Abstract
While glycosyltransferases are known to display unidirectional enzymatic activity, recent studies suggest that some members can also catalyze readily reversible reactions. Recently we found that mammalian sialyltransferase ST3Gal-II can catalyze the formation of CMP-NeuAc from 5′-CMP in the presence of a donor containing the NeuAcα2,3Galβ1,3GalNAc- unit [Chandrasekaran, E V et al. (2008) Biochemistry 47, 320–330]. The present study shows by using [9-3H] or [14C] sialyl mucin core 2 compounds that ST3Gal-II exchanges sialyl residues between CMP-NeuAc and NeuAcα2,3Galβ1,3GalNAc- unit and also radiolabels sialyl residues in gangliosides GD1a and GT1b, but not GM1. Exchange sialylation proceeds with relative ease as evident from a) radiolabeleling of fetuin was ~2 fold that of asialo fetuin when CMP- [9-3H] NeuAc was generated in situ from 5′-CMP and [9-3H] NeuAcα2,3Galβ1,3GalNAcβ1,3Galα-O-Me by ST3Gal-II; b) ST3Gal-II exchanged radiolabels between [14C] sialyl fetuin and [9-3H] NeuAcα2,3Galβ1,3GalNAcβ1,3Galα-O-Me by generating CMP-[14C] and -[9-3H] NeuAc through 5′-CMP; only 20.3% [14C] and 28.0%[3H] remained with the parent compounds after the sialyl exchange. The [9-3H] sialyl tagged MN Glycophorin A, human chorionic gonadotropin β subunit, GlyCAM-1, CD43, fetuin, porcine Cowper's gland mucin, bovine casein macroglycopeptide, human placental glycoproteins and haptoglobin were analysed by using pronase digestion, mild alkaline borohydride treatment, Biogel P6, lectin-agarose and silica gel thin layer chromatography. Sulfated and sialylated O-glycans were found in GlyCAM-1 and human placental glycoproteins. The present technique has the potential to serve as an important tool as it provides a natural tag for the chemical and functional characterization of O-glycan bearing glycoproteins.
Glycoproteins modulate biological function such as signaling, immune response and tissue development by interaction through terminal sugars (1). These sugars or glycans are commonly either N- linked to asparagine, or O- linked to Ser/Thr residues on protein scaffolds. Among these, mucins are O-glycan rich glycoproteins that often contain tandem repeats of peptide sequences characterized by a high content of Ser, Thr and Pro residues (2–4). Each tissue exhibits a unique pattern of mucinous proteins that can be modified under pathological conditions. Such regulation of mucin expression in cancer epithelial cells influences cell adhesion and tumor invasiveness. Moreover, cancer associated mucins that contain incomplete O-glycan chains are highly immunogenic and these are potential targets for immunotherapy. Whereas the study of N-glycans has progressed well, in part, due to the availability of highly-specific endoglycosidases that can release intact N-glycans, similar universal glycosidases for mucin-type O-glycans are not available. New tools for the study of O-glycosylation are thus desirable. The current study addresses this need by presenting a novel strategy to enzymatically radiolabel O-glycans, and also glycolipids.
O-glycosylation is initiated by the attachment of GalNAc via α-linkages to hydroxyl groups of Ser/Thr that are exposed on the protein surface such as at coils, turns or linker regions. This step is catalyzed by a family of specific UDP GalNAc: polypeptide N-acetylgalacto-saminyltransferases (5,6). Further extension of these glycans is regulated, in large measure, by the distribution of glycosyltransferases and sulfotransferases that are primarily localized in the cellular Golgi (7,8). Sialic acid residues are typically found at the terminal non-reducing ends of O-glycans expressed both on cell-surface and secreted glycoproteins. The attachment of these residues is mediated by enzymes belonging to the sialyltransferase family. While these enzymes are typically thought to uni-directionally catalyze the transfer of sialic acid from a sugar nucleotide donor (CMP-NeuAc) to an acceptor substrate, we recently showed that this reaction can be reversible at least for the case of one mammalian/rat sialyltransferase ST3Gal-II (9). Here, using this process called `reverse sialylation', we demonstrated that ST3Gal-II can synthesize CMP-NeuAc from 5′-CMP and NeuAcα2,3Galβ1,3GalNAc- units of O-glycans, and glycolipids. In addition to this, we now report that this enzyme can also catalyze the direct exchange of NeuAc between CMP-NeuAc and the NeuAcα2,3Galβ1,3GalNAc- units of O-glycans and glycolipids, in the absence of exogenous 5'-CMP. While the precise mechanism for this exchange process is yet to be established, this may be partially attributed to the formation of 5'-CMP in the reaction mixture due to the break-down of CMP-NeuAc. These unique catalytic properties of ST3Gal-II could be utilized for the facile radiolabeling in vitro of sialyl residues in mucin-type structures.
MATERIALS AND METHODS
Materials
Rat recombinant ST3Gal-II (α2, 3(O) ST) was purchased from EMD-Chemicals (San Diego, CA) (9,10). CMP-NeuAc was obtained from Sigma (St. Louis. MO). The synthesis of acceptor molecules used in this study is reported elsewhere (11,12). Human chorionic gonadotropin β subunit (HCGβ) was kindly provided by Dr. A. F. Parlow (National Hormone & Peptide Program, Harbor-UCLA Medical Center). GlyCAM-1(200 μg) and CD 43 (28 μg) were gifts from Drs. S. Rosen (UC, San Francisco) and M. Fukuda (Burham Institute, San Diego). Fetuin, bovine casin macroglycopeptide, and human placenta acetone powder were from Sigma. Cowper's gland mucin (CGM) was available from an earlier study (9). Human placental glycoprotein fraction was isolated from the acetone powder by delipidating with CHCl3/CH3OH (2:1v/v) twice. The air-dried material was suspended in water and mixed in the cold room using Speci-Mix for 16h and then centrifuged at 10,000g at 4°C for 1/2h. An aliquot of the supernatant (2 ml) was subjected to Biogel P60 column (100–200 mesh: 1.0×116.0 cm) chromatography using 0.1 M pyridine acetate (pH5.4) as the eluent. The anthrone positive fractions emerging first as a broad peak were pooled and lyophilized to dryness (25mg).
Enzymology studies
All enzymatic sialylation reactions were carried out in Na cacodylate buffer pH 6.0 in the presence of CMP-[9-3H]NeuAc or CMP[14C]NeuAc (NEN-DuPont). Total CMP-NeuAc concentration, in some cases, was adjusted in individual reactions by supplementing with cold CMP-NeuAc. Products formed were subjected to chromatography using either: a) Biogel -P2 or -P6 chromatography (Fine Mesh; 1.0×116.0 cm) columns with 0.1 M pyridine acetate (pH5.4) as the eluent; or b) Lectin- agarose affinity chromatography using columns of 7ml bed volume of WGA-, VVL-, AAL- or ConA-agarose (Vector Lab, Burlingame, CA) under conditions recommended by supplier (9,10). WGA binds terminal GlcNAc residues of glycans and also glycoproteins via sialyl residues. Using synthetic mucin core2 compounds (10), we have noted that WGA binds to mucin core 2 [9-3H]NeuAcα2,3Galβ1,3 (GlcNAcβ1,6)GalNAcα- [1*] and its derivatives containing substituents on GlcNAc such as 6-OSulfo, β1,4 linked Gal or both and β1,4 linked (3-O-Sulfo)Gal; It does not bind to [9-3H]NeuAcα2,3 or α2,6Galβ1,4GlcNAcβ1,6 (Galβ1,3)GalNAcα-. VVL binds the Tn epitope (GalNAcα-O-Ser/Thr) of mucin glycoproteins. ConA binds to complex biantennary N-linked carbohydrate chains. AAL binds α1,6 or α1,3 linked Fuc residues in carbohydrates.
In some cases, peak fractions from chromatography steps containing radioactivity were pooled, lyophilized to dryness, dissolved in small volume of water and stored frozen at −20°C for further experimentation. Thin layer chromatography using Silica gel GHLF (250μm scored 20×20cm; Analtech Newark DE) was also used for further product separation (9,10).
Preparation of radioactive glycans, glycoproteins and glycolipids
[9-3H]NeuAcα2,3Galβ1,3 (GlcNAc β1,6)GalNAcα-O-Al (9-3H[1]) and [9-3H]NeuAcα2,3Galβ1,3GalNAcβ1,3Galα-O-Me (9-3H[3]) were prepared by incubating synthetic acceptors Galβ1,3(GlcNAcβ1,6)GalNAcα-O-Al and Galβ1,3GalNAcβ1,3Galα-O-Me respectively with ST3GalII and CMP-[9-3H]NeuAc as previously described (10). The radiolabeled compound isolated using Biogel P2 chromatography emerged prior to unreacted trisaccharide in both cases. NeuAcα2,3Galβ1,3(GlcNAcβ1, 6) GalNAcα-O-Al and NeuAcα2,3Galβ1,3GalNAcβ1,3Galα-O-Me were synthesized chemically.
[3H] and [14C] labeled bovine brain gangliosides were prepared by exchange sialylation. To prepare tritiated compound, 13mg bovine brain gangliosides (EMD-Chemicals) was incubated with 20μCi of CMP-[9-3H]NeuAc and 0.2 mU of ST3Gal II in 1.2ml of 0.15M NaCa Codylate pH6.0 at 37°C for 20h. [14C] labeled ganglioside was similarly prepared by mixing 25mg of the bovine brain ganglioside mixture with 4μCi CMP-[14C]NeuAc under identical reaction conditions. Following reaction, the radioactive material emerging at the void volume of a Biogel P2 chromatogram was lyophilized to dryness. This [3H] labeled ganglioside mixture was further purified on the Biogel P6 column where it also emerged at the void volume.
A variety of radioactive glycoproteins were prepared using the sialylation properties of ST3Gal-II. [14C] labeled sialyl fetuin was prepared by incubating 30mg fetuin (Sigma) with 1.5μCi CMP-[14C]NeuAc and 0.1U ST3Gal-II in 0.8ml volume of 0.2M Na cacodylate pH 6.0 for 20h at 37°C. All other [9-3H] sialic acid labeled sialoglycoproteins were made by incubating CMP-[9-3H]NeuAc as such, without any addition of cold CMP-NeuAc, with 0.2U ST3Gal-II in a total reaction volume of 1.2 ml in 0.1 M Na cacodylate pH 6.0 for 16 h at 37°C. The labeled glycoproteins were isolated using Biogel P2 chromatography. The reaction mixtures were separated using Biogel-P2 (1.0×116.0 cm) chromatography. The first radioactive peak emerging at the void volume contained radiolabeled glycoprotein. This was lyophilized to dryness and used in the characterization studies.
Proteolytic treatment and separation of glycopeptides
Pronase-digestion of [9-3H] Sialyl glyoproteins was carried out in 1.0 ml of 0.1M Tris-HCl pH 7.0, 1 mM CaCl2, 1% ethanol and 0.1% NaN3 containing 10 mg Pronase CB (EMD-Chemicals) at 37°C for 24h. After the treatment, the samples were kept frozen at −20°C before fractionation on Biogel P6.
Release of O-glycans from protein backbone and their separation
Mild alkaline borohydride treatment of [9-3H] sialyl glyoproteins was performed in Teflon lined screw-capped test tubes using 1.0M Na borohydride in 0.1 N NaOH in a total volume of 1.0 ml. Samples were incubated at 45°C for 24h, excess borohydride was destroyed by adding drops of acetic acid carefully, and storing frozen samples at −20°C before fractionation on Biogel P6. The outcome of mild alkaline borohydride treatment is illustrated by citing two glycoproteins as examples. As anticipated TLC of radio sialylfetuin after this treatment showed one major component representing NeuAcα2,3Galβ1,3GalNAcα-ol and one minor component NeuAcα2,3Galβ1,3(NeuAcα2,6)GalNAcα-ol whereas HCGβ showed four components two being major representing NeuAcα2,3Galβ1, 3GalNAcα-ol and mucin core-2 NeuAcα2,3Galβ1, 3(Galβ1,4 GlcNAcβ1,6)GalNAcα-ol.
Liquid chromatography coupled with Tandem Mass Spectrometry
The LC separation was performed on a C18 reverse-phase column using a linear gradient of acetonitrile in 0.1% formic acid (9, 10). The sample injection volume was 20μl. Negative-ion electrospray ionization (ESI) was used for the detection of sialylated compounds due to its greater sensitivity than positive – ion ESI (9, 10).
Electrophoresis
To release N-glycans, 14C sialyl labeled fetuin, haptoglobin and apotransferrin were treated with 50 U/ml of PNGaseF (Sigma) in 50mM ammonium bicarbonate, 0.2% SDS, 100 mM 2-mercaptoethanol for 20h at 37°C. Enzyme was denatured by 5 min. boiling in sample buffer. SDS-PAGE was then carried out using polyacrylamide gradient gels. Coomassie blue staining of proteins was performed in some cases. In other cases, following transfer to nitrocellulose membrane, radioactive glycoprotein bands were visualized using phosphorimaging.
RESULTS AND DISCUSSION
Exchange of sialic acid between CMP-NeuAc and synthetic mucin core 2 sialyl compounds
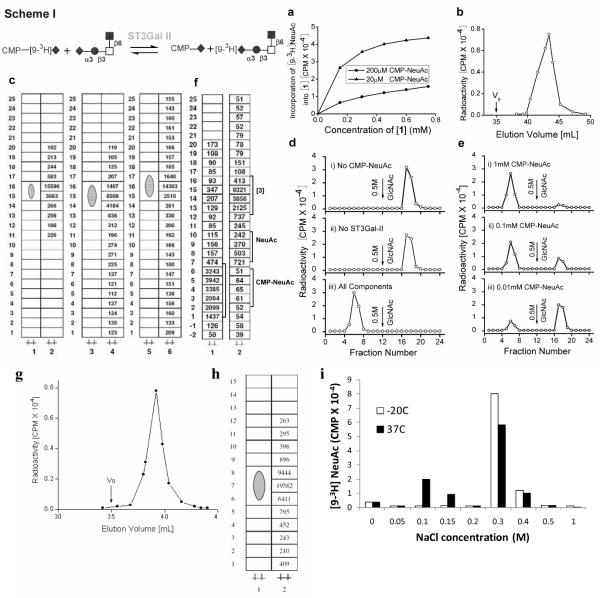
The cloned rat sialyltransferase α2,3(O)ST (ST3Gal-II) mediates α2,3 sialylation of Gal in the Galβ1,3GalNAcα- unit of O-glycans. Additionally, we observed that in the presence of ST3Gal-II and CMP-[9-3H]NeuAc, NeuAcα2,3Galβ1,3(GlcNAcβ1,6)GalNAcα-O-Al [compound 1] gave rise to a radiolabeled product that behaved identical to [1]. Like [1], this product bound to WGA-agarose (a matrix which binds terminal N-acetylglucosamine), with the concentration of the product increasing with concentration of CMP-NeuAc and [1] (Fig. 1a). When the same product was eluted using a Biogel P2 column, a distinct radioactive peak appeared at 40–45mL (Fig. 1b), prior to free CMP-[9-3H]NeuAc which appeared after 60mL. This product, obtained from Biogel column, migrated identically to [1] in TLC plates in three different solvent systems (Fig. 1c).
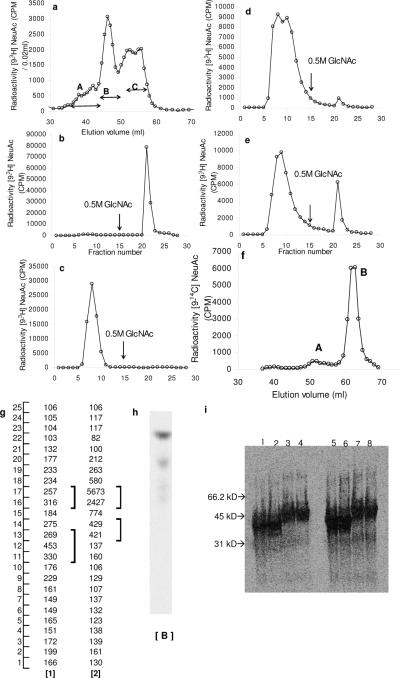
Figure 1. Exchange of Sialyl residues between CMP-[9-3H]NeuAc and NeuAcα2,3Galβ1,3 (GlcNAcβ1,6)GalNAcα-O-Al [1] catalyzed by ST3Gal-II.
(Scheme I symbols: ◆:sialic acid, ●:Gal, ◻:GalNAc, ∎:GlcNAc): a) 0.2μCi [9-3H]CMP-NeuAc was mixed with cold CMP-NeuAc to concentrations of 20μM (low CMP-NeuAc concentration, high specific activity) or 200μM (high concentration, low specific activity). This was added to 0–0.8mM [1] in the presence of 0.16mU enzyme at 37°C for 4h in 100mM NaCa codylate buffer at pH6.0. Reaction volume was 20μl. The product was diluted to 1.0ml using 10mM Hepes pH7.5 containing CaCl2 and MnCl2 and fractioned on a WGA agarose column. Radiolabeled product formed bound WGA agarose affinity column, matrix that binds [1]. b) 0.5mM [9-3H]CMP-NeuAc and 0.3mM [1] were incubated with ST3Gal-II under reaction conditions of panel a and radiolabeled product was isolated using Biogel P2 column. Radiolabeled [1] appeared as a peak between 40–45ml with unreacted [9-3H] CMP-NeuAc appearing at 60mL (not shown). V0 denotes void volume. c) [9-3H]NeuAc labeled product from Biogel P2 column in panel b was subjected to TLC plates in three different solvent systems: 1-propanol/NH4OH/H2O (12/2/5 v/v) developed once (lanes 1,2); CHCl3:CH3OH:H2O (5/4/1/v/v) developed twice (lane 3,4); and ethyl acetate: Pyridine:H2O:Acetic Acid Acetic Acid (5/5/3/1 v/v) developed once (lane 5, 6). In each case, the first lane is the unreacted acceptor while the second lane quantifies the radioactivity associated with product scraped from sections of the TLC plate. d) Reaction mixtures (RM) with the following compositions were incubated at 37°C for 2h in 100mM NaCa codylate buffer, pH6.0: i) RM containing 150μM (0.4μCi) [9-3H]NeuAcα2,3Galβ1,3(GlcNAcβ1,6)GalNAcα-OAl [9-3H[1]] (donor) along with 0.8mU ST3Gal-II but no CMP-NeuAc. ii) RM containing [9-3H[1]] and 1.0mM CMP-NeuAc, but lacking ST3Gal-II. iii) RM containing all components including donor, CMP-NeuAc and enzyme. Products formed were fractionated on a WGA agarose column. Only iii) contained components that did not bind WGA agarose e) WGA agarose affinity chromatography was performed similarly as panel d) with all components in RM except that the amount of CMP-NeuAc was varied. f) TLC of RMs ii) (lane 1) and ii) (lane 2) from panel d) using solvent CHCl3:CH3OH:H2O (5/4/1/v/v) developed twice. Position where radioactivity peak appears is marked by square bracket, along with migration distance for NeuAc, CMP-NeuAc and [9-3H[1]] which were determined in independent run. g. Separation of radiolabeled product from acceptor NeuAcα2,3Galβ1,4(Fucα1,3)GlcNAcβ1,6 (NeuAcα2,3Galβ1,3) GalNAcα-O-Me using Biogel P2 column. h. TLC runs of the radio labeled product and the acceptor using the solvent system 2-propanol/NH4OH/H2O (12/2/5/ v/v). i. CMP-NeuAc was incubated in cacodylade buffer for 4h at either −20°C or 37°C. Dowex-1-formate chromatography was then performed by elution with 3.0mL each of water and various NaCl concentrations as indicated. CMP-NeuAc eluted from the column at 0.3– 0.4M NaCl and free NeuAc at 0.10–0.15M NaCl.
In order to determine if the reverse reaction in scheme-I is feasible, we tested the possibility that CMP-[9-3H]NeuAc can be formed upon addition of ST3Gal-II to a mixture containing cold CMP-NeuAc and tritium labeled compound [1], [9-3H]NeuAcα2,3Galβ1,3(GlcNAcβ1,6) GalNAcα-O-Al (9-3H[1]). For this, 9-3H[1] was prepared using ST3Gal-II, CMP-[9-3H]NeuAc and Galβ1,3(GlcNAcβ1,6)GalNAcα-O-Al (10), and the radiolabeled product was separated from unreacted trisaccharide by Biogel-P2 chromatography. Three different reaction mixtures were then prepared with: i) 9-3H[1] and ST3Gal-II but without CMP-NeuAc, ii) 9-3H[1] and CMP-NeuAc without ST3Gal-II, and iii) everything. When the reaction mixtures were subjected to WGA-agarose affinity chromatography, the radioactive component from i) and ii) but not iii) was bound to the column (Fig. 1d). The data indicate an efficient (>90%) transfer of radioactive [9-3H]NeuAc from 150μM 9-3H[1] to 1.0mM CMPNeuAc in the presence of ST3Gal-II. The extent of enzymatic transfer of [9-3H]NeuAc increased with CMP-NeuAc concentration (Fig. 1e). These observations were also supported by TLC studies (Fig. 1f) where the radioactive component of iii) migrated similar to CMP-NeuAc. The radioactivity from ii) migrated identical to 9-3H[1]. When the disialylated synthetic glycan NeuAcα2,3Galβ1, 4(Fucα1,3)GlcNAcβ1,6 (NeuAcα2,3Galβ1,3)GalNAcα-O-Me [2] was used instead of [1], it gave rise to a radioactive peak at 37–41mL from Biogel P2 column (Fig 1g) and on TLC this radioactive product moved identical to [2] (Fig 1h)The data suggest that ST3Gal-II can catalyze the exchange of NeuAc between CMP-[9-3H]NeuAc and NeuAcα2,3Galβ1,3GalNAc as shown in the forward reaction of scheme-1. Taken together, the data support the feasibility of the sialyl exchange reaction between O-glycans and CMP-NeuAc in presence of ST3Gal-II shown in scheme I.
The mechanism of exchange sialylation is unknown and CMP-NeuAc is resistant to breakdown by NeuAc aldolase, phophomonoesterase, phosphodiesterase and neuraminidase (13). We examined if the breakdown of CMP-NeuAc into 5'-CMP and NeuAc may occur spontaneously under our reaction conditions. To this end, two 80μl mixtures each containing 0.36μm CMP-NeuAc plus CMP-[93H]NeuAc in 0.1M Na cacodylate buffer pH 6.0 containing 2% Triton CF 54 and 10mg/ml BSA were prepared; one was incubated at 37°C for 4 h and the other was stored at −20°C. Both were subjected to Dowex-1-Formate fractionation using stepwise elution of increasing concentration of NaCl. It is evident from Fig 1i that CMP-NeuAc kept at −20°C before subjecting to fractionation was quite stable whereas the one incubated at 37°C for 4h had undergone appreciable (25.3%) breakdown into NeuAc and 5'-CMP. Based on this observation, it may be possible that 5'-CMP formed under our reaction conditions facilitates NeuAc exchange via the “reversible sialylation” mechanism. Additional studies in the future will be needed to confirm this potential mechanism of action.
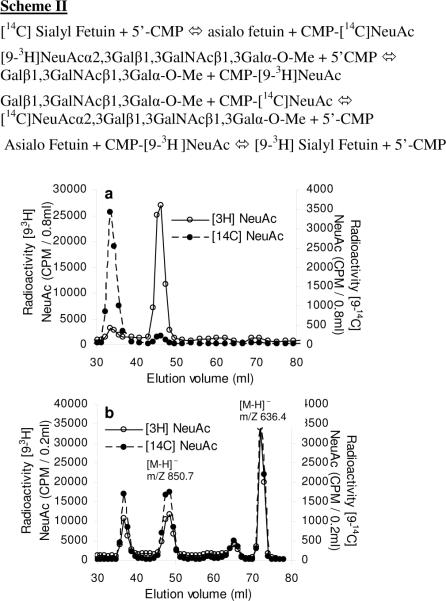
Exchange of [9-3H] and [14C] labeled sialic acid between fetuin and NeuAcα2,3Galβ1,3GalNAcβ1,3Galα-O-Me
We determined if it is possible to use the exchange and reverse sialylation properties of ST3Gal-II to transfer sialic acid between NeuAcα2,3Galβ1,3GalNAcβ1,3Galα-O-Me [3] and fetuin, a major glycoprotein in fetal calf serum. For this reaction, 9-3H[3] was prepared by reacting Galβ1,3GalNAcβ1,3Galα-O-Me with ST3Gal II in the presence of CMP-[9-3H]NeuAc. C-14 labeled fetuin was also prepared by incubating fetuin with CMP-[14C]NeuAc and ST3Gal-II. 9-3H[3] and [14C]fetuin were then mixed with ST3Gal-II, either in the absence (Fig. 2a) or presence (Fig. 2b) of 5′-CMP. After 20h, the reaction mixture was fractionated using Biogel P2 chromatography. Exchange of sialic acid between 9-3H[3] and [14C]fetuin is noted in Fig. 2b in the presence of 5′-CMP. In this panel, 32.3% of the radioactivity from fetuin was transferred to [3]. Also, 18.5% of the [9-3H] sialyl residue from [3] was transferred to [14C]sialyl fetuin. 20.3% [14C] and 28.0% [3H] remained with the parent compounds, while the rest was in the form of either CMP-[14C]NeuAc or CMP-[9-3H]NeuAc. These data are consistent with the reactions outlined in scheme-II, where reverse sialylation results in the formation of radiolabeled CMP-NeuAc. Following this, the exchange of [14C] and [9-3H] sialyl moieties occurs due to the normal sialylation reactions which results in the formation of 3H labeled fetuin and 14C labeled [3].
Figure 2. Enzymatic exchange of sialyl residues between [14C]sialyl fetuin and [9-3H]NeuAcα2,3 Galβ1,3GalNAcβ1,3Galα-O-Me.
In scheme II, 5′-CMP acts as the sialic acid acceptor to form radiolabeled CMP-NeuAc via the reverse sialylation reaction. The newly formed CMP-NeuAc participates in the transfer of sialic acid to fetuin and NeuAcα2,3Galβ1,3GalNAcβ1,3Galα-O-Me via the exchange mechanism. [14C] Sialyl Fetuin (2mg in 100μl water) and 100μl of [93H] NeuAcα2,3Galβ1,3GalNAcβ1,3Galα-O-Me (0.75mM) was diluted into 400μl volume using 0.15M Na cacodylate pH 6.0. 100mU cloned ST3Gal-II was added in the absence (panel a) or presence (panel b) of 10mM 5′-CMP. Reaction proceeded at 37°C for 20h. Reaction mixtures were diluted to 1.0ml with water and fractionated using the Biogel P2 column. The product identity was verified by mass spectrometry as indicated by the molecular weights in the figure.
In order to determine the efficacy of the exchange reaction in the above experiment compared to the efficacy of the forward reaction, we compared the extent to which radioactive sialic acid from the newly formed radioactive CMP-NeuAc in the above scheme is transferred to fetuin versus asialo fetuin. Here, 1mg fetuin (~ 0.016 mol) or 1mg asialo fetuin (~0.018 μmol) in 80 μL of 64 mM NaCacodylate pH 6.0 was incubated with 9-3H[3] (0.037 μmol; 252,000 CPM), 5′-CMP (1.0 μmol or 12mM) and 10 mU of ST3Gal II for 16h at 37°C. The product was separated by Biogel P2 chromatography, and the radioactive fractions corresponding to 9-3H[3], [9-3H] labeled fetuin and CMP-[9-3H]NeuAc were quantified (Table I). Here, in studies with asialo fetuin, we expect to form CMP- [9-3H]NeuAc from 9-3H[3] using reverse sialylation. This would be followed by transfer of a portion of this radioactive sugar-nucleotide to asiaofetuin via the conventional forward sialylation process. Consistent with this, ~11% of the total radioactivity (28,265 CPM) was transferred to asialo fetuin (Table 1). In studies with fetuin, however, we expect to form both CMP- [9-3H]NeuAc from 9-3H[3] and CMP- NeuAc from the sialylated macromolecule. Here, the continuous replenishment of CMP-NeuAc by fetuin is likely to result in greater amounts of CMP-[9-3H]NeuAc release from 9-3H[3] and consequently greater transfer of [9-3H] NeuAc to fetuin. This proposition is consistent with the data in Table 1, where ~54% (135,886 CPM) of the total radioactivity was released from 9-3H [3] and ~21% (53,800 CPM) of the [9-3H]NeuAc was transferred to fetuin. Overall, these results confirm that the exchange/reverse sialylation reaction mediated by ST3Gal II proceeds efficiently. This provides a feasible means to radiolabel sialylated macromolecules.
Table 1.
Extent of exchange versus normal sialylation
| Asialo fetuin | fetuin | |
|---|---|---|
| CPM | CPM | |
| [9-3H]sialylated asialo fetuin or fetuin (Peak I) | 28265 | 53800 |
| Unused [9-3H]sialyl donor [9-3H]NeuAcα2,3 Galβ 1 ,3 GalNAcβ 1, 3Galα-O-Me (Peak II) | 171120 | 115119 |
| Unused CMP- [9-3H]NeuAc (Peak III) | 50570 | 82086 |
| CMP- [9-3H]NeuAc formed (Peaks I+III) | 78835 | 135886 |
Incorporation of [14C] and [9-3H] sialyl residues into gangliosides containing NeuAcα2,3Galβ1,3GalNAc via the exchange reaction
We tested the possibility that the exchange reaction can be used to label gangliosides containing the NeuAcα2,3Galβ1,3GalNAc unit since such molecules may participate in cancer progression (14,15), and since gangliosides are targets of specific immunotherapy in patients with melanoma, colon carcinoma and pancreatic adenocarcinoma (16–19). Further, since serum gangliosides may be more reliable than CA19-9 as an indicator of tumor burden (19), methods to radiolabel them can be useful in the context of cancer.
In the present study, we used a bovine brain ganglioside mixture containing GM1 (18%), GD a (55%) and GT1b (10%) (Fig.3). ST3Gal-II along with either [9-3H] or [14C] labeled CMPNeuAc was added to this ganglioside mixture. After reaction, the radiolabeled ganglioside mixture was analyzed using TLC. Upon comparing Fig 3 lane A2 with lanes A1 and A3, we noted that both [9-3H] and [14C] radioactivities could be localized with GD1a and GT1b but not with GM1. Autoradiography (Lane A1) further showed a band with the mobility closer to GD1a. As this band was minor compared to other bands and since Lane A2 (charring TLC plate) did not show such a band, attempts to identify it were not undertaken. Thus, sialic acid residues in NeuAcα2, 3Galβ1, 3GalNAc- of GD1a and GT1b, but not NeuAcα2, 3Galβ1, 4Glc of GM1, may be exchanged with CMP- NeuAc in the presence of ST3Gal-II. While a small portion of GM1 containing Galβ1, 3GalNAc may be converted into radioactive GD1a via forward sialylation, the labeling of GT1b likely proceeds purely via the exchange mechanism.
Figure 3. Enzymatic exchange of sialyl residues between CMP-NeuAc and gangliosides.
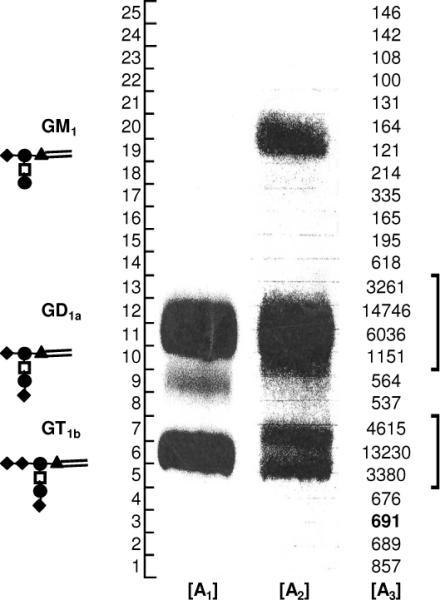
:[9-3H] and [14C] sialic acid were incorporated into bovine brain ganglioside upon addition of either CMP-[14C]NeuAc or CMP-[9-3H]NeuAc to bovine brain gangliosides in the presence of ST3Gal-II. TLC separation used CHCl3:CH3OH:0.2% aqueous CaCl2 (v/v 60:40:9) as the mobile phase. TLC plate containing [14C] sialyl ganglioside mixture was developed by auto radiography (lane A1). TLC plate containing the same sample was also charred with H2S04 in ethanol (lane A2) to locate the gangliosides. Tritium was located on the TLC plates by scraping silica gel from 0.5cm width segments, soaking silica in 2.0ml water and then counting the radioactivity by scintillation counting (lane A3). Structure of gangliosides is shown in cartoon notation (▴:glucose, ◆:sialic acid, ●:Gal, ◻:GalNAc ). Radioactive exchange occurs at the NeuAcα2,3Galβ1,3GalNAc arm of gangliosides.
Characterization of radiolabeled Glycoproteins
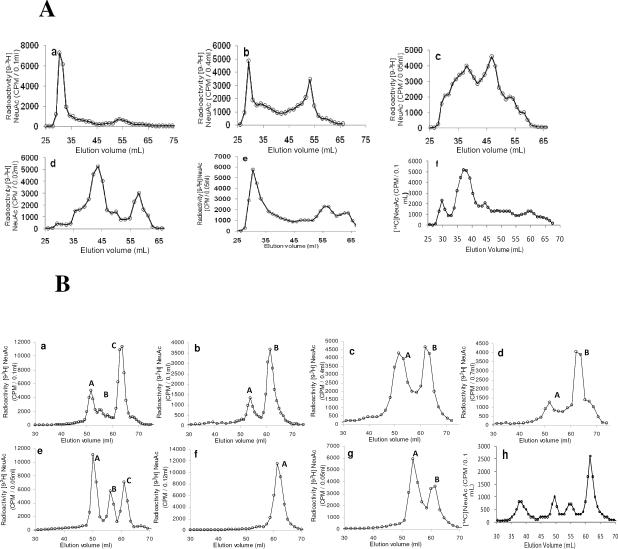
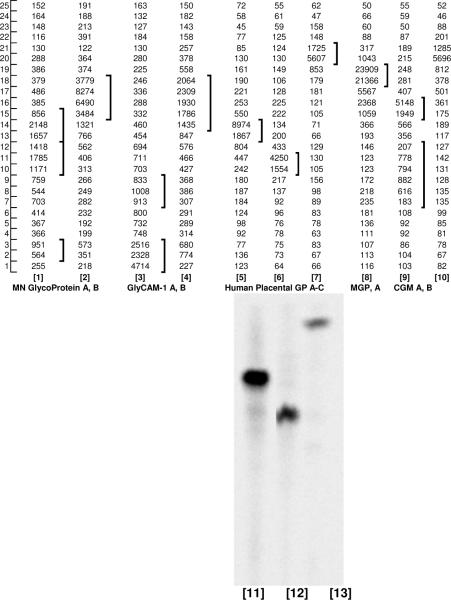
Studies were performed to analyze the nature of sialylated glycoconjugates labeled by ST3Gal II in presence of CMP-NeuAc. Two analysis protocols were followed. Pronase digested glycoproteins were separated using Biogel P6 (Fig. 4A) and this was followed by lectin affinity chromatography. Mild alkaline borohydride treatment of [9-3H] sialyl labeled glyoproteins was used to release O-glycans, the released products were separated using Biogel P6 chromatography (Fig. 4B) and this was followed by TLC analysis. Tables 2 and 3 summarize respectively the results from pronase digestion and mild alkaline borohydride treatment of [9-3H]sialyl labeled glyoproteins.
Figure 4. Characterization [9-3H] Sialyl tagged glycoproteins:
A. Comparing the fragments arising from pronase digestion of [9-3H] Sialyl tagged glycoproteins by Biogel P6 chromatography: a) MN Glycophorin A; b) GlyCAM-1; c) Human placental glycoproteins; d) bovine casein macroglycopeptide (MGP); e) Cowper's gland mucin (CGM) and f) human haptoglobin B. Biogel P6 chromatography of [9-3H]sialylated products arising from mild alkaline borohydride treatment of various glycoconjugates. a) HCGβ, b) MN Glycophorin A, c) GlyCAM-1, d) CD43, e) Human placental glycoproteins (HPG), f) bovine casein macroglycopeptide (MGP) g) Cowper's gland mucin (CGM) and h) human haptoglobin.
Table 2.
Characterization of [9-3H] sialyl labeled glycopeptides obtained following pronase digestion
| [9-3H] sialyl labeled Glycoprotein | [9-3H] sialyl label containing Biogel P6 fractions resulting from Pronase digestion | |
|---|---|---|
| Major Glycopeptide Fractions | Minor Glycopeptide Fractions | |
| I. HCGβ: 31.4% and 10.2% respectively of the glycoprotein binds ConA- and AAL- whereas 100% of it bound WGA-agarose | Four: the largest size one binding to WGA-agarose and the smallest size not binding to WGA-agarose. The middle two fractions contained both WGA binding, non-binding and loosely binding glycopeptides. | None |
| II. MN Glycophorin A | One: binding to WGA-agarose and not binding to VVL-agarose | None |
| III. GlyCAM-1 | Two: the large size one binding to WGA-agarose; The small size one not binding to WGA-agarose | None |
| IV.Fetuin: 100% of glycoprotein binds WGA-agarose | Two: both did not bind to WGA-agarose | One: mostly not binding to WGA-agarose |
| V. Human placental glycoproteins: 98% bound to WGA-agarose | Two: apparently heterogenous | None |
| VI. Bovine casein macroglycopeptide: 60% bound to WGA-agarose | One: large in size | One: small in size |
| VII. Porcine Cowper's gland mucin | One: large in size | Two: smaller in size |
Table 3.
Thin layer chromatographic separation of Biogel P6 fractions obtained from alkaline borohydride treated [9-3H] sialyl labeled glycoproteins
| [9-3H] sialyl labeled glycoprotein | Alkaline borohydride released and separated by Biogel P6 fractions containing [9-3H] sialyl label and their TLC Components | ||
|---|---|---|---|
| A | B | C | |
| I. HCGβ | One major band | One minor and one major bands distinct from A and C | One major band |
| II. MN Glycophorin A | Three distinct bands | One major band distinct from A | |
| III. GlyCAM-1 | Two distinct bands | One band distinct from A | |
| IV. Fetuin | One distinct band | One distinct band | |
| V. Human placental glycoprotein | One distinct band | One distinct band | One distinct band |
| VI. Bovine casein macroglycopeptide | Only one major band | ||
| VII. Porcine Cowper's gland mucin | Two distinct bands | Two distinct bands different from A | |
HCGβ
Human chorianic gonadotropin (HCG) is a glycoprotein hormone produced by placental trophoblasts and trophoblastic tumors. HCG is a heterodimer composed of α-and β-subunits (HCGβ). The α-subunit is shared with the other glycoprotein hormones whereas β-subunit is specific for each hormone. HCGβ contains two Asn linked carbohydrate units (Asn 13 and Asn 30) and four Ser linked oligosaccharides (Ser 121, 127, 132 and 138). The carbohydrate structure of HCGβ derived from pregnancy was found to differ from malignant tumors by Valmu et al (20). They identified core-2 glycans at Ser-121, Ser-127 and Ser-132 and core-1 glycans at Ser-138. They observed that the peptide chain carrying the two O-glycosylation sites Ser-127 and Ser-132 was not specifically cleavable by known proteases. The major N-glycan was biantennary complex type but increased triantennary structures linked to Asn-20 as well as increased fucosylation of Asn-13 bound glycans were identified in cancer. The antibody B152 recognizing mainly the core-2 O-glycans at Ser-132 was shown to be useful for the prediction of Down syndrome pregnancy and in the diagnosis and monitoring of cancer (21).
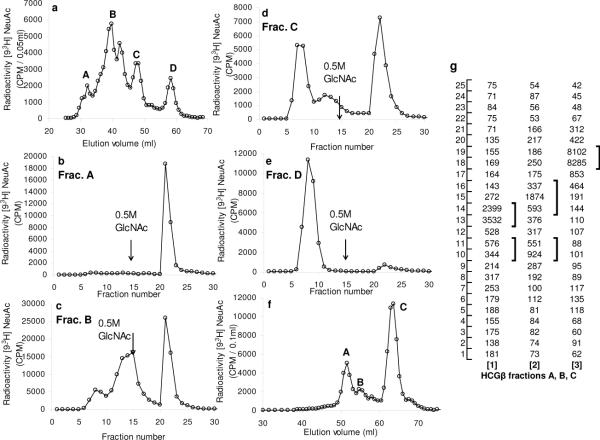
With regard to the above, the present study shows that [9-3H] sialylated HCGβ contained 31.4% Con A-agarose binding and 10.2% AAL-agarose binding glycoproteins. This is consistent with the data that HCGβ contains biantennary complex type N-glycans with some inner core α1, 6 fucosyl residues. An extensive pronase digestion of [9-3H] sialylated HCGβ followed by Biogel P6 chromatography showed four distinct glycopeptide fractions (Fig 5a) probably arising from 3–4 linkage sites containing variable mucin carbohydrate chains as discerned in the present study from their differential binding to WGA-agarose. The largest glycopeptide fraction (A) bound completely (Fig 5b) whereas the smallest one (fraction D) did not bind at all to WGA-agarose (Fig 5e). The two middle fractions (B and C) contained additional WGA-binding glycopeptide fractions (Fig 5c, 5d). Thin layer chromatography of the Biogel P6 fractions (Fig 5f) from mild alkaline borohydride treated [9-3H]sialyl HCGβ indicated the presence of four distinct carbohydrate bands (Fig 5g 1–3). The results suggest that the present radio labeling technique to label mucins may be useful for studying changes in HCGβ due to cancer and other diseases.
Figure 5. Analysis of HCGβ:
WGA-agarose affinity chromatography of the Biogel P6 fractions arising from pronase-digestion of [9-3H] sialyl HCGβ. a. Biogel P6 fractionation of Pronase digested [9-3H]sialyl HCGβ. WGA-agarose chromatography of : b. Pronase fraction A; c. Pronase fraction B; d. Pronase fraction C and e. Pronase fraction D. f. Biogel P6 chromatography of [9-3H]sialylated products arising from mild alkaline borohydride treatment of HCGβ g. TLC analysis of fractions A–C from panel f.
Fetuin
Of the total carbohydrates of fetuin, 21% is located in the O-glycans and the remainder occurring in Asn linked chains (22). It contains three triantennary complex-type sialylated oligosaccharides linked to asparagine and three alkali-labile sialylated O-glycan chains (22). The two mono sialylated disaccharide units (NeuAcα2, 3Galβ1, 3GalNAc) are linked to Ser and Thr residues which are situated in close proximity within no more than 4 amino acid residues (22). The disialylated chain NeuAcα2, 3Galβ1,3 (NeuAcα2, 6)GalNAc is linked to another Ser residue.
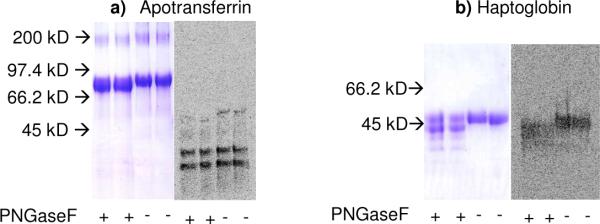
In the present study, pronase digestion of [9-3H]sialyl fetuin gave rise to one minor and two major Biogel P6 glycopeptide fractions, all being included by Biogel P6 (Fig 6a). While [9-3H]sialyl fetuin showed complete binding to WGA-Agarose (Fig 6b), its glycopeptides did not bind to this column, except for the binding of a small portion of the high molecular size fraction (Fig 6c,d and e). Thin layer chromatography of the Biogel P6 fractions from mild alkaline borohydride treated [9-3H]sialyl fetuin (Fig 6f) indicated one major and one minor O-glycan band (Fig 6g and Fig 6h). Treatment of [14C] sialyl fetuin with PNGase F followed by SDS-PAGE (Fig 6i) showed a decrease in the molecular weight of [14C] sialyl fetuin. Further, the radioactive bands had the same intensity before and after PNGase treatment. The data would indicate the specific radiosialyl labeling of O-glycan chains in fetuin by ST3 Gal-II.
Figure 6. WGA-agarose chromatography of [9-3H] Sialyl Fetuin and the Biogel P6 fractions A, B and C arising from pronase-digested [9-3H] Sialyl Fetuin: a).
Biogel P6 fractionation of pronase digested [9-3H]sialyl Fetuin. WGA-agarose chromatography of b) [9-H3]sialyl Fetuin, c) Pronase fraction C, d) Pronase fraction B and e) Pronase fraction A. f) Biogel P6 elution of alkaline borohydride treated [14C] sialyl Fetuin, g) Thin layer chromatography of the Biogel P6 fractions isolated from alkaline borohydride treatment of [9-3H] Fetuin show the presence of one major fraction with radioactivity. h) TLC of dominant fraction identified in panel g (fraction B from panel f) was developed by autoradiography. i) SDS-PAGE of [14C]sialyl fetuin before and after PNGase F treatment. 10 μg (14C-sialylfetuin) was applied to lanes 1,2,3,4, and 20 μg to lanes 5,6,7,8. Lanes 1, 2, 5 and 6 contained PNGase F treated fetuin and 3, 4, 7 and 8 fetuin without PNGase F treatment.
MN Glycophorin A
Glycophorin A (GPA) is a highly glycosylated sialoglycoprotein containing about twelve O-glycans and one N-glycan (23). The blood groups M and N glycophorins differ at the N terminal (Ser1/Gly5 in M antigen, and Leu1/Glu5 in N antigen) and the adjacent amino acid residues (Ser2, Thr3 and Thr4), carry O-linked chains (24). In the present study, [9-3H]sialyl MN GPA gave upon pronase digestion a major glycopeptide fraction excluded by Biogel P6 column: This [9-3H]sialyl glycopeptide fraction showed complete binding to WGA-agarose and complete non-binding to VVL-agarose indicating the clustering of sialylated O-glycans and the absence of Tn epitopes (4).
Alkaline borohydride treatment resulted in one major and one minor Biogel P6 fraction (Fig 4B b). Thin layer chromatography showed one major carbohydrate band and three distinct minor bands (Fig 7 lanes 1and 2). Fukuda et al (25) reported the minor occurrence of novel sialylated large O-glycans in human erythrocyte glycoproteins.
Figure 7. Thin layer chromatography of the Biogel P6 fractions isolated from alkaline borohydride treatment of [9-3H] sialylated glycoproteins:
Lanes 1, 2: MNGlycophorin fractions A, B; Lanes 3, 4: GlyCAM-1 fractions A, B; Lanes 5–7 human placental glycoprotein fractions A, B, C; Lane 8: bovine casein MGP fraction; Lanes 9,10: CGM fractions A, B. Lane 11–13 Fractions A, B, C prepared from [14C] sialyl identical to human placental glycoprotein 5–7 only these were labeled using CMP-[14C] NeuAc and the TLC images were developed by autoradiography.
GlyCAM-1
Hemmerich et al (26,27) identified that GlyCAM-1, the high endothelial venule derived ligand for L-selectin contains 6′-sulfo and 6-sulfo LacNAc as the major disaccharides and NeuAcα2,3(6-SO4) Galβ1,4(Fucα1,3)GlcNAc as the major capping group. The specificities of α1,3-fucosyltransferases FT-III, FT-IV and FT-V (28) and α2,3(N)sialyltransferase (10) indicate the possible existence of the structural unit NeuAc α2,3 Galβ1,4(Fucα1,3) (6-SO4) GlcNAc in GlyCAM-1.
Consistent with these findings, the present study showed that an extensive pronase digestion of [9-3H]sialyl GlyCAM-1 gave rise to two major glycopeptide fractions, one being excluded and the other being included by Biogel P6 column (Table II). These two fractions contain mostly WGA binding and non-binding glycopeptides respectively (Table II). Alkaline borohydride treatment of [9-3H]sialyl GlyCAM-1 led to two major Biogel P6 fractions (Table III; Fig 4B c). TLC of these fractions indicated the presence of three distinct carbohydrate bands in GlyCAM-1 (Fig 7 lanes 3 and 4). Thus GlyCAM-1 differs distinctively from other glycoproteins with respect to O-glycan chains.
CD43
In B-cell precursor (BCP) acute lymphoblastic leukemia, the major selectin ligand on BCP cells is CD43 which is a sialomucin, (29). CD43 was also reported as a ligand for E selectin on CLA+ T cells (30) and in activated T cells (31). In the present study [9-3H]sialyl CD43 on alkaline borohydride treatment gave two Biogel P6 fractions similar to [9-3H]sialyl MN Glycophorin A (Fig 4B d) indicating the presence of disialylated and monosialylated T-hapten structures.
Bovine casein macroglycopeptide (MGP)
Pisano et al (32) identified Thr121, Thr131, Thr133, Thr136, Thr142 and Thr165 as the six sites of O-glycosylation in k-casein macroglycopeptide from bovine milk. No Ser residues are glycosylated. Moreno et al (33) identified by gas chromatographic method GalNAc, Gal and NeuAc as constituents of k-casein macroglycopeptide. In the present study pronase digestion of [9-3H]sialyl casein MGP gave rise to two distinct Biogel P6 fractions (Table II). A clustering of Thr residues (Thr-131, 133, 136, 142) was reported by Pisano et al (32). This would have caused non-cleavage of this peptide portion by pronase and resulted in two distinct Biogel P6 glycopeptide fractions, the major fraction being the large molecular size. Mild alkaline brohydride treatment resulted in one Biogel P6 fraction (Fig 4B f) and TLC of this fraction showed a single component (Fig 7 lane 8) corresponding to the structure NeuAcα2, 3Galβ1, 3GalNAc-ol. These results are consistent with the data reported by others as mentioned above.
Human placental mucin glycoproteins
Rettig et al (34) showed that human placenta expresses the human teratocarcinoma antigen K4, a sulfated and sialylated glycoprotein (160 – 200 KDa). Zimmer et al (35) detected a 36 KDa O-glycosylated sialoglycoprotein in human placenta. Higuchi et al (36) showed the expression of MUC 20 mRNA in human placenta. Human MUC 20 produced in MDCK as well as in HEK 293 indicated a molecular weight of 76 and 79 KDa respectively (35). The present labeling technique was able to reveal more details on the mucin type O-glycan chains of human placental glycoproteins. It showed that [9-3H]sialylated human placental mucin glycoprotein exhibited almost complete binding to WGA-agarose and gave rise to two major heterogenous Biogel P6 fractions upon pronase digestion (Table II). Mild alkaline borohydride treatment resulted in three distinct Biogel P6 fractions (Fig 4B e). When these fractions were subjected to TLC, one distinct component was present in each fraction (Fig 7 lanes 5–7 and 11–13). The TLC mobility of the larger component A was faster than that of the smaller component B, indicating the presence of sulfate group in addition to sialyl group in A (10). The third component C was indentical to NeuAcα2, 3Galβ1, 3 GalNAc-ol.
CGM
Cowper's gland mucin (CGM) is a viscus epithelial glycoprotein (molecular weight ~ 6 × 106 Da) and is the principal constituent of the seminal gel secretion by swine Cowper's gland. Its oligosaccharides linked to Ser/Thr [NeuAcα2, 3 Galβ1, 3 (NeuAcα2, 6)GalNAc and NeuAcα2, 6GalNAc] are distributed all along the polypeptide chains (37). As anticipated with high molecular weight mucins containing clustered carbohydrate chains, pronase treated [9-3H]sialyl CGM gave rise to a major glycopeptide fraction excluded by Biogel P6 column. Mild alkaline borohydride treatment followed by Biogel P6 chromatography showed two fractions (Fig 4B g). Thin layer chromatography showed that CGM contained two distinct major and two minor components (Fig 7 lanes 9 and 10).
Haptoglobin and Apotransferrin
Haptoglobin, known for a long time to contain asparagine linked carbohydrates, has been studied in various types of cancer and other diseases for its N-glycosylation status. Fucosylated haptoglobin in serum has been identified as a marker for pancreatic cancer (38). Fujimura et al. (39) showed recently the presence of mono and disialyl core-1 type O-linked glycans in haptoglobin of prostate cancer sera. In the present study, human plasma haptoglobin (5mg; Calbiochem.) was incubated in 1.2 mL 100 mM Na Cacodylate pH 6.0 containing CMP- [14C]NeuAc (12μM) and ST3 Gal-II (250 mU) for 24 h at 37°C and then subjected to Biogel P6 column chromatography; it was found that 6.4% of [14C]NeuAc was incorporated into haptoglobin. Pronase digestion of 14C sialyl haptoglobin followed by Biogel P6 column chromatography showed a major glycopeptide fraction (46.6% radioactivity) and four minor fractions (Fig 4 A, f). Mild alkaline borohydride treatment of the major glycopeptide fractions followed by Biogel P6 column chromatography showed four distinct radioactive peaks containing 21.9%, 14.7%, 15.1% and 48.3% radioactivity respectively (Fig 4 B h), the pattern apparently being similar to that of human placental mucin glycoproteins (Fig 4B e).
[14C] labeled haptoglobin and [14C] labeled apotransferrin obtained from the sialylation reaction were subjected to PNGase F treatment. When this product was separated using SDS-PAGE followed by Coomassie blue staining and phosphorimaging analysis, it was found that apotransferrin did not get sialylated (Fig 8A). This is reasonable since apotransferrin is known to only contain two N-glycosidic carbohydrate chains. The radiolabeled sialylated product at MW<45kDa detected in this gel is likely a minor contaminant since this was not detected in the Coomassie stained gel. Haptoglobin, on the other hand, was sialylated (Fig 8b). Molecular weight change upon PNGaseF treatment is accompanied by a similar shift in both the Coomassie stained gel and phosphorimaged membrane. Thus, the O-glycans in haptoglobin were specifically labeled by ST3 Gal-II in this study.
Figure 8. Autoradiography of apotransferrin and haptoglobin.
Apotransferrin (panel a) and haptoglobin (panel b) were sialylated using the exchange reaction. These proteins were treated with PNGaseF to remove N-glycans as indicated. 10 μg of each protein was then analyzed using SDS-PAGE with Coomassie blue staining being used to visualize protein and phosphorimaging being applied to monitor 14C-NeuAc incorporation. All samples were analyzed in duplicate.
CONCLUSION
The identification and study of proteins bearing mucin-type O-linked glycans remains challenging both due to the complex nature of their biosynthesis of such structures and due to the lack of well-developed experimental tools (40). While the Bertozzi laboratory has reported a strategy for labeling mucin-type O-linked glyoproteins with a bioorthogonal chemical tag that exploits the presence of a conserved core GalNAc residue in all O-glycans (40), few other tools are available in this field. Such strategies are however necessary for the study of carbohydrate chains that are associated with cancer glycoprotein antigens. The structure of such glycans is tightly regulated primarily based on the tissue-specific repertoire and activity of cellular glycosyltransferase genes, and also based on the availability of sugar nucleotides, and competition between enzymes for acceptor intermediates during glycan elongation. Among the glycosyltransferases, our previous studies show that tumor tissues and cancer cell lines display dominant sialyltrasferase activity towards Galβ1, 3GalNAc (41, 42). Further α1, 2-fucosyltransferase and Gal: 3-0-sulfotransferase acting on Galβ1,3GalNAc are not widely expressed by cancer cells (41–43). Thus, we expect that the NeuAcα2, 3Galβ1, 3GalNAc unit could be a dominant structure in cancer-associated cellular mucins. Based on this knowledge and exploiting the exchange sialylation properties of mammalian sialyltransferase ST3Gal II, we present here a novel strategy to label in vitro glycoproteins containing mucin-type O-glycans. This strategy may also be extended to some specific gangliosides that are involved in disease processes including cancer. As shown in this paper, using this method can be used to radiolabel a range of glycoproteins. Such labeling provides a natural tag for the various structural and functional methodologies that aim to characterize sialyl O-glycan bearing glycoproteins.
Acknowledgments
The study was supported by NIH Grants CA 121294; HL63014 and DOD grant W81XWH-06-1-0013.
Abbreviations
- Al
Allyl
- BSA
Bovine serum albumin
- CMP
Cytidine 5′ monophosphate
- GlyCAM
Glycosylation-dependent cell adhesion molecule
- GM
Monosialo ganglioside
- GD
Disialo ganglioside
- GT
Trisialo ganglioside
- Me
Methyl
- NeuAc
N-acetylneuraminic acid (sialic acid)
- RM
Reaction mixture
- TLC
Thin layer chromatography
- WGA
Wheat germ agglutinin
REFERENCES
- 1.Miyamoto S. Clinical applications of glycomic approaches for the deduction of cancer and other diseases. Current Opinion in Molecular Therapeutics. 2006;8:507–513. [PubMed] [Google Scholar]
- 2.Holingsworth MA, Swanson BJ. Mucins in Cancer: Protection and control of the cell surface. Nat Rev Cancer. 2004;4:45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- 3.Hanisch F-G. O-Glycosylation of the mucin type. J Biol Chem. 2001;382:143–149. doi: 10.1515/BC.2001.022. [DOI] [PubMed] [Google Scholar]
- 4.Baldus SE, Engelmann K, Hanisch F-G. MUC1 and MUCs: A family of human mucins with impact in cancer biology. Crit Rev Clin Lab Sci. 2004;41:189–231. doi: 10.1080/10408360490452040. [DOI] [PubMed] [Google Scholar]
- 5.Brockhausen I. Pathways of O-glycan biosynthesis in cancer cells. Biochim.Biophys Acta. 1999;1473:67–95. doi: 10.1016/s0304-4165(99)00170-1. [DOI] [PubMed] [Google Scholar]
- 6.Van den Steen P, Rudd PM, Dwek RA, Opdenakker G. Concepts and principles of O-linked glycosylation. Crit Rev Biochem Mol Biol. 1998;33:151–208. doi: 10.1080/10409239891204198. [DOI] [PubMed] [Google Scholar]
- 7.Wopereis S, Lefeber DJ, Morava E, Wevers RA. Mechanisms in protein O-glycan biosynthesis and clinical and molecular aspects of protein O-glycan biosynthesis defects: a review. Clin Chem. 2006;52:574–600. doi: 10.1373/clinchem.2005.063040. [DOI] [PubMed] [Google Scholar]
- 8.Tarp MA, Clausen H. Mucin type O-glycosylation and its potential use in drug and vaccine development. Biochim.Biophys Acta. 2008;1780:546–563. doi: 10.1016/j.bbagen.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 9.Chandrasekaran EV, Xue J, Xia J, Locke RD, Matta KL, Neelamegham S. Reversible sialylation: Synthesis of cytidine 5 –monophospho-N-acetylneuraminic acid from cytidine 5 –monophosphate with 2,3-sialyl o-glycan-, glycolipid-, and macromolecule-based donors yields diverse sialylated products. Biochemistry. 2008;47:320–330. doi: 10.1021/bi701472g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chandrasekaran EV, Xue J, Xia J, Chawda R, Piskorz C, Locke RD, Neelamegham S, Matta KL. Analysis of the specificity of sialyltransferases toward mucin core-2, globo and related structures. Identification of the sialylation sequence and the effects of sulfate, fucose, methyl and fluoro substituents of the carbohydrate chain in the biosynthesis of selectin and siglec ligands and noval sialylation by cloned 2, 3(O)sialyltransferase. Biochemistry. 2005;44:15619–15635. doi: 10.1021/bi050246m. [DOI] [PubMed] [Google Scholar]
- 11.Jain R, K., Piskorz CF, Huang BG, Locke RD, Han HL, Koenig A, Varki A, Matta KL. Inhibition of L and P selectin by a rationally synthesized noval core-2 like branched structure containing GalNAc-Lewis X and Neu5Ac 2–3Gal 1–3GalNAc sequences. Glycobiology. 1998;8:707–717. doi: 10.1093/glycob/8.7.707. [DOI] [PubMed] [Google Scholar]
- 12.Xia J, Alderfer JL, Srikrishnan T, Chandrasekaran EV, Matta KL. A convergent synthesis of core-2 branched sialylated and sulfated oligosaccharides. Bioorg Med Chem. 2002;10:3673–3684. doi: 10.1016/s0968-0896(02)00246-8. [DOI] [PubMed] [Google Scholar]
- 13.Warren L. The biosynthesis and metabolism of amino sugars and amino sugar-containing compounds. In: Gottschalk A, editor. Glycoproteins. Elsevier publishing company; New York: 1966. pp. 570–593. [Google Scholar]
- 14.Fuster MM, Esko JD. The sweet and sour of cancer:glycans as noval therapeutic targets. Nat Rev Cancer. 2005;5:526–542. doi: 10.1038/nrc1649. [DOI] [PubMed] [Google Scholar]
- 15.Jeyakumar M, Dwek RA, Butters TD, Platt FM. Storage solutions: treating lysosomal disorders of the brain. Nat Rev Neurosci. 2005;6:1–12. doi: 10.1038/nrn1725. [DOI] [PubMed] [Google Scholar]
- 16.Fredman P, Nilsson O, Svennerholm L, Myrvold H, Persson B, Pettersson S, et al. Colorectal carcinomas have a characteristic ganglioside pattern. Med Biol. 1983;61:45–48. [PubMed] [Google Scholar]
- 17.Morton DL, Ravindranath MH, Irie RF. Tumor gangliosides as targets for active specific immunotherapy of melanoma in man. Prog Brain Res. 1994;101:251–275. doi: 10.1016/s0079-6123(08)61954-8. [DOI] [PubMed] [Google Scholar]
- 18.Ravindranath MH, Bilchik AJ, Chu K, Soh D, Shen P, Morton DL. Anti-GM2 IgM titer inversely correlates with the serum ganglioside level in colon cancer patients after vaccine therapy. Prog Am Assoc Cancer Res. 1999;90:3801. [Google Scholar]
- 19.Chu KU, Ravindranath M, H., Gonzales A, Nishimoto K, Tam WY, Bilchik A, Katopodis N, Morton DL. Gangliosides as targets for immunotherapy for pancreatic adenocarcinoma. Cancer. 2000;88:1828–1836. [PubMed] [Google Scholar]
- 20.Valmu L, Alfthan H, Hotakainen K, Birken S, Stenman U-H. Site-specific glycan analysis of human chorionic gonadotropin -subunit from malignancies and pregnancy by liquid chromatography-electrospray mass spectrometry. Glycobiology. 2006;16:1207–1218. doi: 10.1093/glycob/cwl034. [DOI] [PubMed] [Google Scholar]
- 21.Birken S. Specific measurement of o-linked core-2 sugar containing isoforms of hyperglycosylated human chorionic gonadotropin by antibody B152. Tumor Biology. 2005;26:131–141. doi: 10.1159/000086484. [DOI] [PubMed] [Google Scholar]
- 22.Spiro RG, Bhoyroo VD. Structure of the O-glycosidically linked carbohydrate units of fetuin. J Biol Chem. 1974;249:5704–5717. [PubMed] [Google Scholar]
- 23.Podbielska M, Fredriksson SA, Nilsson B, Lisowska E, Krotkiewski H. ABH blood group antigens in o-glycans of human glycophorinA. Arch Biochem Biophys. 2004;429:145–153. doi: 10.1016/j.abb.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 24.Duk M, Sticher U, Brossmer R, Lisowska E. The differences in significance of 2, 3Gal-linked and 2,6GalNAc-linked sialic acid residues in blood group M- and N-related epitopes recognized by various monoclonal antibodies. Glycobiology. 1994;4:175–181. doi: 10.1093/glycob/4.2.175. [DOI] [PubMed] [Google Scholar]
- 25.Fukuda M, Lauffenburger M, Sasaki H, Rogers ME, Dell A. Structures of noval sialylated o-linked oligosaccharides isolated from human erythrocyte glycophorins. J. Biol Chem. 1987;262:11952–11957. [PubMed] [Google Scholar]
- 26.Hemmerich S, Bertozzi CR, Leffler H, Rosen SD. Identification of the sulfated monosaccharides of GlyCAM -1, an endothelial-derived ligand for L selectin. Biochemistry. 1994;33:4820–4829. doi: 10.1021/bi00182a010. [DOI] [PubMed] [Google Scholar]
- 27.Hemmerich S, Rosen SD. 6-Sulfated sialyl Lewis X is a major capping group of GlyCAM-1. Biochemistry. 1994;33:4830–4835. doi: 10.1021/bi00182a011. [DOI] [PubMed] [Google Scholar]
- 28.Chandrasekaran EV, Jain RK, Larsen RD, Wlasichuk K, DiCioccio RA, Matta KL. Specificity analysis of three clonal and five non-clonal 1,3-lFucosyltransferases with sulfated, sialylated or fucosylated synthetic carbohydrates as acceptors in relation to the assembly of 3 –sialyl-6 –sulfo Lewis x (the L-selectin ligand) and related complex structures. Biochemistry. 1996;35:8925–8933. doi: 10.1021/bi952194e. [DOI] [PubMed] [Google Scholar]
- 29.Nonomura C, Kikuchi J, Kiyokawa N, Ozaki H, Mitznaga K, Ando H, Kanamori A, Kannagi R, Fujimoto J, Muroi K, Furukawa Y, Nakamura M. CD43, but not P-selectin glycoprotein ligand-1, functions as an E-selectin counter-receptor in human pre-B-cell leukemia NALL-1. Cancer Res. 2008;68:790–799. doi: 10.1158/0008-5472.CAN-07-1459. [DOI] [PubMed] [Google Scholar]
- 30.Fuhlbrigge RC, King SL, Sackstein R, Kupper TS. CD43 is a ligand for E-selectin on CLA+ human T cells. Blood. 2006;107:1421–1426. doi: 10.1182/blood-2005-05-2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsumoto M, Atarashi K, Umemoto E, et al. CD43 functions as ligand for E-selectin on activated T cells. J Immunol. 2005;175:8042–8050. doi: 10.4049/jimmunol.175.12.8042. [DOI] [PubMed] [Google Scholar]
- 32.Pisano T, Packer NH, Redmond JW, Williams KL, Gooley AA. Characterization of o-linked glycosylation motifs in the glycopeptides domain of bovine k-casein. Glycobiology. 1994;4:837–844. doi: 10.1093/glycob/4.6.837. [DOI] [PubMed] [Google Scholar]
- 33.Moreno FJ, Villamiel M, Lopez-Fandino R, Olano A. Analysis of monosaccharides in bovine, caprine and ovine k-casein macropeptide by gas chromatography. Chromatographia. 2001;53:525–528. [Google Scholar]
- 34.Rettig WJ, Cordon-Cardo C, Ng JSC, Oettgen HF, Old LJ, Lloyd KO. High-Molecular-Weight glycoproteins of human teratocarcinoma defined by monoclonal antibodies to carbohydrate determinants. Cancer Res. 1985;45:815–821. [PubMed] [Google Scholar]
- 35.Zimmer G, Oeffner F, von Messling V, Tschernig T, Grone H-J, Klenk H-D, Herrler G. Cloning and characterization of gp36, a human mucin-type glycoprotein preferentially expressed in vascular endothelium. Biochem J. 1999;341:277–284. [PMC free article] [PubMed] [Google Scholar]
- 36.Higuchi T, Orita T, Nakanishi S, Katsuya K, Watanabe H, Yamasaki Y, Waga I, Nanayama T, et al. Molecular cloning genomic structure and expression analysis of MUC20, a noval mucin protein, up-regulated in injured kidney. J Biol Chem. 2004;279:1968–1979. doi: 10.1074/jbc.M304558200. [DOI] [PubMed] [Google Scholar]
- 37.Rana SS, Chandrasekaran EV, Kennedy J, Mendicino J. Purification and structures of Oligosaccharide chains in swine trachea and Cowper's gland mucin glycoproteins. J Biol Chem. 1984;259:12899–12907. [PubMed] [Google Scholar]
- 38.Okuyama N, Ide Y, Nakano M, Nakagawa T, Yamanaka K, Moriwaki K, et al. Fucosylated haptoglobin is a novel marker for pancreatic cancer: A detailed analysis of the oligosaccharide structure and a possible mechanism for fucosylation. Int J Cancer. 2006;118:2803–2808. doi: 10.1002/ijc.21728. [DOI] [PubMed] [Google Scholar]
- 39.Fujimura T, Shinohara Y, Tissot B, Pang P-C, Kurogochi M, Saito S, et al. Glycosylation status of haptoglobin in sera of patients with prostate cancer vs. benign prostate disease or normal subjects. Int J Cancer. 2008;122:39–49. doi: 10.1002/ijc.22958. [DOI] [PubMed] [Google Scholar]
- 40.Hang HC, Yu C, Kato DL, Bertozzi CR. A metabolic labeling approach toward proteomic analysis of mucin-type o-linked glycosylation. Proc Natl Acad Sci (USA) 2003;100:14846–14851. doi: 10.1073/pnas.2335201100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chandrasekaran EV, Xue J, Neelamegham S, Matta KL. The pattern of glycosyl- and sulfotransferase activities in cancer cell lines: a predictor of individual cancer-associated distinct carbohydrate structures for the structural identification of signature glycans. Carbohydr Res. 2006;341:983–994. doi: 10.1016/j.carres.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 42.Chandrasekaran EV, Xue J, Piskorz C, F., Locke RD, Toth K, Slokum H, K., Matta KL. Potential tumor markers for human gastric cancer: an elevation of glycan:sulfotransferases and a concomitant loss of α1,2-fucosyltransferase activities. J cancer Res Clin Oncol. 2007;133:599–611. doi: 10.1007/s00432-007-0206-0. [DOI] [PubMed] [Google Scholar]
- 43.Chandrasekaran EV, Jain RK, Rhodes JM, Chawda R, Piskorz C, Matta KL. Characterization of distinct Gal: 3-O-sulfotransferase activities in human tumor epithelial cell lines and of calf lymph node GlcNAc : 6-O-sulfotransferase activity. Glycoconj J. 1999;16:523–536. doi: 10.1023/a:1007074005371. [DOI] [PubMed] [Google Scholar]