Abstract
Objectives
Reduced numbers and activity of circulating progenitor cells are associated with aging and have been linked with coronary artery disease. To determine the impact of aging and atherosclerotic disease on the chemotaxic activity of bone marrow derived cells (BMCs), we examined CXCR4 surface expression on BMCs from aged and atherosclerotic mice.
Methods
CXCR4 expression and cellular mobility were compared between BMCs of young (6-week old) ApoE null mice (ApoE−/−) and aged ApoE−/− mice that had been fed with a high-fat, high-cholesterol diet for 6-months.
Results
Age and atherosclerosis correlated with significantly lower surface expression of CXCR4 that was less inducible by calcium. The impaired calcium response was associated with defective calcium influx and was partially recovered by treatment with the calcium ionophore ionomycin. ApoE−/− mice fed high fat diet for 6-months had defective CXCR4 expression and SDF-1 regulation that is equivalent to that of 24-month old wild type mice. BMCs from aged, atherogenic ApoE−/− mice also displayed defective homing to SDF-1, and the animals had lower serum and bone marrow levels of SDF-1.
Conclusion
Evolution of atherosclerosis in ApoE−/− mice is paralleled by progressive loss of mobility of BMCs with reductions of CXCR4 expression, and reduced levels of SDF-1 in both serum and bone marrow. These changes mute the homing capability of BMCs and may contribute to the progression of atherosclerosis in this model.
Keywords: CXCR4, SDF-1, age, bone marrow, progenitor cells, viability, migration, homing, engraftment
Introduction
Age is an independent risk factor for cardiovascular disease and has been linked with reduced availability and function of progenitor cells that may constitute part of the risk1–3. Defective progenitor cell number and function may also be one reason why autologous cell therapy has produced modest improvements of patient symptoms in clinical trials. In patients with severe coronary artery disease (CAD), the colony-forming capacity and migratory activity of BM-derived endothelial progenitor cells (EPCs) are markedly reduced.3–5 In models of hind limb ischemia, less neovascularization is observed when bone marrow derived cells (BMCs) from CAD patients are transplanted relative to healthy controls.4 Hematopoietic stem cells (HSCs) from aged mice are less efficient at homing and engrafting compared with those from young mice.6 The molecular mechanisms of progenitor cell aging and loss of therapeutic potential are not known.
CXCR4 and its ligand SDF-1 regulate engraftment of hematopoietic cells and endothelial progenitors.7, 8 The SDF-1/CXCR4 interaction is essential for activation of cellular signaling pathways that result in the mobilization of progenitor cells from the bone marrow and homing to sites of injury in peripheral tissues.9, 10 CXCR4+ BMCs exhibit an increased therapeutic potential.11 Inactivation of CXCR4 by neutralizing antibodies has been shown to impair stem/progenitor cell engraftment in the bone marrow.12 Accumulating evidence indicates that the level of CXCR4 surface expression on BMCs determines the efficiency of homing and the subsequent angiogenic response of target tissues.13, 14 Over-expression of CXCR4 in mesenchymal stem cells (MSCs) or CD34+ cells enhances the migration of these cells towards SDF-1 in vitro15 and increases the efficiency of bone marrow transplant in vivo16.
We recently reported that BMCs from aged mice express less surface CXCR4 than BMCs from young mice.17 Since ApoE−/− mice spontaneously develop hypercholesterolemia and atherosclerotic lesions and have significantly shorter lifespan than wild type mice18, we hypothesize that atherogenic ApoE−/− mice have earlier and more severe CXCR4/SDF-1 defects than their wild type (WT) counterparts. Our rationale for the present studies is that atherosclerosis generates an aging phenotype in ApoE−/− mice and this may be associated with correspondingly reduced numbers and functions of CXCR4-positive BM progenitors. It is known that ApoE−/− mice fed with high fat high cholesterol diet develop extensive atherosclerotic plaque, and their lifespan is reduced by more than a year (~50%) relative to non-atherogenic mice. Therefore we compared CXCR4 expression levels in total BMCs from aged ApoE−/− fed high fat/cholesterol or normal chow diets, and young ApoE−/− mice. Here we confirm that CXCR4 expression on BMCs from old ApoE−/− mice is indeed significantly lower and less inducible than that of young ApoE−/− mice without atherosclerosis. An early loss of these functions in ApoE−/− mice fed high fat also correlated with an atherosclerotic phenotype. BMCs from aged ApoE−/− with advanced atherosclerosis displayed impaired responses to SDF-1 in vitro and had a significantly reduced homing capability in vivo. Our results suggest a novel molecular/cellular mechanism that may account in part for the reduced therapeutic efficacy of autologous stems in aged CAD patients.
Materials and Methods
BMCs harvesting and cultured in different condition
All animals were treated in accordance with the NIH Guide for the Care and Use of Laboratory Animals, and the procedures were approved by the Institutional Animal Care and Use Committee. Male WT (C56/BL6) and ApoE−/− mice were from Jackson Laboratory (Bar Harbor, Maine). Young ApoE−/− mice (4–6 weeks) were fed normal chow; aged (7–8 months) ApoE−/− mice were fed a high fat/high cholesterol diet (HF) (Harlan-Teklad, Tampa, FL) beginning at 1 month old. Control aged ApoE−/− mice were also 7–8 months old but maintained on normal chow (NC). Bone marrow harvesting was as described previously.14 After washed with phosphate buffered saline (PBS), BMCs were incubated in PBS, PBS with 1mM CaCl2 or Dulbecco’s Modified Eagle Medium (DMEM, Invitrogen) with/without 10% fetal bovine serum (FBS) (HyClone, Logan, Utah) for 4 hours at 37°C.
FACS analysis
Surface CXCR4 and other markers (Flk1, CD34, lineage, Sca-1) on BMCs were analyzed using fluorescence activated cell sorting (FACS) as described previously.17 All antibodies were from BD Phamingen.
Calcium influx measurement
Calcium influx was analyzed as described previously.17 During flow cytometry analysis, fluorescence of samples was monitored for a 30-second baseline, then sample aspiration was briefly paused, and 2 μl PBS, or CaCl2 (final concentration 1mM), or CaCl2 plus Ionomycin (final concentration 10μM) in Ca2+ flux assay buffer were added into the samples. Ca2+ influx was determined by measuring the change in green fluorescence intensity of the cells over time. The Mean Fluorescence intensity (MFI) of the first 15 second histogram before adding CaCl2 was used to normalize the MFI of every 15 second histogram after adding CaCl2 and plotted as a time course of relative MFI.
Real time RT-PCR
CXCR4 mRNA was quantified by real time-PCR and normalized to hypoxanthine phosphoribosyl transferase 1 (HPRT1) mRNA levels as described.14
Viability and apoptosis of BMCs
BMC viability after 4-hr calcium treatment was quantified using trypan blue and annexin V staining. After treatments, trypan blue staining was performed using a Beckman Coulter vi-cell™ XR cell viability analyzer.
To determine viability of BMCs at conditions of hypoxia and nutrition depletion, BMCs were treated with PBS±1mM CaCl2 for 4hrs, and cultured under hypoxia (0.5% oxygen) in serum-free DMEM supplemented with or without 100ng/ml SDF-1α for 20 hours at 37°C. Apoptosis was quantified after the different treatments using annexin V-FITC kit (ApoAlert®, Clonetech) as described by the manufacturer. Annexin-positive and propidium iodine (PI)-negative cells were considered to be early apoptotic, annexin V and PI double positive cells were considered as late apoptotic and necrotic.
Cell-cell adhesion assay
Human Umbilical Vein Endothelial Cells (HUVEC) (5×104 cells per well) were seeded onto 96-well gelatin-coated plates and cultured for 24 hours. SDF-1α (400ng/ml in 200μl EGM) was added to the HUVEC monolayer and cultured for 30 minutes. After aspiration of the SDF-1 containing medium, BMCs were seeded on top of HUVECs and cultured for 3-hour. BMCs were pretreated with calcium as described above and then exposed to CellTracker™ Green CMFDA (Invitrogen) dyes (2 μM) for 30 min prior to addition to HUVEC monolayers. After the incubation, plates were gently washed, fluorescence representing the attached BMCs on each well was measured with a plate reader, and micrographs were obtained with an inverted fluorescence microscope.
Vertical Collagen Gel Invasion Assay
To measure trans-endothelial migration of BMCs, a vertical collagen gel invasion assay was used as described.19 Briefly, two cover slides were placed 1.5 mm apart facing one another and three sides of the slides were sealed with paraffin. A layer (50 μL) of collagen gel (Millipore) containing 200ng/ml SDF-1α and then second layer (200 μL) of collagen gel alone were poured to form a SDF-1 gradient. HUVECs (1× 105 cells) stained with Red Fluorescence (CellTrack™ Red CMPTX, Invitrogen) were seeded on top of the gel, followed with the testing 4×105 BMCs stained with CellTrack™ Green Fluorescence. After 24-hour culture, the maximum distance of BMCs migration downward from the upper margin of the collagen gel was measured to represent the capacity of invasion.
Bone Marrow Engraftment in vivo
BMC from young and old ApoE−/− mice were harvested, stained with Red Fluorescence (12.5 μM, Invitrogen), and pretreated with PBS alone or PBS + CaCl2 for 4 hr as described above. The cells (6×106 per mouse) were injected into the recipient old and young ApoE−/− mice through the tail vein 24 hrs after the recipients were irradiated at 550cGy. Mice were sacrificed 3 days later. BMCs were recovered from femurs and tibias. Engrafted BMCs were measured by flow cytometry of the recovered BMCs or by direct count of fluorescent cells on the sections of recovered bones. The BMC surface CXCR4 expression on the recovered BMC was determined by flow cytometry as described above.
SDF-1α measurement by ELISA
SDF-1α levels in bone marrow and serum were measured using an SDF-1α Immunoassay Kit from R&D System. Femurs and tibias were frozen in liquid nitrogen, grinded and homogenized in 400 μl RIPA lysis buffer (Sigma). Protein extracts (500μg) or 50μl serum were assayed according to the manufacturer’s recommended protocol.
Statistics
Results are expressed as mean ± standard deviation (SD). Statistically significant differences between groups were compared with one-way analysis of variance (ANOVA) for multi-groups using GraphPad (San Diego, Calif) or 2-tailed unpaired Student t-test for 2 groups only. Significance means P <0.05.
Results
CXCR4 expression of old vs. young ApoE−/− mice
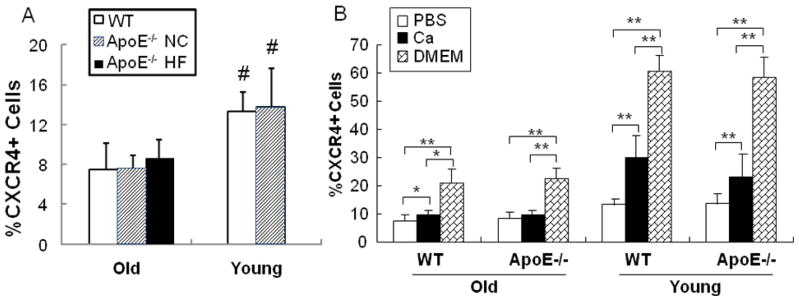
We reported previously that BMCs from 24-month (old) WT mice express less surface CXCR4 in comparison with 2-month old (young) mice.17 Here we compared CXCR4 expression levels in total BMCs from aged ApoE−/− fed high fat/cholesterol or normal chow diets, and young ApoE−/− mice. Young ApoE−/− mice have no atherosclerosis whereas the aortas of the aged ApoE−/− mice under HF diet had severe plaque (Supplemental Fig 1). Old ApoE−/− mice that were fed normal chow developed minor plaque. As shown in Fig 1A, BMCs from 8-month aged ApoE−/− mice from either diet group had significant fewer CXCR4+ cells compared with BMCs from young (n=10, p<0.05). The observation that reduced CXCR4+ cell number occurred in both aged groups irrespective of diet suggest that the changes are related more closely to the ApoE−/− atherosclerotic phenotype rather than diet per se.
Fig 1. Comparison of CXCR4 expression in BMCs from young vs. old WT or ApoE−/− mice.
A). BMC isolated from young and old WT (□)or ApoE−/− mice under either high fat diet (▨) or normal chow (■) were labeled with PE-conjugated anti-CXCR4 mAb. The surface CXCR4 positive BMCs were quantified through flow cytometry and expressed as percentage of total BMCs. # p<0.05 vs. Old groups. B). BMCs were incubated in PBS, PBS plus CaCl2 (1mM) or DMEM containing 10% FBS at 37°C for 4 hrs and CXCR4+ BMCs were then quantified by FACS analysis. n=10. * p<0.05; ** p<0.01.
We also quantified calcium-inducible CXCR4 expression. As shown in Fig 1B, the induction of CXCR4 expression by culturing with 1mM CaCl2 or full medium was muted in BMCaged of ApoE−/− mice to the same degree as in 24-month old wild type mice. BMCs from old ApoE−/− mice fed NC were also unresponsive to calcium (data not shown).
Effect of calcium on CXCR4 expression
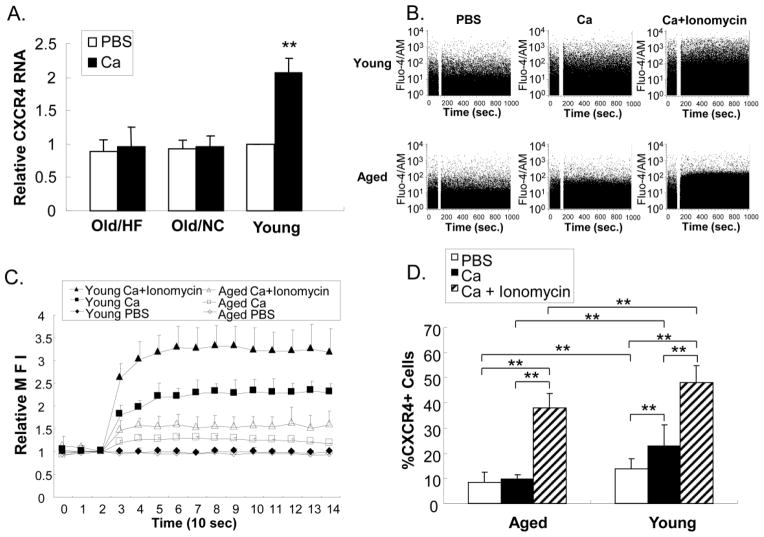
To further characterize the differential BMC responses to calcium, we measured CXCR4 mRNA transcripts before and after calcium-stimulation. Consistent with our results using BMCs from young and old WT mice, there were no differences in the basal levels of CXCR4 transcripts in BMCs from aged and young ApoE−/− mice (Fig 2A). This suggests that the differential surface expression defined in Fig 1 reflects post-transcriptional changes. We found that CXCR4 transcript levels were increased after calcium treatment by 2.07 ± 0.21 fold (n=3, p<0.01) in BMCyoung, whereas the change in BMCaged was not significant (1.10 ± 0.20 and 1.04 ± 0.10 fold for HF and NC, respectively; n=3, p>0.4). To examine whether loss of calcium-induced CXCR4 expression involved defective calcium transport in the aged cells, calcium flux was monitored by flow cytometry. BMCaged had significant lower calcium influx relative to BMCyoung (Fig 2B). Treatment with the calcium ionophore, ionomycin increased calcium influx significantly in both BMCyoung and BMCaged, but the intracellular calcium concentration of BMCaged remained lower than BMCyoung even in the presence of ionomycin (Fig 2C). Ionomycin treatment enhanced CXCR4 surface expression in parallel with enhanced calcium flux in both BMCyoung and BMCaged from ApoE−/− mice (Fig 2D). These results implicate defective calcium influx in BMCaged as responsible at least in part for impaired Ca-induced CXCR4 expression.
Fig 2. Effect of calcium on CXCR4 gene expression.
A). BMCs from young and old ApoE−/− mice under either high fat (HF) diet or normal chow (NC) were isolated and treated with PBS ± calcium for 4 hrs. CXCR4 mRNA levels in the cells were measured by RT-PCR and normalized to HPRT1 transcripts (n=3). B). Time course of calcium influx into BMCs from young and old ApoE−/− mice measured by flow cytometry. The blank space in 2B is the pause time during which calcium was added to the cuvette. C). Quantification of calcium influx into BMCs. Each set point represents the mean fluorescence intensity (MFI) of a 10 sec histogram shown in B. Values were normalized against the basal MFI before adding CaCl2 (n=4). D). CXCR4 surface expression measured by flow cytometry was enhanced by ionomycin. N=4, ** p<0.01.
CXCR4 expression in subpopulations of BMCs
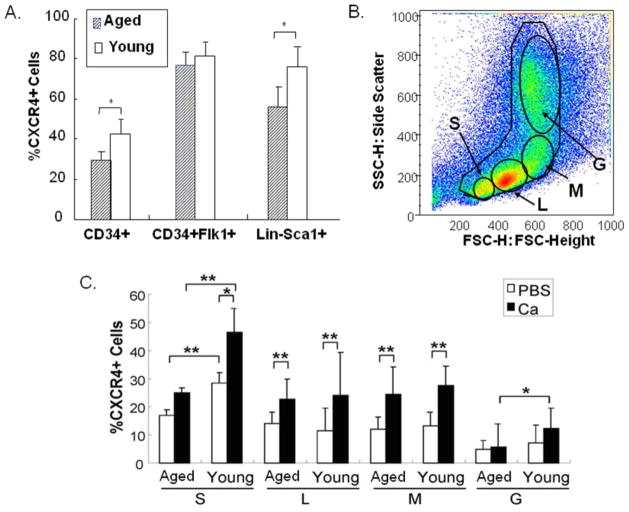
The cell surface CXCR4 expression was further analyzed in BMC subpopulations distinguished by other cell surface markers (Fig 3A) and cell size (Fig 3B). CXCR4 expression was enriched in the CD34+ subpopulations, particularly CD34+Flk1+ and Lin-Sca-1+ subsets (Fig 3A). Similarly, surface CXCR4 in these subpopulations of young mice was significantly higher than that of aged mice, while CXCR4 expression on CD34+Flk1+ subset was not significantly different between young and aged ApoE−/− mice. When the subpopulations of BMCs were arbitrarily grouped according to size (Fig 3C), the “small cell” subpopulations from both young and aged mice were enriched for CXCR4+ cells relative to the larger cells, and there was more CXCR4+ “small cells” in BMCyoung than in BMCaged. Calcium treatment enhanced CXCR4 expression in all subgroups except the small subpopulation of cell and granulocytes in the BMCold group (Fig 3C).
Fig 3. CXCR4 expression in BMC subpopulations.
A). CXCR4 surface expression on CD34+, CD34+Flk1+, and Lin-Sca-1+ subpopulations of BMCs was analyzed by multi-color FACS and compared between young and aged ApoE−/− mice. N= 5, *p<0.05. B). BMCs were gated into 4 subpopulations according to size: small cells (S), lymphocyte enriched (L), monocyte enriched (M), and granulocyte enriched (G). C). Quantification of CXCR4+ cells on the size-dependent subpopulations. N=5. *p<0.05, ** p<0.01
Viability and apoptosis of BMCs
Previous work has shown that CXCR4 expression regulates cell viability as well as mobility15, 16, 20, therefore we quantified necrosis and apoptosis during serum withdrawal with and without calcium stimulation. BMC viability after 4-hr treatment with PBS, PBS+CaCl2, DMEM, or DMEM+FBS was measured by trypan blue staining. The viability of BMC from both young and aged mice was significantly lower in the PBS-treatment groups (p<0.01, n=5), while there was no significant difference in viability between old and young ApoE−/− mice in the other treatment groups (Supplemental Fig 2A). Apoptosis, detected by positive Annexin V staining and negative propidium iodine (PI) staining, was also significantly higher in PBS treated BMCs from young ApoE−/− mice (13.5±0.5%, p<0.05, n=3) compared with the other 3 groups (11.6±1.3%, 10.8±0.6%, and 9.9±0.4%, respectively), while there was no significant difference between old vs. young from the other 3 groups.
To examine these parameters in a simulated disease setting, cells either treated or untreated with Ca2+ were subjected to hypoxia and serum withdrawal ± SDF-1α for 20 hrs. Ca2+-treatments were found to protect young and aged BMCs from both apoptosis and necrosis in the presence or absence of SDF-1α (Supplemental Fig. 2B). Interestingly, BMCaged were more resistant to these death stimuli than BMCyoung.
Adhesion of BMCs on endothelial cells
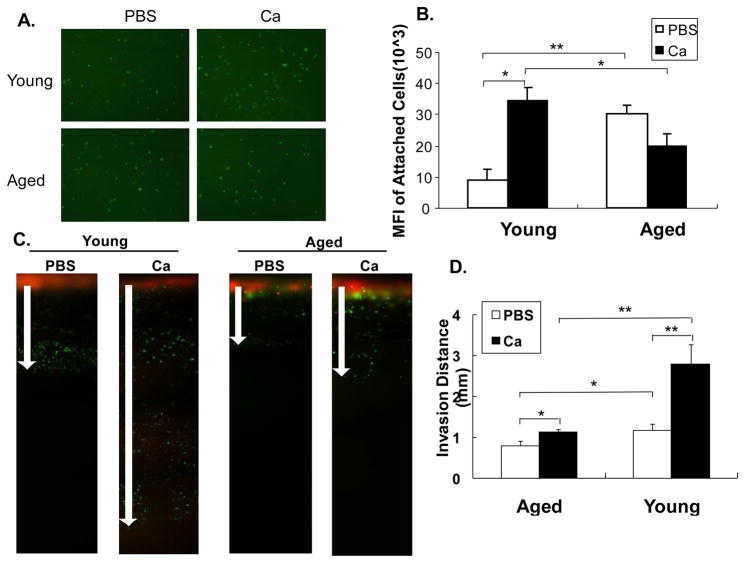
One function of CXCR4 is to direct the homing of circulating cells to tissues that secrete SDF-1. To determine whether this property was undermined by age and atherosclerosis of the host, we quantified adhesion of BMCyoung and BMCaged with and without calcium treatments to a HUVEC monolayer in the presence of SDF-1α (Fig 4A). There were significantly more adherent cells in BMCaged group relative to the young group in the absence of calcium stimulation (Fig 4B). However this situation reversed in calcium-stimulated cells. There were significantly more adherent cells in the calcium stimulated BMCyoung condition than any other condition. Notably, calcium stimulation reduced cell adherence of the BMCold group (Fig 4B).
Fig 4. Adhesion and migration of BMCs.
BMCs from young and aged ApoE−/− mice were treated with PBS or PBS + CaCl2, and then stained with green fluorescent dye as described in Methods. To test the adhesion of BMCs to endothelial cells, BMCs were seeded onto the SDF-1α coated HUVEC monolayers, and cultured for 3 hours. A). Adherent BMCs with Green fluorescence. B). Quantification of the fluorescence intensity representing adherent BMCs. To test the trans-endothelial vertical invasion of BMCs, BMCs with green fluorescence were loaded onto vertical collagen gels pre-seeded with red fluorescent labeled HUVECs and with SDF-1 at the bottom of the gel. C). Cell migration was visualized under a fluorescence microscope. Orange color represents the mixture of BMCs and HUVEC on the top of the gel. Arrows indicate the maximum distance of the migration. D). The maximum migration was quantified as the invasion distance of the each BMC type. N=3, * p<0.05; ** p<0.01.
Impaired migration of BMCs from aged ApoE−/− mice
To determine whether altered surface expression of CXCR4 correlated with changes in BMC mobility, a vertical collagen gel invasion assay was used to measure trans-endothelial migration (Fig 4C). The invasion capacity of BMCaged (0.80 ±0.11mm) was significantly lower than that of BMCyoung (1.17±0.15mm; p<0.05, n=4). Calcium-treatment significantly increased the mobility of BMCyoung (2.79±0.49mm; p<0.01 compared to any other group), while the calcium-enhanced migration of BMCaged was significantly less (1.13±0.06mm; p<0.05 vs. PBS treated, Fig 4D).
Impaired homing capacity of aged ApoE−/− mice in vivo
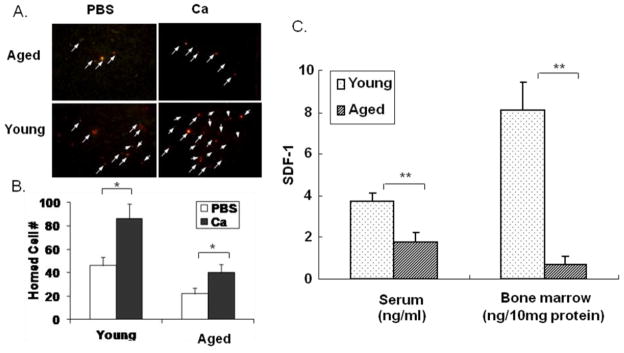
To determine whether impaired CXCR4 expression affects the homing response, BMCs were treated with PBS alone or PBS + CaCl2 as described above, labeled with red fluorescent dye, and injected intravenously into irradiated mice. As shown in Figure 5A and B, BMCs from young donors had significantly higher homing efficiencies than those from aged donors. Calcium-treatment of BMCs increased the homing efficiency of the BMCs from both young and aged donors (Fig 5B). In both cases homing of BMCsaged was significantly less than BMCyoung. In data not shown we found that > 80% of the homed cells in these assays were CXCR4+ in all groups.
Fig 5. BMC homing into bone marrow.
Donor BMCs from old and young ApoE−/− mice were treated with PBS (□) or PBS+ CaCl2 (■), labeled with red fluorescence dye, and injected into irradiated recipient mice. A). Homed BMCs with red fluorescence (arrows pointed) in the bone marrow of the recipient mice were detected under fluorescence microscopy. B). Homed cells were quantified from the sections of recovered bones by counting fluorescent cells under microscope. N=4, * p<0.05. C). Concentrations of SDF-1α in serum and bone marrow from old and young ApoE−/− mice were measured by ELISA (n= 3; **p<0.01).
SDF-1 expression was lower in old mice
Since SDF-1 can modulate the surface expression of CXCR4, we measured SDF-1α concentration in BM and serum of young and aged ApoE−/− mice. As shown in Fig 5C, there was significantly more SDF-1α in both serum and bone marrow of young compared with aged mice.
Discussion
We previously reported loss of CXCR4 expression, calcium inducibility and SDF-1 related functions of BMCs during aging of wild type mice21. Aging and genetic susceptibility are known risk factors for coronary artery disease (CAD). Our studies raise the possibility that depressed levels and functions of CXCR4+ BMCs may contribute to age-related progression of CAD. In the present studies we tested the hypothesis that the progression of atherosclerosis during aging of ApoE−/− mice is paralleled by and perhaps exacerbated by loss of expression and function of CXCR4+ cells in the bone marrow. We demonstrate for the first time markedly decreased surface expression, calcium inducibility and function of CXCR4 in aged atherogenic ApoE−/− mice. The effects could be partially recovered by treatment with the calcium ionophore ionomycin, suggesting defects in calcium handling as a mechanism for reduced CXCR4 expression and lower cell mobilization during aging and progressive disease of ApoE−/− mice. Atherogenic ApoE−/− mice present a phenotype that is equivalent to 24-month old WT mice with respect to CXCR4 expression and cell function. The prematurely aged phenotype also included reduced SDF-1 levels in serum and bone marrow. These are likely to affect the differentiation of cells in the bone marrow as well as mobilization and homing. Consistent with these disease related changes it was recently reported that diabetic mice also have reduced expression of SDF-1 in bone marrow.21
Our results are in accordance with the cellular repair hypothesis of atherosclerosis.2, 3 In this hypothesis endothelial progenitor cells from the bone marrow serve as a surveillance system, continuously repairing the endothelium and sealing damaged areas of the vessel wall thereby preventing lesions that can initiate atherosclerosis. The hypothesis is supported by experiments wherein the injection of bone marrow cells from young healthy ApoE−/− mice prevent the progression of atherosclerosis whereas the same cell types from aged ApoE−/− mice do not2. BMC therapy in combination with metabolic intervention ameliorated functional activity via decreased cellular senescence and increased telomerase and CXCR4 activities.22 Our results suggest that loss of CXCR4 expression and function parallels atherosclerosis in the ApoE−/− model and, as a consequence BMCs from ApoE−/− mice with severe atherosclerosis do not migrate effectively towards SDF-1 (Fig 4), and do not home efficiently in vivo (Fig 5). It has been reported that CXCR4 expression regulates cell viability as well as mobility.15, 16, 20 Our results are also consistent with this. We found that cells from aged mice were highly sensitive to serum withdrawal, and the effect was improved by calcium treatment (Supplemental Fig 2). Our results suggest that the entire CXCR4/SDF-1 network becomes physically and functionally defective during aging, and the effects are markedly exacerbated by concurrent atherosclerosis. We also found that CD34+Flk1+ and Lin-Sca-1+ cells were particularly affected by the aged/atherogenic condition (Fig 3). These cells may be part of the endothelial/hematopoietic progenitor cell populations within the bone marrow that are required for repair and maintenance of endothelial integrity.21, 23 Loss of number, function or homing of these cells may promote endothelial dysfunction. CXCR4 expression in Lin-Sca-1+c-Kit+ cells has been shown to be increased during fetal development and may be required for remodeling and maturation of cardiovascular networks.24 Similarly we found more pronounced effects in the "small cell" sub-population. This population of small cells has EPC-like characteristics and is reduced in parallel with aging and hyperlipidemia.25, 26 It seems possible that defects in CXCR4 expression and function in these and other BMCs contributes to the endothelial dysfunction that has been reported in aged ApoE−/− mice27, 28, as well as in aged human subjects.29
Multifactorial alterations in progenitor cell functions, including shortened telmoeres and increased inflammation and oxidative stress, have been described during aging and cardiovascular disease. Colony-forming capacity, proliferative potential, and migratory activity of BM-derived stem/progenitor cells from aged patients are markedly reduced.3, 4, 30 Expression of cell cycle regulators p53 and p21 and growth factors HGF, IGF-1, and VEGF decline significantly during aging of MSCs.31 Oxidative stress has been implicated in these effects; it is well known that aging is accompanied by increased oxidative stress.30 Particularly relevant to our studies is the age-related loss of neovascularization by BMCs from aged, diseased subjects in ischemic hindlimb models and defective homing of HSCs from aged mice.4, 6
In conclusion, atherosclerotic aged ApoE−/− mice are impaired in the expression and function of CXCR4 and its ligand, SDF-1 in select populations of bone marrow cells. The dysfunctions of BMCs from atherosclerotic, aged mice include reduced engraftment and repair potential and are likely to severely handicap the therapeutic actions of these cells in the setting of autologous bone marrow cell therapy. Enhanced CXCR4 in aged BMCs, either by gene therapy or chemical treatment, could be a way to restore the diminished activities of BMCs from old donors.
Supplementary Material
Acknowledgments
Funding. This work was supported by grants from America Heart Association #0855311 (HY); National Institutes of Health RO1 # HL072924 and HL092499 (KAW) and State of Florida James & Esther King Biomedical Research Program, Team Science Project # 07KT-02 (HY, KAW), New Investigator Research grant # 09KN-18 (MZ), Walter G. Ross Distinguished Chair of Vascular Biology (KAW), and China Scholarship #2008632079 (QX).
Footnotes
Conflict of Interest. None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1–7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 2.Rauscher FM, Goldschmidt-Clermont PJ, Davis BH, Wang T, Gregg D, Ramaswami P, Pippen AM, Annex BH, Dong C, Taylor DA. Aging, Progenitor Cell Exhaustion, and Atherosclerosis. Circulation. 2003;108:457–463. doi: 10.1161/01.CIR.0000082924.75945.48. [DOI] [PubMed] [Google Scholar]
- 3.Heiss C, Keymel S, Niesler U, Ziemann J, Kelm M, Kalka C. Impaired Progenitor Cell Activity in Age-Related Endothelial Dysfunction. Journal of the American College of Cardiology. 2005;45:1441–1448. doi: 10.1016/j.jacc.2004.12.074. [DOI] [PubMed] [Google Scholar]
- 4.Heeschen C, Lehmann R, Honold J, Assmus B, Aicher A, Walter DH, Martin H, Zeiher AM, Dimmeler S. Profoundly Reduced Neovascularization Capacity of Bone Marrow Mononuclear Cells Derived From Patients With Chronic Ischemic Heart Disease. Circulation. 2004;109:1615–1622. doi: 10.1161/01.CIR.0000124476.32871.E3. [DOI] [PubMed] [Google Scholar]
- 5.Liguori A, Fiorito C, Balestrieri ML, Crimi E, Bruzzese G, Williams-Ignarro S, D’Amora M, Sommese L, Grimaldi V, Minucci PB, Giovane A, Farzati B, Ignarro LJ, Napoli C. Functional impairment of hematopoietic progenitor cells in patients with coronary heart disease. Eur J Haematol. 2008;80:258–264. doi: 10.1111/j.1600-0609.2007.01007.x. [DOI] [PubMed] [Google Scholar]
- 6.Morrison SJ, Wandycz AM, Akashi K, Globerson A, Weissman IL. The aging of hematopoietic stem cells. Nature medicine. 1996;2:1011–1016. doi: 10.1038/nm0996-1011. [DOI] [PubMed] [Google Scholar]
- 7.Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, Rovner A, Ellis SG, Thomas JD, DiCorleto PE. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. The Lancet. 2003;362:697–703. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- 8.Jin DK, Shido K, Kopp HG, Petit I, Shmelkov SV, Young LM, Hooper AT, Amano H, Avecilla ST, Heissig B, Hattori K, Zhang F, Hicklin DJ, Wu Y, Zhu Z, Dunn A, Salari H, Werb Z, Hackett NR, Crystal RG, Lyden D, Rafii S. Cytokine-mediated deployment of SDF-1 induces revascularization through recruitment of CXCR4+ hemangiocytes. Nature medicine. 2006;12:557–567. doi: 10.1038/nm1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lapidot T. Mechanism of human stem cell migration and repopulation of NOD/SCID and B2mnull NOD/SCID mice. The role of SDF-1/CXCR4 interactions. Ann N Y Acad Sci. 2001;938:83–95. doi: 10.1111/j.1749-6632.2001.tb03577.x. [DOI] [PubMed] [Google Scholar]
- 10.De Falco E, Porcelli D, Torella AR, Straino S, Iachininoto MG, Orlandi A, Truffa S, Biglioli P, Napolitano M, Capogrossi MC, Pesce M. SDF-1 involvement in endothelial phenotype and ischemia-induced recruitment of bone marrow progenitor cells. Blood. 2004;104:3472–3482. doi: 10.1182/blood-2003-12-4423. [DOI] [PubMed] [Google Scholar]
- 11.Seeger FH, Rasper T, Koyanagi M, Fox H, Zeiher AM, Dimmeler S. CXCR4 Expression Determines Functional Activity of Bone Marrow-Derived Mononuclear Cells for Therapeutic Neovascularization in Acute Ischemia. Arterioscler Thromb Vasc Biol. 2009;29:1802–1809. doi: 10.1161/ATVBAHA.109.194688. [DOI] [PubMed] [Google Scholar]
- 12.Foguenne J, Di Stefano I, Giet O, Beguin Y, Gothot A. Ex vivo expansion of hematopoietic progenitor cells is associated with downregulation of alpha4 integrin- and CXCR4-mediated engraftment in NOD/SCID beta2-microglobulin-null mice. Haematologica. 2009;94:185–194. doi: 10.3324/haematol.13206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang D, Fan GC, Zhou X, Zhao T, Pasha Z, Xu M, Zhu Y, Ashraf M, Wang Y. Over-expression of CXCR4 on mesenchymal stem cells augments myoangiogenesis in the infarcted myocardium. J Mol Cell Cardio. 2008;44:281–292. doi: 10.1016/j.yjmcc.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu Q, Shao H, Eton D, Li J, Li J, Yang B, Webster KA, Yu H. Extracellular calcium increases CXCR4 Expression on Bone Marrow-derived Cells and Enhances Pro-Angiogenesis Therapy. J Cell Mol Med. 2009;13:3764–3773. doi: 10.1111/j.1582-4934.2009.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kahn J, Byk T, Jansson-Sjostrand L, Petit I, Shivtiel S, Nagler A, Hardan I, Deutsch V, Gazit Z, Gazit D, Karlsson S, Lapidot T. Overexpression of CXCR4 on human CD34+ progenitors increases their proliferation, migration, and NOD/SCID repopulation. Blood. 2004;103:2942–2949. doi: 10.1182/blood-2003-07-2607. [DOI] [PubMed] [Google Scholar]
- 16.Porecha NK, English K, Hangoc G, Broxmeyer HE, Christopherson KW., 2nd Enhanced functional response to CXCL12/SDF-1 through retroviral overexpression of CXCR4 on M07e cells: implications for hematopoietic stem cell transplantation. Stem Cells Dev. 2006;15:325–333. doi: 10.1089/scd.2006.15.325. [DOI] [PubMed] [Google Scholar]
- 17.Shao H, Xu Q, Wu Q, Ma Q, Salgueiro L, Wang J, Eton D, Webster KA, Yu H. Defective CXCR4 Expression in Aged Bone Marrow Cells Impairs Vascular Regeneration. J Cell Mol Med. doi: 10.1111/j.1582-4934.2010.01231.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piedrahita JA, Zhang SH, Hagaman JR, Oliver PM, Maeda N. Generation of mice carrying a mutant apolipoprotein E gene inactivated by gene targeting in embryonic stem cells. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:4471–4475. doi: 10.1073/pnas.89.10.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoon CH, Hur J, Park KW, Kim JH, Lee CS, Oh IY, Kim TY, Cho HJ, Kang HJ, Chae IH, Yang HK, Oh BH, Park YB, Kim HS. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: the role of angiogenic cytokines and matrix metalloproteinases. Circulation. 2005;112:1618–1627. doi: 10.1161/CIRCULATIONAHA.104.503433. [DOI] [PubMed] [Google Scholar]
- 20.Bhakta S, Hong P, Koc O. The surface adhesion molecule CXCR4 stimulates mesenchymal stem cell migration to stromal cell-derived factor-1 in vitro but does not decrease apoptosis under serum deprivation. Cardiovasc Revasc Med. 2006;7:19–24. doi: 10.1016/j.carrev.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 21.Orlandi A, Chavakis E, Seeger F, Tjwa M, Zeiher A, Dimmeler S. Long-term diabetes impairs repopulation of hematopoietic progenitor cells and dysregulates the cytokine expression in the bone marrow microenvironment in mice. Basic Research in Cardiology. 2010 doi: 10.1007/s00395-010-0109-0: 1-10. In press. [DOI] [PubMed] [Google Scholar]
- 22.Balestrieri ML, Lu SJ, de Nigris F, Giovane A, Williams-Ignarro S, D’Armiento FP, Feng Q, Fiorito C, Testa G, Pastore L, Cacciatore F, Mancini FP, Servillo L, De Rosa G, Pagliarulo C, Rienzo M, Minucci PB, Farzati B, Salvatore F, Rengo F, Ignarro LJ, Giordano A, Baker A, Lanza R, Napoli C. Therapeutic angiogenesis in diabetic apolipoprotein E-deficient mice using bone marrow cells, functional hemangioblasts and metabolic intervention. Atherosclerosis. 2010;209:403–414. doi: 10.1016/j.atherosclerosis.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 23.Yoder MC, Ingram DA. The definition of EPCs and other bone marrow cells contributing to neoangiogenesis and tumor growth: Is there common ground for understanding the roles of numerous marrow-derived cells in the neoangiogenic process? Biochimica et Biophysica Acta (BBA) - Reviews on Cancer. 2009;1796:50–54. doi: 10.1016/j.bbcan.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ciriza J, Garcia-Ojeda ME. Expression of migration-related genes is progressively upregulated in murine Lineage-Sca-1+c-Kit+ population from the fetal to adult stages of development. Stem Cell Res Ther. 2010;1:14. doi: 10.1186/scrt14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kucia M, Reca R, Campbell FR, Zuba-Surma E, Majka M, Ratajczak J, Ratajczak MZ. A population of very small embryonic-like (VSEL) CXCR4+SSEA-1+Oct-4+ stem cells identified in adult bone marrow. Leukemia. 2006;20:857–869. doi: 10.1038/sj.leu.2404171. [DOI] [PubMed] [Google Scholar]
- 26.Zhu S, Liu X, Li Y, Goldschmidt-Clermont PJ, Dong C. Aging in the atherosclerosis milieu may accelerate the consumption of bone marrow endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2007;27:113–119. doi: 10.1161/01.ATV.0000252035.12881.d0. [DOI] [PubMed] [Google Scholar]
- 27.Wassmann S, Werner N, Czech T, Nickenig G. Improvement of Endothelial Function by Systemic Transfusion of Vascular Progenitor Cells. Circ Res. 2006;99:E74–83. doi: 10.1161/01.RES.0000246095.90247.d4. [DOI] [PubMed] [Google Scholar]
- 28.Cola MS, Gava AL, Meyrelles SS, Vasquez EC. Endothelial dysfunction of resistance vessels in female apolipoprotein E-deficient mice. Lipids Health Dis. 2010;9:51. doi: 10.1186/1476-511X-9-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al-Shaer MH, Choueiri NE, Correia MLG, Sinkey CA, Barenz TA, Haynes WG. Effects of aging and atherosclerosis on endothelial and vascular smooth muscle function in humans. International Journal of Cardiology. 2006;109:201–206. doi: 10.1016/j.ijcard.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 30.Stolzing A, Jones E, McGonagle D, Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev. 2008;129:163–173. doi: 10.1016/j.mad.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Wilson A, Shehadeh LA, Yu H, Webster KA. Age-related molecular genetic changes of murine bone marrow mesenchymal stem cells. BMC Genomics. 2010;11:229. doi: 10.1186/1471-2164-11-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.