Abstract
Objective
Mental fatigue, a poorly understood symptom of sports-related concussion, ideally requires assessment across multiple modalities. Our study aimed to examine mental fatigue effects among ten neurologically normal, athletically active students undergoing typical concussion testing. It is our intention to ultimately address the question whether fatigue effects due to mild traumatic brain injury (mTBI) may become confounded with fatigue effects due to testing effort.
Methods
Fourteen athletically active and neurologically normal volunteers were initially recruited from Penn State University. Self-reported fatigue, neuropsychological performance, and electroencephalographic (EEG) activity were measured throughout the whole testing duration. EEG measures in frequency domain (e.g., relative power of theta, alpha & beta bands) were examined over the course of neuropsychological (NP) test administration.
Results
Predicted fatigue effects over the course of testing included: (a) increased self-reported fatigue; (b) increased errors on the Stroop Interference Test; (c) significantly increased relative power of theta activity during the Stroop Interference Test in frontal-central and parietal regions; and (d) migration of alpha activation from the occipital to anterior (left parietal and pre-central) regions during the Stroop Interference task administered at the beginning compared with the end of testing.
Conclusions
Results supported predictions related to subjective fatigue and cognitive performance and offered partial support for predictions related to EEG activation patterns over the course of administering the NP testing.
Significance
Neurologically intact and athletically active college students demonstrate effects related to fatigue after undergoing a typical sports concussion assessment battery, including an increase in subjectively experienced fatigue, a decrease in cognitive task performance accuracy and associated modulations in EEG activity. This finding should be considered by clinical practitioners while evaluating the symptoms of concussion and making a decision regarding the return-to-sport participation.
Keywords: Mental fatigue, EEG, Stroop Interference Test
1. Introduction
Fatigue is believed to be a gradual and cumulative process associated with a disinclination for effort, reduced efficiency and alertness, and impaired mental performance (Grandjean, 1970, 1988; cf: Lal & Craig, 2001). Mental fatigue is defined as a state of reduced mental alertness that impairs performance. Recently, several studies reported on the feasibility of measuring mental fatigue via participants’ performance, based on EEG data in sustained attention experiments (Makeig & Jung, 1995; Makeig & Inlow, 1993; Lal et al., 2002; Jones & Harrison, 2001). More recently, advanced use of EEG to measure mental fatigue using multi-class support vector machines (SVM) with confidence estimates has been proposed (Shen et al., 2008). Although numerous physiological indicators are available to measure level of alertness, the EEG signal may be one of the most predictive and reliable.
A recent conference on sports concussion in Zurich (2008) emphasized the need to develop a better understanding of fatigue, a common symptom after sports concussion (McCrory et al., 2005; Moser et al., 2007). Guidelines for managing post-concussion fatigue increasingly have highlighted the need for refraining from both physical and mental exertion after concussion to prevent a return of symptoms and to allow brain functioning to recover (Gioia, 2008). Despite its frequency and importance as a symptom of brain injury, no comprehensive study of fatigue relevant to sports concussion has been conducted to date.
Fatigue may result from sleep deprivation, physical exhaustion, or boredom, among other factors. All of these processes share the feature of reduced activation or arousal in the central nervous system, which leads to decreased alertness and increased drowsiness (Lal & Craig, 2001). Fatigue can be characterized by a sensation of weariness, reduction in motivation, attenuation in efficiency, or impairments in vigilance and task performance (Grandjean, 1970; Mosso, 1904); it is a multidimensional construct with subjective, behavioral, and physiological components (Chaudhuri & Behan, 2004; DeLuca, 2007; Grandjean, 1970; Mosso, 1904). A comprehensive characterization of fatigue thus requires the assessment of all three domains.
In the assessment of different functional domains after sports concussion, self-reports are used to characterize the subjective experience of symptoms (Hutchison et al., 2009; McCrea et al., 2003), neuropsychological tests are used to measure cognitive functioning (Belanger & Vanderploeg, 2005), and electroencephalographic activity (EEG) or cerebral blood flow (CBF) are used to characterize changes in neurophysiology (Chen et al., 2004; Dupuis et al., 2000; Nuwer et al., 2005). In sports concussion investigations, as well as other studies of mild traumatic brain injury caused by other factors, fatigue is typically evaluated in only one domain or, less commonly, in two domains simultaneously (Broglio & Puetz, 2008; Stulemeijer et al., 2006).
The only study to date that has evaluated post-concussion symptoms, including fatigue, across multiple modalities did not include a control group of non-injured, neurologically normal individuals (Thompson, 2007). This consideration becomes particularly important given that the neuropsychological test batteries used to identify post-concussion symptoms often require considerable cognitive effort, which can itself be fatiguing. Thus, fatigue effects due to brain injury may become confounded with fatigue effects due to testing effort. Evaluating a non-injured sample to establish a baseline for typical fatigue effects would represent an important first step in developing a better understanding of fatigue as a symptom among concussed athletes.
Characterizing possible fatigue effects that might arise during a neuropsychological concussion assessment battery requires not only the measurement of fatigue across multiple domains but the measurement, within each domain, of aspects relevant to such effects. In the domain of subjectively experienced fatigue, for example, widely used self-report measures for assessing fatigue exist (e.g. Fisk et al., 1994; Krupp et al., 1989; Stein et al., 2001; Whitehead, 2009). However, all of these measures elicit information about the experience of fatigue during daily activities. A better alternative for the assessment of more immediate effects of fatigue on neuropsychological test performance would involve assessing fatigue during the testing session.
Prior research on cognitive functioning has shown that fatigue disrupts lower-level cognitive processes such as alertness as well as higher-level cognitive processes involving executive functioning (Beebe et al., 2003; Durmer & Dinges, 2005). On neuropsychological tests, increasing fatigue is associated with decrements in task performance, including increased error rate and decreased efficiency (Grandjean, 1970; Lal & Craig, 2001). Studies investigating fatigue have used tests requiring sustained attention, such as psychomotor vigilance tasks, as well as tests assessing executive function, like the Stroop test (Jones & Harrison, 2001; Lim & Dinges, 2008).
The Stroop test is especially relevant to investigating fatigue in sports concussion. First, it is widely used in concussion assessment batteries, where has been shown to be sensitive to concussion effects (Rosenbaum et al., 2006). Second, it calls upon both lower- and higher-level cognitive abilities, with the more automatic Word condition requiring the former, and the more effortful Interference condition requiring the latter.
In the physiological domain, changes in brain electrical activity or metabolic rate are often used to monitor fatigue. Although previous research has used a variety of physiological parameters to assess levels of alertness or fatigue, EEG continues to be among the most promising and reliable (Lal & Craig, 2001; Papadelis et al., 2007). The EEG signal represents postsynaptic cortical neuronal potentials, which summate in the cortex and are captured as they propagate through the scalp. This electrical activity in the brain is defined in terms of frequency bands, including delta (0–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), and beta (12–20 Hz). Different aspects of a specific frequency band can be measured, such as amplitude (power) and frequency (cycles per second) within the bandwidth (Stern et al., 2000).
Changes in EEG spectral activity during drowsiness, as well as in response to fluctuations in vigilance, have been long observed (De Gennaro et al., 2007; Loomis et al., 1937; Santamaria & Chiappa, 1987). In studies that have examined EEG patterns, the most consistent findings have been increases in delta and theta rhythms as drowsiness and fatigue build up, especially in frontal and central areas, and decreases in beta rhythms as alertness declines, especially in posterior regions (De Gennaro et al., 2007; Makeig & Jung, 1995; Matousek & Petersen, 1983; Lal & Craig, 2002; Santamaria & Chiappa, 1987; Tinguely et al., 2006). Increased theta activity, in particular, has been correlated with decrements in cognitive task performance (Corsi-Cabrera et al., 2003; Horváth et al., 1976; Makeig & Jung, 1995).
Findings related to alpha rhythms are less consistent. Investigators have found both decreases and increases in alpha activity with the onset of drowsiness and attenuation of alertness (Cajochen et al., 1995; Makeig & Jung, 1995; Torsvall & Akerstedt, 1987). The direction of change appears to depend upon various factors, including scalp recording site and time of measurement. One pattern commonly seen with drowsiness onset is the attenuation or disappearance of alpha activity in occipital areas accompanied or followed by the enhancement of alpha activity in central and frontal areas (Lal & Craig, 2001; Santamaria & Chiappa, 1987).
Incorporating the above considerations, we designed a study examining subjective, cognitive, and physiological indicators of fatigue in a nonconcussed, neurologically normal, and athletically active group of participants undergoing a typical neuropsychological concussion test battery. Our goal was to create a template for studying multimodal fatigue effects in the context of such an assessment that could then be applied to clinical populations, e.g., concussed individuals. We reasoned that, if multimodal fatigue effects were evident in this normal population, further study would be warranted in concussed athletes, given that such effects might be expected to be even more pronounced in these individuals who are known to be especially susceptible to fatigue.
Our study focused on fatigue as the main outcome variable across three domains. To capture the subjective experience of fatigue, we used a self-report measure that asks about fatigue experienced in relation to neuropsychological testing. To assess cognitive or mental fatigue, we used the Stroop test, a neuropsychological task shown to be sensitive to concussion (Rosenbaum et al., 2006). To examine physiological changes related to fatigue, we administered the concussion assessment battery while recording EEG activity simultaneously, including theta, alpha, and beta bandwidths across four regions (frontal, central, parietal, and occipital) in both brain hemispheres.
After undergoing a typical 90-minute assessment battery, we hypothesized that participants would show an increase in subjectively experienced fatigue from pretest to posttest on the self-report measure. On the neuropsychological measure, which was administered at three time points, we hypothesized that participants would show improved or maintained speed on both Stroop conditions due to practice effects, but that they would exhibit decreased accuracy due to fatigue only on the more effortful Interference condition. We further anticipated that participants would show changes in EEG power density or peak frequency from pretest to posttest contingent upon neuropsychological task, bandwidth, and brain region.
First, we expected participants to display increases in theta power in frontal and central regions from pretest to posttest under both Stroop conditions due to the effects of increasing fatigue, but we hypothesized that these effects would be more pronounced for the cognitively demanding Interference condition. Second, we expected participants to display decreases in beta peak frequency in posterior regions due to decreasing vigilance from pretest to posttest under both Stroop conditions, but again we hypothesized that these effects would be more pronounced for the Interference condition. Finally, we expected participants to show a pretest to posttest attenuation of occipital alpha power accompanied by a concomitant enhancement of alpha power in central and frontal areas under both Stroop conditions.
2. Method
2.1. Subjects
Data were collected from 14 athletically active and neurologically normal undergraduate students recruited from the Pennsylvania State University. Average age was 21.4 years (SD = 1.3) and average estimated IQ, based upon Wechsler Test of Adult Reading (WTAR; The Psychological Corporation, 2001) test scores, was 108.9 (SD = 6.4). The sample was 70% male and 30% female. The reported racial/ethnic composition was 70% Caucasian American, 10% African American, 10% Asian American, and 10% mixed. Overall, subject percentages can be presented as 10% multiples, given the usable sample consisted of 10 subjects. All participants had become involved in sports between ages four and eight years old and had maintained their involvement, at either the recreational or collegiate level, as students at the university.
All participants were right-handed (Chapman & Chapman, 1987), and none were taking medications known to affect EEG measurement. We ensured that participants were neurologically normal through a telephone screening questionnaire. Participants were excluded if they reported a prior history of (a) traumatic brain injury, (b) learning disability or attention deficit hyperactivity disorder, (c) alcohol or drug abuse or dependence, (d) a neurological condition, or (e) a psychiatric disorder. Participants were also excluded if English was not their first language. All participants reported that they had slept 7–8 hours the night before testing. Half of the subjects were tested between 10:00am and noon, and the other half were tested between noon and 2:00pm. Subjects were requested to avoid taking any beverages containing caffeine at least 3 hours prior to testing.
2.2. Measures and Apparatus
Beatty Pretest and Posttest Fatigue Rating (Beatty; Beatty, 1996)
The Beatty is a 16-item measure of cognitive fatigue (7 items), physical fatigue (8 items), and overall fatigue (1 item) which is administered before and after a neuropsychological test battery in order to determine how much change has occurred in these domains over the course of testing. For the pretest fatigue measure, items related to cognitive fatigue and physical fatigue are rated on a five-point Likert scale ranging from 1 (not at all) to 5 (a great deal). For the posttest fatigue measure, respondents rate how much difficulty they are having on these same items after testing has finished as compared to before testing started, using a five-point Likert scale ranging from 1 (much less) to 3 (the same) to 5 (much more). Dependent variables were cognitive, physical, and total fatigue raw scores at pretest and posttest.
The Stroop Neuropsychological Screening Test (Stroop; Trenerry et al., 1989)
The Stroop test is a measure of processing speed, attention, and cognitive control which assesses lower-level cognitive abilities, such as sustaining attention, as well as higher-level cognitive abilities, such as initiating or inhibiting responses, shifting between response modes, and monitoring cognitive performance. In the Word condition, participants are asked to read aloud 112 color words that are printed in colored ink. In the Interference condition, they are asked to state aloud the ink color in which the 112 color words are printed. Dependent variables were total time and total errors for the Word and Interference conditions.
2.3. EEG recording
EEG data were acquired using Ag/AgCl electrodes mounted in a 64 channels electrode helmet. This Quik-Cap elastic nylon cap holds 64 Ag-AgCL electrodes from Neuromedical Supplies, Sterling, VA. According to company reference/specs, the configuration of the electrode arrangement in the cap is based on the 10% system. Recording diameter is 6 mm and cavity depth is 5 mm for each electrode. Conducting gel (Electro-gelTM, Electro-Cap International Inc., Eaton, OH) was injected into each electrode to connect the recording surface of the electrode with the scalp. The ground electrode was located 10% anterior to FZ, with linked earlobes serving as references, and impedances were kept below 5 kΩ. It should be noted that there is no ‘gold standard” for reference electrode/sites in EEG studies. It is possible that the type of referencing (i.g., common reference, linked ears, linked mastoid, forehead, virtual reference free montage, etc. commonly reported in EEG literature) may influence topographical distribution of EEG signal. The EEG signals were amplified using a programmable DC coupled broadband SynAmps amplifier (NeuroScan, Inc., El Paso, TX). The EEG data were sampled at 250 Hz and then segmented in 500-ms epochs. Data were digitized and stored offline.
2.4. Procedures
Participants were recruited and screened by phone and informed that they would be reimbursed for their participation ($10/hour). If participants met inclusionary criteria and agreed to participate in the study, a testing appointment was scheduled. Neuropsychological testing and EEG recording were conducted by Ph.D.’s level graduate students.
Testing and recording took place in an electrically shielded experimental room. On the day of testing, the assessor reviewed study protocols with participants and obtained informed consent in accordance with Institutional Review Board guidelines. Participants were then asked to fill out questionnaires while they were connected to the EEG monitor, which took approximately 30 minutes. They were instructed to limit eye, facial, and limb movements during testing and recording sessions. Neuropsychological testing commenced, with EEG activity recorded simultaneously and continuously throughout each test.
The neuropsychological test battery took approximately 90 minutes to complete. Participants were administered a measure of cognitive performance (Stroop) at the beginning, middle, and end of the battery, and a measure of subjective fatigue (Beatty) at the beginning and end of the battery. In addition to these measures, the battery included computerized tests and pencil-and-paper measures typically used in sports concussion: ImPACT, Hopkins Verbal Learning Test-Revised, Brief Visuospatial Memory Test-Revised, Trail Making Tests A & B, and the Computerized Assessment of Response Bias (CARB).
2.5. EEG Data Processing and Analysis
To reduce the number of independent variables (Oken & Chiappa, 1986), we collapsed 62 EEG channels into four topographical regions of interest (frontal, central, parietal, and occipital) in each hemisphere: (a) frontal right (FP2, AF4, F6, F4, F2) and left (FP1, AF3, F5, F3, F1); (b) central right (FC6, FC4, FC2, C6, C4, C2, CP6, CP4, CP2) and left (FC5, FC3, FC1, C5, C3, C1, CP5, CP3, CP1); (c) parietal right (P6, P4, P2, PO6, PO4) and left (P5, P3, P1, PO5, PO3); and (d) occipital right (CB2, O2) and left (CB1, O1). We combined data for left and right hemispheres in each region as we were not predicting differential hemispheric activation. We excluded the temporal area and the delta bandwidth to reduce the chance of including muscle artifacts.
Data were processed offline with EEGLAB 5.03 software (Delorme and Makeig, 2004) using Matlab open source toolbox (Mathworks, Natick, USA). Epochs were baseline normalized and then visually screened for unique, nonstereotypic artifacts such as eye blinks, eye movements, heartbeats, and muscle activity, which were eliminated. To compute power spectra during cognitive testing, Fast Fourier Transform power calculations were performed within each epoch and then averaged across them for each frequency band (theta, alpha, and beta). To ensure homogeneous data processing, relative power for each frequency band was used instead of absolute power.
3. Results
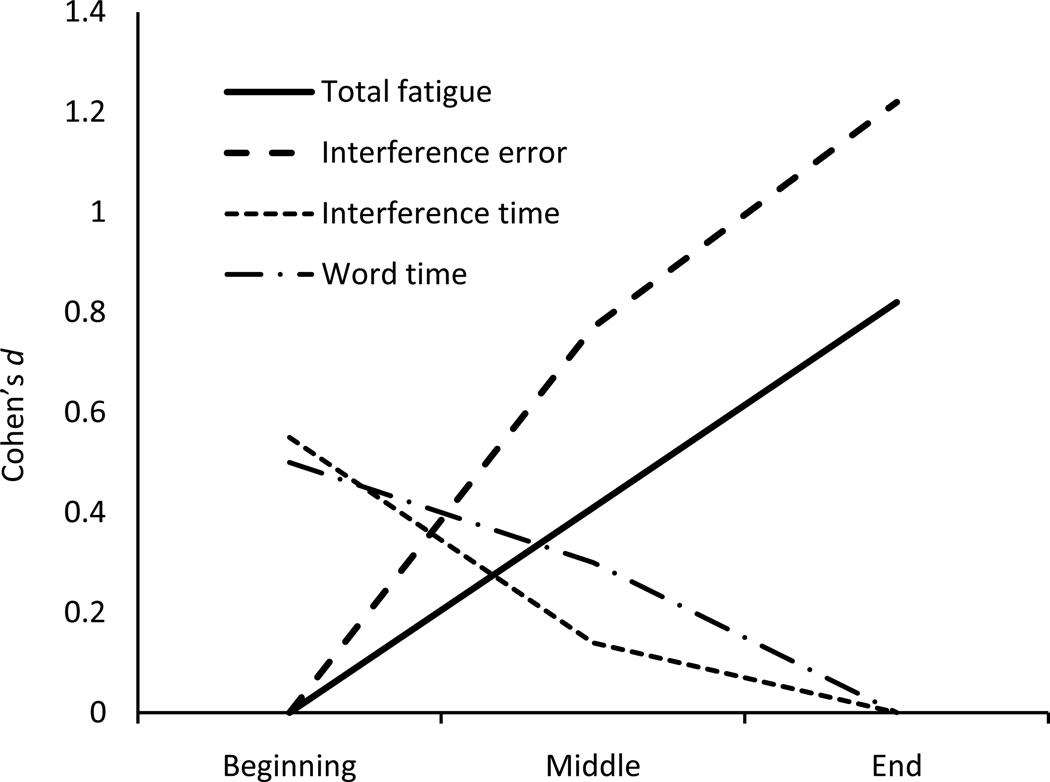
Results from the fatigue rating scales and neuropsychological measures are shown in Table 1 and Figure 1. A standard statistical t-test including degrees of freedom (n=9 in parentheses) values was used to examine the differences in NP data as testing was progressed from pretest to post-test. Consistent with hypotheses, participants reported a significant increase in overall fatigue from pretest to posttest, t(9) = 2.90, p < .05. For the subscales, reported increases in physical fatigue were significant, t(9) = 2.32, p < .05, whereas reported increases in cognitive fatigue approached significance, t(9) = 2.03, p = .07. Also consistent with predictions, participants showed a significant decrease in time required to complete the task administered at the beginning compared with the end of the test battery on both the Stroop Word task, t(9) = 3.15, p < .05, and the Interference task, t(9) = 3.36, p < .01. Furthermore as expected, a significant increase occurred from the administration at the beginning compared with the end in total errors on the Interference task, t(9) = 2.49, p < .05, but not on the Word task, t(9) = 1.40, p > .10.
Table 1.
Reported Fatigue and Neuropsychological Test Scores at Pretest and Posttest
| Measure administered | Pretest | Posttest | Test statistic | |
|---|---|---|---|---|
| M (SD) | M (SD) | t-test | p-value | |
| Self-reported fatigue: | ||||
| Beatty Pretest and Posttest Fatigue Rating | ||||
| Cognitive fatigue | 9.5 (2.8) | 11.4 (4.7) | 2.03 | .073 |
| Physical fatigue | 10.9 (3.3) | 13.7 (4.8) | 2.32 | .046* |
| Total fatigue | 22.6 (6.1) | 29.3 (10.3) | 2.90 | .018* |
| Neuropsychological test performance: | ||||
| Stroop Color-Word (Word) and Color-Word Interference (Interference) | ||||
| Word total time | 50.9 (7.9) | 47.3 (6.4) | 3.15 | .012* |
| Interference total time | 101.4 (25.5) | 88.4 (21.6) | 3.36 | .008** |
| Word total errors | 0.0 (0.0) | 0.3 (0.7) | 1.40 | >.10 |
| Interference total errors | 0.5 (0.7) | 1.9 (1.6) | 2.49 | .034* |
p < .05.
p < .01.
Figure 1.
Cohen’s standardized differences (Cohen’s d) at beginning, middle, and end of test battery for fatigue and neuropsychological performance variables. To show increasing or decreasing trends, Cohen’s d was calculated for Beatty total fatigue (Total fatigue), Stroop Color-Word Interference total errors (Interference error), and total completion time for both Stroop Color-Word (Word time) and Color-Word Interference (Interference time) conditions. Note: To show the increasing trend in total fatigue and in total errors, Cohen’s d was calculated using the formula (mean at other times – mean at beginning)/pooled standard deviation. To show the decreasing trend on total completion times, Cohen’s d was calculated using the formula (mean at other times – mean at end)/pooled standard deviation.
The results from EEG analyses are shown in Figure 2. Consistent with predictions, there were significant changes in theta power activation during the Stroop Interference condition administered at the beginning compared with the end of the testing session in frontal, F(2, 18) = 13.78, p < .01, central, F(2, 18) = 7.30, p < .01, and parietal, F(1.4, 12.5) = 5.16, p < .05 areas. Effect sizes were greatest in frontal areas (η2 = .61) and declined across central (η2 = .45) and parietal (η2 = .36) areas. As shown in Figure 2, there was a significant increase of theta power during the Stroop Interference condition as the testing progressed. Specifically, this theta increment was obvious initially at central areas with further diffusion to frontal electrode sites. This finding is consistent with previous reports suggesting increase of theta power as a function of progressive mental fatigue (Makeig & Inlow, 1993). The effect across time for theta power was not significant during the Stroop Word condition.
Figure 2.
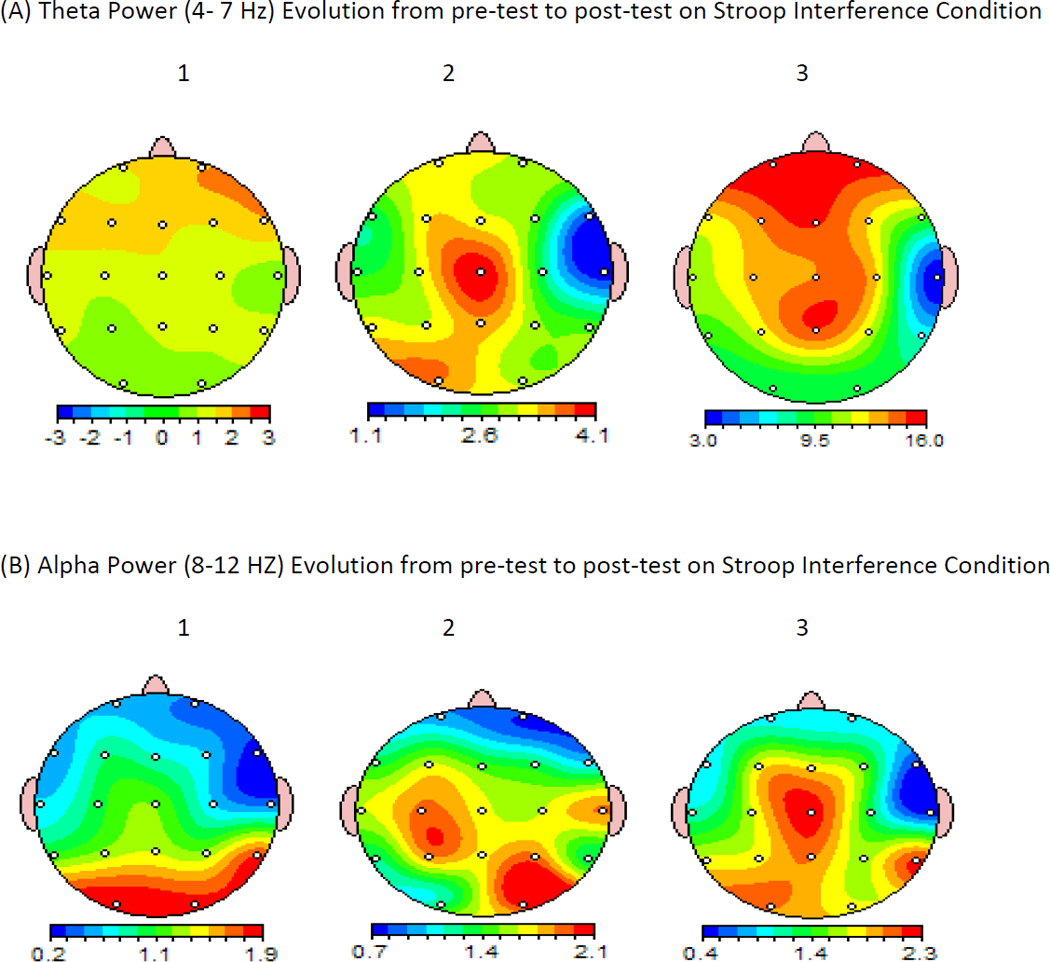
Topographical distribution of the Grand Averaged Evolution of theta (4–7 Hz) and alpha (8–12 Hz) EEG power during the Stroop Interference task administered at the beginning (1), middle (2), and at the end (3) of the testing battery. Note a progressive enhancement of theta power at frontal-central and parietal areas (A). Also notice a migration of alpha power from predominantly occipital areas during the Stroop test administered at the beginning of the battery, to anterior (left parietal and pre-central) regions when the Stroop was administered at the end of the battery (B). The maps were calculated using EEGLab adjusted for 19 footage sites on the scalp according 10% system.
For beta peak frequency, significant linear trends (p < .05) emerged in central and parietal regions for the Stroop Interference condition associated small effect sizes (η2 = .41 and .32, respectively). Follow-up t tests showed significant decreases in beta peak frequency in both parietal, t(9) = 2.42, p < .05, and central, t(9) = 2.69, p < .05, areas from pretest to posttest. No significant changes in beta power occurred over time during the Stroop Word condition.
Alpha power showed a significant change in central, F(1.3, 11.4) = 8.97, p < .01, and parietal, F(1.3, 11.4) = 22.91, p < .01, regions during the Stroop Interference condition administered at the three time points, with a correspondingly large effect size in the parietal area, η2 = .70, and a moderate effect size in the central area, η2 = .50. Significant linear trends (p<.05) also appeared in frontal and occipital areas on this same task, but with somewhat smaller effect sizes (η2 = .34 and .30 respectively). Follow-up t tests showed increases in alpha power in frontal, t(9) = 2.46, p < .05, and occipital, t(9) = 2.58, p < .05, regions during the Stroop Interference condition administered at the beginning compared with the end of the test battery. As displayed in Figure 2, there was a shift of topographical distribution of alpha power from predominantly occipital areas to parietal and then to central areas with localized focus at the Cz electrode site. A similar pattern of alpha shift from posterior to anterior regions was previously suggested as an index of mental fatigue (Trejo et al., 1995). No significant increases in alpha power occurred for any regions on the Stroop Word condition.
4. Discussion
In this study, we examined subjective, cognitive, and physiological indicators of fatigue in a nonconcussed, neurologically normal sample of athletically active students undergoing a typical neuropsychological concussion assessment battery in conjunction with EEG recording. In support of the first hypothesis, participants reported a significant increase in subjectively experienced fatigue from pretest to posttest. In support of the second hypothesis, participants demonstrated significantly faster performance under both conditions of the Stroop task from pretest to posttest, but they exhibited significantly decreased accuracy on the more effortful Interference condition.
Predictions regarding EEG activity were partially supported. As predicted in the first hypothesis, participants showed significant increases in theta power in frontal and central regions from pretest to posttest under the more effortful Stroop Interference condition, with effect sizes increasing linearly from posterior to anterior regions. For the less effortful Word condition, theta power also increased significantly from pretest to posttest but only in frontal regions, and the effect size was less marked than for the Interference condition. As predicted in the second hypothesis, participants displayed significant decreases in beta peak frequency in posterior (parietal) regions from pretest to posttest. However, these changes were apparent only upon examining linear trends and follow-up tests, and they occurred only under the more effortful Interference condition and not under the less effortful Word condition.
Finally, as predicted in the third hypothesis, participants showed significant increases in alpha power in central areas but only under the Stroop Interference condition. Although they also displayed increases in frontal areas, these changes emerged only upon examining linear trends and follow-up tests. Furthermore, contrary to prediction, the greatest increases in alpha power appeared in parietal areas. Also contrary to prediction, participants actually showed a significant, albeit slight, increase in alpha power in occipital areas rather than the predicted pretest to posttest attenuation.
In this study we were concerned with decrement in cognitive functions arising from sustained mental work in a controlled laboratory experiment. We refer to these decrements as cognitive fatigue to distinguish them from effects of sleepiness, motivation, or practice effects (learning). We accept the definition of cognitive fatigue as the unwillingness of alert, motivated participants to continue performance of mental work (Trejo et al., 1995).
It may be argued that 90 minutes of neurocognitive assessment may not be sufficient to induce mental fatigue. However, it should be noted that in another EEG study, evidence of fatigue from sustained mental effort (sustained information processing for a 70-minute intelligence test) induced EEG changes consistent with mental fatigue (Smith et al., 1999). Specifically, theta power increased most robustly at Fz electrode site. No changes were observed in beta power band. However, Kiroy et al. (1996) found an increase in both theta and beta power in participants who performed mental tasks, something that may reflect compensatory mechanisms for their low vigilance level.
A number of EEG studies have demonstrated increased theta power after mental effort, something that may reflect alteration of vigilance. However, basing such an interpretation on one EEG variable is unlikely to be as reliable as detecting simultaneous changes that occur at other frequency bands, as in our study. We added location of alteration concomitant with cognitive performance measures (Lal et al., 2003). Therefore, we analyzed EEG at various frequency bands, spatial locations, and also included behavioral data and subjective reports.
Alteration of theta should also be considered as a function of task difficulty. For example, increased frontal theta has been observed under conditions requiring more focused attention (Gevins et al., 1979) or time pressure (Slobounov et al., 2000). The Fm theta increases as a function of task difficulty and practice (Gevins et al., 1997). In essence, an Fm-theta peak of 6.5Hz is the equivalent of the power of frontal midline (Fz, CZ) theta, which was observed in a number of previous EEG studies. Specifically, an increase in visuomotor task complexity systematically induced the increase of Fm-theta power (Slobounov et al., 2000), similar to other cognitive (Smith et al., 1999) and visuomotor (Karni et al., 1998) tasks. Overall, it was suggested that midline Fm-theta might be a good index of task complexity, which is related with changes in focused attention during task performance. It should be noted, however, that practice should induce performance enhancement, unlike what occurred with our results, so changes in EEG were more likely to reflect fatigue, not practice effects.
An inverse relationship between alpha and task difficulty has been reported, and interpreted as alpha sensitivity to task difficulty, cortical idling or disengagement (Buzsaki, 2006). The subjective reports of increased fatigue available in our study, along with increased errors on the Stroop Interference task over time allow for greater clarity in interpretation. Given these concomitant reports it seems most likely that the increased alpha in anterior regions that occurred on the Stroop from the beginning to the end of the battery would seem to be most likely due to increased fatigue.
The degree of convergence between initial hypotheses and experimental results supports several conclusions. First, neurologically intact and athletically active college students demonstrate effects related to fatigue after undergoing a typical sports concussion assessment battery, including an increase in subjectively experienced fatigue and a decrease in cognitive task performance accuracy, as has been found in other studies (Beebe et al., 2003; Grandjean, 1970; Lim & Dinges, 2008). Also as shown in prior work, changes in EEG activity associated with increasing fatigue and decreasing alertness appear after undergoing such a battery, including an increase in frontal theta power and a concomitant decrease in posterior beta peak frequency (Corsi-Cabrera et al., 2003; Horváth et al., 1976; Lal & Craig, 2002; Makeig & Jung, 1995). Although participants reported a slightly greater increase in physical than cognitive fatigue from pretest to posttest, this difference was extremely small and not likely to be meaningful.
A second conclusion from our work is that the Stroop Interference condition, like other executive functioning tasks, appears to be differentially sensitive to fatigue effects (Beebe et al., 2003; Jones & Harrison, 2001). Performance accuracy declined significantly from pretest to posttest only under the more effortful Stroop Interference condition but not under the less effortful Word condition. The differential effects of fatigue on these two tasks were further borne out by different EEG activation patterns during them. Under the Interference condition, theta power, which is associated with fatigue, increased to a significantly greater degree in frontal, central, and even parietal regions. On the Word condition, however, it increased only in frontal areas and to a much lesser degree.
A third inference from our study relates to the decrease in beta peak frequency in parietal regions that emerged on the Interference task but not on the Word task. Although contrary to our prediction, this finding is consistent with the idea that the Interference task is more sensitive to effects associated with decreasing alertness. Whereas the Word task is overlearned and largely automatic, and thus may be less susceptible to declines in vigilance, the Interference task is both novel and cognitively demanding, and thus may be more susceptible to decreasing alertness. These findings may have particular relevance when attempting to use neuropsychological tasks along with EEG recording to better characterize fatigue as a symptom in sports concussion.
Differences in task-related demands might also explain the Stroop-related activation patterns for alpha power, which were the least supported of our hypotheses. Rather than decreasing in occipital areas while simultaneously or subsequently increasing in frontal and central areas, as we predicted for our participants and as commonly occurs with the onset of fatigue (Lal & Craig, 2001; Santamaria & Chiappa, 19870), alpha power increased across all four topographical regions, with the greatest increases in parietal followed by central areas. However, these increases occurred only under the Stroop Interference condition.
In conclusion, the results of our study offer evidence that nonconcussed and athletically active students experience the effects of increased fatigue in multiple modalities, including subjective, cognitive, and physiological domains, over the course of a sports concussion assessment battery. Fatigue effects should thus be taken into account--either controlled for or further explored--when administering similar test batteries to concussed athletes. With this examination, we hope to provide an initial reference point for interpreting fatigue effects in future multimodal studies examining this symptom in concussed college athletes.
Highlights.
Prolonged administration of neuropsychological testing induces increased self-reported and performance-related fatigue.
Modulation of EEG activity during neuropsychological testing is an index of fatigue rather than a training effect.
Fatigue effect should be taken into consideration during administration of a neuropsychological test battery in clinical populations.
Acknowledgements
We would like to thank Dr. Cao Cheng for EEG data analysis. This research was partly supported by NIH, NIND grant R01 NS056227-02 “Identification of Athletes at Risk for Traumatic Brain Injury”, awarded to Dr. Slobounov.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beebe DW, Groesz L, Wells C, Nichols A, McGee K. The neuropsychological effects of obstructive sleep apnea: a meta-analysis of norm-referenced and case-controlled data. Sleep. 2003;26:298–307. doi: 10.1093/sleep/26.3.298. [DOI] [PubMed] [Google Scholar]
- Bellanger HG, Vanderploeg RD. The neuropsychological impact of sports-related concussion: A meta-analysis. J Intern Neuropsych Soc. 2005;11:345–357. doi: 10.1017/s1355617705050411. [DOI] [PubMed] [Google Scholar]
- Broglio SP, Puetz TW. The effect of sport concussion on neurocognitive function, self-report symptoms and postural control: A meta-analysis. Sports Med. 2008;3:53–67. doi: 10.2165/00007256-200838010-00005. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Rhythms of the brain, Cycle 12" coupling of systems by oscillations. Oxford University Press; 2006. p. 341. [Google Scholar]
- Cajochen C, Brunner DP, Kräuchi K, Graw P, Wirz-Justice A. Power density in theta/alpha frequencies of the waking EEG progressively increases during sustained wakefulness. Sleep. 1995;18:890–894. doi: 10.1093/sleep/18.10.890. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP. The measurement of handedness. Brain Cogn. 1987;6:175–183. doi: 10.1016/0278-2626(87)90118-7. [DOI] [PubMed] [Google Scholar]
- Chaudhuri A, Behan PO. Fatigue in neurological disorders. Lancet. 2004;363:978–988. doi: 10.1016/S0140-6736(04)15794-2. [DOI] [PubMed] [Google Scholar]
- Chen JK, Johnston KM, Frey S, Petrides M, Worsley K, Ptito A. Functional abnormalities in symptomatic concussed athletes: An fMRI study. NeuroImage. 2004;22:68–82. doi: 10.1016/j.neuroimage.2003.12.032. [DOI] [PubMed] [Google Scholar]
- Corsi-Cabrera M, Sánchez AI, del-Río-Portilla Y, Villanueva Y, Pérez-Garcia E. Effect of 38 hours of total sleep deprivation on the waking EEG in women: sex differences. Int J Psychophysiol. 2003;50:213–224. doi: 10.1016/s0167-8760(03)00168-5. [DOI] [PubMed] [Google Scholar]
- De Gennaro L, Marzano C, Veniero D, Moroni F, Fratello F, Curcio G, et al. Neurophysiological correlates of sleepiness: a combined TMS and EEG study. NeuroImage. 2007;33:1277–1287. doi: 10.1016/j.neuroimage.2007.04.013. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Meth. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- DeLuca J. The Neuropsychology of fatigue and its influence in clinical populations; Symposium conducted at the National Academy of Neuropsychology 27th Annual Conference Scottsdale; AZ, USA. 2007. [Google Scholar]
- Dupuis F, Johnston KM, Lavoie M, Lepore F, Lassonde M. Concussions in athletes produce brain dysfunction as revealed by event-related potentials. Neuroreport. 2000;11:4087–4092. doi: 10.1097/00001756-200012180-00035. [DOI] [PubMed] [Google Scholar]
- Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2005;25:117–129. doi: 10.1055/s-2005-867080. [DOI] [PubMed] [Google Scholar]
- Fisk JD, Pontegract A, Ritvo PG, Archibald CJ, Murray TJ. The impact of fatigue on patients with multiple sclerosis. Can J Neurol Sci. 1994;21:9–14. [PubMed] [Google Scholar]
- Gevins A, Smith ME, McEvoy L, Yu D. High resolution EEG mapping of cortical activation related to working memory: effects of task difficulty, and practice. Cereb. Cortex. 1997;7:374–385. doi: 10.1093/cercor/7.4.374. [DOI] [PubMed] [Google Scholar]
- Gioia G. Concussion management on the field and return to play decisions: A new approach; Symposium conducted at the Third International Conference on Concussion in Sport; Zurich. 2008. [Google Scholar]
- Grandjean EP. Fatigue. Am Indust Hyg Ass J. 1970;31:401–411. doi: 10.1080/0002889708506267. [DOI] [PubMed] [Google Scholar]
- Horváth M, Frantik E, Kopriva K, Meissner J. EEG theta activity increase coinciding with performance decrement in a monotonous task. Activitas Nervosa Superior. 1976;18:207–210. [PubMed] [Google Scholar]
- Hutchison M, Mainwaring LM, Comper P, Richards DW, Bisschop SM. Differential emotional responses of varsity athletes to concussion and musculoskeletal injuries. Clin J Sports Med. 2009;19:13–19. doi: 10.1097/JSM.0b013e318190ba06. [DOI] [PubMed] [Google Scholar]
- Jones K, Harrison Y. Frontal lobe function, sleep loss and fragmented sleep. Sleep Med Rev. 2001;5:463–475. doi: 10.1053/smrv.2001.0203. [DOI] [PubMed] [Google Scholar]
- Karni A, Meyer G, Rey-Hipolito C, Jezzard P, Adams R, Turner R. The acquisition of skilled motor performance: fast and slow experience-driven changes in primary motor cortex. Proc. Natl. Acad. Sci. 1998;3:861–868. doi: 10.1073/pnas.95.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiroy VN, Warsawskaya LV, Voynov VB. EEG after prolong mental activity. Int J. Neurosci. 1996;1–2:31–43. doi: 10.3109/00207459608986349. [DOI] [PubMed] [Google Scholar]
- Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The Fatigue Severity Scale: Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch of Neurol. 1989;46:1121–1123. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- Lal SKL, Craig A. A critical review of the psychophysiology of driver fatigue. Biol Psych. 2001;55:173–194. doi: 10.1016/s0301-0511(00)00085-5. [DOI] [PubMed] [Google Scholar]
- Lal SKL, Craig A. Driver fatigue: Electroencephalography and psychological assessment. Psychophysiol. 2002;39:313–321. doi: 10.1017/s0048577201393095. [DOI] [PubMed] [Google Scholar]
- Lim J, Dinges DF. Sleep deprivation and vigilant attention. Ann NY Acad Sci. 2008;1129:305–322. doi: 10.1196/annals.1417.002. [DOI] [PubMed] [Google Scholar]
- Loomis AL, Newton H, Hobart GA. Cerebral states during sleep, as studied by human brain potentials. J Exp Psych. 1937;21:127–144. [Google Scholar]
- Makeig S, Jung TP. Changes in alertness are a principal component of variance in the EEG spectrum. NeuroReport. 1995;7:213–216. [PubMed] [Google Scholar]
- Makeig S, Inlow M. Lapses in alertness: coherence of fluctuations in performance and EEG spectrum. Electroencephalogr Clin Neurophysiol. 1993;86:23–25. doi: 10.1016/0013-4694(93)90064-3. [DOI] [PubMed] [Google Scholar]
- Markand ON. Alpha rhythms. J Clin Neurophysiol. 1990;7:163–189. doi: 10.1097/00004691-199004000-00003. [DOI] [PubMed] [Google Scholar]
- Matousek M, Petersen I. A method for assessing alertness fluctuations from EEG spectra. Electroencephalogr Clin Neurophysiol. 1983;55:108–113. doi: 10.1016/0013-4694(83)90154-2. [DOI] [PubMed] [Google Scholar]
- McCrea M, Guskiewiez KM, Marshall SW, Varr W, Randolph C, Cantu RC, et al. Acute effects and recovery time following concussion in collegiate football players: The NCAA Concussion Study. J Amer Med Ass. 2003;290:2556–2563. doi: 10.1001/jama.290.19.2556. [DOI] [PubMed] [Google Scholar]
- McCrory P, Johnston K, Meeuwisse W, Aubry M, Cantu R, Dvorak J, et al. Summary and agreement statement of the 2nd International Conference on Concussion in Sport, Prague 2004; Brit J Sports Med; 2005. pp. 78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosso A, Drummon TrM, Drummond WB. New York: G. P. Putnam’s Sons; 1904. Available from Google Books web site http://books.google.com/books. [Google Scholar]
- Moser RS, Iverson GL, Echemendia RJ, Lovell MR, Schatz P, Webbe FM, et al. Neuropsychological evaluation in the diagnosis and management of sports-related concussion. Arch Clin Neuropsychol. 2007;22:909–916. doi: 10.1016/j.acn.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Nuwer MR, Hovda DA, Schrader LM, Vespa PM. Routine and quantitative EEG in mild traumatic brain injury. Clin Neurophysiol. 2005;116:2001–2025. doi: 10.1016/j.clinph.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Oken BS, Chiappa KH. Statistical issues concerning computerized analysis of brainwave topography. Ann Neurol. 1986;19:493–497. doi: 10.1002/ana.410190511. [DOI] [PubMed] [Google Scholar]
- Papadelis C, Chen Z, Kourtidou-Papadeli C, Bamidis PD, Chouvarda I, Bekiaris E, et al. Monitoring sleepiness with on-board electrophysiological recordings for preventing sleep-deprived traffic accidents. Clin Neurophysiol. 2007;118:1906–1922. doi: 10.1016/j.clinph.2007.04.031. [DOI] [PubMed] [Google Scholar]
- The Psychological Corporation. Wechsler Test of Adult Reading. San Antonio, TX: Harcourt Assessment; 2001. [Google Scholar]
- Rosenbaum AM, Arnett PA, Bailey CM, Echemendia RJ. Neuropsychological assessment of sports-related concussion: Measuring clinically significant change. In: Slobounov SM, Sebastianelli WJ, editors. Foundations of Sports-Related Brain Injuries. New York: Springer; 2006. pp. 137–169. [Google Scholar]
- Santamaria J, Chiappa KH. The EEG of drowsiness in normal adults. J Clin Neurophysiol. 1987;4:327–382. doi: 10.1097/00004691-198710000-00002. [DOI] [PubMed] [Google Scholar]
- Shen KQ, Li XP, Ong CJ, Shao SY, Wilder-Smith EP. EEG based mental fatigue using multi-class support vector machines with confidence estimate. Clin Neurophysiol. 2008;119/7:1524–1533. doi: 10.1016/j.clinph.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Slobounov SM, Fukada K, Simon R, Rearick M, Ray W. Neurophysiological and behavioral indices of time pressure effects on visiomotor task performance. Cogn Brain Res. 2000;9:287–298. doi: 10.1016/s0926-6410(00)00009-4. [DOI] [PubMed] [Google Scholar]
- Smith ME, McEvoy LK, Gevins A. Neurophysiological indices of strategy development and skill acquisition. Cogn. Brain. Res. 1999;7:389–404. doi: 10.1016/s0926-6410(98)00043-3. [DOI] [PubMed] [Google Scholar]
- Stein KD, Martin SC, Hann DH, Jacobsen PB. A multidimensional assessment of fatigue for use with cancer patients. Cancer Practice. 2001;6:143–152. doi: 10.1046/j.1523-5394.1998.006003143.x. [DOI] [PubMed] [Google Scholar]
- Stern RM, Ray WJ, Quigley KS. Psychophysiological recording. New York: Oxford University Press; 2000. [Google Scholar]
- Stulemeijer M, van der Werf S, Eleijenberg G, Biert J, Brauer J, Vos PE. Recovery from mild traumatic brain injury: A focus on fatigue. J Neurol. 2006;253:1041–1047. doi: 10.1007/s00415-006-0156-5. [DOI] [PubMed] [Google Scholar]
- Thompson J. Dissertation Abstracts International. Vol. 68. 2007. Concussions in sport: Investigation of assessment measures and functional deficits (Doctoral dissertation Penn State University; p. 3000. [Google Scholar]
- Tinguely G, Finelli LA, Landolt HP, Borbély AA, Achermann P. Functional EEG topography in sleep and waking: State-dependent and state-independent features. Neuroimage. 2006;32:283–292. doi: 10.1016/j.neuroimage.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Torsvall L, Akerstedt T. Sleepiness on the job: Continuously measured EEG changes in train drivers. Electroencephalogr Clin Neurophysiol. 1987;66:502–511. doi: 10.1016/0013-4694(87)90096-4. [DOI] [PubMed] [Google Scholar]
- Trejo LJ, Kramer AF, Arnold A. Event-related potentials as indices of display-monitoring performance. Biol Psych. 1995;40:33–71. doi: 10.1016/0301-0511(95)05103-1. [DOI] [PubMed] [Google Scholar]
- Trenerry MR, Crosson B, DeBoe J, Leber WR. Stroop Neuropsychological Screening Test. Odessa, FL: Psychological Assessment Resources; 1989. [Google Scholar]
- Whitehead L. The measurement of fatigue in chronic illness: A systematic review of unidimensional and multidimensional fatigue measures. J Pain Sympt Manag. 2009;37:108–128. doi: 10.1016/j.jpainsymman.2007.08.019. [DOI] [PubMed] [Google Scholar]