Abstract
The purpose of this study was to evaluate the impact of free fatty acids (FFA), namely oleic (OA) and linoleic (LA) ones, on meibomian lipid films (MLF) using a Langmuir trough (LT) and a Brewster angle microscope (BAM). Human meibum was collected from healthy volunteers. A Tris-buffered saline (TBS, pH 7.4) was used as the control aqueous subphase for LT experiments. Then, varying amounts of OA and LA were dissolved in TBS to make FFA-containing subphases. Predetermined amounts of meibum were loaded onto the surface of the (TBS/±FFA) subphases to form MLF. Then, surface pressure-area (π/A) isotherms of MLF were recorded. Standard rheological parameters such as rigidity, elasticity, and hysteresis, were computed. In a separate experiment, OA and LA were premixed with meibum at different weight ratios prior their spreading onto the control TBS subphase, and the (π/A) isotherms of the resulting mixed films of meibum and FFA were studied and analyzed in the same fashion as described above.
When studied at the normal corneal temperature of 34°C with the (TBS/−FFA) subphase, meibum formed stable films. When (TBS/+FFA) subphase was used, both FFA quickly disrupted the MLF, acting in a similar fashion. BAM revealed that the most dramatic changes in the structure of MLF occurred in the range of OA concentrations between 5 and 15 μM. However, this effect was apparent even with 2.5 μM OA. When OA was premixed with meibum, but was absent from the subphase, it caused gradual concentration-dependent changes in the (π/A) isotherms, but the MLF did not disappear from the surface.
Thus, tested FFA showed a remarkable ability to disrupt, and/or prevent the formation of, human MLF, which could contribute to the onset of those forms of dry eye disease that are associated with enhanced activity of lipolytic enzymes, such as chronic blepharitis.
Keywords: lipids, tear film, dry eye, meibomian gland dysfunction, fatty acids, Langmuir trough, Brewster angle microscopy
Introduction
The human ocular surface is protected from the outer environment by a thin aqueous film, which is called the tear film (TF). The TF is a complex and dynamic structure formed from lipids, carbohydrates, proteins, salts, and water (Van Haeringen, 1981). Anatomically, the outermost part of the TF is composed of an oily secretion produced by the holocrine lipid-secreting Meibomian glands (MG) that are embedded in the tarsal plates of the upper and lower eyelids in single rows along their edges, while the underlying aqueous part of the TF is produced by lachrymal glands. The Meibomian gland secretion (MGS) is also often called “meibum” (Andrews, 1970; Brauninger et al., 1972; McCulley and Shine, 1997). The outermost lipid membrane-like part of the TF, so-called TF lipid layer (TFLL), is thought to (1) reduce evaporation of the aqueous tears, (2) lubricate the eyelids during blinking, and (3) serve as a barrier against microorganisms (Tiffany, 1987). The anti-evaporatory function of the TFLL is considered to be critical for protection of the human ocular surface, especially of its most sensitive and delicate part – cornea – from desiccating. Earlier experiments showed that MGS is a very diverse lipid mixture that is composed predominantly of non-polar lipids such as wax esters and cholesteryl esters (Butovich, 2009a; Nicolaides et al., 1981), and even more complex compounds whose exact nature is currently being investigated.
Changes in the quantity or composition of meibum can result in instability of the TF, compromising the health of the ocular surface and leading to pathological conditions such as dry eye disease (DED) (McCulley and Shine, 1998; Shine and McCulley, 1993, 1996). DED affects millions of people worldwide, increasing the risk of ocular infections, causing moderate to severe discomfort, and negatively impacting the everyday activities of affected people.
In certain circumstances detrimental changes in the quality of meibum can occur due to a condition known as MG dysfunction (MGD) –one of the leading causes of DED. These changes have been linked to the fluctuations in the lipid composition of meibum (Bron and Tiffany, 1998; Joffre et al., 2008; McCulley and Shine, 2004; Shine and McCulley, 1996, 2000) that could adversely affect the integrity and stability of the TF, compromising the health of the ocular surface (McCulley and Shine, 2001).
Finding a connection between the lipid composition of the TFLL and the onset of DED has been the goals of many studies. A comprehensive chemical analysis of meibum samples collected from healthy subjects has demonstrated a very complex nature of Meibomian lipids (Butovich, 2009b; Nicolaides et al., 1981). Many lipid classes have been identified in meibum, including free fatty acids (FFA)(Butovich, 2010). An earlier publication compared the overall fatty acid composition of meibomian lipids collected from MGD, DED, and control patients (Joffre et al., 2008). The authors reported that a common, moderately long FFA oleic acid (OA) (a C18:1-FA) was a predominant monounsaturated fatty acid in the meibomian lipids in all study groups (Joffre et al., 2008). Interestingly, McCulley and Shine reported that the amount of FFA is increased in the meibomian lipids from patients with chronic blepharitis, probably due to the activity of bacterial hydrolases (Butovich et al., 2007; Shine and McCulley, 2000).
To the best of our knowledge, the direct impact of FFA on human meibomian lipid films (MLF) has never been tested experimentally1. We hypothesized that moderately long FFA, being very potent surface active agents, could disintegrate, or prevent the formation of, MLF, if present in sufficient concentrations. Thus, to test this possibility, we decided to employ a Langmuir trough, a biophysical apparatus designed to study thin films of various molecules at the air-water interface (Gunstone, 2007). The advantage of this technique is that a lipid film formed from meibum at the surface of an aqueous subphase resembles the TFLL, and can be subjected to the same type of repetitive stress (compression and expansion) that the TFLL is subjected to during normal blinking.
In previous publications, basic rheological parameters such as viscosity, rigidity (or two-dimensional elasticity), and hysteresis of MLF have been evaluated by using the Langmuir trough methodology (Butovich et al., 2010; Leiske et al., 2010; Millar et al., 2009; Mudgil and Millar, 2008; Mudgil et al., 2006). In addition, a Brewster angle microscope (BAM) was used for visualization of the microscopic structure of MLF (Kaercher et al., 1993). The combination of both techniques was to provide a more comprehensive overview of the TFLL in vitro. Thus, the purpose of our study was to evaluate the direct impact of FFA on MLF in vitro.
Materials and Methods
Collection of meibum samples
The meibum collection protocol, consent forms and data accumulation methods were approved by the University of Texas Southwestern Medical Center Institutional Review Board and were conducted in accordance with the declaration of Helsinki. All meibum donors gave informed consent. HIPPA regulations were followed. Meibum samples were obtained from healthy subjects who underwent clinical assessment for ocular diseases. A previously described protocol was followed (Butovich, 2008, 2009a). Spectroscopy and high performance liquid chromatography (HPLC) grade chloroform and methanol used in the study were obtained from Burdick&Jackson (Muskegon, MI) and Sigma-Aldrich (St. Louis, MO). A pre-weighed 1.5ml Total Recovery vial (Waters Corp., Milford, MA) was filled with ~1ml of chloroform:methanol mixture (2:1, v/v). To collect meibum, a cotton swab was applied over the eyelids margin to remove any remnants of aqueous tears. Then, meibum was expressed from the subject’s two lower eyelids using a cotton swab moving in the rolling fashion toward the eyelids’ edges. A polished platinum spatula was used to collect the lipid samples from the MG orifices without scraping the surface of the eyelid. Then, the lipid material was dissolved in the solvent and placed under a stream of nitrogen to evaporate the solvent. To determine the dry weight of collected meibum, the vial with the dry sample was reweighed after complete evaporation of the solvent. The dry weights of the samples depended on the donor and varied between 0.5 and 1 mg.
High performance liquid chromatography–mass spectrometry of the meibomian free fatty acids
To compare the study meibum samples with previously analyzed samples to assure consistency. The Meibomian lipid samples were evaluated by HPLC-mass spectrometry (HPLC-MS) as described earlier (Butovich, 2008, 2009a). Meibomian FFA were analyzed using a procedure described earlier (Butovich, 2010). HPLC separation of FFA from other meibomian lipids was performed on a Waters Alliance 2695 HPLC Separations Module (Waters Corp). The HPLC system was interfaced to a LCQ Deca XP Max ion trap mass spectrometer equipped with an atmospheric pressure chemical ionization (APCI) ion source (Thermo Electron, Waltham, MA). The analytes were separated on a reverse phase C18 Hypersil Gold column (2.1 × 150 mm, 5 μm, Thermo Fisher Scientific, Hampton, NH) in a gradient eluent composed of acetonitrile, iso-propanol, and 0.01% M acetic acid in water (Butovich, 2010). FFA were detected as adducts with acetic acid in negative ion mode; thus, the observed species were of (M + CH3COO)− nature. Linoleic acid (LA), OA, and other FFA were purchased from Nu-Chek Prep, Inc. (Elysian, MN) and Sigma-Aldrich. The data were analyzed using a XCalibur software (v.1.4, ThermoElectron) and a SigmaPlot v.11 software package (Systat Software, Inc., San Jose, CA).
Langmuir trough experiments with lipid films
MilliQ-grade (Millipore, Billerica, MA) deionized water (18MΩ) was used for making aqueous buffers. A thermostated miniature Langmuir trough (NIMA 102M, NIMA Technology Ltd, Coventry, England) was utilized to conduct the lipid films experiments (Butovich et al., 2010). Software provided by NIMA (NIMA v.5.16) was used to run the experiments. The temperature of the Langmuir trough was maintained using a refrigerating/heating circulating water thermostat (RTE-7, Thermo Fisher Scientific) and was monitored using a solid-state thermometer (model TMP, NIMA). The trough was housed inside a Plexiglas® cabinet (model CSL, NIMA) to protect the lipid films from dust and air drafts, and to allow more stable experimental conditions.
Surface pressure-Area (π/A) measurements
Standard protocols for Langmuir trough experiments (Grunfeld, 2004; Korchowiec et al., 2006; Millar et al., 2006; Mudgil and Millar, 2008; Tragoulias et al., 2005) and the manufacture’s recommendations were followed while conducting Langmuir trough experiments. The Wilhelmy plate was made of Whatman, Chr 1 filter paper. During the experiments, the researchers wore polyethylene gloves to avoid contamination of the samples and instruments with skin lipids. The trough was cleaned with chloroform prior to, and after each, experiment. A 0.9% (w/v) NaCl (saline) solution in 50 mM Tris-HCL pH titrated to 7.4 (TBS) was used as a reference aqueous subphase to resemble the acidity, pH and salinity of human tears (Fischer and Wiederholt, 1978). Then, a series of aqueous TBS-based subphases with varying amounts of dissolved FFA was prepared. The range of tested FFA concentrations was 0 to 30 μM. After filling the trough with TBS with or without FFA, the subphase was allowed to pre-equilibrate at 34°C for 60 minutes. The trough barriers were closed after the initial equilibration period and the surface was checked for any surface contamination which would manifest itself as an increase in the surface pressure in response to closing barriers. If such increase was detected, a vacuum probe was used to aspirate the impurities from the surface. Then, the barriers were closed again and the surface pressure was re-checked for any residual contaminations. A 1mg/ml stock solution of meibum in chloroform (app. 1.5mM, for an average MW of a meibomian lipid estimated to be ~700 (Butovich et al., 2010) was carefully loaded on top of the subphase using a HPLC Hamilton micro syringe. A typical amount of loaded meibum was 16 μg, or 0.2 μg/cm2 of the trough area. Then, the solvent was allowed to evaporate for 30 minutes and multiple (π/A) isotherms of MLF were recorded in cycles (Butovich et al., 2010). In a typical experiment, MLF were compressed between the areas of 80 and 25 cm2 at a rate of 20 cm2/min. The barriers were opened and closed repeatedly a few times while recording the π/A isotherms until the lipid film stabilized. These few initial curves were excluded from the study and those that showed consistency and reproducibility were included.
In a separate experiment, a series of mixed solutions of OA and meibomian lipids in chloroform with different weight ratios (10:0, 8:2, 6:4, 4:6, 2:8, 0:10, respectively) was prepared. Then, the mixtures were deposited onto the surface of the TBS subphase and studied as described above for pure meibum.
The standard parameters used to analyze the experimental data were initial surface pressure (πin), maximum surface pressure (πm), collapse pressure (πc), lift-off area (AL), hysteresis (ΔΔG), and two-dimensional in-plane elasticity (Cs−1). A detailed explanation of the mathematical analysis is described in our previous publication (Butovich et al., 2010). The surface pressure of the subphase before loading meibomian lipids was set to be an arbitrary zero. When MLF forms, πin increases. Both FFA tested in these experiments were reported in human meibum (Butovich, 2009a, 2010; Butovich et al., 2007; McCulley and Shine, 2001).
Brewster angle microscopy experiments
A micro-BAM (Model 3, NIMA) was used for lipid films visualization at the air-water interface in the Langmuir trough. The microscope was equipped with a laser (wavelength 659 nm, power output 30 mW), a polarizer, an analyzer, and a CCD video camera with 3.6 × 4 mm field of view and a 6 μm resolution. The p-polarized light beam was pointed at the air-water interface at approximately 53.1° (the incident Brewster angle). Under this condition, the surface of pure water appeared to be black (zero reflectivity), while the lipids loaded onto the air-water interface appeared as whitish to grayish complex surface structures. The whiteness of the structures depended on the degree of lipid organization – the brighter the image, the more dense and oriented the lipid structures (Henon, 1991).
Results
Free fatty acids in meibum
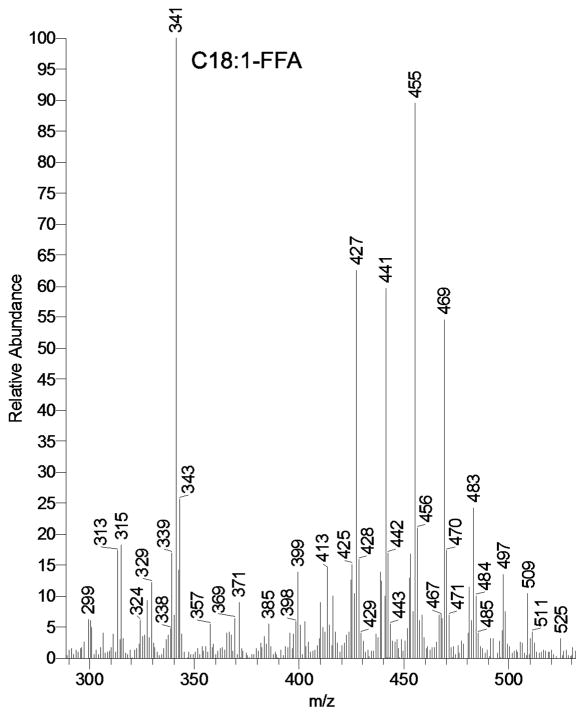
Meibomian lipids collected from healthy individuals demonstrated the presence of various FFA (Figure 1). The acids ranged from C16 to, at least, C32. Both saturated and unsaturated FFA were observed. Their quantitation was achieved by using calibration curves obtained for authentic FFA ranging from C12 to C24. Chemical standards of the longer FFA of interest were not available at the time. Thus, their estimation in meibum was conducted by extrapolating the results obtained with available standards (to be published elsewhere). However, OA and LA, which are in the focus of this report, were easily detectable and quantifiable in human meibum. The presence of endogenous OA in tested samples was found to be in the range between 0.01% and 0.03% (weight/weight), while the amounts of endogenous LA were typically an order of magnitude lower.
Figure 1.
Mass spectrum of free fatty acids present in human meibomian gland secretions recorded in negative ion mode. Free fatty acids were observed as acetic acid adducts (M + CH3COO)−
Meibomian lipid films on the TBS subphase
At physiologically normal corneal temperature of 34±1°C, TBS with no added lipids showed no presence of any surface active contaminants on the surface of the aqueous subphase. When meibum was added in the amount sufficient to form a monolayer (0.2 μg/cm2) (Butovich et al., 2010), a typical π/A isotherm of meibum was observed (Figure 2, dotted curve). The highest surface pressure (πm) recorded in these experiments was ~12 mN/m. The π/A isotherms did not produce any abrupt changes in their slopes no matter the πm.
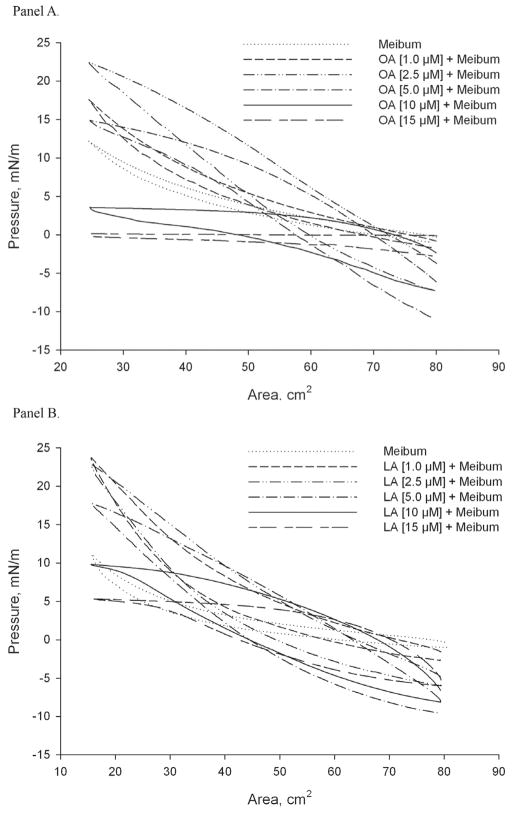
Figure 2. Effects of free fatty acids on the meibomian lipid film.
Panel A. π/A isotherms of meibomian lipids spread over an oleic acid-containing TBS aqueous subphase. Increasing concentrations of oleic acid in the TBS subphase negatively affected the π/A isotherm of meibomian lipid films. Note a drop in π at large surface areas caused by changes in the composition of the initially formed surface films, induced by the lateral forces of the closing barriers and solubilization of meibum by oleic acid.
Panel B. π/A isotherms of human meibomian lipids spread over a linoleic acid-containing TBS aqueous subphase. The amount of meibum loaded onto the subphase was 16 μg/80 cm2 of the trough’s area.
Effects of FFA dissolved in the TBS subphase
To determine the impact of FFA on MLF, π/A isotherms were recorded using a series of TBS subphases with various amounts of FFA dissolved in them. The experiments were conducted at 34±1°C. For the current series of experiments, the standard amount of human meibum loaded onto the surface was set to be 0.2 μg/cm2. As expected, both OA and LA showed remarkable surface activity. Note that because of the much higher presence of OA in meibum (Figure 1), and close similarities in properties of OA and LA, only the effects of OA were evaluated in detail. Used in the concentrations of 1 μM and 2.5 μM, OA raised the πm of MLF to ~22 mN/m, while LA – to the equally high ~24 mN/m. However, when the concentration of FFA in the subphase exceeded 2.5 μM, a decrease in the πm of MLF was observed. The effects of mono- (OA) and di-unsaturated (LA) FFA on MLF were found to be similar and concentration-dependent, increasing gradually from 0 to 15 μM (Figure 2).
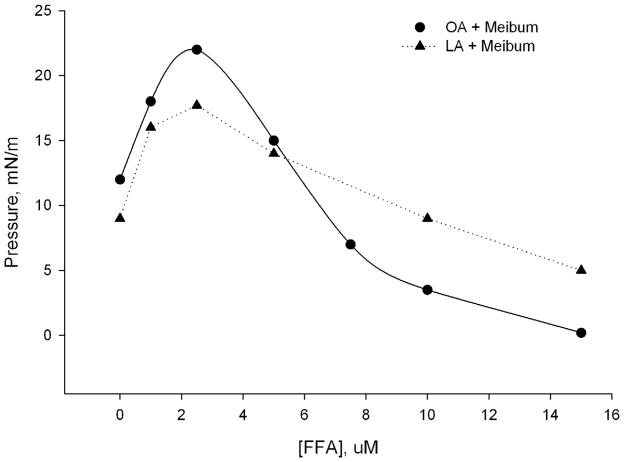
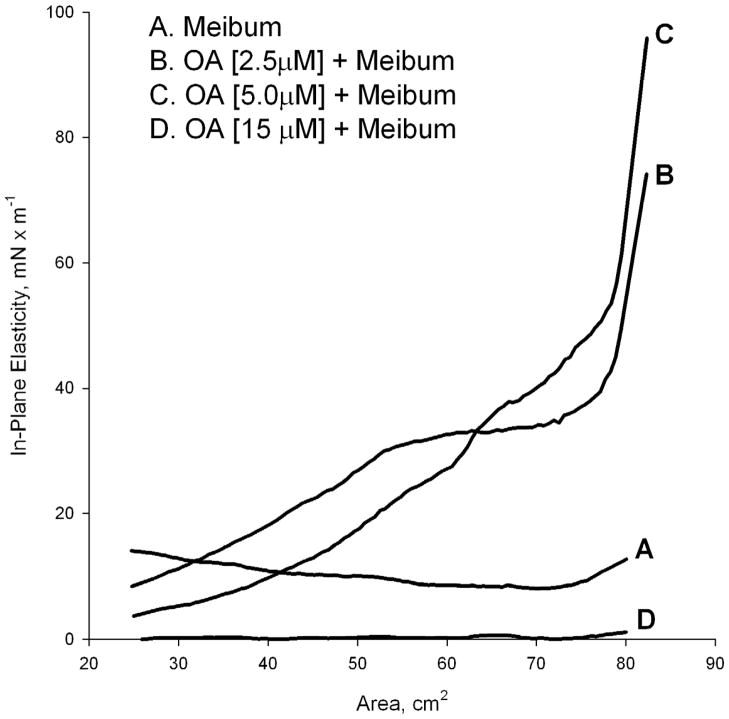
The decrease in πm was proportional to the concentration of exogenously added FFA in the subphase (Figure 3). As the FFA concentration increased in the subphase, the π/A isotherms of meibum showed a change in the shape and hysteresis (Figure 2). The in-plane elasticity Cs−1 was calculated using a method described in our previous publication (Butovich et al., 2010). When using a 15 μM OA in the TBS subphase, Figure 4 (trace D), the MLF had almost zero in-plane elasticity regardless the surface area. This situation, however, changed once the OA concentration diminished. At OA concentrations of 2.5 and 5 μM, Cs−1 achieved values as high as 100mN/m at low compression ratios (between 80 and 70 cm2 of the trough area). However, a quick and progressive decrease of Cs−1 was observed as soon as the barriers started to close, reaching values between 8 and 3 mN/m at a surface area of 25 cm2. Without OA, the values of Cs−1 of MLF were relatively constant and stayed in the range of 15±5 mN/m no matter the compression ratio (trace A). In addition, the in-plane elasticity of MLF without FFA was highest when the level of compression increased, while in the experiments in which FFA was present in the subphase in concentrations of 2.5, 5.0, and 15 μM, the opposite was found. The effects of LA were found to be similar to those of OA. However, the presence of free LA in meibum is about 15% (or less) of that of OA (Figure 1, MS signals m/z 339 and 341, correspondingly). Also, the physicochemical properties of OA and LA are quite similar. Thus, the data only for the major FFA, namely OA, are shown.
Figure 3.
Maximum surface pressure (πm) of meibomian lipid films measured with varying concentrations of free fatty acids in the TBS aqueous subphase. Data were taken from Figures 2 and 3. When present in the subphases in concentrations above 2.5 μM, free fatty acids disrupt the meibomian lipid films in a concentration-dependent manner.
Figure 4.
In-plane elasticity Cs−1 of meibomian lipid films measured using varying amounts of oleic acid in the TBS aqueous subphase. The data from Figure 2 were used. When OA was present in the TBS subphases in the indicated concentrations, the π/A isotherms of meibomian lipids were strongly affected. Our findings suggest that oleic acid diminishes stability of meibomian lipid films, causing their disintegration and/or collapsing.
Hysteresis (ΔΔG) of a control sample of meibum (16 μg/80 cm2, in the FFA-free TBS subphase, Figure 2) was calculated to be ~7.6 μJ. In the presence of 2.5 μM OA, ΔΔG assumed a considerably higher value of 21 μJ.
Lipid films formed from exogenous free fatty acids and meibum mixed in different weight ratios
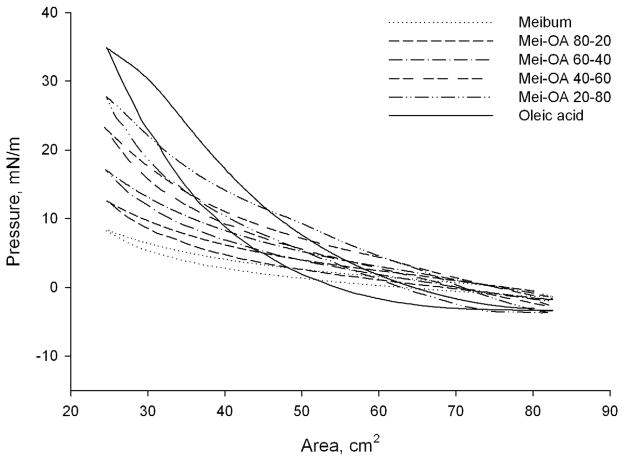
To evaluate the effects of FFA on MLF when loaded directly onto the surface of an aqueous subphase (instead of being dissolved in the subphase), a series of mixed chloroformic solutions of OA and human meibum was prepared. The tested molar ratios of OA and meibomian lipids were 10:0, 8:2, 6:4, 4:6, 2:8, and 0:10, respectively. When spread onto the surface of the TBS subphase, the π/A isotherms of these mixtures (Figure. 5) differed significantly from those of pure meibum when OA was dissolved in the subphase.
Figure 5. π/A isotherms of a series of mixed films of oleic acid and meibomian lipids formed on the surface of a control, FFA-free TBS subphase.
Oleic acid and meibum were pre-mixed to form solutions with their molar ratios in the mixtures varying from 10:0 to 0:10. When loaded onto the surface of the TBS subphase, π/A isotherms of the mixed films differed significantly from those of pure meibum and oleic acid dissolved in the subphase. Note that, unlike the data presented in Figures 2 and 3, no significant drop in the initial surface pressure π was observed in these experiments, apparently because of the inability of oleic acid to solubilize large, and very hydrophobic, meibomian lipids in these conditions.
The π/A isotherms of pure OA without meibum (Figure 5) had much higher hysteresis compared with pure meibum. The values of ΔΔG for 10:0, 6:4, 2:8, and 0:10 mixtures of OA and meibum were 25.4, 10.6, 7.2, and 6.5 μJ. Also, upon compression the values of π rose faster than those of pure meibum. The properties of mixed films of OA and meibum depended on their weight ratio and gradually changed when their composition changed from pure meibum to pure OA, without signs of abrupt collapsing.
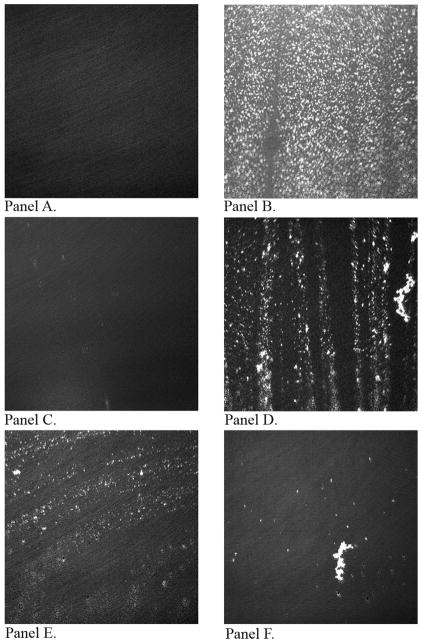
The BAM visualization of the MLF topography suggested that when the level of FFA exceeds 2.5 μM, a progressive removal of the meibomian lipids from the surface occurred. In Figure 6, a series of BAM images of MLF taken in different conditions are shown. Figure 6, Panel A, shows the TBS surface with no lipids and surface contaminants. Then, following the addition of 0.2 μg/cm2 of meibum on the surface of TBS, a lipid film is formed, in which denser, more organized, lipid aggregates appear as brighter dots surrounded by darker areas, most likely filled with more loosely organized lipid structures (Figure 6, Panel B). Next Figure 6, Panel C, illustrates the condition when OA was mixed with TBS (TBS-OA) at a concentration of 15 μM (with no meibum loaded). Here, OA formed what appears to be “micro-droplets”, clearly showing that OA reached the limit of its solubility in tested conditions. These droplets were in constant movement, occupying the TBS surface for a short period of time followed by their expulsion back into the subphase. However, the next Figure 6, Panel D, illustrates an interesting phenomenon: when TBS with 2.5 μM OA was used as subphase, and 0.2 μg/cm2 meibomian lipids were loaded on its surface in a standard manner, the MLF started to disintegrate and disappear from the surface shortly after the addition of an aliquot of meibomian lipids, leaving large portions of the surface unprotected with MLF and exposed to the air. An increase in the OA concentration in the subphase to 5 μM, and then to 15 μM, further potentiated this effect (Fig. 6, Panels E and F).
Figure 6.
BAM images of meibomian lipid films. Panel A. Control TBS subphase with no meibomian lipids, oleic acid, or surface contaminants. Panel B. Meibomian lipid film at the surface of the control FFA-free TBS (surface density of lipids 0.2 μg/cm2). Panel C. TBS subphase with 15 μM oleic acid (no meibum loaded). Panel D. Meibomian lipid film at on the surface of the TBS with 2.5 μM oleic acid. Panel E. Meibomian lipid film at on the surface of the TBS with 5 μM oleic acid. Panel F. Meibomian lipid film at on the surface of the TBS with 15 μM oleic acid. Target surface density of lipids 0.2 μg/cm2.
Discussion
It is believed that the integrity of the TFLL is based on a delicate equilibrium between many parameters which include, but are not limited to, chemical composition of meibum and aqueous tears, ocular surface temperature, and amount of meibum (Butovich et al., 2010; Shine and McCulley, 1996, 2000). If a deviation from what is known as “normal conditions” occurs, we can expect that these could lead to the development of an ocular pathology, e.g. DED.
In our earlier Langmuir trough experiments, the effects of temperature and meibum loads (in μg/cm2), on the properties of MLF were studied (Butovich et al., 2010). The purpose of the current project was to model and quantitatively evaluate the consequences of a change in the amount of FFA in the tear film.
McCulley and Shine reported that the amount of FFA contained in human MGS could be increased in patients with DED (McCulley and Shine, 2001). The effects of such increase in the levels of FFA in MGS on the TFLL, and its consequences for ocular homeostasis in general, are related to the chemical properties of FFA. Very long chain FFA with carbon chains longer than C18 found in meibum are poorly soluble in water, and because of their lower density, tend to float on the surface when mixed with water (Gunstone, 2007). However, moderately long FFA with carbon chains ≤18 are partly soluble in water, especially at physiological pH and above. Moreover, as a consequence of their amphiphilic nature [caused by the fact that one part of the molecule (its carboxyl group) is hydrophilic and lipophobic, and the other part (alkyl chain) is hydrophobic and lipophilic], moderately long FFA have very high surface activity (Gunstone, 2007).
The surfactant properties of FFA vary with their concentration. Very small amounts of these surfactants dissolved in water in the amounts below critical micelle concentration produce true molecular and/or ionic solutions. However, when the concentration is increased, an interesting change occurs: the surfactant molecules reversibly assemble into supramolecular aggregates called micelles. The presence of micelles of amphiphilic compounds in water facilitates the wetting and removal of water-insoluble molecules by incorporation in micelles (Gunstone, 2007).
Chijiiwa studied the effects of OA on the cholesterol monomer activity in micellar bile salt solutions (Chijiiwa, 1987) and concluded that the micellar solubility of cholesterol in the bile solution is increased by the addition of OA. Thus, the addition of OA decreases the monomer activity of cholesterol and drives cholesterol monomers away from aqueous phase to micellar aggregates. Also, the same author reported that OA inhibited the release of cholesterol monomers from mixed micelles (Chijiiwa, 1987).
In our experiments, a similar effect was observed. The solubility of meibomian lipids appeared to be increasing with the addition of small amounts of OA into the subphase. Based on the BAM images (Figure 6) and the recorded π/A isotherms, there is evidence that the integrity of the lipid film formed by the meibomian lipids is highly affected by OA mixed into the subphase. With the FFA-free TBS subphase, BAM clearly demonstrated the formation of MLF on the surface of the aqueous subphase. However, after dissolving FFA in the TBS subphase in concentrations higher than 2.5 μM, MLF started to disintegrate and disappear from the surface, most likely because of its solubilization by OA. This effect was concentration-dependent and at a FFA concentration close to 15 μM, MLF was almost completely disintegrated and/or removed from the surface. However, at low concentrations of OA (i.e. less than 2.5 μM), the topography of MLF remained virtually unchanged. We speculate that the formation of mixed micelles composed of meibomian lipids and OA was partly responsible for these effects (Figure 7). The absence of the solubilization effect of OA pre-mixed with meibum was clearly due to the very low physical amount of OA in such films (between 1.4 and 5.6 μg of OA was tested for the meibum loads of up to 13 μg per trough), which was insufficient to solubilize very large and hydrophobic meibomian lipids even at the highest molar ratio of OA to meibum tested (4:1). On the other hand, the OA amounts in the FFA-containing subphases (17 μg to 510 μg, in 60 mL of a subphase with OA concentrations ranging from 1 to 30 μM), were apparently large enough to cause disintegration of the MLF and/or solubilization of meibomian lipids. Thus, it appears that the amounts of FFA in (aqueous) tears might be more important to the stability of the MLF and TFLL than their amounts in meibum itself, as it is very unlikely that meibum will ever contain enough FFA to cause self-solubilization. Shine and McCulley suggested that unsaturated fatty acids, especially those with a carbon chain length of ≤18 carbons, may be important in determining meibum consistency (Shine and McCulley, 2000). However, our in vitro experiments demonstrated that the presence of such FFA at concentration higher than 2.5 μM in the aqueous subphase might not be beneficial for the TFLL. In fact, the elevated presence of FFA in the aqueous subphase (such as the tear film) may lead to a partial removal of the TFLL, leaving the aqueous tears exposed, and predisposed to DED. As no data is currently available on the typical quantities of FFA in aqueous tears, we have initiated experiments to quantify their presence in human tears under various conditions, the results of which will be reported elsewhere. However, in other biological fluids and tissues, FFA were estimated to be present in concentrations approaching 1 mM. For example, the normal FFA concentration in rat blood was found to be ~ 400 μM (Rodriguez et al., 2010), i.e. even higher than the concentrations tested in our experiments.
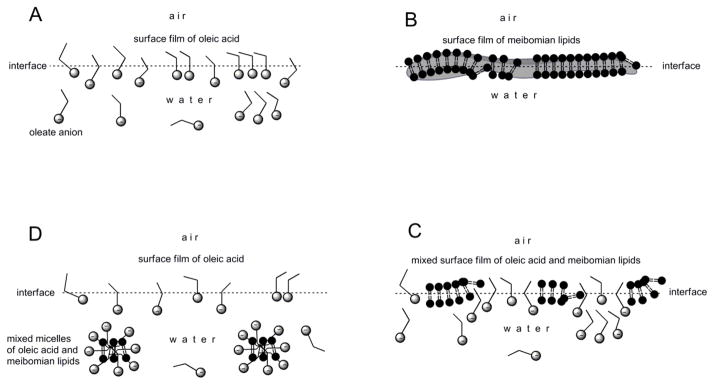
Figure 7. Solubilization effects of free fatty acids dissolved in a TBS subphase on meibomian lipid films (shown clockwise from Panel A to Panel D).
Panel A. Due to its ionization at physiological pH, highly surface active free fatty acids (e.g. unsaturated OA) form a loose, unstable surface film, which coexists in dynamic equilibrium with their molecules and anions dissolved in the bulk of the aqueous subphase. The effects are expected to strongly depend on the chemical nature of the free fatty acid, its concentration, and temperature of the solution.
Panel B. Very diverse, but generally very hydrophobic, meibomian lipids form irregular surface films with varying thicknesses, states of solidification, and degrees of aggregation. Their topography is expected to change with temperature and the meibum loads (in weight/area) (Butovich et al., 2010).
Panel C. When exposed to free fatty acids, meibomian lipid films start to disintegrate and the air-water interface gradually becomes populated by a mixture of meibomian lipids and highly surface active free fatty acids.
Panel D. When the free fatty acid concentration in a TBS subphase is high (>10−5 M), meibomian lipid films completely disintegrate and/or cannot be formed, due to the high surface activity and solubilizing effects of the fatty acid anions.
In conclusion, our in vitro experiments demonstrated that FFA, if dissolved in the aqueous subphase even in small, physiologically relevant micromolar concentrations, could adversely impact meibomian lipid films, causing their collapsing and/or disintegration. This effect(s) could contribute to the onset of those forms of dry eye disease that are associated with enhanced activity of lipolytic enzymes, such as chronic blepharitis.
Research Highlights.
Meibomian lipids form the lipid layer of the human tear film.
Tear film and its lipid layer protect the human ocular surface.
Meibomian lipids were studied in a Langmuir trough and by Brewster angle microscopy.
Micromolar concentrations of free fatty acids disintegrate meibomian lipid films.
Acknowledgments
This study was supported by NIH Grant RO1EY019480 (to I.B) and an unrestricted grant from the Research to Prevent Blindness foundation (New York, NY).
Abbreviations
- BAM
Brewster angle microscope
- πc
collapse pressure
- DED
dry eye disease
- FFA
free fatty acids
- ΔΔG
hysteresis
- πin
initial surface pressure
- Cs−1
in-plane elasticity
- J
N × m, Joule
- AL
lift-off area
- LA
linoleic acid
- πm
maximum surface pressure
- MGD
meibomian gland dysfunction
- MGS
meibomian gland secretion
- MG
Meibomian glands
- MLF
meibomian lipid film
- OA
oleic acid
- π/A
surface pressure-area
- TFLL
tear film lipid layer
- TBS
Tris-buffered saline
- TF
tear film
Footnotes
A preliminary report on the effect of FFA on MLF was presented at ARVO 2009 conference in Fort Lauderdale, FL, USA
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrews JS. Human tear film lipids. I. Composition of the principal non-polar component. Exp Eye Res. 1970;10:223–227. doi: 10.1016/s0014-4835(70)80032-x. [DOI] [PubMed] [Google Scholar]
- Brauninger GE, Shah DO, Kaufman HE. Direct physical demonstration of oily layer on tear film surface. Am J Ophthalmol. 1972;73:132–134. doi: 10.1016/0002-9394(72)90315-7. [DOI] [PubMed] [Google Scholar]
- Bron AJ, Tiffany JM. The meibomian glands and tear film lipids. Structure, function, and control. Adv Exp Med Biol. 1998;438:281–295. doi: 10.1007/978-1-4615-5359-5_40. [DOI] [PubMed] [Google Scholar]
- Butovich IA. On the presence and role of polar lipids in meibum. Invest Ophthalmol Vis Sci. 51:6908–6910. doi: 10.1167/iovs.10-6328. author reply 6910-6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovich IA. On the lipid composition of human meibum and tears: comparative analysis of nonpolar lipids. Invest Ophthalmol Vis Sci. 2008;49:3779–3789. doi: 10.1167/iovs.08-1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovich IA. Lipidomic analysis of human meibum using HPLC-MSn. Methods Mol Biol. 2009a;579:221–246. doi: 10.1007/978-1-60761-322-0_11. [DOI] [PubMed] [Google Scholar]
- Butovich IA. The Meibomian puzzle: combining pieces together. Prog Retin Eye Res. 2009b;28:483–498. doi: 10.1016/j.preteyeres.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovich IA. On the presence and role of polar lipids in meibum. Invest Ophthalmol Vis Sci. 2010;51:6908–6910. doi: 10.1167/iovs.10-6328. author reply 6910-6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovich IA, Arciniega JC, Wojtowicz JC. Meibomian lipid films and the impact of temperature. Invest Ophthalmol Vis Sci. 2010;51:5508–5518. doi: 10.1167/iovs.10-5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovich IA, Uchiyama E, Di Pascuale MA, McCulley JP. Liquid chromatography-mass spectrometric analysis of lipids present in human meibomian gland secretions. Lipids. 2007;42:765–776. doi: 10.1007/s11745-007-3080-2. [DOI] [PubMed] [Google Scholar]
- Chijiiwa K. Oleic acid modulates the partitioning of cholesterol from micellar bile salt solution. Lipids. 1987;22:121–124. doi: 10.1007/BF02534864. [DOI] [PubMed] [Google Scholar]
- Fischer FH, Wiederholt M. The pH dependency of sodium and chloride transport in the isolated human cornea. Invest Ophthalmol Vis Sci. 1978;17:810–813. [PubMed] [Google Scholar]
- Grunfeld F. Langmuir-Blodgett Troughs, Operating Manual. 6. Nima Technology Ltd; Coventry, England: 2004. [Google Scholar]
- Gunstone FD, Harwood John L, Padley FB. The Lipid handbook. 3. Taylor & Francis Group, LLC; Boca Raton, Florida: 2007. [Google Scholar]
- Henon S. Microscope at the Brewster angle: Direct observation of first-order phase transitions in monolayers. Review of Scientific Instruments. 1991;62:936–939. [Google Scholar]
- Joffre C, Souchier M, Gregoire S, Viau S, Bretillon L, Acar N, Bron AM, Creuzot-Garcher C. Differences in meibomian fatty acid composition in patients with meibomian gland dysfunction and aqueous-deficient dry eye. Br J Ophthalmol. 2008;92:116–119. doi: 10.1136/bjo.2007.126144. [DOI] [PubMed] [Google Scholar]
- Kaercher T, Honig D, Mobius D. Brewster angle microscopy. A new method of visualizing the spreading of Meibomian lipids. Int Ophthalmol. 1993;17:341–348. doi: 10.1007/BF00915741. [DOI] [PubMed] [Google Scholar]
- Korchowiec B, Paluch M, Corvis Y, Rogalska E. A Langmuir film approach to elucidating interactions in lipid membranes: 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine/cholesterol/metal cation systems. Chem Phys Lipids. 2006;144:127–136. doi: 10.1016/j.chemphyslip.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Leiske DL, Raju SR, Ketelson HA, Millar TJ, Fuller GG. The interfacial viscoelastic properties and structures of human and animal Meibomian lipids. Exp Eye Res. 90:598–604. doi: 10.1016/j.exer.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Leiske DL, Raju SR, Ketelson HA, Millar TJ, Fuller GG. The interfacial viscoelastic properties and structures of human and animal Meibomian lipids. Exp Eye Res. 2010;90:598–604. doi: 10.1016/j.exer.2010.02.004. [DOI] [PubMed] [Google Scholar]
- McCulley JP, Shine W. A compositional based model for the tear film lipid layer. Trans Am Ophthalmol Soc. 1997;95:79–88. discussion 88–93. [PMC free article] [PubMed] [Google Scholar]
- McCulley JP, Shine WE. Meibomian secretions in chronic blepharitis. Adv Exp Med Biol. 1998;438:319–326. doi: 10.1007/978-1-4615-5359-5_45. [DOI] [PubMed] [Google Scholar]
- McCulley JP, Shine WE. The lipid layer: the outer surface of the ocular surface tear film. Biosci Rep. 2001;21:407–418. doi: 10.1023/a:1017987608937. [DOI] [PubMed] [Google Scholar]
- McCulley JP, Shine WE. The lipid layer of tears: dependent on meibomian gland function. Exp Eye Res. 2004;78:361–365. doi: 10.1016/s0014-4835(03)00203-3. [DOI] [PubMed] [Google Scholar]
- Millar TJ, Mudgil P, Butovich IA, Palaniappan CK. Adsorption of human tear lipocalin to human meibomian lipid films. Invest Ophthalmol Vis Sci. 2009;50:140–151. doi: 10.1167/iovs.08-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar TJ, Tragoulias ST, Anderton PJ, Ball MS, Miano F, Dennis GR, Mudgil P. The surface activity of purified ocular mucin at the air-liquid interface and interactions with meibomian lipids. Cornea. 2006;25:91–100. doi: 10.1097/01.ico.0000164779.87795.3c. [DOI] [PubMed] [Google Scholar]
- Mudgil P, Millar TJ. Adsorption of apo- and holo-tear lipocalin to a bovine Meibomian lipid film. Exp Eye Res. 2008;86:622–628. doi: 10.1016/j.exer.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Mudgil P, Torres M, Millar TJ. Adsorption of lysozyme to phospholipid and meibomian lipid monolayer films. Colloids Surf B Biointerfaces. 2006;48:128–137. doi: 10.1016/j.colsurfb.2006.01.017. [DOI] [PubMed] [Google Scholar]
- Nicolaides N, Kaitaranta JK, Rawdah TN, Macy JI, Boswell FM, 3rd, Smith RE. Meibomian gland studies: comparison of steer and human lipids. Invest Ophthalmol Vis Sci. 1981;20:522–536. [PubMed] [Google Scholar]
- Rodriguez AM, Sanchez J, Tobaruela A, Priego T, Pico C, Palou A. Time-course effects of increased fatty acid supply on the expression of genes involved in lipid/glucose metabolism in muscle cells. Cell Physiol Biochem. 2010;25:337–346. doi: 10.1159/000276566. [DOI] [PubMed] [Google Scholar]
- Shine WE, McCulley JP. Role of wax ester fatty alcohols in chronic blepharitis. Invest Ophthalmol Vis Sci. 1993;34:3515–3521. [PubMed] [Google Scholar]
- Shine WE, McCulley JP. Meibomian gland triglyceride fatty acid differences in chronic blepharitis patients. Cornea. 1996;15:340–346. doi: 10.1097/00003226-199607000-00002. [DOI] [PubMed] [Google Scholar]
- Shine WE, McCulley JP. Association of meibum oleic acid with meibomian seborrhea. Cornea. 2000;19:72–74. doi: 10.1097/00003226-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Tiffany JM. The lipid secretion of the meibomian glands. Adv Lipid Res. 1987;22:1–62. doi: 10.1016/b978-0-12-024922-0.50005-9. [DOI] [PubMed] [Google Scholar]
- Tragoulias ST, Anderton PJ, Dennis GR, Miano F, Millar TJ. Surface pressure measurements of human tears and individual tear film components indicate that proteins are major contributors to the surface pressure. Cornea. 2005;24:189–200. doi: 10.1097/01.ico.0000138837.52694.37. [DOI] [PubMed] [Google Scholar]
- Van Haeringen NJ. Clinical biochemistry of tears. Surv Ophthalmol. 1981;26:84–96. doi: 10.1016/0039-6257(81)90145-4. [DOI] [PubMed] [Google Scholar]