Abstract
Background
Environmental pollutants are thought to be one of major triggers of atopic dermatitis (AD).
Objective
We attempted to evaluate the clinical effects of environment with low indoor pollutant levels on AD management.
Methods
Fifty-one children (mean age 1.7 years) with moderate to severe AD who failed to show improvement with conventional management were recruited. Disease severity was assessed by SCORAD (Scoring of AD) indices. They were admitted in a low pollutant oom for 3-4 days (mean 3.3 days) which was designed to keep low levels of dust, house dust mites, micro-organisms, and indoor air pollutants such as total volatile organic compounds (TVOCs), particulate matter (PM), and so on. Air pollutant levels in the low pollutant room were lower than primary standards defined by the Korean Ministry of Environment. we compared disease severity on admission and after discharge, and the pollutant levels of each patient's home and low pollutant room.
Results
The SCORAD was significantly reduced from 42.0 ± 11 .5 to 29.8 ± 8.9 (p < 0.001) by management in a low pollutant room. PM2.5, PM10, formaldehyde, TVOCs, carbon dioxide, bacterial suspensions, and indoor molds were significantly higher in the patient's home than low pollutant room. Out of 29 patients who deteriorated after discharge to their home, 8 patients were admitted again, and their SCORAD was rapidly decreased from 53.1 ± 16.2 to 39.2 ± 9.8 (p = 0.036).
Conclusion
Indoor air pollutants are likely to affect AD in susceptible individuals. Environmental control to lower indoor air pollutant levels might be necessary for better management of AD in some patients.
Keywords: Atopic dermatitis, Childhood, Environment, Air pollution, Indoor
INTRODUCTION
Atopic dermatitis (AD) is a chronic, pruritic inflammatory skin disease and is one of the most common allergic diseases in children. The prevalence of AD has recently increased worldwide, with an incidence of 10-20% of children in Westernized countries [1]. Although AD has been correlated with hereditary factors [2-4], the rapidly growing incidence of AD in recent years cannot be explained by genetic factors alone. In other words, the mechanisms for AD development involve complex interactions between susceptibility genes and environmental factors [5-8].
The role of the indoor environment in the development of AD has been studied. For example, a recent study reported that indoor redecoration activities before birth and in the first years of life are associated with the development of AD in early childhood [9]. Exposure to volatile organic compounds (VOCs) can damage the epidermal barrier and enhance adverse effects of house dust mites on sensitized subjects with AD [10]. Formaldehyde and nitrogen dioxide (NO2) at domestic concentrations cause skin barrier function impairment in patients with AD [11]. Environmental pollutants seem to activate non-specific inflammation, lead to mechanical responses, or modify the T helper (Th) 2 response to allergens [12]. In addition, Staphylococcus aureus is also thought to be one of the major environmental triggers of AD [13].
Although previous studies have been carried out to identify adverse environmental effects on AD symptoms [9-11], few reports have been published regarding the relationship between the indoor air pollutant levels and AD symptoms. Therefore, we performed this study to evaluate clinical effects of an indoor environment with low pollutant levels on disease severity in children with AD.
MATERIALS AND METHODS
Study subjects
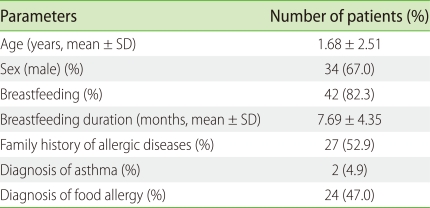
In this study we recruited 51 children with AD whose symptoms did not improve with conventional management in our outpatient clinic. Out of 51 children, 52.9% of patients have family histories of allergy. In past medical history, 4.9% of patients have diagnosed with asthma, and 47% had food allergy. About 82% (42 children) were breastfed; the mean duration of breastfeeding was 7.7 months (Table 1).
Table 1.
Demographic characteristics of the participants (n = 51)
AD was diagnosed by using Hanifin and Rajka's criteria [14], and the severity of AD was assessed by the SCORAD (SCORing of Atopic Dermatitis), ranging from 0 to 103 [15]. Those who were enrolled in this study had moderate to severe AD, which was determined by SCORAD indices ≥ 15. AD severity was assessed on the day of hospitalization, at discharge and in the outpatient clinic within a month after discharge. This study was approved by the Institutional Review Board of Samsung Medical Center, and written informed consent was obtained from all parents prior to participation in this study.
Low pollutant room
We prepared a low pollutant room in our pediatric wards which was designed to have low levels of dust, house dust mite, micro-organisms, and indoor air pollutants such as total VOCs (TVOCs), formaldehyde, carbon monoxide (CO), carbon dioxide (CO2), and NO2. The room was constructed and decorated with environment-friendly building materials. In addition, an air curtain (Spi System; Samsung Electronics, Korea) was installed above the entrance, and an air purifier-ventilator (Indoor Air Quality, IAQ100DV; Samsung Electronics, Korea) was put in the room.
Patients were admitted to the low pollutant room for 3-4 days (mean 3.3 days). During admission, they were educated and managed according to the guideline developed in our hospital. During stay in the low pollutant room, systemic anti-inflammatory treatment was not used, even in severe patients. After discharge, they were instructed to continue management in the same way they did in the hospital.
Indoor air quality measurement
We assessed indoor air quality by measuring the levels of known pollutants in the indoor environment, such as particulate matter (PM2.5 and PM10), formaldehyde, TVOC (benzene, toluene, ethylbenzene, xylene, and styrene), CO, CO2, NO2, bacterial suspension, and indoor molds in the low pollutant room regularly once a week. Indoor air quality in the living room of each patient's home was also assessed in the same way. These measurements were performed according to the guideline of the Korean Ministry of Environment [16]. Primary standards for particulate matter, formaldehyde, TVOC, and those for bacterial suspension were also defined by the guideline of the Korean Ministry of Environment [17]. In addition, the standard for indoor molds was decided according to the World Health Organization (WHO) [18].
Statistical analyses
Data were statistically analyzed with SPSS version 13.0 (SPSS, USA). Wilcoxon Signed Rank test was used to compare SCORAD indices before and after hospitalization. The t test and analysis of variance were applied to compare the pollutant levels of patients' homes and the low pollutant room. A p value less than 0.05 was considered to be statistically significant.
RESULTS
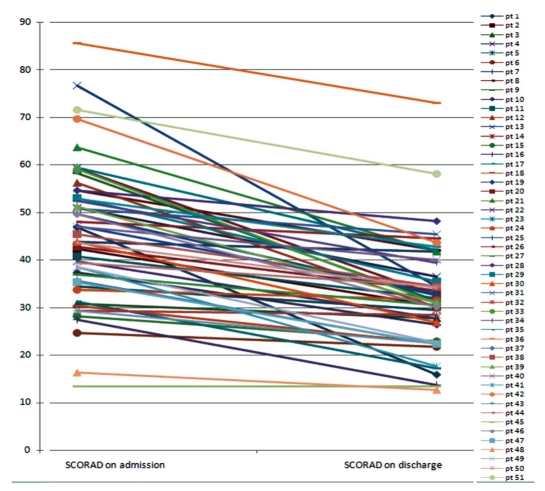
Out of 51 children (males: 34, females: 17), the mean age was 1.7 years old. The SCORAD was reduced from 42.0 ± 11.5 to 29.8 ± 8.9 by hospitalization in the low pollutant room. The Wilcoxon Signed Rank test showed significant improvements of symptom scores in patients with AD (p < 0.001, Fig. 1).
Fig. 1.
Atopic dermatitis severity score before and after hospitalization.
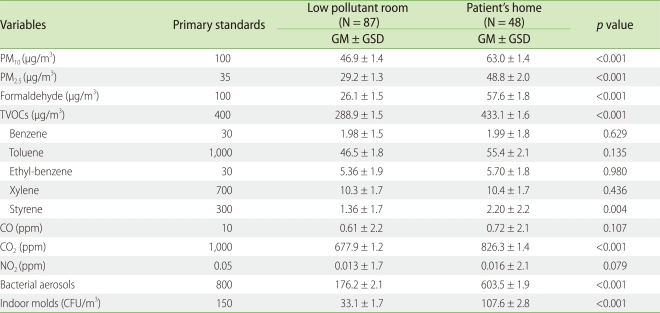
Analyses of individual environmental factors, such as PM2.5 (p < 0.001), PM10 (p < 0.001), formaldehyde (p < 0.001), TVOCs (p < 0.001), styrene (p = 0.004), CO2 (p < 0.001), bacterial suspensions (p < 0.001), and indoor molds (p < 0.001) showed significantly higher levels in patients' rooms compared to the low pollutant room. There was no significant statistical difference in the levels of benzene, toluene, ethyl-benzene, xylene, CO and NO2 (Table 2).
Table 2.
Comparison of indoor air quality between the patient's home and low pollutant room
t-test was used to compare the indoor air pollutant level between low pollutant room and patient's home. GM: geometric mean, GSD: geometric standard deviation, TVOCs: total volatile organic compounds, CFU: colony forming unit.
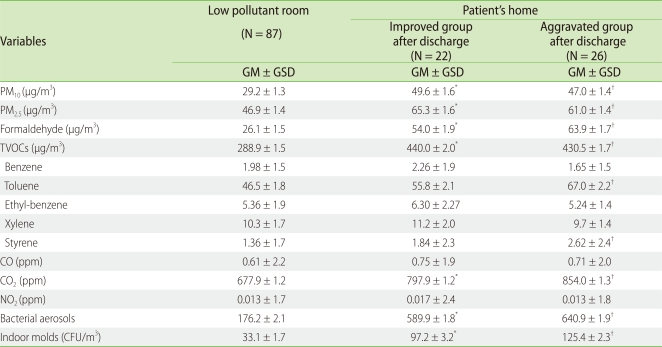
At the first out-patient clinic visit within a month after discharge, out of 51 patients, 22 patients maintained the improvement in their symptoms, but skin lesions of the remaining 29 patients were aggravated. Excluding 3 patients who had clear reasons for aggravation such as ingestion of restricted food, herpes virus infection, and the contact with irritants, we divided 48 patients into two groups, the improved and aggravated groups, after discharge. No statistical significance of indoor environment was shown between the aggravated and improved groups (Table 3).
Table 3.
Comparison of indoor air quality among low pollutant room, home of patients who were improved after discharge and home of patients who were aggravated after discharge
Analysis of variance was used to compare the indoor air pollutant levels. *p < 0.05 between low pollutant room and home of improved group after discharge. †p < 0.05 between low pollutant room and home of aggravated group after discharge. GM: geometric mean, GSD: geometric standard deviation, TVOCs: total volatile organic compounds, CFU: colony forming unit.
Out of 29 patients in the aggravated group, 8 patients were readmitted to the low pollutant room, and their SCORAD indices rapidly decreased from 53.1 ± 16.2 to 39.2 ± 9.8 (p = 0.036). Out of 8 patients, 4 patients had recently undertaken indoor renovation activities; 2 patients had performed house painting, and 2 patients had replaced wallpaper. Among 8 patients, 6 patients live in apartments, while 2 patients reside in row houses. Current paternal smoking and wall molds were found in 4 and 3 patients, respectively. Two patients lived between 200 m and 500 m away from major thoroughfares with four or more lanes, while five patients lived less than 200 m away.
DISCUSSION
We hypothesized that AD symptom severity might be affected by the indoor environment. If there are AD patients who are exposed to inappropriate home environment in terms of indoor air quality, their symptoms would be controlled more effectively in a low pollutant environment and might be aggravated again when they are re-exposed. We selected AD patients whose symptoms did not improve by conventional treatment in outpatient clinic because they could be possible candidates. We attempted to observe the differences in change of symptom severity and compared indoor air pollutant levels between their home and the low pollutant room. As a result, we found in this study that some patients showed alteration in their symptom severity by the change in indoor air pollutant levels, although our hypothesis is not applicable in all patients.
Previous studies have shown that indoor environmental factors increase the risk for AD and allergic diseases. Herbarth et al. [9] has reported that participants experiencing recent indoor renovation show a high odds ratios of 1.9 (95% CI: 1.4-2.7) for eczema. They also reported that inflammatory response mediators such as interleukin (IL)-8 and monocyte chemoattractant protein-1 in children's blood increased when renovations were conducted [19]. Lehmann et al. [20] demonstrated that exposure to indoor VOCs may differentiate T cells into responsive Th2 cells. In addition, even the typical domestic concentration of formaldehyde can cause skin barrier function impairment in patients with AD [11]. A moderate increase in long-term exposure to background ambient air pollutants, such as ozone and PM10, was associated with an increased prevalence of AD in children [21]. However, these previous investigations have been limited by a lack of evidence showing clinical effects of the environment with low levels of pollutants on atopic diseases.
Other than chemical pollutants, a strong association between indoor bioaerosols and AD severity has been reported. Higher levels of environmental S. aureus may contribute to disease severity and persistence in AD patients [13]. Molds on indoor walls increased the risk of early infantile AD [5], and dampness in the child's home may be an important risk factor for AD [8]. The itching in AD is significantly dependent on meteorological conditions [22]. However, others reported that the risk for AD is higher in children living in damp homes but this trend did not reach statistical significance [23]. Patients with AD have skin barrier dysfunction and increased transepidermal water loss. This impairment of skin barrier function causes increased allergen absorption, which contributes to the cutaneous hyper-reactivity of AD [24].
In this study, only a small number of patients participated, and treatment during hospitalization was not well controlled. In addition, some patients showed association between clinical symptoms and indoor air pollutant levels, while others did not. Therefore, it is too early to conclude that control of indoor air quality is mandatory for the management of AD. However, our hypothetical experience suggests that better indoor environment helps to improve AD symptoms in a certain group of patients. PM2.5, PM10, TVOCs, formaldehyde, indoor bacterial aerosols, and indoor molds might be responsible for aggravation of AD lesions in these susceptible individuals. If it is true, an effort should be made to minimize exposure to indoor air pollutants using a ventilation system, air cleaner, and environmentally friendly materials for better management of AD.
In conclusion, indoor air pollutants are likely to affect AD in susceptible individuals. Environmental control to lower indoor air pollutant levels might be necessary for better management of AD in some patients. Further research is required to clarify the role of indoor air quality in improving or aggravating AD symptoms.
ACKNOWLEDGEMENTS
This study was financially supported by the Ministry of Environment of Korea.
References
- 1.Williams H, Stewart A, von Mutius E, Cookson W, Anderson HR International Study of Asthma and Allergies in Childhood (ISAAC) Phase One and Three Study Groups. Is eczema really on the increase worldwide? J Allergy Clin Immunol. 2008;121:947–954.e15. doi: 10.1016/j.jaci.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Bisgaard H, Halkjaer LB, Hinge R, Giwercman C, Palmer C, Silveira L, Strand M. Risk analysis of early childhood eczema. J Allergy Clin Immunol. 2009;123:1355–1360.e5. doi: 10.1016/j.jaci.2009.03.046. [DOI] [PubMed] [Google Scholar]
- 3.Peroni DG, Piacentini GL, Bodini A, Rigotti E, Pigozzi R, Boner AL. Prevalence and risk factors for atopic dermatitis in preschool children. Br J Dermatol. 2008;158:539–543. doi: 10.1111/j.1365-2133.2007.08344.x. [DOI] [PubMed] [Google Scholar]
- 4.Purvis DJ, Thompson JM, Clark PM, Robinson E, Black PN, Wild CJ, Mitchell EA. Risk factors for atopic dermatitis in New Zealand children at 3.5 years of age. Br J Dermatol. 2005;152:742–749. doi: 10.1111/j.1365-2133.2005.06540.x. [DOI] [PubMed] [Google Scholar]
- 5.Wang IJ, Guo YL, Weng HJ, Hsieh WS, Chuang YL, Lin SJ, Chen PC. Environmental risk factors for early infantile atopic dermatitis. Pediatr Allergy Immunol. 2007;18:441–447. doi: 10.1111/j.1399-3038.2007.00550.x. [DOI] [PubMed] [Google Scholar]
- 6.Miyake Y, Ohya Y, Tanaka K, Yokoyama T, Sasaki S, Fukushima W, Ohfuji S, Saito K, Kiyohara C, Hirota Y Osaka Maternal and Child Health Study Group. Home environment and suspected atopic eczema in Japanese infants: the Osaka Maternal and Child Health Study. Pediatr Allergy Immunol. 2007;18:425–432. doi: 10.1111/j.1399-3038.2007.00545.x. [DOI] [PubMed] [Google Scholar]
- 7.Sebök B, Schneider I, Harangi F Primary Care Paediatricians in Baranya County. Familiar and environmental factors influencing atopic dermatitis in the childhood. J Eur Acad Dermatol Venereol. 2006;20:418–422. doi: 10.1111/j.1468-3083.2006.01490.x. [DOI] [PubMed] [Google Scholar]
- 8.McNally NJ, Williams HC, Phillips DR. Atopic eczema and the home environment. Br J Dermatol. 2001;145:730–736. doi: 10.1046/j.1365-2133.2001.04474.x. [DOI] [PubMed] [Google Scholar]
- 9.Herbarth O, Fritz GJ, Rehwagen M, Richter m, Röder S, Schlink U. Association between indoor renovation activities and eczema in early childhood. Int J Hyg Environ Health. 2006;209:241–247. doi: 10.1016/j.ijheh.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Huss-Marp J, Eberlein-König B, Breuer K, Mair S, Ansel A, Darsow U, Krämer U, Mayer E, Ring J, Behrendt H. Influence of short-term exposure to airborne Der p 1 and volatile organic compounds on skin barrier function and dermal blood flow in patients with atopic eczema and healthy individuals. Clin Exp Allergy. 2006;36:338–345. doi: 10.1111/j.1365-2222.2006.02448.x. [DOI] [PubMed] [Google Scholar]
- 11.Eberlein-König B, Przybilla B, Kühnl P, Pechak J, Gebefügi I, Kleinschmidt J, Ring J. Influence of airborne nitrogen dioxide or formaldehyde on parameters of skin function and cellular activation in patients with atopic eczema and control subjects. J Allergy Clin Immunol. 1998;101:141–143. doi: 10.1016/S0091-6749(98)70212-X. [DOI] [PubMed] [Google Scholar]
- 12.Peden DB. The epidemiology and genetics of asthma risk associated with air pollution. J Allergy Clin Immunol. 2005;115:213–219. doi: 10.1016/j.jaci.2004.12.003. quiz 20. [DOI] [PubMed] [Google Scholar]
- 13.Leung AD, Schiltz AM, Hall CF, Liu AH. Severe atopic dermatitis is associated with a high burden of environmental Staphylococcus aureus. Clin Exp Allergy. 2008;38:789–793. doi: 10.1111/j.1365-2222.2008.02964.x. [DOI] [PubMed] [Google Scholar]
- 14.Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol Suppl (Stockh) 1980;92:44–47. [Google Scholar]
- 15.Staldera JF, Taïebb A Consensus Report of the European Task Force on Atopic Dermatitis. Severity scoring of atopic dermatitis: the SCORAD index. Dermatology. 1993;186:23–31. doi: 10.1159/000247298. [DOI] [PubMed] [Google Scholar]
- 16. http://www.law.go.kr/DRF/MDRFLawService.jsp?OC=me&ID=10302.
- 17. http://www.law.go.kr/DRF/MDRFLawService.jsp?OC=me&ID=8375.
- 18. http://www.euro.who.int/pubrequest.
- 19.Herberth G, Gubelt R, Röder S, Krämer U, Schins RP, Diez U, Borte M, Heinrich J, Wichmann HE, Herbarth O, Lehmann I LISAplus study group. Increase of inflammatory markers after indoor renovation activities: the LISA birth cohort study. Pediatr Allergy Immunol. 2009;20:563–570. doi: 10.1111/j.1399-3038.2008.00819.x. [DOI] [PubMed] [Google Scholar]
- 20.Lehmann I, Rehwagen M, Diez U, Seiffart A, Rolle-Kampczyk U, Richter M, Wetzig H, Borte M, Herbarth O Leipzig Allergy Risk Children Study. Enhanced in vivo IgE production and T cell polarization toward the type 2 phenotype in association with indoor exposure to VOC: results of the LARS study. Int J Hyg Environ Health. 2001;204:211–221. doi: 10.1078/1438-4639-00100. [DOI] [PubMed] [Google Scholar]
- 21.Pénard-Morand C, Charpin D, Raherison C, Kopferschmitt C, Caillaud D, Lavaud F, Annesi-Maesano I. Long-term exposure to background air pollution related to respiratory and allergic health in schoolchildren. Clin Exp Allergy. 2005;35:1279–1287. doi: 10.1111/j.1365-2222.2005.02336.x. [DOI] [PubMed] [Google Scholar]
- 22.Vocks E, Busch R, Frohlich C, Borelli S, Mayer H, Ring J. Influence of weather and climate on subjective symptom intensity in atopic eczema. Int J Biometeorol. 2001;45:27–33. doi: 10.1007/s004840000077. [DOI] [PubMed] [Google Scholar]
- 23.Yang CY, Cheng MF, Hsieh YL. Effects of indoor environmental factors on risk for atopic eczema in a subtropical area. J Toxicol Environ Health A. 2000;61:245–253. doi: 10.1080/00984100050136562. [DOI] [PubMed] [Google Scholar]
- 24.Sator PG, Schmidt JB, Hönigsmann H. Comparison of epidermal hydration and skin surface lipids in healthy individuals and in patients with atopic dermatitis. J Am Acad Dermatol. 2003;48:352–358. doi: 10.1067/mjd.2003.105. [DOI] [PubMed] [Google Scholar]