Abstract
CD1d-binding glycolipids exert potent adjuvant effects on T-dependent Ab responses. The mechanisms include cognate interaction between CD1d-expressing B cells and TCR-expressing Type I CD1d-restricted Natural Killer T cells (NKT). However, the critical NKT-derived factors that stimulate B cells are poorly understood. We tested the hypothesis that CD1d-driven CD40L expression by NKT cells influences humoral immunity. Bone marrow chimeras with CD40L+/+ or CD40L−/− NKT cells were immunized with Ag plus CD1d ligand before measuring Ab responses. CD40L−/− NKT cells stimulated higher endpoint Ab titers than controls expressing CD40L. In contrast, immunization of CD40L−/− mice revealed that CD40L−/− NKT cells could not provide B cell help when Th cells lacked CD40L. We report that CD40L−/− NKT cells can provide help for Ab production and do so cooperatively with CD40L+/+ Th cells. We suggest that the manner in which NKT cells provide B cell help is distinct from that of Th cells.
Keywords: Antibody, B lymphocyte, CD40L, NKT cell
Introduction
Type I CD1d-restricted NKT cells regulate adaptive immunity. In response to professional APC’s presenting CD1d/glycolipid complexes, the semi-invariant TCR on NKT cells is activated and leads to cytokine production. NKT cell activation influences cellular [1] and humoral [2–4] immunity. Consequently NKT cells have been implicated in beneficial immune responses to cancer [5] and infectious pathogens [6], and in harmful immune responses in asthma [7].
There has been progress in understanding the mechanism by which NKT cells are induced to provide B cell help. NKT cells activated by the CD1d ligand alpha-galactosylceramide (α-GC) enhanced specific Ab response to a co-administered T-dependent Ag [2–4]. Cognate interaction between CD1d-expressing B cells and NKT cells appears necessary for NKT-enhanced Ab responses [8–10], but non-cognate help may also occur [11].
CD40L, a TNF super-family member, is best known for its up-regulation on Th cells following activation by class II/peptide and co-stimulatory molecules [12]. CD40 engagement by CD40L exerts multiple effects on B cells including germinal center formation, immunoglobulin isotype switch, somatic hyper-mutation, B cell memory and long-lived plasma cell differentiation [13]. The importance of CD40L/CD40 interactions in B cell help is underscored by spontaneous CD40L mutations which cause hyper-IgM syndrome, resulting in a failure in isotype switch, somatic hyper-mutation and B cell memory [14].
Several reports implicate CD40L in NKT-enhanced adaptive immunity [2, 4, 11, 15]. In two independent studies, NKT cells induced limited T-dependent Ab responses in class II-null mice lacking Th cells [2, 4]. In one study agonistic anti-CD40 mAb was required for Ab production [4]. In another, α-GC had no adjuvant effect on Ab responses in mixed bone marrow chimeras whereby NKT cells were CD40L+/+ and B cells were CD40−/− [11]. Despite the available evidence, the absence of an in vivo system whereby only NKT cells lack CD40L expression has thus far impeded determining specifically whether NKT-expressed CD40L regulates humoral immunity.
Herein through immunization of CD40L−/− mice we confirm that CD40L−/− NKT cells cannot provide B cell help when Th cells lack CD40L expression. We then demonstrate through the use of mixed bone marrow chimeras that CD40L−/− as well as CD40L+/+ NKT cells provide help for Ab production. These results demonstrate that CD40L−/− NKT provide B cell help for Ab production, but must do so cooperatively with CD40L+/+ Th cells.
Materials and Methods
Mice
C57Bl/6 (CD45.2+/+ and CD45.1+/+) and CD40L−/− mice were from the National Cancer Institute (Bethesda, MD) and the Jackson Laboratory (Bar Harbor, ME) respectively. Jα18−/− mice were bred in the Animal Resource Center at the University of Oklahoma Health Sciences Center (OUHSC). All procedures were approved by the OUHSC Institutional Animal Care and Use Committee.
Flow Cytometry
Splenocytes and thymocytes were isolated as described previously [3]. Cells were incubated with media (containing: FCS, FcR-block; fluorochrome-conjugated mAbs; and CD1d tetramer), before washing, fixation and analysis with a Becton-Dickinson FACScalibur (Palo Alto, CA) [3]. Reagents were purchased from: BD Biosciences, San Jose, CA (anti-CD1d, -CD40L, -TCRβ); eBioscience, San Diego, CA (anti-CD4, -CD8, -CD11c and -CD19) and BioXcell, Lebanon, NH (anti-FcγR). CD1d/α-GC tetramers were provided by the NIAID Tetramer Facility (Emory University, Atlanta, GA).
Immunizations and serum collection
Mice were immunized s.c. (dose divided over both flanks) with 10 µg NP-KLH (Biosearch Technologies, Novato, CA) in 200 µl sterile endotoxin-free PBS or NP-KLH mixed with 4 µg of α-galactosylceramide (α-GC, Axorra, Plymouth Meeting, PA) in PBS. Mice were bled at d 28 post-immunization and sera obtained. On d 28 mice were bled and then boosted with 10 µg of NP-KLH and bled again on d 35.
ELISA
Endpoint anti-NP Ig titers in serum were measured as described previously [3].
Bone Marrow Chimeras
Six weeks old C57Bl/6 CD45.1+/+ mice were irradiated in split doses (700 then 500 Rad, 18 h apart). After a further 4 h, 106 donor bone marrow cells were transferred by the i.v. route to irradiated recipients. Donor cells consisted of 50:50 mixtures of: (i) Jα18−/− and C57Bl/6 cells; (ii) Jα18−/− and CD40L−/− cells. Recipients were engrafted for 12 wk before immunization.
Results
CD40L−/− NKT cells do not provide B cell help in the absence of CD40L+/+ Th cells
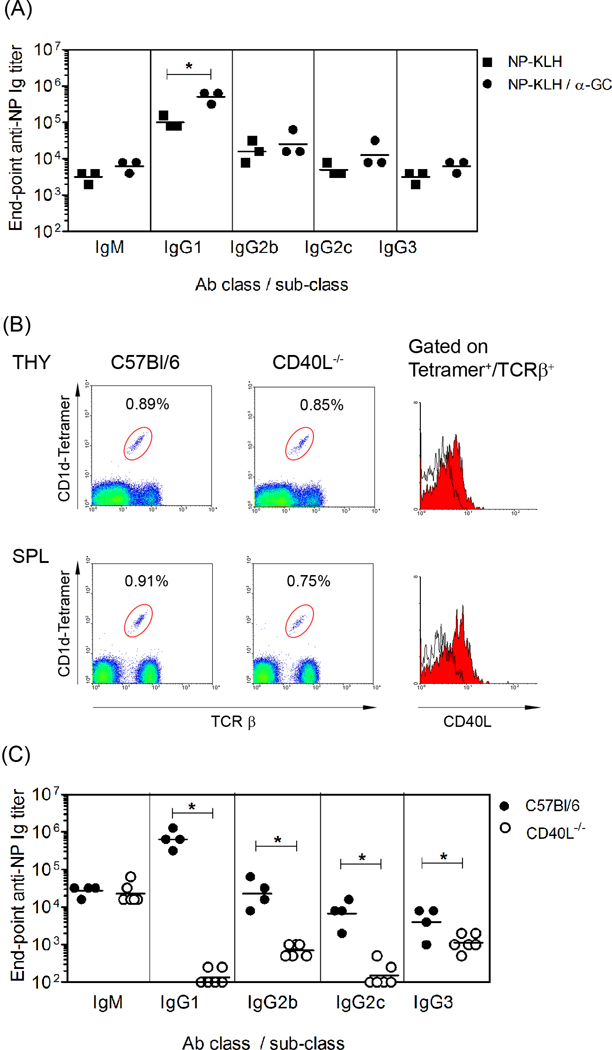
As reported previously, the CD1d ligand α-GC exerts a potent adjuvant effect on specific Ab responses to T-dependent Ags (Figure 1A) ([3, 4, 9, 16]). When C57Bl/6 mice were immunized with NP-KLH alone or NP-KLH plus α-GC, NP-specific Ab titers were higher in the group receiving α-GC. The effect was significant in IgG1 titers as compared to IgM, IgG2b, IgG2c and IgG3 titers. Since CD40L is required for B cell help, experiments were performed to determine if NKT cells could stimulate Ab production in CD40L−/− mice.
Figure 1. NKT cells do not provide B cell help in CD40L−/− mice.
(A) C57Bl/6 mice were immunized with NP-KLH or NP-KLH plus α-GC. After 28 days, all mice received a booster vaccine (NP-KLH). Sera were collected on day 35 and endpoint IgM, IgG1, IgG2b, IgG2c and IgG3 titers determine by ELISA. (B) Thymocytes and splenocytes were obtained from CD40L−/− and C57Bl/6 mice and then analyzed by flow cytometry. Dot plots (left) show CD1d tetramer+/TCRβ+ cells. Histograms (right) show expression of CD40L by gated CD1d tetramer+/TCRβ+ cells. Data show representative analyses from two CD40L−/− mice and numerous (>50) C57Bl/6 mice. (C) C57Bl/6 and CD40L−/− mice were immunized with NP-KLH plus α-GC on d 0 and boosted with NP-KLH on day 28 before bleeding on day 35. Endpoint IgM, IgG1, IgG2b, IgG2c and IgG3 titers in the sera collected on day 35 were then determined by ELISA. Each data point in (A) and (C) represents an individual mouse and line indicates geometric mean titer. Statistically significant differences between groups were determined using Mann Whitney U test.
Flow cytometry analysis revealed that thymic and splenic cells from CD40L−/− mice had a comparable frequency of TCRβ+, CD1d-tetramer-binding NKT cell to C57Bl/6 controls (Figure 1B). Comparable numbers of thymocytes and splenocytes were also recovered from C57Bl/6 and CD40L−/− mice. CD40L expression was detected on NKT cells from C57Bl/6 mice but not CD40L−/− mice.
Following immunization with NP-KLH plus α-GC, CD40L−/− mice produced NP-specific IgM, but largely failed to produce IgG (Figure 1C). End-point NP-specific IgG1, IgG2b, IgG2c and IgG3 titers were significantly lower in CD40L−/− mice than in C57Bl/6 mice. These data show that CD40L−/− NKT cells did not provide B cell help when Th cells lacked CD40L expression.
CD40L−/− NKT cells provide B cell help in the presence of CD40L+/+ Th cells
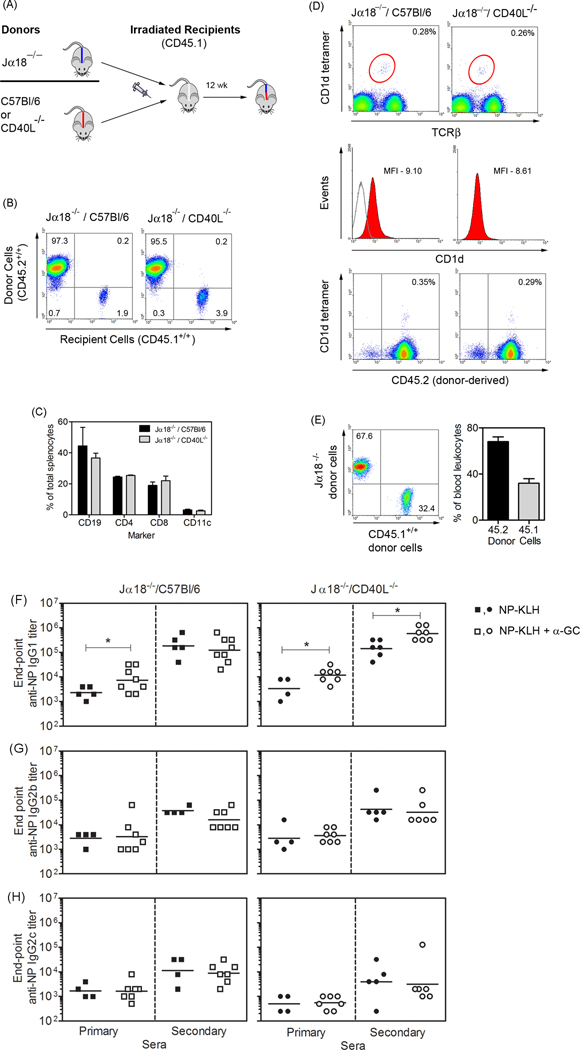
Mixed bone marrow chimeric mice were designed so that NKT cells were unable to express CD40L (Figure 2A). Flow cytometry revealed that >95% of splenocytes in the chimeric mice were donor-derived (Figure 2B). The Jα18−/−/C57Bl/6 and Jα18−/−/CD40L−/− chimeras had a similar frequency of B cell, T cells, and DCs (Figure 2C). The chimeras also had equivalent re-constitution of donor-derived NKT cells and expression of CD1d (Figure 2D). Re-constitution of NKT cells to the frequency observed in C57Bl/6 mice did not occur, but intact function and ability to enhance Ab responses has been demonstrated by our group [17].
Figure 2. NKT-derived CD40L is dispensable for Ab production.
(A) Outlines the strategy used for generating mixed bone marrow chimeras. Jα18−/− mice have a gene deletion in the TCR locus and do not rearrange the Vα14/Jα18 invariant TCR found on Type I NKT cells. (B) Spleens were obtained from immunized Jα18−/−/C57Bl/6 and Jα18−/−/CD40L−/− chimeric mice and analyzed by flow cytometry for CD45.2+/+ donor cells and residual CD45.1+/+ recipient cells. (C) Graph shows expression of B cell, T cell and dendritic cell markers for each of the chimeras. (D) Dot-plots in upper panels show CD1d tetramer+/TCRβ+ NKT cells. Histograms in middle panels show CD1d expression. Anti-CD1d and isotype control mAb binding are represented by filled and empty histograms respectively. Dot-plots in lower panels show donor-derived (CD45.2+/+) versus recipient-derived (CD45.2−/−) tetramer-binding cells. Data in B-D are representative of three of each chimera. (E) CD45.2+/+ (C57Bl/6) mice were lethally irradiated and engrafted with a 50/50 mixture of donor bone marrow cells from Jα18−/− mice (CD45.2+/+) and CD45.1+/+ mice. After 12 weeks peripheral blood leukocytes were analyzed by flow cytometry for CD45.2 and CD45.1 expression. Graph shows relative percentages of CD45.1 and CD45.2 cells in the periphery of 5 re-constituted recipients. (F-H) Jα18−/−/C57Bl/6 and Jα18−/−/CD40L−/− mixed chimeras were immunized as indicated. Sera were collected on d 28 (primary) and 35 (secondary) and ELISA assays were performed to analyze NP-specific (F) IgG1, (G) IgG2b and (H) IgG2c titers. Each data point represents an individual mouse and the lines indicate geometric mean titer. Two outliers were removed from the NP-KLH, primary sera group in (F). Data shown are representative of 2 independent experiments. Statistically significant differences between control and experimental mice were determined using Mann Whitney U test.
Measuring relative engraftment of donor Jα18−/− versus C57BL/6 or CD40L−/− cells directly was not possible because all donor strains were CD45.2+/+. C57Bl/6 (CD45.2+/+) mice were therefore irradiated and transferred with a 50/50 mix of donor Jα18−/− (CD45.2+/+) and CD45.1+/+ donor cells. Engraftment was such that a 68/32 average ratio of CD45.2+/+/CD45.1+/+ cells was observed (Figure 2E). This shows that while NKT cells in Jα18−/−/CD40L−/− chimeras could not express CD40L, the capacity for expression by Jα18−/−-derived non-NKT cells was minimally affected.
Following immunization, endpoint anti-NP IgG1, IgG2b and IgG2c titers were measured (Figure 2F-H). IgG2a was not assayed since C57Bl/6 mice express IgG2c rather than IgG2a [18]. In the Jα18−/−/C57Bl/6 mice, α-GC enhanced the primary IgG1 response similar to that observed in C57Bl/6 mice [3, 4], but had little impact on the secondary Ab response (Figure 2F). In contrast, α-GC significantly enhanced the primary and secondary IgG1 response following the booster in Jα18−/−/CD40L−/− mice (Figure 2F). Perturbation of CD40L expression by non-NKT cells was not problematic because Ab responses in NP-KLH-immunized Jα18−/−/C57BL/6 and Jα18−/−/CD40L−/− chimeras were comparable. IgG1 was the dominant Ab titer, and α-GC did not significantly alter IgG2b or IgG2c titers (Figure 2G-H), consistent with data in C57Bl/6 mice. This shows that CD40L on NKT cells is dispensable for NKT-enhanced Ab production and may even limit Ab production.
Discussion
We have demonstrated that NKT cells, despite dependence on interaction with B cells via CD1d/TCR interaction [8–10] do not provide B cell help in a manner dependent on CD40L expression by NKT cells. We reported previously that CD40 ligation with agonistic mAbs could result in limited Ab production in Ag/α-GC-immunized class II−/− mice lacking Th cells [4]. We originally interpreted the result to suggest that CD40L on NKT cells could contribute to enhanced Ab responses. However, in Jα18−/−/CD40L−/− chimeras, CD40L−/− NKT cells provided adequate help for Ab production following immunization with NP-KLH α-GC and the response was modestly enhanced as compared to controls. These two observations allow refinement of previous interpretations such that CD40L−/− NKT cells provide help for Ab production, but a CD40L signal from another source (Th cells) is required.
The enhancing effect of CD40L−/− NKT cells on the secondary Ab response may appear paradoxical because CD40L is required for induction of B cell memory and isotype switch to produce IgG. However, the amount of CD40L expression and therefore the number of CD40 molecules engaged on the B cell determines its fate. A heightened engagement of CD40 with agonistic mAbs inhibited B cell memory [19]. Constitutive expression of CD40L in transgenic mice led to diminished germinal center formation following immunization [20]. In this context, our data may be explained by a model in where CD40L expression by NKT cells is dispensable for NKT-enhanced Ab production and high expression of CD40L exerts a limiting effect on the response.
Since CD40L expression by NKT cells was dispensable for the α-GC-enhanced Ab response, we tested other candidate molecules known to be expressed by NKT cells. Using a similar mixed bone marrow chimera approach to that described herein, we were unable to assign a contribution to the inducible co-stimulator (ICOS) molecule (data not shown), or to NKT-derived IL-4 or IFNγ [17]. ICOS-blocking mAbs were administered to the Jα18−/−/C57Bl/6 and the Jα18−/−/CD40L−/− chimeras since ICOS−/− mice are NKT-deficient. Under those conditions, there was no effect of ICOS blockade on the Ab response, but incomplete ICOS blockade could not be excluded. In a recently published study, we showed that NKT-derived IL-4 and IFNγ were dispensable for IgG1 responses against anthrax toxin, but could exert modulatory effects on IgG2c [17].
The surprising lack of effect of NKT expressed CD40L, ICOS or secreted IL-4 or IFNγ suggests either functional redundancy, whereby Th cells provide adequate amounts of several signals provided by NKT cells, or that NKT cells provide B cell help in a manner distinct from Th cells. This could include use of different receptor pairings between the B cell and NKT cell, different soluble factors including other cytokines, or a mechanism whereby NKT cells boost Th cell priming to increase B cell help. Thus far available information suggests room for each of these hypotheses since cognate and non-cognate interactions seem to be important for NKT-enhanced B cell help [2, 4, 8, 9, 11].
In previous studies, adoptive transfer of CD1d+/+ versus CD1d−/− B cells into B cell-deficient µMT mice was used to demonstrate that CD1d expression by B cells was required for NKT-mediated help [9]. Production of Ab specific for NP-haptenylated α-GC was compromised in B7.1−/− and CD28−/− mice [10], but the lack of NKT development in those mice precluded using a bone marrow chimeric system. Bone marrow chimera approaches also showed that if class II and CD1d were expressed on different B cells then NKT cells could still provide B cell help [11], with the interpretation favoring non-cognate interactions. We therefore favor a hypothesis whereby CD1d expression by B cells is important for eliciting help from NKT cells but propose that help is not delivered directly, but perhaps through effects of NKT cells on Th function. Delineating these mechanisms is a current goal of our laboratory and will impact efforts to include NKT-activating ligands in vaccines.
Acknowledgements
This work was supported by grants to M.L.L. from the National Institutes of Health (1RO1AI078993) and the Oklahoma Center for the Advancement of Science and Technology (H-07 144S). We acknowledge generous support from the NIAID Tetramer Facility (Emory University, Atlanta, GA) for supplying CD1d tetramers.
Non-standard abbreviations
- NKT
natural killer T cell
- α-GC
alpha-galactosylceramide
- KLH
keyhole limpet hemocyanin
- NP
nitrophenol
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
H.S. designed and performed experiments, analyzed data and wrote the paper. S.J. performed experiments. M.L. designed the project, analyzed data and wrote the paper.
Conflict of Interest Disclosure
The authors declare no competing financial interests.
References
- 1.Fujii S, Shimizu K, Kronenberg M, Steinman RM. Prolonged IFN-gamma-producing NKT response induced with alpha-galactosylceramide-loaded DCs. Nature immunology. 2002 Sep;3(9):867–874. doi: 10.1038/ni827. [DOI] [PubMed] [Google Scholar]
- 2.Galli G, Pittoni P, Tonti E, Malzone C, Uematsu Y, Tortoli M, et al. Invariant NKT cells sustain specific B cell responses and memory. Proceedings of the National Academy of Sciences of the United States of America. 2007 Mar 6;104(10):3984–3989. doi: 10.1073/pnas.0700191104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Devera TS, Shah HB, Lang GA, Lang ML. Glycolipid-activated NKT cells support the induction of persistent plasma cell responses and antibody titers. European journal of immunology. 2008 Apr;38(4):1001–1011. doi: 10.1002/eji.200738000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lang GA, Exley MA, Lang ML. The CD1d-binding glycolipid alpha-galactosylceramide enhances humoral immunity to T-dependent and T-independent antigen in a CD1d-dependent manner. Immunology. 2006 Sep;119(1):116–125. doi: 10.1111/j.1365-2567.2006.02413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, et al. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science (New York, NY. 1997 Nov 28;278(5343):1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 6.Kobrynski LJ, Sousa AO, Nahmias AJ, Lee FK. Cutting edge: antibody production to pneumococcal polysaccharides requires CD1 molecules and CD8+ T cells. J Immunol. 2005 Feb 15;174(4):1787–1790. doi: 10.4049/jimmunol.174.4.1787. [DOI] [PubMed] [Google Scholar]
- 7.Akbari O, Stock P, Meyer E, Kronenberg M, Sidobre S, Nakayama T, et al. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nature medicine. 2003 May;9(5):582–588. doi: 10.1038/nm851. [DOI] [PubMed] [Google Scholar]
- 8.Barral P, Eckl-Dorna J, Harwood NE, De Santo C, Salio M, Illarionov P, et al. B cell receptor-mediated uptake of CD1d-restricted antigen augments antibody responses by recruiting invariant NKT cell help in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2008 Jun 11;105(25):8345–8350. doi: 10.1073/pnas.0802968105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lang GA, Devera TS, Lang ML. Requirement for CD1d expression by B cells to stimulate NKT cell-enhanced antibody production. Blood. 2008 Feb 15;111(4):2158–2162. doi: 10.1182/blood-2007-10-117309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leadbetter EA, Brigl M, Illarionov P, Cohen N, Luteran MC, Pillai S, et al. NK T cells provide lipid antigen-specific cognate help for B cells. Proceedings of the National Academy of Sciences of the United States of America. 2008 Jun 17;105(24):8339–8344. doi: 10.1073/pnas.0801375105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tonti E, Galli G, Malzone C, Abrignani S, Casorati G, Dellabona P. NKT-cell help to B lymphocytes can occur independently of cognate interaction. Blood. 2009 Jan 8;113(22):370–376. doi: 10.1182/blood-2008-06-166249. [DOI] [PubMed] [Google Scholar]
- 12.Noelle RJ, Roy M, Shepherd DM, Stamenkovic I, Ledbetter JA, Aruffo A. A 39-kDa protein on activated helper T cells binds CD40 and transduces the signal for cognate activation of B cells. Proceedings of the National Academy of Sciences of the United States of America. 1992 Jul 15;89(14):6550–6554. doi: 10.1073/pnas.89.14.6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elgueta R, Benson MJ, de Vries VC, Wasiuk A, Guo Y, Noelle RJ. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunological reviews. 2009 May;229(1):152–172. doi: 10.1111/j.1600-065X.2009.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allen RC, Armitage RJ, Conley ME, Rosenblatt H, Jenkins NA, Copeland NG, et al. CD40 ligand gene defects responsible for X-linked hyper-IgM syndrome. Science (New York, NY. 1993 Feb 12;259(5097):990–993. doi: 10.1126/science.7679801. [DOI] [PubMed] [Google Scholar]
- 15.Nishimura T, Kitamura H, Iwakabe K, Yahata T, Ohta A, Sato M, et al. The interface between innate and acquired immunity: glycolipid antigen presentation by CD1d-expressing dendritic cells to NKT cells induces the differentiation of antigen-specific cytotoxic T lymphocytes. Int Immunol. 2000 Jul;12(7):987–994. doi: 10.1093/intimm/12.7.987. [DOI] [PubMed] [Google Scholar]
- 16.Devera TS, Aye LM, Lang GA, Joshi SK, Ballard JD, Lang ML. CD1d-dependent B-cell help by NK-like T cells leads to enhanced and sustained production of Bacillus anthracis lethal toxin-neutralizing antibodies. Infection and immunity. 2010 Apr;78(4):1610–1617. doi: 10.1128/IAI.00002-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Devera TS, Joshi SK, Aye LM, Lang GA, Ballard JD, Lang ML. Regulation of Anthrax Toxin-Specific Antibody Titers by Natural Killer T Cell-Derived IL-4 and IFNgamma. PloS one. 2011;6(8) doi: 10.1371/journal.pone.0023817. e23817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin RM, Brady JL, Lew AM. The need for IgG2c specific antiserum when isotyping antibodies from C57BL/6 and NOD mice. Journal of immunological methods. 1998 Mar 15;212(2):187–192. doi: 10.1016/s0022-1759(98)00015-5. [DOI] [PubMed] [Google Scholar]
- 19.Erickson LD, Durell BG, Vogel LA, O'Connor BP, Cascalho M, Yasui T, et al. Short-circuiting long-lived humoral immunity by the heightened engagement of CD40. The Journal of clinical investigation. 2002 Mar;109(5):613–620. doi: 10.1172/JCI14110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bolduc A, Long E, Stapler D, Cascalho M, Tsubata T, Koni PA, et al. Constitutive CD40L expression on B cells prematurely terminates germinal center response and leads to augmented plasma cell production in T cell areas. Journal of immunology. 2010 Jul 1;185(1):220–230. doi: 10.4049/jimmunol.0901689. [DOI] [PMC free article] [PubMed] [Google Scholar]