Abstract
Here we demonstrate that ciliated protozoa can jettison mitochondria as intact organelles, releasing their contents to the extracellular space either in a soluble form, or in association with membrane vesicles at the cell periphery. The response is triggered by lateral clustering of GPI-anchored surface antigens, or by heat shock. In the first instance, extrusion is accompanied by elevated levels of intracellular calcium and is inhibited by Verapamil and BAPTA-AM arguing strongly for the involvement of calcium in triggering the response. Cells survive mitochondrial discharge raising the interesting possibility that extrusion is an early evolutionary adaptation to cell stress.
Keywords: Mitochondria, ciliate protozoa, heat shock, GPI-anchor, calcium, organelle extrusion
1.0 Introduction
Mitochondria generate much of the cell’s energy currency in the form of ATP. At the same time, they play critically important roles in a variety of other intracellular processes including calcium homeostasis, maintenance of the cell’s redox state, carbohydrate and lipid metabolism, cell growth, and apoptosis (Graier et al., 2007; Rego and Oliveira, 2003). They are capable of fusion and fission and associate with other intracellular organelles, most notably the endoplasmic reticulum (ER) (Bereiter-Hahn, 1990; Detmer and Chan, 2007; Kornmann et al., 2009). Indeed, mitochondrial constituents have been demonstrated to be present outside the cell suggesting that these organelles are far more dynamic than previously thought (Lyamzaev et al., 2008; Nakajima et al., 2008; Yousefi et al., 2008; Yousefi et al., 2009).
Numerous studies over the past decade have identified mitochondrial proteins in association with the plasma membrane, membrane vesicles (exosomes), and tissue fluids of mammals. These include subunits of ATP synthase (Kenan and Wahl, 2005; Martinez et al., 2003; Moser et al., 2001), superoxide dismutase (Mancini et al., 2006), pyruvate dehydrogenase (Macdonald, 2004), subunit 2 of NADH dehydrogenase (Gingrich et al., 2004), HSP60 (Jean-Eric Alard, 2007; Knowlton, 2007; Stefano et al., 2009) and others (Bhowmick et al., 2009; Falabella et al., 2009; Mark A. Baker, 2004; Sorice et al., 2004). In some instances, these extracellular mitochondrial proteins have been shown to perform unexpected functions. For example, F1ATP synthase associated with the plasma membrane of endothelial cells is capable of generating ATP outside the cell, and can act as a receptor for angiostatin (Kenan and Wahl, 2005). In the same vein, the β-catalytic chain of ATP synthase, and the mitochondrial chaperone HSP60 have been identified as cell surface receptors for high-density lipoproteins such as apolipoprotein A-I in various cell types (Bocharov et al., 2000; Martinez et al., 2003).
While other roles have been ascribed to ectopically localized mitochondrial proteins, the mechanism(s) responsible for their release from living cells remains a central unanswered question. The majority of these proteins normally associate with the inner mitochondrial membrane and lack canonical signal peptides that could potentially direct them to the plasma membrane or constitutive secretory pathway. Although it is now recognized that some proteins can reach the exterior of the cell without transiting the ER and golgi (Hermo and Jacks, 2002; Tveit et al., 2009), how this occurs is still unknown.
Aside from proteins, recent reports have described the catapult-like release of mitochondrial DNA from mammalian granulocytes (Yousefi et al., 2008; Yousefi et al., 2009). Eosinophils and neutrophils appear capable of jettisoning mitochondrial DNA in response to reactive oxygen stress associated with bacterial infection. While dying neutrophils release chromatin to the surrounding tissue space (Brinkmann et al., 2004), mitochondrial DNA appears to be extruded from live cells and is thought to play an essential role in trapping microbial pathogens and limiting their spread (Yousefi et al., 2008; Yousefi et al., 2009). Mitochondrial DNA has also been shown to associate with exosomes released from cultured astrocytes and glioblastoma cells (Guescini et al., 2009). Exosomes are believed to play a general role in intercellular information exchange, and could potentially carry mitochondrial genes from cell-to-cell (Cocucci et al., 2009; Fevrier and Raposo, 2004; Johnstone, 2006; Lotvall and Valadi, 2007). Transfer of mitochondrial DNA from stem cells to cells with nonfunctional mitochondria as a means of rescuing aerobic respiration has, in fact, been described (Spees et al., 2006). To date the mechanisms responsible for the release of mitochondrial DNA from mammalian granulocytes and stem cells are completely unknown.
The shedding of GPI-linked surface antigens from ciliated protozoa following their lateral clustering in the plasma membrane led us to examine the nature of material released in two ciliate species, Tetrahymena thermophila, an established model for basic research, and Ichthyophthirius multifillis, a commercially important pathogen of freshwater fish. Along with membrane aggregates, we were surprised to find large numbers of mitochondria within the extracellular space. Extrusion of mitochondria appeared to be calcium dependent and was triggered by heat shock as well. The mechanism(s) underlying mitochondrial extrusion in these cells, and its presumed benefit for cell physiology are discussed.
2.0 Materials and Methods
2.1 Construction and maintenance of cell lines
The coding region of the 52 kDa i-antigen gene of Ichthyophthirius strain G5 (IAG52B[G5]; AAK94941), was placed under the control of an endogenous, cadmium-inducible (MTT1) gene promoter and introduced into T. thermophila either at the β-tubulin-1 (BTU1) locus, or on high-copy number ribosomal DNA vectors as previously described (Gaertig and Kapler, 1999; Bruns and Cassidy-Hanley, 1999; Shang et al., 2002). Transgenic cell lines were grown to late log/early stationary phase in Neff medium (0.25% proteose peptone, 0.25% yeast extract, 0.55% glucose, and 0.033 mM FeCl3) at 30° C with constant shaking, then treated with 2 μg/ml CdCl2 for 12–16 hr to induce expression of the transgene. Following induction cells were harvested by centrifugation at 300 × g for 3 min at RT. Transformed cells displayed normal swimming behavior and were rapidly immobilized by monoclonal antibody, G3-61, which is specific for the IAG52B[G5] gene product. The calcium reporter construct, GCamP2 (Nakai et al., 2001) was cloned into the shuttle vector, pXS76, and introduced downstream of the endogenous MTT1 promoter in T. thermopila cell lines harboring the IAG52B[G5] i-antigen gene at the BTU1 locus. Ichthyophthirius multifiliis strain G5 was maintained on juvenile channel catfish as previously described (Clark et al., 2001).
2.2 I-antigen cross-linking and heat shock
Tetrahymena cell lines grown in Neff medium were resuspended in buffer A containing 10 mM Tris-HCl, 1 mM CaCl2 (pH 7.4) pre-warmed to 30° C, and treated with hydridoma culture supernatant containing mouse monoclonal antibody, G3-61, at a final dilution of 1:100 (Lin T.L., 1996). Negative controls for each experiment were treated identically but without the addition of primary antibody. In the case of Ichthyophthirius, infective theronts were resuspended in 10 mM Hepes, 1 mM CaCl2 buffer (pH 7.4) prior to antibody treatment. For heat shock, wild type Tetrahymena strain CU428 as well as transgenic cell lines expressing the 52 kDa parasite i-antigen were incubated at 40°C for 1hr in growth media. Following heat shock cell pellets and culture supernatant fractions were by differential centrifugation.
2.3 Confocal Imaging
Cells were fixed in cold 50mM Hepes buffer (pH7.4) containing 4% paraformaldehyde for 1hr at 4° C. Cells were allowed to gravity settle, then washed in 50mM Hepes buffer (pH 7.4) and blocked in phosphate buffered saline (PBS) containing 1% BSA (pH 7.6) for 15min at RT. Samples were then incubated with primary antibodies for 1hr at RT, washed in PBS and incubated 1hr in either FITC-, rhodamine-, or Alexa 633-conjugated secondary antibodies as indicated in the text (Invitrogen). Cells were again washed and mounted in ProLong® Gold anti-fade reagent containing DAPI (Invitrogen). Images were acquired with a Leica SP5 confocal microscope using a 63X water objective. Sequential scanning was used in all double-labeling experiments.
2.4 Electron Microscopy
For visualization of mitochondrial extrusion by negative stain, cells were washed in buffer A, placed on Formvar-coated grids and treated with mAb G3-61 at a final dilution of 1:100. After 1hr at RT, grids were drained on filter paper to remove cells and stained with 2% uranyl acetate and/or 1% K-PTA (potassium phosphotungstate) using standard protocols. Samples containing wild-type T. thermophila treated with an irrelevant antibody served as negative controls. For TEM, cells or high-speed pellets from cell-free culture supernatant fractions were fixed in 4% glutaraldehyde, 0.2M sodium cacodylate (pH 7.4) for 40 min at RT. Samples were then washed in 0.1M sodium cacodylate (pH 7.4) at 4°C, and post-fixed in 2% OsO4 for 1hr at RT. Samples were then dehydrated and infiltrated with epon/araldehyde. Sections were cut with a Reichert microtome (Leica) prior to staining with uranyl acetate and lead citrate. TEM and negatively stained images were taken with a Technai12 electron microscope using an accelerating voltage of 80–100 KV. Emission was set at 2 or 4 and magnifications ranged from 3,000X – 100,000X. For immuno-EM, samples were treated with rabbit polyclonal antisera against the 52 kDa i-antigen, then fixed in 40 mM Hepes buffer (pH7.4) containing 0.15% glutaraldehyde and 4% paraformaldehyde for 1hr on ice, and washed twice in buffer alone. Samples were dehydrated and embedded in LR White. Infiltrated cells were transferred to beam capsules with fresh resin and cured for 24 hr at 50° C prior to sectioning. For i-antigen localization, thin sections were incubated with 1:50 dilutions of secondary gold-labeled anti-rabbit IgG (Jackson Labs) for 12–16 hr at 4° C. For localization of mitochondrial ATPsynthase, sections were incubated with 1:25 dilutions of mouse mAbs against ATPsynthase-ComplexV (Invitrogen) as above, followed by secondary gold-labeled anti-mouse IgG (Jackson Labs) at 1:100 dilutions. In all cases, antibodies were diluted in PBS containing 1% fish gelatin (Ted Pella).
2.5 Western blotting
Control and antibody-treated cells were harvested by low-speed centrifugation as above. Protease inhibitor cocktail (5 X final concentration [Roche]) was added to cell pellets, and cells were lysed in an equal volume of 2 X SDS sample buffer (Sambrook, 1989). Culture supernatant fractions were re-centrifuged to eliminate any contaminating cells, and TCA precipitated by the addition of 1/10 volume 0.15% DOC followed by 1/10 volume of 70% TCA for 30 min on ice. Precipitates were spun down at 13,000×g for 20 min at 4° C, washed in acetone, air dried, and dissolved in 1X SDS sample buffer (pH8.0) containing protease inhibitor cocktail (Roche). Samples were fractionated on 12% SDS-polyacrylamide gels and transferred to nitrocellulose filters (Trans-Blot pure, 0.45μm; Bio-Rad) using standard protocols. Following transfer, membranes were incubated in 150 mM NaCl, 50 mM Tris.HCl (pH 7.4) containing 10% non-fat milk (blocking buffer) at 4° C overnight. Blots were then incubated in the same buffer containing 0.1% Tween-20 and primary antibody diluted as indicated in the text. After 1 hr at RT, membranes were washed in blocking buffer and incubated in the same buffer with HRP-labeled secondary antibody. Membranes were washed again signals developed in Super signal West Pico Luminol/Enhancer Solution and Stable Peroxide Solution mixed 1:1 (Pierce Biotechnologies) before image capture on film (Kodak BioMAX MS) or with a CCD camera (BioRad, Chemigenius).
2.6 PCR
Cells were treated with mAb G3-61 to induce mitochondrial extrusion. Cell pellets and cell-free culture supernatant fractions were collected as above. One-tenth volume of isopropanol containing 1μg/ml UltraPure™ Glycogen (Invitrogen) was then added and samples were incubated at −80° C to precipitate nucleic acid. Precipitates were air dried, diluted in 1x commercially supplied PCR buffer (Invitrogen), and used as the template in PCR reactions. Positive control DNA was prepared from 106 cells diluted in 500 μl PCR buffer, and lysed in buffer containing 50 mM KCl, 10 mM Tris.HCl (pH 8.0), 2.5 mM MgCl2, 0.45% Tween 20, 0.45% NP-40, and Proteinase K at a final concentration of 1 μg/μl for 1 hr at 55° C. Proteinase K was inactivated by incubation at 95° C for 10 min prior to amplification. PCR was carried out in 50 μl reaction volumes using either 5 μl experimental template, or 2.5μl control template. For mitochondrial DNA amplification forward and reverse primers were (CTCTACCACTGAGCTACTTA) and (AAAAATCTGATAAAACTGTT), respectively. For micronuclear DNA amplification, forward and reverse primers were (GATTAATTTACTAGGCACTA) and (TGTTCTCTAAATAGGATTGC), respectively.
2.7 Sytox orange staining and cell survival measurements
Transenic T. thermophila expressing the 52 kDa parasite i-antigen were harvested and resuspended in 1ml buffer A containing 0.01 μM Sytox Orange stain (Invitrogen). After incubation for 5min at 30°C, cells were mounted on coverslips and examined by confocal laser scanning microscopy. Monoclonal antibody, G3-61, was then added directly to the cells as they were being observed. For heat shocked samples, cells were harvested and resuspended in buffer A prior to heat shock at 40°C. After 1 hr, Sytox orange was added and cells were monitored as above.
For cell survival measurements, recombinant Tetrahymena expressing the 52 kDa parasite i-antigen were treated with 1:100 dilution of mAb G3-61 in Neff medium for varying periods of time. Between 125–150 individual cells from control and treated samples were hand-isolated with a fine-bore pipette and transferred hanging drops of fresh medium in Petri dishes. Cultures were maintained at 30°C overnight and survival was assessed based on cells’ ability to divide. Statistical analyses were performed using LR statistics type 3 (P value > 0.2405).
2.8 Intercellular calcium measurements
Double transgenic Tetrahymena (2.5 × 105 cells) co-expressing the 52 kDa parasite i-antigen and GCamP2 calcium reporter were transferred to a cuvette in 2 ml buffer A and fluorescence measurements taken with a PTI spectrofluorometer equipped with Felix 32 software. For inhibitor studies, Verapamil or Bapta AM were added to cultures 1 hr prior to antibody treatment. Cells were then washed into 10mM Tris pH 7.4 and fluorescence measurements taken immediately following addition of mAb-G361.
3.0 Results
3.1 Membrane vesicle shedding in Tetrahymena and Ichthyophthirius
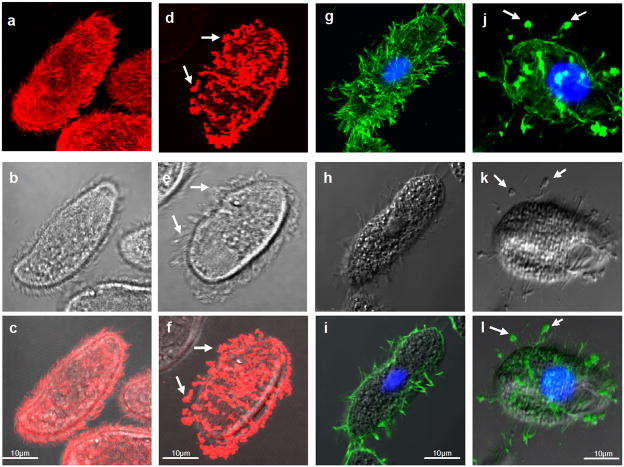
Holotrichous ciliates have abundant GPI-anchored surface proteins known as immobilization antigens (i-antigens) that vary their expression in response to environmental stimuli (Clark, 2003). As shown originally in Paramecium (Kacser, 1957), antibody-driven clustering of these antigens triggers rapid loss of cell movement, and massive rearrangement of the proteins at the cell surface (YB and TGC, unpublished). In the free-living ciliate, Tetrahymena thermophila, and its parasitic cousin, Ichthyophthirius multifiliis, i-antigens aggregate at the cell surface following antibody binding and coalesce at the tips of cilia prior to being shed into the culture medium (Figure 1). At the ultrastructural level, antigen clustering is accompanied by the formation of 50–100 nm vesicles on plasma and ciliary membranes that assemble into large aggregates, which are then shed (Figure S1).
Figure 1. I-antigen shedding from Ichthyophthirius and Tetrahymena.
Panels (a–f): Ichthyophthirius theronts were treated with mAb G3-61 and secondary goat anti-mouse IgG coupled to rhodamine. In (a–c), cells were fixed prior to addition of primary antibody, while in (d–f) cells were treated with primary antibody 30 min prior to fixation. Note the uniform labeling of cilia in (a) and (c), and punctate staining at the tips of cilia in (d) and (f). Panels (g–l): Recombinant Tetrahymena expressing the 52 kDa i-antigen of Ichthyophthirius treated with mAb G3-61 and secondary FITC-conjugate as above. Cells were fixed before (g–i) or 30 min after addition of primary antibody (j–l). Again, note the uniform labeling of ciliary and plasma membranes in (g) and (i), and the punctate staining at the tips of cilia in (j). All confocal images are merged Z-series except for (i) which is a Z-section; middle panels are bright field (b, e) or phase contrast (h, k) images, and bottom panels are merged confocal and bright field or phase contrast images, respectively. Arrows show membrane aggregates at the ciliary tips. Scale bar = 10 μm.
3.2 Mitochondrial extrusion in response to i-antigen clustering
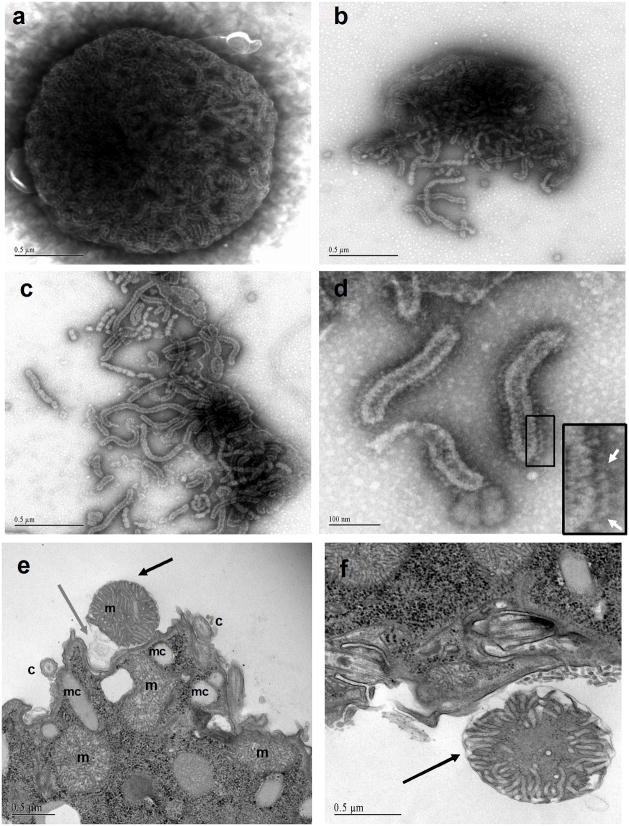
To gain insight into the nature of the material released from cells in response to antigen cross-linking, T. thermophila were placed on Formvar-coated grids, treated with the i-antigen-specific mAb, G3-61, and the cells removed prior to examination of grid surfaces using negative strain. As expected, membrane aggregates resembling those seen by transmission electron microscopy (TEM) were visible in these samples (data not shown). Surprisingly, however, numerous electron dense structures ranging from 1.2–1.7 μm in diameter were also present on the grids (Figure 2). These structures contained outer membranes enclosing tubular endomembrane systems. In many instances the outer membranes were broken open splaying inner tubules onto the grids (Figure 2b–d). At high magnification, the walls of the tubules were studded with characteristic lollypop-shaped structures that bore all the hallmarks of mitochondrial ATP synthase (Figure 2d; inset). To verify that mitochondria were, in fact, being jettisoned from cells in response to i-antigen cross-linking, cells were fixed at different times following mAb addition and examined by TEM. Along with membrane vesicle aggregates, structures readily identifiable as mitochondria were visible outside cells, with the number peaking roughly 2 hr following antibody addition (Figure 2e–f; S1). In some instances mitochondria in the extracellular space were positioned near a pocket in the plasma membrane with veils of membrane extending from the organelle towards the cell surface (Figure 2e; S1). However, plasma membranes appeared intact and there were few if any examples of mitochondria in transit across the lipid bilayer. Based on the size of these organelles, the thickness of the sections and the relative dimensions of the cells, we estimate that 5–20% of total mitochondria were being released.
Figure 2. Material released from antibody-treated T. thermophila.
Transgenic Tetrahymena expessing the 52 kDa i-antigen gene product were exposed to mAb G3-61 on EM grids, and then removed prior to staining of the grids with uranyl acetate. Panel (a) shows a single mitochondrion with inner membrane tubular network. Panels (b–d): Fragmented mitochondria with released inner membrane crystae. Note in panel (d) that inner membrane tubules are inside out and show the characteristic lollipop structures of the F1 subunits of ATP-synthase (arrows, inset). For (a–c) scale bars = 0.5 μm; and for d scale bar = 100nm. Panels (e and f) are transmission electron micrographs showing mitochondria (black arrows) in the extracellular space. Note the membrane veils extending from the extruded organelle in (e) (gray arrow). In all cases, plasma and ciliary membranes were intact as were intracellular mitochondria (m-mitochondria, c-cilia, mc-mucocycts, bb-basal bodies, mv-membrane vesicles). Scale bars = 0.5 μm.
3.3 Redistribution of mitochondrial proteins to the cell surface
To determine whether other ciliates were capable of releasing mitochondria, infective theronts of the common fish parasite, Ichthyophthirius multifiliis, were examined by confocal microscopy before and after i-antigen cross-linking using anti-HSP60 antibodies as a mitochondrial probe. As shown in Figure 3, prior to cross-linking HSP60 was localized within tubular mitochondria at the anterior end of theronts (Figure 3c; and, S3), but was redistributed within a short time of the stimulus often co-localizing with the i-antigens on the cell surface (Figure 3e–g; and, S3). Confocal imaging showed a similar redistribution of mitochondrial ATP synthase following i-antigen cross-linking in T. thermophila (Figure 3i–p). Initially the protein was confined to discrete rows of mitochondria within the cortex (Figure 3k), but became redistributed to the cell surface following i-antigen clustering and in many instances co-localized with i-antigens in membrane blebs at the tips of cilia (Figure 3m–o). Immunogold labeling of thin sections of the same samples confirmed the presence of mitochondrial ATP synthase outside cells within extruded mitochondria and in association with aggregated vesicles on ciliary and plasma membranes (Figure S2). In thick sections, mitochondria were visible either in close proximity to, or embedded within membrane aggregates following i-antigen clustering (Figure S2). These mitochondria were often small and appeared fragmented.
Figure 3. Mitochondrial proteins co-localize with plasma membranes and membrane aggregates.
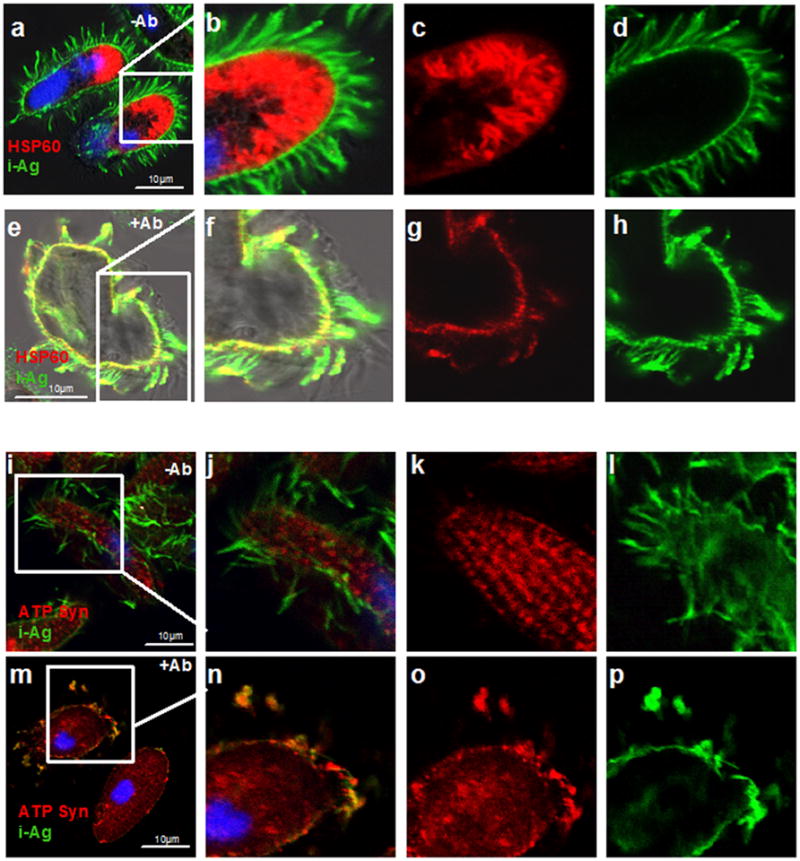
Panels (a–h): Confocal fluorescence images of control (a–d) and mAb G3-61-treated (e–h) Ichthyophthirius multifiliis theronts that were fixed and double labeled with antibodies against the native 52kDa i-antigen and Tetrahymena HSP-60. Following mAb G3-61 treatment, HSP60 was rearranged to the cell periphery and co-localized with i-antigen on plasma and ciliary membranes (e and f) (see also S2). Panels (b) and (f) are merged images of (c) and (d), and (g) and (h), respectively. Panels (i-p): Confocal images of control (i–l) and mAb G3-61-treated T. thermophila (m–p) that were fixed and double-labeled with antibodies against i-antigen and ATP synthase. Controls showed characteristic staining of mitochondria along cortical ciliary rows (k) and uniform labeling of i-antigen on ciliary and plasma membranes (l). Antigen clustering led to dramatic redistribution of mitochondrial staining to the plasma membrane and plasma membrane blebs that in many instances co-localized with i-antigens (n). Panels (j) and (n) are merged images of (k) and (l), and (o) and (p), respectively. DAPI-stained nuclei appear blue.
3.4 Cells survive mitochondrial extrusion
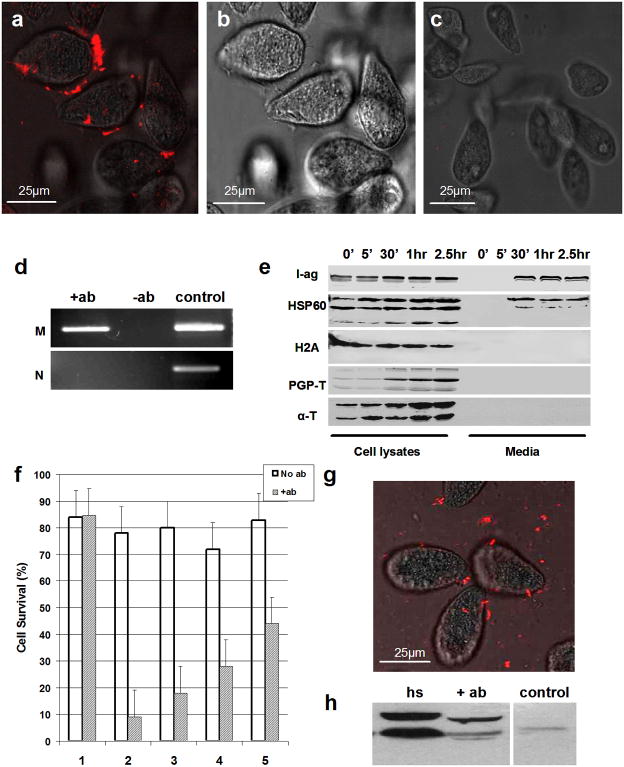
To determine whether mitochondria were simply being released from dead or dying cells, T. thermophila were incubated with the membrane impermeable, DNA intercalating dye, Sytox orange, before or after antibody treatment. While intracellular labeling was absent in both control and antibody-treated groups, bright punctuate staining was clearly visible at the cell periphery within minutes of antibody addition and appeared to localize at the distal tips of cilia over time (Figure 4). The presence of mitochondrial DNA outside cells was verified by PCR using primers that span a region of the T. thermophila mitochondrial genome, and material from cell-free culture supernatant fractions as the template (Figure 4d). PCR on the same samples using primers specific for T. thermophila genomic DNA were negative. Consistent with these findings, Western blots showed clear evidence of HSP60 in cell-free culture supernatant fractions within 30 min of antibody treatment, and an absence of the abundant cytosolic and nuclear proteins, α-tubulin and histone H2A, respectively (Figure 4e).
Figure 4. Mitochondrial DNA and protein in the extracellular space.
Panels (a–c): Fluorescence (a) and bright field (b) images of live transgenic Tetrahymena stained with Sytox orange 40 min after treatment with mAb G3-61. Control (untreated) cells from the same culture are shown in (c). Panel (d): PCR primers for mitochondrial (M) and nuclear (N)-specific DNA sequences were used to amplify material from cell-free culture supernatant fractions of cells before and after antibody treatment (−ab and +ab, respectively). Right lane shows PCR products amplified from total cellular DNA as a positive control. Panel (e): Western blot of cells and media collected at varying times following treatment of transgenic T. thermophila with mAb G3-61. Fractions were probed with antibodies specific for parasite i-antigens, HSP60, histone H2A, polyglycylated-tubulin, and a-tubulin (from top to bottom, respectively). Panel (f): Long-term survival of transgenic T. thermophila treated for 1 hr with mAb G3-61. Single control (no Ab) and antibody-treated cells were transferred to fresh Neff medium in 96 well plates and scored for survival based on their ability to proliferate (group 1). Survival of control and antibody-treated cells preincubated for 30 min 20 mM taxol (group 2); 10 mM oryzalin (group 3); 20 mM nocadozol (group 4); and, 25 mM cytochalasin B (group 5) was scored in the same way. Results show the average % survival for each group in three independent experiments (error bars = SD; N = 250 cells per experiment). No statistically significant difference in survival was seen between antibody-treated and control cells for group 1 (P value = 0.89). Differences in survival between antibody-treated and untreated cells were nevertheless highly statistically significant in all drug-treated groups (i.e. 2–5) (P values <0.0001). Data analysis was carried out using logistical regression and Bonferroni adjustment for multiple pair-wise comparisons. Panel (g): Merged bright field and fluorescence confocal images of live transgenic Tetrahymena subjected to heat shock for 1 hr and stained with Sytox orange. Panel (h): Western blot of cell-free culture supernatant fractions from heat shocked (hs); mAb G3-61-treated (+ab); and, control cultures probed with rabbit polyclonal antibodies against HSP60. Cells were heat shocked or treated with mAb G3-61 for 1 hr in each case.
The lack of intracellular Sytox orange staining together with the results of Western blotting and PCR were a strong indication that cells were intact and had not undergone lysis in response to i-antigen clustering. Furthermore, while cells stopped swimming within minutes of antibody treatment, they regained motility several hours later indicating that mitochondrial extrusion had no long-term affect on cell viability. This was tested at the single cell level by transferring individual T. thermophila treated with mAb G3-61 for 1 hr to fresh media in 96 well plates and then monitoring their ability to divide over the next 24 hrs. Robust logarithmic growth was seen in >80% of single cell clones with no statistically significant differences between control and antibody-treated groups (Figure 4f; group 1).
3.5 Induction of mitochondrial extrusion by heat shock
While Tetrahymena clearly survived mitochondrial discharge, Western blots of whole cell lysates showed elevated levels of HSP60 and tubulin following i-antigen clustering (Figure 4e). Increased expression of both proteins is a hallmark of heat shock and oxidative stress in a variety of cell types including ciliates (McMullin and Hallberg, 1987; Ron and Zeuthen, 1980), and it seemed reasonable that membrane reorganization resulting from i-antigen clustering could elicit a stress response in these cells. With the idea that stress might act as a trigger to induce mitochondrial extrusion, we examined the effect of heat shock on wild type and transgenic Tetrahymena cultures. Remarkably a shift from 30° to 40° C (the standard heat shock temperature for T. thermophila) caused rapid loss of forward swimming movement and the appearance of both HSP60, and Sytox orange staining outside cells within minutes of the stimulus (Figure 4g,h). Indeed, when the relative levels of HSP60 in culture supernatant fractions from antibody-treated and heat shocked cells were compared, heat shock appeared to be an even stronger stimulus for mitochondrial extrusion under the conditions used (Figure 4h). As in the case of antibody treatment, cells survived heat shock with no apparent deleterious effect (data not shown).
3.6 Cytoskeletal perturbation alters cell survival in response to surface antigen cross-linking
The ability of T. thermophila to survive i-antigen clustering and heat shock while at the same time releasing mitochondria to the extracellular space raised the interesting possibility that cells extrude mitochondria to cope with the damaging affects of stress. With the idea that the cytoskeleton might play a critical role in mitochondrial release, we attempted to block extrusion with inhibitors of microtubule or microfilament function and then measured the effect on cell survival. As shown in Figure 4f, pretreatment with the microfilament destabilizing agent, cytochalasin B, or with the microtubule poisons oryzalin, nocodazol, or taxol, had no effect on control (i.e. non-antibody treated) cells, but resulted in a steep decline in cell viability following i-antigen cross-linking. Although suggestive, it was difficult to know whether cell death resulted from a failure to extrude mitochondria in this case since dying cells released mitochondrial DNA and protein to the surrounding medium in a completely indiscriminate manner.
3.7 Mitochondrial extrusion is [Ca++]-dependent
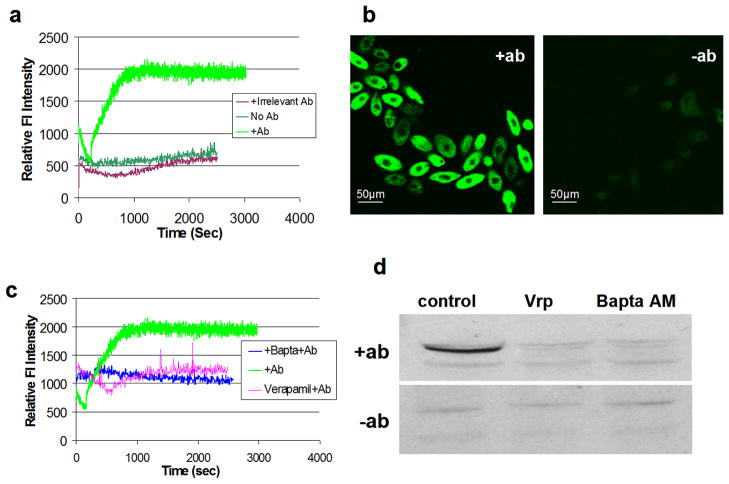
Previous studies have suggested an important role for calcium as a second messenger in the downstream events surrounding i-antigen clustering (Ramanathan et al., 1983). To test whether Ca++-signaling was involved in mitochondrial extrusion as well, relative levels of intracellular Ca++ were measured in T. thermophila before and after i-antigen cross-linking using the circularly permutated GFP-calcium reporter, GCamP2 (Nakai et al., 2001). As shown in Figure 5, addition of mAb G3-61 to cells expressing the GFP reporter resulted in a dramatic and sustained increase in intracellular Ca++ beginning immediately upon antibody addition and lasting an hour or more (Figure 5a,b). Elevation of intracellular Ca++ in response to i-antigen cross-linking was blocked by pretreatment of cells with the L-type calcium channel inhibitor Verapamil (50 μM), or with the calcium chelator, BaptaAM (2 μM) (Figure 5c). To determine whether these drugs also inhibited mitochondrial extrusion, cells were pretreated with either Verapamil or BaptaAM and the release of HSP60 into culture supernatant fractions in response to antibody treatment was determined. As shown in Figure 5d, both drugs prevented the appearance of HSP60 in these fractions arguing strongly for the involvement of elevated intracellular Ca++ in mitochondrial release.
Figure 5. Mitochondrial extrusion is Ca++-dependent.
Panel (a): Spectrofluorometric measurements were made with T. thermophila that were double transgenic for the calcium reporter, GcamP2, and the 52 kDa i-antigen of Ichthyophthirius multifiliis. Cells treated with mAb G3-61 at time zero showed several fold increase in GFP fluorescence as a result of elevated intracellular [Ca++] compared to controls treated with an irrelevant antibody or no antibody at all. Panel (b): Confocal images of the same cells before (right) and after (left) mAb G3-61 treatment. Panel (c): Cells were incubated in Bapta AM or Verapamil for 1 hr and transferred to fresh medium containing mAb G3-61. Elevation of GFP fluorescence was strongly inhibited in both cases. Panel (d): Western blots of cell-free culture supernatant fractions from T. thermophila that were treated with mAb G3-61 for 1 hr (upper panel) or left untreated (lower panel). Blots were probed with rabbit polyclonal antibodies against HSP60. Experimental samples were pre-incubated in 50 mM Verapamil (Vrp) or 2 mM Bapta AM prior to mAb addition.
4.0 Discussion
Taken together, these findings offer the first direct evidence that mitochondria can be jettisoned from eukaryotic cells as intact organelles and that their contents can mix with aggregated vesicles on ciliary and plasma membranes following their release. The association of mitochondrial DNA and protein with the surface membranes of ciliates is reminiscent of recent reports localizing mitochondrial proteins to plasma membranes (Bocharov et al., 2000; Falabella et al., 2009; Kenan and Wahl, 2005; Macdonald, 2004; Martinez et al., 2003; Moser et al., 2001; Sorice et al., 2004) and exosomes (Guescini et al., 2009; Staubach et al., 2009) of mammalian cells, and of similar reports describing the catapult-like release of mitochondrial DNA from mammalian granulocytes (Yousefi et al., 2008; Yousefi et al., 2009). While it remains to be determined whether the same mechanisms are involved in each case, the release of mitochondrial constituents, and their association with surface membrane components of cells from such widely diverged taxa (that is, ciliates and mammals) would argue that the phenomenon is deeply rooted in evolution and has broad significance for cellular physiology as a whole.
The triggers for mitochondrial extrusion in ciliates include clustering of GPI-anchored surface proteins and heat shock. While the signaling cascades involved in each case have yet to be identified, both stimuli would be expected to alter biophysical properties of the plasma membrane, in particular, membrane fluidity. Immobilization antigens are highly abundant GPI-anchored surface proteins (Clark, 2003), and are associated with detergent-resistant membranes (DRMs) in both Ichthyophthirius (YB and TGC, unpublished) and Tetrahymena (Zhang and Thompson, 1997). Synonymous with “lipid rafts”, DRMs are thought to form liquid-ordered microdomains in the plasma membrane whose coalescence via i-antigen clustering would be expected to increase membrane fluidity locally (Mukherjee and Maxfield, 2004). By the same token, heat shock is known to increase membrane fluidity on a more global scale in a wide variety of cell types (Balogh et al., 2005; Shigapova et al., 2005). Increased membrane fluidity can, in turn, activate voltage-gated calcium channels (Dai et al., 2009) and could account for the elevation in intracellular [Ca++] seen here. L-type calcium channels are present in ciliates and are believed to play a central role in the control of ciliary beat (Andrivon, 1988). The fact that Verapamil (a classical L-type calcium channel inhibitor) blocked elevation of intracellular [Ca++] in response to antibody treatment would clearly suggest the involvement of such channels in the overall response.
While increased levels of intracellular Ca++ appear to be required for mitochondrial extrusion in this case, the precise role that calcium plays is unknown. On the one hand, calcium can act as a second messenger in a variety of downstream signaling pathways that control cytoskeletal function and cell survival. At the same time, Ca++ is stored by mitochondria, and excessive levels can damage mitochondrial function leading to reactive oxygen stress (Feissner et al., 2009). As shown here, mitochondrial extrusion in ciliates is triggered by various forms of stress, and strong associations between the production of reactive oxygen species (ROS) and ectopic localization of mitochondrial constituents in mammalian cells have been reported (Lyamzaev et al., 2008; Nakajima et al., 2008). Release of damaged mitochondria from phenylhydrazine-treated reticulocytes was reported as early as 1968 (Simpson and Kling, 1968). More recently, embryonic fibroblasts undergoing TNF-α induced apoptosis have been shown to take up damaged mitochondria into vacuoles that fuse with the plasma membrane(Nakajima et al., 2008). The process is accompanied by the accumulation of ROS and the release of mitochondrial fragments to the extracellular space prior to cell death (Nakajima et al., 2008). HeLa cells treated with respiratory chain inhibitors and uncouplers of oxidative phosphorylation appear to undergo a similar phenomenon (termed “mitoptosis”) in which damaged mitochondria cluster around the nucleus and gather into a vacuole in which they decompose. The vacuole, or “mitoptotic body”, eventually protrudes from the cell and releases mitochondrial constituents to the extracellular space (Lyamzaev et al., 2008). Elimination of decomposing mitochondria in this case is dependent on the formation of ROS (Lyamzaev et al., 2008). Indeed, Skulachev and co-workers have argued that oxidative stress not only triggers the response, but that cells are protected from additional stress through the removal of damaged mitochondria. Finally, the release of mitochondrial DNA from mammalian neutrophils and eosinophils following cytokine priming and the engagement of complement and Toll-like receptors by their respective ligands, also appears to be dependent on the production of ROS (Yousefi et al., 2008; Yousefi et al., 2009). To determine whether ciliates jettison mitochondria as a means of coping with damaging effects of stress, we attempted to block extrusion using inhibitors of cytoskeletal function. Interestingly, we found that induction of i-antigen clustering in the presence of such drugs resulted in cell death, a result that is at least consistent with this hypothesis.
Finally, with regard to the mechanisms responsible for extrusion, there were no instances in which mitochondria were seen transiting the plasma membrane suggesting that the process itself is rapid. Occasionally extracellular mitochondria were positioned near invaginations in the plasma membrane, and there were numerous examples of extracellular mitochondria containing membrane veils that extended towards the plasma membrane (Figure 2; S1). The presence of intact organelles outside cells would tend to rule out the possibility that mitochondria are released via fusion of their outer membranes with the plasma membrane. This leaves only a limited number of models that could account for the phenomenon. One might involve transient opening of the plasma membrane allowing mitochondria to squeeze through. Alternatively, mitochondria could be forced through the plasma membrane in the absence of a fusigenic event (reminiscent of cell-to-cell spread of Listeria moncytogenes (Tilney and Portnoy, 1989)) emerging on the outside surrounded either by their original outer membrane, or a by a third layer contributed by the plasma membrane. Efforts to resolve this question are currently underway.
5.0 Conclusions
Cross-linking of GPI-anchored cell surface proteins, as well as heat shock, have been shown to induce ciliated protozoa to discharge mitochondria into the extracellular space. The initiating trigger for the response appears to be sustained elevation of intracellular [Ca++]. While the mechanisms responsible for mitochondrial extrusion in these cells is unknown, the phenomenon is reminiscent of the rapid release of mitochondrial DNA, and the association of mitochondrial proteins with the outer membranes of mammalian cells suggesting that the phenomenon as a whole may be deeply rooted in evolution.
Supplementary Material
Panel (a) shows the periphery of a cell 30 min following i-antigen cross-linking with mAb G3-61. Membrane vesicles (mv) forming a large aggregate are visible at the base of a cilium. Panel (b) is a thin section through a 100,000 X g pellet of a cell-free culture supernatant fraction derived from cells treated with mAb G3-61. Large aggregates of membrane vesicles along with fragmented mitochondria were readily detected in such fractions. Panels (c, d): Mitochondria in the extracellular space 1 hr following treatment of T. thermophila with mAb G3-61. Cilia (c); mitochondria (m); membrane vesicles (mv); basal body (bb); dense core mucocysts (mc).
Panels (a) and (b): T. thermophila treated for 60 min with polyclonal rabbit antisera against the 52 kDa i-antigen were prepared for transmission EM and thin sections incubated in either gold-labeled secondary (anti-rabbit) IgG to localize i-antigens (panel b), or primary mouse antibodies against ATPsynthase followed by secondary gold-labeled anti-mouse IgG to localize the mitochondrial protein (panel a). Note the labeling of both mitochondria and membrane vesicles in panel (a) inset. Panel (c): Thick section through the cell cortex showing two mitochondria in the extracellular space near membrane vesicle aggregates. Panel (d): Thick section showing what appears to be a single mitochondrion emerging from the cell near the base of a cilium.
Immunocytochemical localization of i-antigens and mitochondrial HSP60 in control (top panels) and mAb G3-61-treated theronts (bottom panels). In each case the panels show optical (Z) sections from the top of cells to the bottom of cells (left to right) emphasizing the movement of HSP60 from the interior in controls, to the cell surface in antibody treated samples. DAPI-stained nuclei are shown in blue.
Highlights.
Ciliated protozoa can jettison intact mitochondria to the extracellular space
Extrusion is triggered by clustering of GPI-linked surface proteins or heat shock
Discharge appears dependent on sustained elevation of intracellular calcium
Mt DNA and protein associate with the plasma membrane during the response
Acknowledgments
We thank Dr. Mandayam Parthasarathy for identifying mitochondria in negatively stained EM images; Ms. Shannon Caldwell & Mr. Richard Medwell for their assistance with TEM and immuno-EM, respectively; Mr. Mozammal Hossein for maintenance of parasites; Dr. Martin Gorosky for his gift of plasmid, pXS76, and mAb, AXO 49; Dr. Tom Fox for the gift of anti-HSP antibodies; Dr. Michael Kotlikoff for providing the GCamP2 reporter construct; Dr. David Russell for the use of his spectrofluorometer; Prof. Tian Long Lin for preparation of immobilizing mAb G3-61; and, Drs. Donna Cassidy-Hanley; David Holowka; and Margaret Bynoe for their helpful discussions. TGC is a founding member of Tetragenetics, Inc. Ithaca, NY and is a member of its scientific advisory board. This work was supported in part by NIH grant 5P40RR019688-06 from the National Center for Research Resources to TGC.
7.0 Role of the Funding Source.
The funding source (NIH grant 5P40RR019688-06) provided salary support for YB but had no input on study design; the collection, analysis, and interpretation of data; in the writing of the report; or the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alard JE, Dueymes M, Youinou P, Jamin C. Modulation of endothelial cell damages by anti-Hsp60 autoantibodies in systemic autoimmune diseases. Autoimmun Rev. 2007;6:438–443. doi: 10.1016/j.autrev.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Andrivon C. Membrane control of ciliary movement in ciliates. Biol Cell. 1988;63:133–142. doi: 10.1016/0248-4900(88)90052-4. [DOI] [PubMed] [Google Scholar]
- Balogh G, Horvath I, Nagy E, Hoyk Z, Benko S, Bensaude O, Vigh L. The hyperfluidization of mammalian cell membranes acts as a signal to initiate the heat shock protein response. Febs J. 2005;272:6077–6086. doi: 10.1111/j.1742-4658.2005.04999.x. [DOI] [PubMed] [Google Scholar]
- Bereiter-Hahn J. Behavior of mitochondria in the living cell. Int Rev Cytol. 1990;122:1–63. doi: 10.1016/s0074-7696(08)61205-x. [DOI] [PubMed] [Google Scholar]
- Bhowmick IP, Kumar N, Sharma S, Coppens I, Jarori GK. Plasmodium falciparum enolase: stage-specific expression and sub-cellular localization. Malar J. 2009;8:179. doi: 10.1186/1475-2875-8-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocharov AV, Vishnyakova TG, Baranova IN, Remaley AT, Patterson AP, Eggerman TL. Heat shock protein 60 is a high-affinity high-density lipoprotein binding protein. Biochem Biophys Res Commun. 2000;277:228–235. doi: 10.1006/bbrc.2000.3663. [DOI] [PubMed] [Google Scholar]
- Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- Bruns PJ, Cassidy-Hanley D. Biolistic transformation of macro- and micronuclei. In: Asai DJ, Forney JD, editors. Tetrahymena thermophila: Methods in cell biology. Vol. 60. Academic Press; London: 1999. pp. 501–512. [DOI] [PubMed] [Google Scholar]
- Clark TG, Forney JD. Free-living and parasitic ciliates. In: Craig A, Scherf A, editors. Antigeni variation. Academic Press; London: 2003. [Google Scholar]
- Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: artefacts no more. Trends Cell Biol. 2009;19:43–51. doi: 10.1016/j.tcb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Dai S, Hall DD, Hell JW. Supramolecular assemblies and localized regulation of voltage-gated ion channels. Physiol Rev. 2009;89:411–452. doi: 10.1152/physrev.00029.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detmer SA, Chan DC. Functions and dysfunctions of mitochondrial dynamics. Nat Rev Mol Cell Biol. 2007;8:870–879. doi: 10.1038/nrm2275. [DOI] [PubMed] [Google Scholar]
- Falabella P, Riviello L, De Stradis ML, Stigliano C, Varricchio P, Grimaldi A, de Eguileor M, Graziani F, Gigliotti S, Pennacchio F. Aphidius ervi teratocytes release an extracellular enolase. Insect Biochem Mol Biol. 2009;39:801–813. doi: 10.1016/j.ibmb.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Feissner RF, Skalska J, Gaum WE, Sheu SS. Crosstalk signaling between mitochondrial Ca2+ and ROS. Front Biosci. 2009;14:1197–1218. doi: 10.2741/3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fevrier B, Raposo G. Exosomes: endosomal-derived vesicles shipping extracellular messages. Curr Opin Cell Biol. 2004;16:415–421. doi: 10.1016/j.ceb.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Findly RC, Gillies RJ, Shulman RG. In vivo phosphorus-31 nuclear magnetic resonance reveals lowered ATP during heat shock of Tetrahymena. Science. 1983;219:1223–1225. doi: 10.1126/science.6828852. [DOI] [PubMed] [Google Scholar]
- Gaertig J, Kapler G. Transient and stable DNA transformation of Tetrahymena thermophila by electroporation. In: Asai DJ, Forney JD, editors. Tetrahymena thermophila: Methods in cell biology. Vol. 60. Academic Press; London: 1999. pp. 485–500. [DOI] [PubMed] [Google Scholar]
- Gingrich JR, Pelkey KA, Fam SR, Huang Y, Petralia RS, Wenthold RJ, Salter MW. Unique domain anchoring of Src to synaptic NMDA receptors via the mitochondrial protein NADH dehydrogenase subunit 2. Proc Natl Acad Sci U S A. 2004;101:6237–6242. doi: 10.1073/pnas.0401413101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graier WF, Frieden M, Malli R. Mitochondria and Ca(2+) signaling: old guests, new functions. Pflugers Arch. 2007;455:375–396. doi: 10.1007/s00424-007-0296-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guescini M, Genedani S, Stocchi V, Agnati LF. Astrocytes and Glioblastoma cells release exosomes carrying mtDNA. J Neural Transm. 2010;117:1–4. doi: 10.1007/s00702-009-0288-8. [DOI] [PubMed] [Google Scholar]
- Gupta S, Knowlton AA. HSP60 trafficking in adult cardiac myocytes: role of the exosomal pathway. Am J Physiol Heart Circ Physiol. 2007;292:H3052–H3056. doi: 10.1152/ajpheart.01355.2006. [DOI] [PubMed] [Google Scholar]
- Hermo L, Jacks D. Nature’s ingenuity: bypassing the classical secretory route via apocrine secretion. Mol Reprod Dev. 2002;63:394–410. doi: 10.1002/mrd.90023. [DOI] [PubMed] [Google Scholar]
- Johnstone RM. Exosomes biological significance: A concise review. Blood Cells Mol Dis. 2006;36:315–321. doi: 10.1016/j.bcmd.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Kacser GHBAH. Studies on the antigens of Paramecium aurelia with the aid of fluorescent antibodies. J Gen Microbiol. 1957;17:68–74. doi: 10.1099/00221287-17-1-68. [DOI] [PubMed] [Google Scholar]
- Kenan DJ, Wahl ML. Ectopic localization of mitochondrial ATP synthase: a target for anti-angiogenesis intervention? J Bioenerg Biomembr. 2005;37:461–465. doi: 10.1007/s10863-005-9492-x. [DOI] [PubMed] [Google Scholar]
- Kornmann B, Currie E, Collins SR, Schuldiner M, Nunnari JK, Weissman JS, Walter P. An ER-mitochondria tethering complex revealed by a synthetic biology screen. Science. 2009;325:477–481. doi: 10.1126/science.1175088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin TL, Clark TG, Dickerson HW. Passive immunization of channel catfish (Ichtalurus punctatus) against the ciliated protozoan parasite Ichthyophthirius multifiliis by use of murine monoclonal antibodies. Infect Immun. 1996;64:4085–4090. doi: 10.1128/iai.64.10.4085-4090.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotvall J, Valadi H. Cell to cell signalling via exosomes through esRNA. Cell Adh Migr. 2007;1:156–158. doi: 10.4161/cam.1.3.5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyamzaev KG, Nepryakhina OK, Saprunova VB, Bakeeva LE, Pletjushkina OY, Chernyak BV, Skulachev VP. Novel mechanism of elimination of malfunctioning mitochondria (mitoptosis): formation of mitoptotic bodies and extrusion of mitochondrial material from the cell. Biochim Biophys Acta. 2008;1777:817–825. doi: 10.1016/j.bbabio.2008.03.027. [DOI] [PubMed] [Google Scholar]
- Macdonald JP, Kirby JA, Jones DEJ. Apoptosis as a mechanism for cell surface expression of the autoantigen pyruvate dehydrogenase complex. Clin Exp Immunol. 2004;136:559–567. doi: 10.1111/j.1365-2249.2004.02484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini A, Borrelli A, Schiattarella A, Fasano S, Occhiello A, Pica A, Sehr P, Tommasino M, Nuesch JP, Rommelaere J. Tumor suppressive activity of a variant isoform of manganese superoxide dismutase released by a human liposarcoma cell line. Int J Cancer. 2006;119:932–943. doi: 10.1002/ijc.21904. [DOI] [PubMed] [Google Scholar]
- Mark A, Baker DJ, Jennifer RL, Ly D, De Pinto V, Lawen A. VDAC1 is a transplasma tembrane NADH-ferricyanide reductase. J Biol Chem. 2004;279:4811–4819. doi: 10.1074/jbc.M311020200. [DOI] [PubMed] [Google Scholar]
- Martinez LO, Jacquet S, Esteve JP, Rolland C, Cabezon E, Champagne E, Pineau T, Georgeaud V, Walker JE, Terce F, Collet X, Perret B, Barbaras R. Ectopic beta-chain of ATP synthase is an apolipoprotein A-I receptor in hepatic HDL endocytosis. Nature. 2003;421:75–79. doi: 10.1038/nature01250. [DOI] [PubMed] [Google Scholar]
- McMullin TW, Hallberg RL. A normal mitochondrial protein is selectively synthesized and accumulated during heat shock in Tetrahymena thermophila. Mol Cell Biol. 1987;7:4414–4423. doi: 10.1128/mcb.7.12.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser TL, Kenan DJ, Ashley TA, Roy JA, Goodman MD, Misra UK, Cheek DJ, Pizzo SV. Endothelial cell surface F1-F0 ATP synthase is active in ATP synthesis and is inhibited by angiostatin. Proc Natl Acad Sci U S A. 2001;98:6656–6661. doi: 10.1073/pnas.131067798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, Maxfield FR. Membrane domains. Annu Rev Cell Dev Biol. 2004;20:839–866. doi: 10.1146/annurev.cellbio.20.010403.095451. [DOI] [PubMed] [Google Scholar]
- Nakai J, Ohkura M, Imoto K. A high signal-to-noise Ca(2+) probe composed of a single green fluorescent protein. Nat Biotechnol. 2001;19:137–141. doi: 10.1038/84397. [DOI] [PubMed] [Google Scholar]
- Nakajima A, Kurihara H, Yagita H, Okumura K, Nakano H. Mitochondrial extrusion through the cytoplasmic vacuoles during cell death. J Biol Chem. 2008;283:24128–24135. doi: 10.1074/jbc.M802996200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan R, Saimi Y, Peterson JB, Nelson DL, Kung C. Antibodies to the ciliary membrane of Paramecium tetraurelia alter membrane excitability. J Cell Biol. 1983;97:1421–1428. doi: 10.1083/jcb.97.5.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rego AC, Oliveira CR. Mitochondrial dysfunction and reactive oxygen species in excitotoxicity and apoptosis: implications for the pathogenesis of neurodegenerative diseases. Neurochem Res. 2003;28:1563–1574. doi: 10.1023/a:1025682611389. [DOI] [PubMed] [Google Scholar]
- Ron A, Zeuthen E. Tubulin synthesis and heat shock-induced cell synchrony in Tetrahymena. Exp Cell Res. 1980;128:303–309. doi: 10.1016/0014-4827(80)90066-x. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press; New York: 1989. [Google Scholar]
- Shang Y, Song X, Bowen J, Corstanje R, Gao Y, Gaertig J, Gorovsky MA. A robust inducible-repressible promoter greatly facilitates gene knockouts, conditional expression, and overexpression of homologous and heterologous genes in Tetrahymena thermophila. Proc Natl Acad Sci U S A. 2002;99:3734–3739. doi: 10.1073/pnas.052016199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigapova N, Torok Z, Balogh G, Goloubinoff P, Vigh L, Horvath I. Membrane fluidization triggers membrane remodeling which affects the thermotolerance in Escherichia coli. Biochem Biophys Res Commun. 2005;328:1216–1223. doi: 10.1016/j.bbrc.2005.01.081. [DOI] [PubMed] [Google Scholar]
- Simpson CF, Kling JM. The mechanism of mitochondrial extrusion from phenylhydrazine-induced reticulocytes in the circulating blood. J Cell Biol. 1968;36:103–109. [PubMed] [Google Scholar]
- Sorice M, Circella A, Cristea IM, Garofalo T, Di Renzo L, Alessandri C, Valesini G, Esposti MD. Cardiolipin and its metabolites move from mitochondria to other cellular membranes during death receptor-mediated apoptosis. Cell Death Differ. 2004;11:1133–1145. doi: 10.1038/sj.cdd.4401457. [DOI] [PubMed] [Google Scholar]
- Spees JL, Olson SD, Whitney MJ, Prockop DJ. Mitochondrial transfer between cells can rescue aerobic respiration. Proc Natl Acad Sci U S A. 2006;103:1283–1288. doi: 10.1073/pnas.0510511103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staubach S, Razawi H, Hanisch FG. Proteomics of MUC1-containing lipid rafts from plasma membranes and exosomes of human breast carcinoma cells MCF-7. Proteomics. 2009;9:2820–2835. doi: 10.1002/pmic.200800793. [DOI] [PubMed] [Google Scholar]
- Stefano L, Racchetti G, Bianco F, Passini N, Gupta RS, Bordignon PP, Meldolesi J. The surface-exposed chaperone, Hsp60, is an agonist of the microglial TREM2 receptor. J Neurochem. 2009;110:284–294. doi: 10.1111/j.1471-4159.2009.06130.x. [DOI] [PubMed] [Google Scholar]
- Tilney LG, Portnoy DA. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol. 1989;109:1597–1608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tveit H, Akslen LK, Fagereng GL, Tranulis MA, Prydz K. A Secretory Golgi Bypass Route to the Apical Surface Domain of Epithelial MDCK Cells. Traffic. 2009;10:1685–1695. doi: 10.1111/j.1600-0854.2009.00984.x. [DOI] [PubMed] [Google Scholar]
- Yousefi S, Gold JA, Andina N, Lee JJ, Kelly AM, Kozlowski E, Schmid I, Straumann A, Reichenbach J, Gleich GJ, Simon HU. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat Med. 2008;14:949–953. doi: 10.1038/nm.1855. [DOI] [PubMed] [Google Scholar]
- Yousefi S, Mihalache C, Kozlowski E, Schmid I, Simon HU. Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death Differ. 2009;16:1438–1444. doi: 10.1038/cdd.2009.96. [DOI] [PubMed] [Google Scholar]
- Zhang X, Thompson GA., Jr An apparent association between glycosylphosphatidylinositol-anchored proteins and a sphingolipid in Tetrahymena mimbres. Biochem J. 1997;323:197–206. doi: 10.1042/bj3230197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Panel (a) shows the periphery of a cell 30 min following i-antigen cross-linking with mAb G3-61. Membrane vesicles (mv) forming a large aggregate are visible at the base of a cilium. Panel (b) is a thin section through a 100,000 X g pellet of a cell-free culture supernatant fraction derived from cells treated with mAb G3-61. Large aggregates of membrane vesicles along with fragmented mitochondria were readily detected in such fractions. Panels (c, d): Mitochondria in the extracellular space 1 hr following treatment of T. thermophila with mAb G3-61. Cilia (c); mitochondria (m); membrane vesicles (mv); basal body (bb); dense core mucocysts (mc).
Panels (a) and (b): T. thermophila treated for 60 min with polyclonal rabbit antisera against the 52 kDa i-antigen were prepared for transmission EM and thin sections incubated in either gold-labeled secondary (anti-rabbit) IgG to localize i-antigens (panel b), or primary mouse antibodies against ATPsynthase followed by secondary gold-labeled anti-mouse IgG to localize the mitochondrial protein (panel a). Note the labeling of both mitochondria and membrane vesicles in panel (a) inset. Panel (c): Thick section through the cell cortex showing two mitochondria in the extracellular space near membrane vesicle aggregates. Panel (d): Thick section showing what appears to be a single mitochondrion emerging from the cell near the base of a cilium.
Immunocytochemical localization of i-antigens and mitochondrial HSP60 in control (top panels) and mAb G3-61-treated theronts (bottom panels). In each case the panels show optical (Z) sections from the top of cells to the bottom of cells (left to right) emphasizing the movement of HSP60 from the interior in controls, to the cell surface in antibody treated samples. DAPI-stained nuclei are shown in blue.