Abstract

An efficient synthesis of chloroisosulochrin was accomplished using a novel ortho-selective chlorination of a phenol with sulfuryl chloride and 2,2,6,6-tetramethylpiperidine as the key step. Further elaboration by a biomimetic route converted chloroisosulochrin to dihydromaldoxin, maldoxone (lactone formed by dehydration of dihydromaldoxin), and maldoxin and isosulochrin to dechlorodihydromaldoxin and dechloromaldoxin.
Liu, Che and co-workers recently reported the isolation of the cytotoxic natural product chloropestolide A (3) and several congeners from the fermentation of the plant endophytic fungus Pestalotiopsis fici.1 These compounds were suggested to arise by an inverse electron demand Diels-Alder reaction between maldoxin (1b)2 as the diene and isopropenylallene 2, which was also isolated from Pestalotiopsis fici, as the dienophile (see Scheme 1).1 We decided to synthesize maldoxin (1b) and then explore the regio- and stereoselectivity of the Diels-Alder reaction of maldoxin with a simpler isopropenylallene.3
Scheme 1.
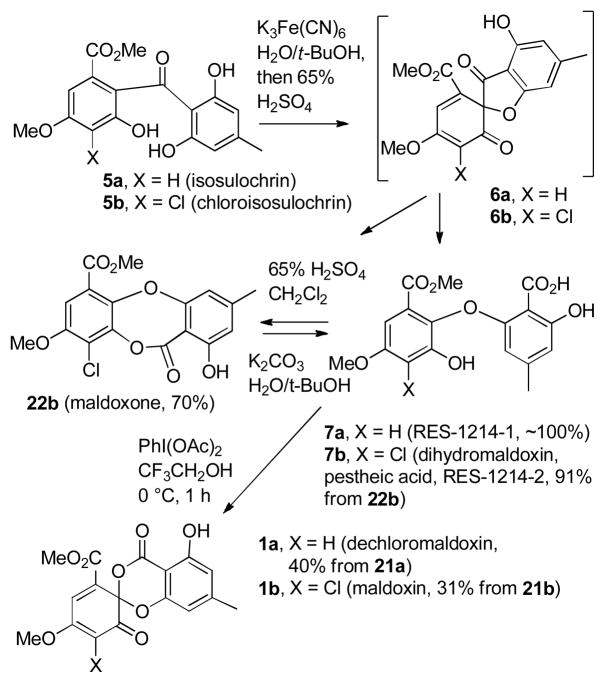
The biosynthesis of maldoxin (1b) may proceed by oxidative cleavage of the anthraquinone fragilin (4b) and methylation of the carboxylic acid to give chloroisosulochrin (5b) (see Scheme 2).4 Oxidative cyclization could give spirofuran-3-one 6b, which could be hydrolyzed to give dihydromaldoxin (RES-1214-2, pestheic acid, 7b).2,4,5 A second oxidative cyclization could then lead to maldoxin (1b).2 A similar oxidative cleavage of physcion (4a) would give isosulochrin (5a),4,6 which could be oxidatively cyclized to give dechlorodihydromaldoxin (RES-1214-1, 7a).2,5 Although only dihydromaldoxin was isolated by Liu and Che from Pestalotiopsis fici,1 isosulochrin (5a), chloroisosulochrin (5b), and dihydromaldoxin (pestheic acid, 7b) were all isolated from Pestalotiopsis theae,4 both dechloro-dihydromaldoxin (RES-1214-1, 7a) and dihydromaldoxin (RES-1214-2, 7b) were isolated from Pestalotiopsis sp. RES-1214,5 and dechlorodihydromaldoxin (7a), dihydromaldoxin (7b), and maldoxin (1b) were all isolated from an unidentified Xylaria species.2 The isolation of both chlorinated and unchlorinated 5 and 7 from the same source suggests that chlorination might occur after oxidative cleavage of anthraquinone 4. RES-1214-1 (7a) and -2 (7b) selectively inhibit ET-1 binding to endothelin type A receptor (ETA receptor) with IC50 values of 1.5 μM and 20 μM, respectively.5 They also inhibit the increase in intracellular Ca2+ concentration elicited by 1 nM ET-1 in A10 cells.5
Scheme 2.
Biosynthesis of Maldoxin
We planned to synthesize chloroisosulochrin (5b) and convert it to maldoxin (1b) by the biomimetic sequence shown in Scheme 2. The oxidative cyclization of 2,2′-dihydroxybenzophenones analogous to 5 to give spirofuranones analogous to 6, and acid-catalyzed hydrolysis of the spirofuranones to provide 2-(2-hydroxyphenoxy)-benzoic acids analogous to 7 is well precedented.7 The oxidative cyclization of 2-(4-hydroxyphenoxy)benzoic acids to give benzo[d][1.3]dioxin-4-ones spiro fused at the 4 position to 2,5-cyclohexadienones is known,8 but the analogous preparation of benzo[d][1.3]dioxin-4-ones such as maldoxin that are spiro fused at the 6 position to 2,4-cyclohexadienones is unknown, possibly because of the susceptibility of such dienones to dimerization.9
We started with resorcinol 8,10 an intermediate in Katoh’s synthesis of geodin. Selective methylation of the less hindered hydroxy group of 8 provided phenol 9a in 92% yield (see Scheme 3). We needed to selectively chlorinate 9a ortho to the phenol. Unfortunately, ortho chlorination of complex phenols has proved to be very challenging.11 Our initial attempt with NCS and benzoyl peroxide in CCl4 provided a disappointing 1:8 mixture of the desired ortho chlorophenol 9b and the para chlorophenol 10. Gnaim and Sheldon reported that phenol can be selectively ortho chlorinated with SO2Cl2 and primary or secondary amines in toluene.12 They suggested that the high regioselectivity with phenol results from the in situ formation of an N-chloroamine, which hydrogen bonds to the phenol forming a complex that delivers chlorine intramolecularly to the ortho position. We were disappointed to find that chlorination of 9a with SO2Cl2 and t-butylamine in toluene provided only a 1:2.6 mixture of 9b and 10, although these conditions provide an 11.4:1 ortho/para mixture from phenol itself.12b Presumably, intermolecular chlorination to give 10 occurs more rapidly with the more nucleophilic aromatic ring of 9a. We reasoned that hindered secondary amines would form a more hindered and therefore less reactive N-chloroamine that would favor chlorination via the hydrogen bonded complex that leads to 9b. We were pleased to find that the selectivity increased as we changed to more hindered secondary amines, increasing to 1.8:1 with dipropylamine, to 3.3:1 with diisopropylamine and to 3.9:1 with 2,2,6,6-tetramethylpiperidine. Fortunately the products are easily separated so that using the latter amine we were able to obtain 9b (58%) and 10 (15%) on a gram scale. We observed a similar effect of amine bulk on the chlorination selectivity with phenol 11, which gave a 0.8:1, 1.8:1, 2.4:1, or 2.6:1 mixture of 1213 and 1314 with sulfuryl chloride and t-butylamine, dipropylamine, diisopropylamine, or 2,2,6,6-tetramethylpiperidine, respectively.
Scheme 3.
Selective Ortho Chlorination
We proceeded in parallel with both maldoxin precursor 9b and the unchlorinated phenol 9a, which should give rise to dechloromaldoxin and allow us to explore the effect of the chlorine atom on the Diels-Alder reaction. Protection of phenols 9a/b with MOMCl and DIPEA afforded MOM ethers 14a (91%) and 14b (92%) (see Scheme 4). Deprotonation15 of bis MOM ether 15 with n-BuLi and addition of the aryllithium to 14a/b afforded alcohols 16a (90%) and 16b (92%), which were oxidized by Dess-Martin periodinane to give benzophenones 17a (88%) and 17b (90%).
Scheme 4.
Preparation of Hydroxymethyl Ketone 18
Not surprisingly, the hydrogenolysis of the benzyl ether of 17a/b proved to be challenging. Product 18a/b is a hydroxy ketone that can cyclize to form hemiketal 19a/b, which is readily hydrogenolyzed to form dihydroisobenzofuran 20a/b, which still has two benzylic oxygen substituents that are susceptible to hydrogenolysis. Hydrogenolysis of 17a was best accomplished over 20% Pd(OH)2/C with 1 atm of H2 in MeOH for 2 h to give 18a (80%). However, 20a was isolated in 97% yield when the reaction was run for 5 h. Hydrogenation of chloride 17b over 20% Pd(OH)2/C afforded a mixture of hydroxy ketone 18b and hemiketal 19b, which was rapidly reduced to 20b. The effect of substituents on the equilibration of related hydroxy ketones and hemiketals has been previously noted.16 Chloride 17b was best hydrogenolyzed over 5% Pd/BaSO417 with 1 atm of H2 in EtOH for 50 min to give 18b. However, 20b was isolated when the reaction was run for 2 h. Replacing the benzyl protecting group with a 2-naphthylmethyl group should solve the overreduction problem because hydrogenolysis of the 2-naphthylmethyl group is much faster than that of a benzyl group.18 Unfortunately, chlorination of the 2-naphthylmethyl analog of 9a proceeded in only 40% yield with only 1.6:1 ortho/para selectivity and addition of the lithium reagent prepared from 15 to the 2-naphthylmethyl analog of 14b proceeded in only 68% yield.
The standard three-step sequence shown in Scheme 5 converted hydroxy ketones 18a and 18b to keto esters 21a (65% from 17a) and 21b (80% from 17b). The four-step conversion of the benzyl ethers of 17a/b to the methyl esters of 21a/b is cumbersome, but was used because we were unable to add the aryllithium reagent prepared from 15 to the aldehyde analogous to 14a with a CO2Me instead of a CH2OBn group. Deprotection of all three MOM ethers of 21a/b with TsOH in MeOH at reflux cleanly afforded isosulochrin (5a)6 and chloroisosulochrin (5b).4
Scheme 5.
Completion of the Chloroisosulochrin Synthesis
Oxidation7 of 5a with potassium ferricyanide in aqueous t-butanol afforded spirofuranone 6a, which was hydrolyzed7 with 65% sulfuric acid to give diaryl ether dechlorodihydromaldoxin2,4 (7a) in quantitative yield. A similar oxidation of 5b afforded spirofuranone 6b, but hydrolysis of 6b with 65% sulfuric acid initially gave a mixture of maldoxone2 (22b) and dihydromaldoxin (7b) that was converted to maldoxone in 70% yield at longer reaction times. Hydrolysis of maldoxone with K2CO3 in aqueous t-butanol afforded dihydromaldoxin2,4,5 (7b) in 91% yield. Oxidative cyclization of 7a with PhI(OAc)2 in CF3CH2OH at 0 °C afforded dechloromaldoxin (1a) in 40% overall yield for the four-step sequence from 21a. The structure of 1a was confirmed by X-ray crystallography. A similar oxidative cyclization of 7b afforded maldoxin (1b) in 31% overall yield for the five-step sequence from 21b. The Diels–Alder reactions of both dihydromaldoxin (1a) and maldoxin (1b) with an isopropenylallene will be described elsewhere.3
In conclusion, an efficient synthesis of chloroisosulochrin (5b) was accomplished using a novel ortho-selective chlorination of a phenol with sulfuryl chloride and 2,2,6,6-tetramethylpiperidine as the key step. Further elaboration by a biomimetic route converted chloroisosulochrin to dihydromaldoxin, maldoxone, and maldoxin and isosulochrin to dechlorodihydromaldoxin and dechloromaldoxin.
Supplementary Material
Scheme 6.
Biomimetic Conversion of Chloroisosulochrin to Maldoxin
Acknowledgments
We are grateful to the National Institutes of Health (GM-50151) for support of this work. We thank the National Science Foundation for grant CHE-0521047 for a new X-ray diffractometer.
Footnotes
Supporting Information Available: Complete experimental procedures, copies of 1H and 13C NMR spectral data, and CIF file and drawing of X-ray crystal structure of 1a. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Liu L, Liu S, Jiang L, Chen X, Guo L, Che Y. Org Lett. 2008;10:1397–1400. doi: 10.1021/ol800136t. [DOI] [PubMed] [Google Scholar]; (b) Liu L, Li Y, Liu S, Zheng Z, Chen X, Zhang H, Guo L, Che Y. Org Lett. 2009;11:2836–2839. doi: 10.1021/ol901039m. [DOI] [PubMed] [Google Scholar]; (c) Liu L, Bruhn T, Guo L, Götz DCG, Brun R, Stich A, Che Y, Bringmann G. Chem - Eur J. 2011;17:2604–2613. doi: 10.1002/chem.201003129. [DOI] [PubMed] [Google Scholar]; (d) Liu L. Mycology. 2011;2:37–45. [Google Scholar]
- 2.Adeboya MO, Edwards RL, Lassøe T, Maitland DJ, Shields L, Whalley AJS. J Chem Soc, Perkin Trans. 1996;1:1419–1425. [Google Scholar]
- 3.Yu M, Snider BB. Manuscript in preparation [Google Scholar]
- 4.Shimada A, Takahashi I, Kawano T, Kimura Y. Z Naturforsch B. 2001;56:797–803. [Google Scholar]
- 5.Ogawa T, Ando K, Aotani Y, Shinoda K, Tanaka T, Tsukuda E, Yoshida M, Matsuda Y. J Antibiot. 1995;48:1401–1406. doi: 10.7164/antibiotics.48.1401. [DOI] [PubMed] [Google Scholar]
- 6.(a) Assante G, Camarda L, Nasini G. Gazz Chim Ital. 1980;110:629–631. [Google Scholar]; (b) Hamasaki T, Kimura Y. Agric Biol Chem. 1983;47:163–165. [Google Scholar]
- 7.Hendrickson JB, Ramsay MVJ, Kelly TR. J Am Chem Soc. 1972;94:6834–6843.Sala T, Sargent MV. J Chem Soc, Perkin Trans. 1981;1:855–869.Sala T, Sargent MV. J Chem Soc, Perkin Trans. 1981;1:877–882.Coomber MF, Sargent MV, Skelton BW, White AH. J Chem Soc, Perkin Trans. 1989;1:441–448.Pulgarin C, Tabbachi R. Helv Chim Acta. 1989;72:1061–1065.and references cited therein.
- 8.Hassall CH, Lewis JR. J Chem Soc. 1961:2312–2315. [Google Scholar]
- 9.(a) Quideau S, Pouységu L. Org Prep Proc Int. 1999;31:617–680. [Google Scholar]; (b) Gagnepain J, Méreau R, Dejugnac D, Léger JM, Castet F, Deffieux D, Pouységu L, Quideau S. Tetrahedron. 2007;63:6493–6505. [Google Scholar]; (c) Dory YL, Roy AL, Soucy P, Deslongchamps P. Org Lett. 2009;11:1197–1200. doi: 10.1021/ol8026768. [DOI] [PubMed] [Google Scholar]
- 10.Katoh T, Ohmuri O, Iwasaki K, Inoue M. Tetrahedron. 2002;58:1289–1299. [Google Scholar]
- 11.Mal D, Dey S. Tetrahedron. 2006;62:9589–9602. [Google Scholar]
- 12.(a) Smith K, Butters M, Nay B. Tetrahedron Lett. 1988;29:1319–1322. [Google Scholar]; (b) Gnaim JM, Sheldon RA. Tetrahedron Lett. 1995;36:3893–3896. [Google Scholar]; (c) Gnaim JM, Sheldon RA. Tetrahedron Lett. 2004;45:8471–8473. [Google Scholar]
- 13.Plattner JJ, Fung AKL, Parks JA, Pariza RJ, Crowley SR, Pernet AG, Bunnell PR, Dodge PW. J Med Chem. 1984;27:1016–1026. doi: 10.1021/jm00374a014. [DOI] [PubMed] [Google Scholar]
- 14.Akselson ØW, Skattebøl L, Hansen TV. Tetrahedron Lett. 2009;50:6339–6341. [Google Scholar]
- 15.Lambert GJ, Duffley RP, Dalzell HC, Razdan RK. J Org Chem. 1982;47:3350–3353. [Google Scholar]
- 16.(a) Smith JG, Fogg DE, Munday IJ, Sandborn RE, Dibble PW. J Org Chem. 1988;53:2942–2953. [Google Scholar]; (b) Epsztajn J, JóŸwiak A, Szcześniak AK. Tetrahedron. 1993;49:929–938. [Google Scholar]
- 17.Nikam SS, Kornberg BE, Johnson DR, Doherty AM. Tetrahedron Lett. 1995;36:197–200. [Google Scholar]
- 18.Gaunt MJ, Yu J, Spencer JB. J Org Chem. 1998;63:4172–4173. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.