Abstract
Data from human genetics, histopathology, and animal models reveal a major role for the complement system in the development of age-related macular degeneration (AMD). Genetic variations in the complement factor H (CFH) gene are associated with an elevated risk of AMD. In this study we sought to determine whether eyes from donors with a high risk genotype (homozygosity for the histidine allele at codon 402) exhibit altered levels of membrane attack complex (MAC) in the choroid, compared to eyes with a low risk genotype (homozygosity for tyrosine). Proteins were extracted from the RPE/choroid of 18 donors (10 low risk and 8 high risk) and levels of MAC were assessed using an ELISA assay. Eyes from donors homozygous for the histidine allele showed 69% higher levels of MAC than those homozygous for the tyrosine allele (p<0.05), independent of whether the eyes showed signs of early AMD. Our results provide evidence that high-risk CFH genotypes may affect AMD risk by increased deposition of MAC around the aging choriocapillaris.
The relationship between variations in genes encoding members of the complement cascade and age-related macular degeneration (AMD) is well established. AMD-associated variations include polymorphisms in C3 (Maller, et al., 2007; Yates, et al., 2007), the C2/CFB locus (Gold, et al., 2006), and, most notably, complement factor H (CFH)(Edwards, et al., 2005; Hageman, et al., 2005; Haines, et al., 2005; Klein, et al., 2005).
In view of the role of CFH as an inhibitor of the alternative pathway of complement activation, it seems likely that some polymorphisms affecting protein sequence would result in loss of function, and thus increased complement activation (Anderson, et al., 2010). We and others have described the deposition of terminal complement complexes in the eyes of donors with AMD (Abrera-Abeleda, et al., 2006; Hageman, et al., 2005; Seth, et al., 2008; Skeie, et al., 2010). Complement complexes deposited on and around the choriocapillaris may place stress on the choroidal endothelium and could be related to the choriocapillaris loss observed in early AMD that is associated with abundance and size of drusen (Mullins, et al., 2011). While the assumption that CFH polymorphisms alter the inhibitory function of CFH appears reasonable, there is little empirical evidence to date that show altered complement inhibition in the human choroid. While C-reactive protein levels have been found elevated in donor eyes homozygous for the high risk allele (Johnson, et al., 2006) and the abundance of systemic complement components have been shown to vary with AMD affection status and genotype (see for example (Hecker and Edwards, 2011; Sivaprasad, et al., 2007)), the levels of the membrane attack complex (MAC) have not been quantified in eyes with high and low risk CFH genotypes.
In the current study we sought to evaluate the impact of the Y402H CFH polymorphism on choroidal deposition of the MAC in aged human eyes. Other components of complement activation are deposited in aging Bruch’s membrane/choroid, and some of these have effects on RPE and/or choroidal behavior (Nozaki, et al., 2006; Skeie, et al., 2010). MAC formation is likely the most crucial outcome of complement activation as it results from uninhibited C5 convertase activity and failure of vitronectin or CD59 to impair C9 polymerization, and has the potential to injure the RPE and/or choriocapillaris.
Human donor eyes were obtained from the Iowa Lions Eye Bank (Iowa City, IA). Eyes were obtained within 8 hours of death, an interval in which protein content is largely unchanged (Ethen, et al., 2006). All experiments were performed in accordance with the Declaration of Helsinki and with consent of the donors’ families. Eyes were dissected and a 6mm-diameter trephine punch was collected from the inferotemporal, juxtamacular RPE-choroid complex. Punches of RPE and choroid were snap frozen in liquid nitrogen and stored at −80ºC until processed for protein. For immunohistochemistry, a 6mm macular punch was collected, fixed, and embedded as described previously (Mullins, et al., 2011).
Genotyping was performed on either whole blood obtained at time of enucleation or from a fragment of extraocular muscle using established techniques (Qiagen DNeasy kit, Valencia CA). Genotyping was performed on subjects using TaqMan pre-designed SNP genotyping assays (SNP rs1061170, Applied Biosystems) in a high-throughput micro-fluidic system (Fluidigm, San Fransisco, CA).
Samples were selected for ELISA analysis on the basis of CFH genotype. Protein from donors homozygous for the Y402H high risk allele (“HH”, n=8) and from donors homozygous for the low risk allele (“YY”, n=10) were processed for MAC quantification. Punches of RPE-choroid were raised in 60μL of PBS with 1% Triton X-100 with protease inhibitors (Roche Complete Mini tablets, Indianapolis, IN) and homogenized using disposable pestles. Following centrifugation on a benchtop microcentrifuge, protein concentrations of supernatants were determined using a commercial protein assay kit (BioRad DC Protein Assay, Hercules, CA). Thirty micrograms of total protein were then loaded in each well of a MAC ELISA kit (Quidel MicroVue SC5b-9 Plus Kit) in triplicate, according to the manufacturer’s instructions. The concentration of MAC was determined in each sample based on absorbance and by linear interpolation using standards in the kit. For three samples (two YY and one HH) in which the loaded amount of protein was slightly lower than 30μg, the final protein content was adjusted accordingly. MAC levels were assessed both as a fraction of total protein and as mass extracted from each punch. To determine fraction of MAC to total protein, the MAC concentration was divided by the total protein content. To determine the mass of MAC extracted from each punch, the MAC concentration was multiplied by the volume of diluent used to extract the protein.
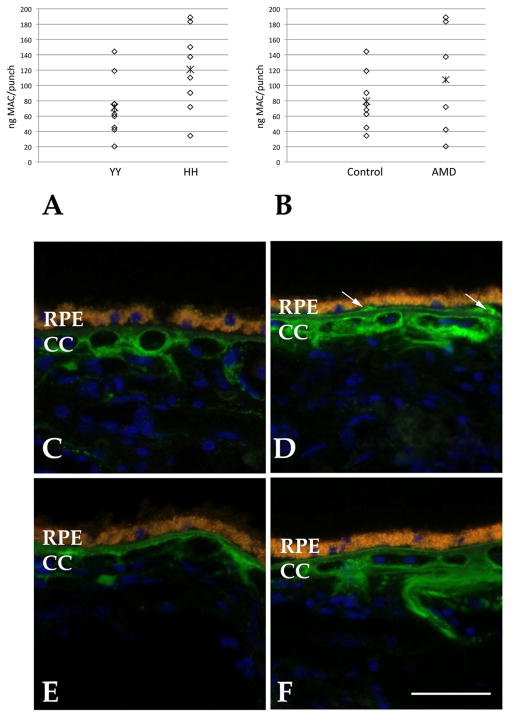
The MAC levels were assessed between the two genotypes. We observed an approximately 69% increase in MAC content in the choroids of donors homozygous for the histidine allele. Statistical analysis was performed using a permutation test with 10,000 permutations (Streitherg and Röhmel, 1986). Results showed a marginally statistically significant elevation in MAC in HH as compared to YY homozygotes (p=0.039). (Figure 1A).
Figure 1. MAC associated with CFH genotype in the human RPE-choroid.
A, B Graphs showing normalized levels of MAC in human RPE-choroid. Levels of MAC were significantly elevated in donor eyes homozygous for the high risk (HH) allele compared to the low risk (YY) allele (A). The presence of early AMD was associated with a trend toward increased levels of MAC but this did not reach statistical significance. Asterisks indicate mean value. C–F, Distribution of the membrane attack complex (green fluorescence) in donor maculae. In most cases, MAC is localized to domains surrounding the choriocapillaris in both HH (C, D) and YY (E, F) homozygotes. Drusen (arrows) are often immunoreactive. Yellow-orange fluorescence indicates RPE lipofuscin and blue fluorescence is due to the nuclear counterstain diamidino-phenol-indole. CC, choriocapillaris. Scale bar = 50μm.
In addition to CFH genotype, premortem clinical information was available for most of the donors. None of the eyes in this study manifested late atrophic or exudative AMD, whereas 6 had early AMD (as defined previously (Mullins, et al., 2011)), 9 were unaffected controls, and no data were available for 3. When data were analyzed on the basis of AMD affection status for the 15 eyes for which these data were available, increased MAC content was observed on average (35% higher than control levels; Figure 1B). In contrast to CFH genotype, however, this trend was not significant in our data set (p=0.97). Interestingly, although the sample size is small, the samples from donors with AMD showed a bimodal distribution, with both the highest and lowest values in the group being present in the RPE-choroid from AMD samples. Further study will be necessary to confirm and explore the clinical significance of this observation.
In summary, we observed that donor eyes homozygous for high and low risk CFH variants showed different levels of choroidal membrane attack complex, with histidine homozygotes possessing over 60% elevated MAC compared to tyrosine homozygotes. There are limitations to our study. Our approach using human donor eyes does not discriminate between circulating and localized MAC. However, in light of the distribution of MAC in the human macula—in which most of the protein appears matrix associated rather than in vascular lumens—it is likely that the circulating component makes a relatively small contribution (Figure 1C–F). This may be in contrast to abundant plasma proteins like C-reactive protein. Overall, this study lends support to the notion that the Y402H polymorphism in CFH is associated with decreased complement cascade inhibition in the choroid. Strategies to attenuate complement complex formation in AMD are now in trials (recently reviewed, (Yehoshua, et al., 2011)). Our results suggest that these modalities may relieve the vascular and RPE loss observed in AMD, especially in patients with high risk CFH genotypes.
Acknowledgments
Supported in part by NEI EY017451 (RFM), NEI 016822 (EMS), Foundation Fighting Blindness (RFM), the Macula Vision Research Foundation (RFM), the Hansjoerg E.J.W. Kolder Professorship for Best Disease Research (RFM) and the Howard Hughes Medical Institute (EMS)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrera-Abeleda M, Nishimura C, Smith J, Sethi S, McRae J, Murphy B, Silvestri G, Skerka C, Józsi M, Zipfel P, et al. Variations in the complement regulatory genes factor H (CFH) and factor H related 5 (CFHR5) are associated with membranoproliferative glomerulonephritis type II (dense deposit disease) J Med Genet. 2006;43:582–9. doi: 10.1136/jmg.2005.038315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DH, Radeke MJ, Gallo NB, Chapin EA, Johnson PT, Curletti CR, Hancox LS, Hu J, Ebright JN, Malek G, et al. The pivotal role of the complement system in aging and age-related macular degeneration: hypothesis re-visited. Prog Retin Eye Res. 2010;29(2):95–112. doi: 10.1016/j.preteyeres.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards A, Ritter R, Abel K, Manning A, Panhuysen C, Farrer L. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308:421–4. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- Ethen CM, Reilly C, Feng X, Olsen TW, Ferrington DA. The proteome of central and peripheral retina with progression of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2006;47(6):2280–90. doi: 10.1167/iovs.05-1395. [DOI] [PubMed] [Google Scholar]
- Gold B, Merriam JE, Zernant J, Hancox LS, Taiber AJ, Gehrs K, Cramer K, Neel J, Bergeron J, Barile GR, et al. Variation in factor B (BF) and complement component 2 (C2) genes is associated with age-related macular degeneration. Nat Genet. 2006;38(4):458–62. doi: 10.1038/ng1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hageman G, Anderson D, Johnson L, Hancox L, Taiber A, Hardisty L, Hageman J, Stockman H, Borchardt J, Gehrs K, et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci U S A. 2005;102:7227–32. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines J, Hauser M, Schmidt S, Scott W, Olson L, Gallins P, Spencer K, Kwan S, Noureddine M, Gilbert J, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419–21. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- Hecker LA, Edwards AO. Genetic control of complement activation in humans and age related macular degeneration. Adv Exp Med Biol. 2011;703:49–62. doi: 10.1007/978-1-4419-5635-4_4. [DOI] [PubMed] [Google Scholar]
- Johnson PT, Betts KE, Radeke MJ, Hageman GS, Anderson DH, Johnson LV. Individuals homozygous for the age-related macular degeneration risk-conferring variant of complement factor H have elevated levels of CRP in the choroid. Proc Natl Acad Sci U S A. 2006;103(46):17456–61. doi: 10.1073/pnas.0606234103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R, Zeiss C, Chew E, Tsai J, Sackler R, Haynes C, Henning A, Sangiovanni J, Mane S, Mayne S, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–9. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maller JB, Fagerness JA, Reynolds RC, Neale BM, Daly MJ, Seddon JM. Variation in complement factor 3 is associated with risk of age-related macular degeneration. Nat Genet. 2007;39(10):1200–1. doi: 10.1038/ng2131. [DOI] [PubMed] [Google Scholar]
- Mullins RF, Johnson MN, Faidley EA, Skeie JM, Huang J. Choriocapillaris vascular dropout related to density of drusen in human eyes with early age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52(3):1606–12. doi: 10.1167/iovs.10-6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki M, Raisler BJ, Sakurai E, Sarma JV, Barnum SR, Lambris JD, Chen Y, Zhang K, Ambati BK, Baffi JZ, et al. Drusen complement components C3a and C5a promote choroidal neovascularization. Proc Natl Acad Sci U S A. 2006 doi: 10.1073/pnas.0408835103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth A, Cui J, To E, Kwee M, Matsubara J. Complement-associated deposits in the human retina. Invest Ophthalmol Vis Sci. 2008;49(2):743–50. doi: 10.1167/iovs.07-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaprasad S, Adewoyin T, Bailey TA, Dandekar SS, Jenkins S, Webster AR, Chong NV. Estimation of systemic complement C3 activity in age-related macular degeneration. Arch Ophthalmol. 2007;125(4):515–9. doi: 10.1001/archopht.125.4.515. [DOI] [PubMed] [Google Scholar]
- Skeie JM, Fingert J, Russell S, Stone EM, Mullins RF. Complement Component C5a Activates ICAM-1 Expression on Human Choroidal Endothelial Cells. Invest Ophthalmol Vis Sci. 2010;51:5336–42. doi: 10.1167/iovs.10-5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streitherg B, Röhmel J. Exact distributions for permutations and rank tests: an introduction to some recently published algorithms. Statistical Software Newsletter. 1986;12:10–17. [Google Scholar]
- Yates JR, Sepp T, Matharu BK, Khan JC, Thurlby DA, Shahid H, Clayton DG, Hayward C, Morgan J, Wright AF, et al. Complement C3 variant and the risk of age-related macular degeneration. N Engl J Med. 2007;357(6):553–61. doi: 10.1056/NEJMoa072618. [DOI] [PubMed] [Google Scholar]
- Yehoshua Z, Rosenfeld PJ, Albini TA. Current Clinical Trials in Dry AMD and the Definition of Appropriate Clinical Outcome Measures. Semin Ophthalmol. 2011;26(3):167–80. doi: 10.3109/08820538.2011.577132. [DOI] [PubMed] [Google Scholar]