.20.3.348. [DOI] [PubMed] [Google Scholar]
- 29.Carter BZ, Mak DH, Morris SJ, Borthakur G, Estey E, Byrd AL, Konopleva M, Kantarjian H, Andreeff M. XIAP antisense oligonucleotide (AEG35156) achieves target knockdown and induces apoptosis preferentially in CD34(+)38 (−) cells in a phase 1/2 study of patients with relapsed/refractory AML. Apoptosis. 2010 doi: 10.1007/s10495-010-0545-1. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim DW, Kim KO, Shin MJ, Ha JH, Seo SW, Yang J, Lee FY. siRNA-based targeting of antiapoptotic genes can reverse chemoresistance in P-glycoprotein expressing chondrosarcoma cells. Mol Cancer. 2009;8:28–38. doi: 10.1186/1476-4598-8-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shrikhande SV, Kleeff J, Kayed H, Keleg S, Reiser C, Giese T, Büchler MW, Esposito I, Friess H. Silencing of X-linked inhibitor of apoptosis (XIAP) decreases gemcitabine resistance of pancreatic cancer cells. Anticancer Res. 2006;26:3265–73. [PubMed] [Google Scholar]
- 32.Schimmer AD, Estey EH, Borthakur G, Carter BZ, Schiller GJ, Tallman MS, Altman JK, Karp JE, Kassis J, Hedley DW, Brandwein J, Xu W, et al. Phase I/II trial of AEG35156 X-linked inhibitor of apoptosis protein antisense oligonucleotide combined with idarubicin and cytarabine in patients with relapsed or primary refractory acute myeloid leukemia. J Clin Oncol. 2009;27:4741–6. doi: 10.1200/JCO.2009.21.8172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lecis D, Drago C, Manzoni L, Seneci P, Scolastico C, Mastrangelo E, Bolognesi M, Anichini A, Kashkar H, Walczak H, Delia D. Novel SMAC-mimetics synergistically stimulate melanoma cell death in combination with TRAIL and Bortezomib. Br J Cancer. 2010;102:1707–16. doi: 10.1038/sj.bjc.6605687. [DOI] [PMC free article] [PubMed] [Google Scholar]


