Abstract
Purpose of review
To summarize recent advances in androgen biosynthesis and metabolism in peripheral tissues (e.g. liver and prostate) and how these can be exploited therapeutically.
Recent findings
Human liver catalyzes the reduction of circulating testosterone (T) to yield four stereoisomeric tetrahydrosteroids. Recent advances have assigned the enzymes responsible for these reactions and elucidated their structural biology. Data also suggests that for 5α-dihydrotestosterone (5α-DHT), conjugation reactions (phase II) may precede ketosteroid reduction (phase I) reactions. Human prostate is the site of benign prostatic hyperplasia and prostate cancer which occur in the aging male. While the importance of local androgen biosynthesis in these diseases is accepted, recent advances have identified enzymes that regulate ligand access to the androgen receptor; a “backdoor pathway” to 5α-DHT that does not require T acting as an intermediate; and the finding that castrate resistant prostate cancer has undergone an adaptive response to androgen deprivation, which involves intra-tumoral T and 5α-DHT biosynthesis that can be targeted using inhibitors of (CYP17-hydroxylase/17,20-lyase), aldo-keto reductase 1C3, and 5α-reductase type 1 and type 2.
Summary
Enzyme isoforms responsible for the biosynthesis and metabolism of androgens in liver and prostate have been identified and those responsible for the biosynthesis of androgens in castrate resistant prostate cancer can be therapeutically targeted.
Keywords: castrate resistant prostate cancer, aldo keto reductase, abiraterone acetate, 5α/β-reductase
Introduction
Androgen biosynthesis (testosterone (T) production in the Leydig cells of the testis under Luteinizing Hormone (LH) control; and 5α-dihydrotestosterone (5α-DHT) production under local control in peripheral tissues) is essential for sex-determination and mature sexual development in man. In the absence of these androgens hypogonadism and pseudohermaphroditism occurs [1, 2]. As males age there is a slow decline in circulating testosterone which can lead to deleterious effects (e.g. loss of libido, osteoporosis and loss of muscle mass). These are symptoms of the so called “andropause” which do not affect all men equally [3•, 4].
Diseases of the prostate e.g. benign prostatic hyperplasia (BPH) and prostate cancer are prevalent in the aging male, and are androgen dependent at the time when circulating T from the gonads wanes. This suggests that the local or intracrine production of androgens may drive these diseases [5, 6]. Importantly, BPH affects 90% of all men above the age of 80; while prostate cancer is the second leading cause of cancer mortality in the US population and generally occurs in men > 50 years of age. It is estimated that 200,000 new cases of prostate cancer will be diagnosed annually in the USA and that this will result in 40,000 deaths per year.
Androgen insufficiency syndromes can be treated by providing replacement androgen therapy (androgen receptor agonists) while BPH and prostate cancer can be treated by androgen deprivation e.g. the use of agents that deprive the androgen receptor (AR) of its ligand. These agents include inhibitors of steroidogenesis and androgen receptor antagonists. The former approach requires a detailed knowledge of the extra-gonadal biosynthesis and metabolism of androgens and the discrete enzymes involved. The purpose of this article is to review recent breakthroughs in this knowledge and how it can be used to improve therapy.
Hepatic Androgen Metabolism
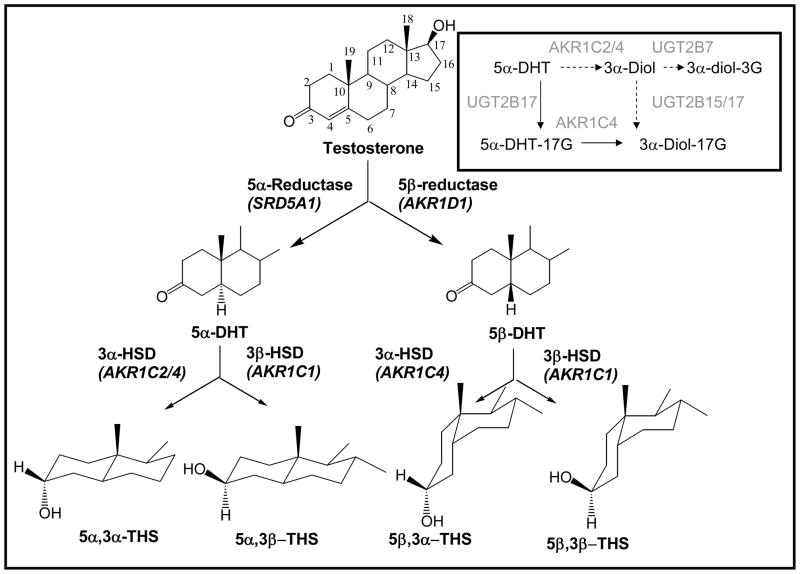
Testosterone is a C19 Δ4-3-ketosteroid and undergoes clearance in the liver by sequential reduction by either 5α-reductase type 1 (SRD5A1) or steroid 5β-reductase (AKR1D1) to yield 5α-DHT and 5β-DHT, respectively [2, 7]. Each of these dihydrosteroids are further reduced by 3α or 3β-hydroxysteroid dehydrogenases (HSD) to yield four stereoisomeric tetrahydrosteroids e.g. 5α-androstane-3α,17β-diol (3α-diol), 5α-androstane-3β,17β-diol (3β-diol), 5β-androstane-3α,17β-diol and 5β-androstane-3β,17β-diol, Figure 1. It is now recognized that human liver contains four aldo-keto reductase (AKR) isoforms (AKR1C1-AKR1C4) which are NADPH-dependent ketosteroid reductases. These enzymes display different ratios of 3α-/3β-HSD activity [8, 9], and couple with either SRD5A1 or AKR1D1 to produce the four isomeric products observed. For example, SRD5A1 and AKR1C1 couple to produce 3β-diol and SRD5A1 and AKR1C2 couple to produce 3α-diol [9].
Figure 1.
Reductive androgen metabolism in human liver. The reductive enzymes responsible for the metabolism of testosterone to its four stereoisomeric tetrahydrosteroids are shown (genes are italicized). The inset shows the preferred pathway of 5α-DHT metabolism with glucuronidation (a phase II reaction) preceding 3-ketosteroid reduction (a phase I reaction). AKR1C4 is shown preferentially reducing 5α-DHTG to 3α-diol-17-G since it has the highest catalytic efficiency for this reaction among the AKR1C isoforms: 5α-DHT = 5α-dihydrotestosterone; 3α-diol = 5α-androstane-3α,17β-diol; 3α-diol-3G = 3α-diol-3α-glucuronide; 5α-DHTG = 5α-DHT-17β-glucuronide; 3α-diol-17G = 3α-diol-17β-glucuronide.
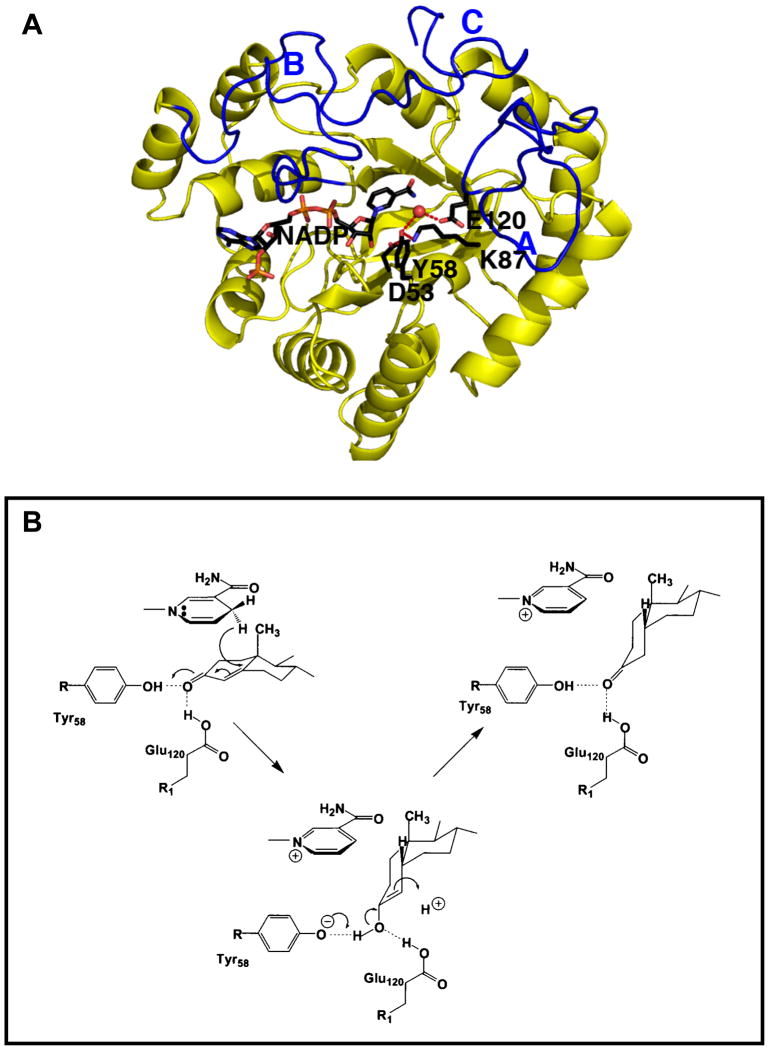
Until recently no structural information existed on any mammalian steroid double bond reductase. Recently the structures of several AKR1D1•NADP+•Steroid (testosterone, progesterone, cortisol, 5β-dihydroprogesterone, finasteride) ternary complexes were reported [10•–13]. This enzyme has the (α/β)8-barrel structural motif shared by the AKR superfamily, and contains three large loops at the back of the structure that determine substrate specificity, Figure 2A. The cofactor binding site is highly conserved within the superfamily and straddles the lip of the barrel in an extended anti-conformation that determines 4-proR-hydride transfer to C5 of the steroid. By conducting hydride transfer to the β-face of the steroid the A/B cis-ring junction is introduced and generates a 90° bend into the planar steroid structure, Figure 1. This AKR has a substitution in its catalytic tetrad where E120 replaces a histidine. The structure reveals that this substitution allows the steroid substrate to penetrate further into the active site so that the hydride donor (NADPH) and C5 of the steroid are optimally aligned. In addition, the side-chain of E120 is in a fully protonated anti-conformation which forms hydrogen bonds with the C3-ketone of the steroid, and in concert with the catalytic tyrosine (Y58) promotes enolization of the ketosteroid, Figure 2B. Thus the mechanism of steroid double- bond reduction has been revealed for the first time.
Figure 2.
Structure and mechanism of human steroid 5β-reductase (AKR1D1). (A) (α/β)8-Barrel structure of an AKR1D1•NADP+ binary complex. NADP+ is in a stick presentation, a water molecule (red ball) is hydrogen bonded to Y58 and E120 of the catalytic tetrad which also contains (D53 and K87); and the three loops A, B and C which comprise the steroid binding site are in blue. (B) Catalytic mechanism of steroid double bond reduction.
Finasteride (a selective mechanism-based inactivator of SRD5A2) is a competitive inhibitor of 5β-reductase Ki = 2.1 μM. However, finasteride is not a mechanism based inactivator of AKR1D1 and the structure of the AKR1D1•NADP+•Finasteride complex explains why this is the case [12••]. The NADPH is on the wrong side of the steroid pocket in AKR1D1 and thus reduction of the Δ1,2-ene of finasteride observed in SRD5A2 to produce a bisubstrate analog is not possible. The Ki value of finasteride for AKR1D1 is only one order of magnitude higher than that observed for SRD5A1 suggesting that high doses of this drug would inhibit both enzymes. In this instance finasteride would inhibit its own metabolism. 5β-Pregnanes produced by AKR1D1 are ligands for the orphan nuclear receptors PXR and CAR which when activated induce CYP3A4 [14, 15]. This CYP isoform is the major enzyme involved in finasteride metabolism [16].
The route of hepatic androgen metabolism so far described leads to tetrahydroandrostanes which are conjugated as either glucuronides or sulfates for excretion; i.e., reduction of the ketosteroid (phase 1 reactions) precede conjugation reactions of the alcohol (phase 2 reactions). In recent studies, evidence for the reverse sequence was found to exist [17••]. AKR1C1-AKR1C4 which reduce 5α- and 5β-DHT to their corresponding 3α- and 3β-diols were found to have high catalytic efficiencies towards 5α-DHT-17β-glucuronide or 5β-DHT-17β-sulfate. The products of the reactions were verified by liquid chromatography-mass spectrometry (LC-MS). Interestingly, although uridine glucuronsyl transferase (UGT)2B7 preferentially catalyzes the 3-′OH glucuronidation of 3α-diol and is highly expressed in liver [18] no 3′-glucuronide is formed in the circulation, suggesting the dominant pathway is conjugation at the 17β-position followed by reduction of the 3-ketone [17], Figure 1 (inset). Others have proposed that steroid metabolism could occur on steroid conjugates [19, 20] but these recent studies revive this issue with strong supporting data.
Prostate Androgen Biosynthesis and Metabolism
BPH and prostate cancer are two separate diseases. The former originates in the transitional zone where outgrowth of the stromal and epithelial cells leads to obstruction of the urinary bladder and difficulty in voiding the urine. By contrast prostate cancer is a disease of the peripheral zone (outer prostate) and aggressive disease can metastasize into the adjacent lymph glands and bone. Both are dependent upon intra-tumoral conversion of T [Kd = 10−9 M for the androgen receptor (AR)] to the higher-affinity ligand 5α-DHT (Kd = 10−11 M for AR) and can be treated by blocking 5α-DHT synthesis. For this approach to be effective requires a detailed knowledge of androgen biosynthesis in both disease states with the understanding that there may be paracrine influences between epithelial and stromal cells.
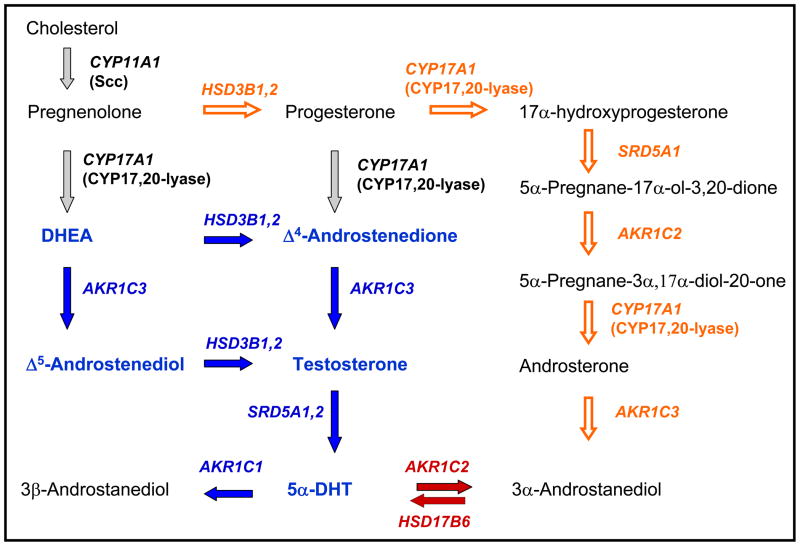
The route to 5α-DHT production in the prostate starting from circulating dihydroepiandrosterone (DHEA) is shown in Figure 3. Major progress in this area has led to the precise annotation of the enzyme isoforms involved in the process as follows: (i) DHEA is converted to Δ4-androstene-3,17-dione via 3β-hydroxysteroid dehydrogenase/ketosteroid isomerase type 1 and type 2 (HSD3B1 and HSD3B2) [21, 22]; (ii) Δ4-androstene-3,17-dione is reduced to T by 17β-HSD type 5 (AKR1C3) [23–26]; (iii) T is reduced to 5α-DHT by both 5α-reductase type 1 and type 2 (SRD5A1 and SRD5A2) where the type 2 enzyme plays the more dominant role [2, 27]; (iv) 5α-DHT is reduced by 3α-HSD type 3 (AKR1C2) to produce 3α-diol and by 3(20α)-HSD (AKR1C1) to produce 3β-diol [9, 23, 28, 29]; and (v) 17β-hydroxysteroids can be conjugated by glucuronidation by UGT2B15/17 [18, 30••].
Figure 3.
Androgen biosynthesis in the prostate. Traditional pathway converting DHEA to 5α-DHT is shown in blue. Arrows and genes in blue show altered expression in castrate resistant prostate cancer (CRPC). Arrows and genes in red show the molecular switch that regulates ligand access to the AR. AKR1C2 is also overexpressed in CRPC. Arrows in grey show de novo routes to DHEA and Δ4-androstene-3,17-dione that may contribute to adaptive androgen biosynthesis in CRPC. Open orange arrows show the backdoor pathway to 5α-DHT.
Several features are worthy of mention. First, the pathway from DHEA is emphasized since in the aging male the influence of adrenal androgens acting as precursors becomes pronounced. Second, AKR1C3 is the peripheral 17-ketosteroid reductase responsible for testosterone production. It has been shown to be expressed in prostate at the RNA, protein and functional level [24, 25, 31]. The enzyme is more highly expressed in epithelial cells than stromal cells and it is upregulated in prostate cancer [23]. By contrast although Leydig cell specific 17β-HSD type 3 (HSD17B3) transcripts have been detected in the prostate its role in testosterone production in this tissue remains to be elucidated [32•]. Third, the inability of finasteride to reduce prostatic volume by more than 30% and intraprostatic 5α-DHT levels by greater than 90%, suggests that 5α-DHT still forms in sufficient amounts to sustain prostate size [33]. Fourth, AKR1C2 is the dominant 3-ketosteroid reductase that will convert 5α-DHT (Kd = 10−11 M AR) to 3α-diol (Kd =10−6 M) and will prevent 5α-DHT from binding to its receptor [9, 23, 29]. Fifth, the highly related AKR1C1 (98 % sequence identity) instead converts 5α-DHT to 3β-diol, where 3β-diol is a pro-apototic ligand for estrogen receptor β [9, 34, 35].
Studies in castrate dogs [36], the tamar wallaby [37, 38], and humans [39] all support the back conversion of 3α-diol to 5α-DHT with growth consequences for the prostate. Five different short-chain dehydrogenase genes (HSD17B10, HSD17B6, RDH5, DHRS9, RODH4) have been invoked as being the oxidative 3α-HSDs responsible for this back conversion. However, detailed kinetic analysis using mammalian expression systems and expression profiling have provided unequivocal evidence that the enzyme responsible is “RoDH like 3α-HSD” (HSD17B6) [40]. Remarkably, HSD17B6 is more highly expressed in stromal versus epithelial cells providing a paracrine influence on the later. These data beg the question as to the circulating source of 3α-diol. Recently, a “backdoor pathway” to 5α-DHT was proposed starting with adrenal steroidogenesis to generate progesterone [41, 42]. In this pathway progesterone is converted to 17α-hydroxy-progesterone by CYP17α-hydroxylase/17,20 lyase and then reduced by SRD5A1 to yield 5α-pregnane-17α-ol-3,20-dione. This is then reduced by AKR1C2 to yield 5α-pregnane-3α,17α-diol-20-one. Subsequent reaction by CYP17α-hydroxylase/17,20-lyase would produce androsterone which is then reduced by AKR1C3 to 3α-diol. Thus a new molecular switch that controls ligand access to the AR has been identified as AKR1C2 which reduces 5α-DHT to 3α-diol (in epithelial cells) and HSD17B6 which oxidizes 3α-diol back to 5α-DHT (in stromal cells) [43, 44••].
Targeting the Androgen Axes in Prostate Disease
Finasteride was originally used to treat BPH but despite the beneficial affects on reducing prostatic volume and intraprostatic 5α-DHT levels symptomatic relief was disappointing [33]. This led to the concept that dutasteride a dual SRD5A1 and SRD5A2 inhibitor would provide greater benefit [45•]. In aside by side comparison of finasteride and dutasteride symptomatic relief seems comparable [33]. While compounds such as terazosin and tamsulosin (α-adrenergic receptor antagonists) may be superior compounds to increase urine flow [46] they do not halt the natural progression of the disease that can be achieved with 5α-reductase inhibitors.
Androgen ablative therapy is a major treatment for prostate cancer. The disease can be treated by surgical castration and prostatectomy or by the combined use of a LH-RH agonist (leuprolide) and an AR antagonist (bicalutamide/flutamide). Leuprolide blocks LH production at the level of the anterior pituitary and reduces circulating T levels to those seen in the castrate male (<0.2 ng/mL) [47]. By contrast bicalutamide prevents binding of 5α-DHT to the AR with an EC50 = 160 nM [48•]. Dual inhibition of both SRD5A1 and SRD5A2 with dutasteride may also be effective in preventing or delaying the growth of prostate cancer and is the focus of the ongoing 4-yr REduction by DUtasteride of prostate Cancer Events (REDUCE). Irrespective of the treatment regimen, in about 30–40% of cases the cancer can re-emerge (2–3 yr later) with a tell-tale increase in prostatic specific antigen (PSA). This recurrent cancer has been described as Castrate Resistant Prostate Cancer (CRPC). Consensus has built that CRPC is not androgen-independent and that both adaptive responses in AR signaling [49–51] and increased dependence on intra-tumoral androgen biosynthesis [32••, 52•, 53] may drive the disease and by-pass the form of castration used. Recently, a phase I/II clinical trial of abiraterone acetate (a CYP17α-hydroxylase/17,20-lyase inhibitor) in advanced CRPC was shown to reduce PSA levels and bone metastases in a dramatic fashion [54••, 55••]. These studies added weight to the importance of intra-tumoral formation of androgens [54••]. Abiraterone acetate would not only block the adrenal production of DHEA but also de novo synthesis of DHEA in the prostate and together these mechanisms would prevent the formation of T and 5α-DHT in the tumor.
Evidence for the importance of intra-tumoral formation of androgens in CRPC has come from studies in patients and xenograft models. Affymetrix microarray analysis and confirmatory real-time PCR in both primary prostate tumors and “androgen independent prostate cancer” showed the following fold increases in gene expression in the latter disease AR (5.8) HSD3B2 (1.8); AKR1C3 (5.3); SRD5A1 (2.1); SRD5A2 (0.54); AKR1C2 (3.4 x); AKR1C1 (3.1 x) and UGTB15 (3.5x). The increase in AKR1C3 expression was also confirmed by immunohistochemistry [53]. In a subsequent study, levels of testosterone and 5α-DHT were measured in prostate cancer and CRPC using stable-isotope dilution LC-MS and this was related to transcript level. Interestingly, prostate cancer gave 0.23 ng/g T and 2.75 ng/g 5α-DHT; but in CRPC the levels were reversed to 0.74 ng/g T and 0.25 ng/5α-DHT. These changes were reflected by increases in the transcripts for CYP17A1, HSD3B1, AKR1C3 and decreases in SRD5A2 [32••]. Taken together there seems to be an adaptive response for CRPC to synthesize there own androgens and that there may be a shift towards the production of T over 5α-DHT.
The dependence of CRPC on intra-tumoral androgens has also been modeled in LNCaP (AR positive cancer cell line) xenografts in castrate immunodeficient mice [52, 56••, 57••]. A caveat of this model is that CYP17α-hydroxylase/lyase is not expressed in the murine adrenal gland and these tumors may be under additional selection pressure to produce their own androgens. Nevertheless ex vivo experiments on the recurrent tumors provided evidence of the conversion of [14C]-acetate into 5α-DHT and the conversion of [3H]-progesterone into pregnane intermediates in the “backdoor” pathway to 5α-DHT, Figure 4 [52, 56••]. These experiments have been recapitulated to show that T production is accompanied by increases in StaR (steroidogenic acute regulatory protein) and side-chain cleavage enzyme (CYP11A1) which suggest that that de novo synthesis from cholesterol could be occurring [52, 58•]. In other studies on the LNCaP xenograft model a coordinated response to increase cholesterol influx and cholesterol biosynthesis was observed (including altered expression of LDL-r, SR-BI, HMGCoA reductase, ACT1,2 and ABCA1 proteins) [56•]. Thus CRPC can find away to produce its own androgens.
The dramatic response to abiraterone acetate suggests a role for targeting androgen biosynthesis in CRPC. Because this drug inhibits CYP17α-hydroxylase/17,20-lyase there are concerns that this will have the unintended consequence to cause the accumulation of the mineralocorticoid desoxycoticosterone in the adrenal. This will be exacerbated by the lack of cortisol to cause feedback inhibition of ACTH a the level of the anterior pituitary. Mineralocorticoid excess is currently prevented by the co-administration of dexamethasone [55••]. The success of abiraterone acetate has led to the development of both steroidal and nonsteroidal inhibitors of this enzyme [59, 60••]. One such compound VN/124-1 not only inhibits the target enzyme but also increases the rate of AR degradation [60••]. An alternative attractive target for CRPC which is down stream from CYP17 is AKR1C3 which catalyzes the final step in prostate T biosynthesis. Compounds that target this enzyme would not have to be co-administered with dexamethasone. Indomethacin and indomethacin analogs that do not inhibit COX-1 and COX-2 are lead compounds in targeting AKR1C3 [61•]. Importantly, these compounds do not inhibit the highly related AKR1C1 and AKR1C2. All these AKR1C isoforms are inhibited by the N-phenylantharnilic acids including flufenamic acid [62]. Recently, flufenamic acid analogs were reported to be AR antagonists [63•]. Thus another strategy for the treatment of CRPC would be to develop flufenamic acid analogs that target AKR1C3 and AR but leave AKR1C1 and AKR1C2 unaffected.
Conclusions
Reductive androgen metabolism in extragonadal tissues is now understood relative to the genes and enzyme isoforms involved. In liver a case can be made for reduction occurring after phase II conjugation. In castrate resistant prostate cancer adaptive local androgen biosynthesis may surmount androgen deprivation and the enzymes involved may be drug targets for advanced disease.
Acknowledgments
Supported in part from 1R01-CA90744, 1R01-DK47015, P30-ES013508 and the Prostate Cancer Foundation (to TMP).
Footnotes
This work was supported in part by NIH grants: 1RO1-CA90744, 1RO1-DK47015, P30-ES013508 and by a Challenge Grant from the Prostate Cancer Foundation.
References and recommended reading
Papers of particular interest published within the annual period of review have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Geissler WM, Davis DL, Wu L, et al. Male pseudohermaphroditism caused by mutations of testicular 17β-hydroxysteroid dehydrogenase 3. Nat Genet. 1994;7:34–39. doi: 10.1038/ng0594-34. [DOI] [PubMed] [Google Scholar]
- 2.Russell DW, Wilson JD. Steroid 5α-reductase two genes/two enzymes. Ann Rev Biochem. 1994;63:25–61. doi: 10.1146/annurev.bi.63.070194.000325. [DOI] [PubMed] [Google Scholar]
- 3•.Beg S, Al-Khoury L, Cunningham GR. Testosterone replacement in men. Curr Opin Endocrinol Diabetes Obes 1. 2008;15:364–70 . doi: 10.1097/MED.0b013e328305081a. Provides a balanced review of the pros and cons of androgen replacement therapy and recommends that therapy should be closely monitored in men over age 50. [DOI] [PubMed] [Google Scholar]
- 4.Myers JB, Meacham RB. Androgen replacement therapy in the aging male. Rev Urol. 2003;5:216–26. [PMC free article] [PubMed] [Google Scholar]
- 5.Labrie F, Belanger A, Simard J. Intracrinology. Autonomy and freedom of peripheral tissues. Annuals Endocrinology. 1995;56:23–29. [PubMed] [Google Scholar]
- 6.Labrie F, Luu-The V, Lin SX, et al. Intracrinology: role of the family of 17β-hydroxysteroid dehydrogenases in human physiology and disease. J Mol Endocrinol. 2000;25:1–16. doi: 10.1677/jme.0.0250001. [DOI] [PubMed] [Google Scholar]
- 7.Kondo K, Kai M, Setoguchi Y, et al. Cloning and expression of cDNA of humanΔ4-3-oxosteroid-5β-reductase and substrate specificity of the expressed enzyme. Eur J Biochem. 1994;219:357–363. doi: 10.1111/j.1432-1033.1994.tb19947.x. [DOI] [PubMed] [Google Scholar]
- 8.Penning TM, Burczynski ME, Jez JM, et al. Human 3α-hydroxysteroid dehydrogenase isoforms (AKR1C1-AKR1C4) of the aldo keto reductase superfamily: functional plasticity and tissue distribution reveals roles in the inactivation and formation of male and female sex hormones. Biochem J. 2000;351:67–77. doi: 10.1042/0264-6021:3510067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steckelbroeck S, Jin Y, Gopishetty S, et al. Human cytosolic 3α-hydroxysteroid dehydrogenases of the aldo-keto reductase superfamily display significant 3β-hydroxysteroid dehydrogenase activity: Implications for steroid hormone metabolism and action. J Biol Chem. 2003;279:10784–10795. doi: 10.1074/jbc.M313308200. [DOI] [PubMed] [Google Scholar]
- 10••.Di Costanzo L, Drury J, Penning TM, Christianson DW. Crystal structure of human liver Δ4-3-ketosteroid 5β-reductase (AKR1D1) and implications for substrate binding and catalysis. J Biol Chem. 2008;283:16830–9. doi: 10.1074/jbc.M801778200. Describes the first crystal structure of a human steroid double-bond reductase and mechanism of catalysis. This was selected as paper of the week by the journal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Costanzo L, Drury J, Christianson DW, Penning TM. Structure and catalytic mechanism of human steroid 5β-reductase (AKR1D1) Mol Cell Endocrinol. 2009;301:191–198. doi: 10.1016/j.mce.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12••.Drury JE, Di Constanzo L, Penning TM, Christianson DW. Inhibition of human steroid 5β-reductase (AKR1D1) by finasteride and structure of the enzyme-inhibitor complex. J Biol Chem. 2009;284:19786–19790. doi: 10.1074/jbc.C109.016931. Describes the first crystal structure of a steroid double-bond reductase with finasteride bound and explains why it is not a mechanism based inactivator of AKR1D1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faucher F, Cantin L, Luu-The V, Breton R. The crystal structure of human Δ4-3-ketosteroid 5β-reductase defines the functional role of the residues of the catalytic tetrad in the steroid double bond reduction mechanism. Biochemistry. 2008;47:8261–8270. doi: 10.1021/bi800572s. [DOI] [PubMed] [Google Scholar]
- 14.Bertilsson G, Heidrich J, Svensson K, et al. Identification of a human nuclear receptor defines a new signaling pathway for CYP3A induction. Proc Natl Acad Sci USA. 1998;95:12208–12213. doi: 10.1073/pnas.95.21.12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moore LB, Parks DJ, Jones SA, et al. Orphan nuclear receptors constitutive androstane receptor and pregnane × receptor share xenobiotic and steroid ligands. J Biol Chem. 2000;275:15122–15127. doi: 10.1074/jbc.M001215200. [DOI] [PubMed] [Google Scholar]
- 16.Huskey SW, Dean DC, Miller RR, et al. Identification of human cytochrome P450 isozymes responsible for the in vitro oxidative metabolism of finasteride. Drug Metab Dispos. 1995;23:1126–1135. [PubMed] [Google Scholar]
- 17••.Jin Y, Duan L, Lee SH, et al. Human cytosolic hydroxysteroid dehydrogenases of the aldo-ketoreductase superfamily catalyze reduction of conjugated steroids: implications for phase I and phase II steroid hormone metabolism. J Biol Chem. 2009;284:10013–10022. doi: 10.1074/jbc.M809465200. Provides evidence that ketosteroid reductases of the aldo-keto reductase superfamily have high catalytic efficiency for steroid conjugates and indicates that phase 1 enzyme reactions may occur after phase 11 conjugation reactions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bélanger A, Pelletier G, Labrie F, et al. Inactivation of androgens by UDP-glucuronosyltransferase enzymes in humans. Trends Endocrinol Metab. 2003;14:473–479. doi: 10.1016/j.tem.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Hochberg RB, Ladany S, Welch M, Liebermann S. Cholesterol and cholesterol sulfate as substrates for the adrenal side-chain cleavage enzyme. Biochemistry. 1974;13:1938–1945. doi: 10.1021/bi00706a025. [DOI] [PubMed] [Google Scholar]
- 20.Jacobsohn GM, Hochberg RB. 17β-Hydroxysteroid Dehydrogenase from Human Red Blood Cells. J Biol Chem. 1968;243:2985–2994. [PubMed] [Google Scholar]
- 21.Chang BL, Zheng SL, Hawkins GA, et al. Joint effect of HSD3B1 and HSD3B2 genes is associated with hereditary and sporadic prostate cancer susceptibility. Cancer Res. 2002;62:1784–9. [PubMed] [Google Scholar]
- 22.Simard J, Ricketts ML, Gingras S, et al. Molecular Biology of the 3s-Hydroxysteroid dehydrogenase/5-4 isomerase gene family. Endocrine Reviews. 2005;26:525–582. doi: 10.1210/er.2002-0050. [DOI] [PubMed] [Google Scholar]
- 23.Bauman DR, Steckelbroeck S, Peehl DM, Penning TM. Transcript profiling of the androgen signal in normal prostate, benign prostatic hyperplasia, and prostate cancer. Endocrinology. 2006;147:5806–5816. doi: 10.1210/en.2006-0627. [DOI] [PubMed] [Google Scholar]
- 24.Fung KM, Samara EN, Wong C, et al. Increased expression of type 2 3α-hydroxysteroid dehydrogenase/type 5 17β-hydroxysteroid dehydrogenase (AKR1C3) and its relationship with androgen receptor in prostate carcinoma. Endocr Relat Cancer. 2006;13:169–80. doi: 10.1677/erc.1.01048. [DOI] [PubMed] [Google Scholar]
- 25.Lin HK, Steckelbroeck S, Fung KM, et al. Characterization of a monoclonal antibody for human aldo-keto reductase AKR1C3 (type 2 3α-hydroxysteroid dehydrogenase/type 5 17β-hydroxysteroid dehydrogenase); immunohistochemical detection in breast and prostate. Steroids. 2004;69:795–801. doi: 10.1016/j.steroids.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 26.Penning TM, Steckelbroeck S, Bauman DR, et al. Aldo-keto reductase (AKR) 1C3: role in prostate disease and the development of specific inhibitors. Mol Cell Endocrinol. 2006;248:182–91. doi: 10.1016/j.mce.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 27.Thomas LN, Douglas RC, Lazier CB, et al. Type 1 and type 2 5α-reductase expression in the development and progression of prostate cancer. Eur Urol. 2008;53:244–252. doi: 10.1016/j.eururo.2007.10.052. [DOI] [PubMed] [Google Scholar]
- 28.Jin Y, Penning TM. Multiple steps determine the overall rate of the reduction of 5α-dihydrotestosterone catalyzed by human type 3 3α-hydroxysteroid dehydrogenase: implications for the elimination of androgens. Biochemistry. 2006;45:13054–13063. doi: 10.1021/bi060591r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rizner T, Lin H-K, Peehl DM, et al. Human type 3 3α-hydroxysteroid dehydrogenase (AKR1C2) and androgen metabolism in prostate cells. Endocrinology. 2003;144:2922–2932. doi: 10.1210/en.2002-0032. [DOI] [PubMed] [Google Scholar]
- 30•.Barbier O, Belanger A. Inactivation of androgens by UDP-glucuronosyltransferases in the human prostate. Best Pract Res Clin Endocrinol Metab. 2008;22:259–70. doi: 10.1016/j.beem.2008.01.001. Provides a detailed review of androgen glucurondiation in human prostate. [DOI] [PubMed] [Google Scholar]
- 31.Lin HK, Jez J, Schlegel BP, et al. Expression and characterization of recombinant type 2 3α-hydroxysteroid dehydrogenase (HSD) from human prostate: demonstration of bifunctional 3α/17β-HSD activity and cellular distribution. Mol Endocrinol. 1997;11:1971–1984. doi: 10.1210/mend.11.13.0026. [DOI] [PubMed] [Google Scholar]
- 32••.Montgomery RB, Mostaghel E, Vessella R, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68:4447–4454. doi: 10.1158/0008-5472.CAN-08-0249. This study relates levels of intraprostatic androgens measured by liquid chromatography mass spectrometry to transcript levels of genes involved in androgen metabolism in castrate resistant prostate (CRPC) cancer. It shows that T: 5α-DHT ratios are reversed in CRPC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nickel JC. Comparison of clinical trials with finasteride and dutasteride. Rev Urol. 2004;6 (Suppl 9):S31–S39. [PMC free article] [PubMed] [Google Scholar]
- 34.Weihua Z, Lathe R, Warner M, Gustafsson JA. An endocrine pathway in the prostate, ERβ, AR, 5α-androstane-3β,17β-diol, and CYP7B1, regulates prostate growth. Proc Natl Acad Sci USA. 2002;99:13589–13594. doi: 10.1073/pnas.162477299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weihua Z, Makela S, Andersson LC, et al. A role for estrogen receptor β in the regulation of growth of the ventral prostate. Proc Natl Acad Sci USA. 2001;98:6330–6335. doi: 10.1073/pnas.111150898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jacobi GH, Moore RJ, Wilson JD. Studies on the mechanism of 3α-androstanediol-induced growth of the dog prostate. Endocrinology. 1978;102:1748–1758. doi: 10.1210/endo-102-6-1748. [DOI] [PubMed] [Google Scholar]
- 37.Shaw G, Renfree M, Leihy MW, et al. Prostate formation in a marsupial is mediated by the testicular androgen 5α-androstane-3α,17β-diol. Proc Natl Acad Sci USA. 2000;97:12256–12259. doi: 10.1073/pnas.220412297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilson JD, Auchus R, Leihy MW, et al. 5α-Androstane-3α,17β-diol is formed in tammar wallaby pouch young testes by a pathway involving 5α-pregnane-3α,17α-diol-20-one as a key intermediate. Endocrinology. 2003;144:575–580. doi: 10.1210/en.2002-220721. [DOI] [PubMed] [Google Scholar]
- 39.Horst HJ, Dennis M, Kaufmann J, Voigt KD. In vivo uptake and metabolism of 3-h 5α-androstane-3α,17β-diol and of 3-h 5α-androstane-3β,17β-diol by human prostatic hypertrophy. Acta Endocrinol (Copenh) 1975;79:394–402. [PubMed] [Google Scholar]
- 40.Bauman DR, Steckelbroeck S, Williams MV, et al. Identification of the major oxidative 3α-hydroxysteroid dehydrogenase in human prostate that converts 5α-androstane-3α,17β-diol to 5α-dihydrotestosterone: A potential therapeutic target for androgen dependent disease. Mol Endocrinol. 2006;20:444–458. doi: 10.1210/me.2005-0287. [DOI] [PubMed] [Google Scholar]
- 41.Auchus R. The backdoor pathway to dihydrotestosterone. Trends Endocrinol Metab. 2004;15:432–438. doi: 10.1016/j.tem.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 42.Ghayee HK, Auchus R. Basic concepts and recent developments in human steroid hormone biosynthesis. Rev Endocr Metab Disord. 2007;8:289–300. doi: 10.1007/s11154-007-9052-2. [DOI] [PubMed] [Google Scholar]
- 43.Penning TM, Bauman D, Jin Y, Rizner TL. Identification of the molecular switch that regulates access of 5α-DHT to the androgen receptor. Mol Cell Endocrinol. 2007;265–266:77–82. doi: 10.1016/j.mce.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44••.Penning TMJY, Rizner TL, Bauman DR. Pre-receptor regulation of the androgen receptor. Mol Cell Endocrinol. 2008;281:1–8. doi: 10.1016/j.mce.2007.10.008. Reviews the evidence that the ligand for the AR is regulated by a 3-ketosteroid reductase (AKR1C2) and a 3α-hydroxysteroid oxidase (HSD17B6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45•.Rittmaster R. 5α-Reductase inhibitors in benign prostatic hyperplasia and prostate cancer risk reduction. Best Pract Res Clin Endocrinol Metab. 2008;22:389–402. doi: 10.1016/j.beem.2008.01.016. Reviews the efficacy of finasteride and dutasteride for the long term suppression of 5α-DHT in prostate disease. [DOI] [PubMed] [Google Scholar]
- 46.Schwinn DA, Roehrborn C. Alpha1-adrenoceptor subtypes and lower urinary tract symptoms. Int J Urol. 2008;15:193–199. doi: 10.1111/j.1442-2042.2007.01956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oefelein M. Time to normalization of serum testosterone after 3-month leutenizing hormone releasing hormone agonist administered in the neoadjuvant setting: implications for dosing schedule and neoadjuvant study consideration. J Urol. 1998;160:1685–88. [PubMed] [Google Scholar]
- 48•.Tran C, Ouk S, Clegg NJ, et al. Development of a second generation anti-androgen for the treatment of advanced prostate cancer. Science. 2009;324:787–790. doi: 10.1126/science.1168175. Describes the development of diarylthiohydantoins as superior AR antagonists to bicalutamide and their potential use to treat advanced prostate cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49•.Attar RM, Takimoto C, Gottardis MM. Castration-resistant prostate cancer: locking up the molecular escape routes. Clin Cancer Res. 2009;15:3251–3255. doi: 10.1158/1078-0432.CCR-08-1171. Reviews how dysregulation of the AR, AR mutation and changes in steroidogenic pathways contribute to castrate resistant prostate cancer. [DOI] [PubMed] [Google Scholar]
- 50.Knudsen KE, Scher H. Starving the addiction: new opportunities for durable suppression of AR signaling in prostate cancer. Clin Cancer Res. 2009;15:4792–4798. doi: 10.1158/1078-0432.CCR-08-2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yuan X, Balk S. Mechanisms mediating androgen receptor reactivation after castration. Urol Oncol. 2009;27:36–41. doi: 10.1016/j.urolonc.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52••.Locke J, Guns ES, Lubik AA, et al. Androgen levels increase by intratumoral de novo steroidogenesis during the progression of castration-resistant prostate cancer. Cancer Res. 2008;68:6407–15. doi: 10.1158/0008-5472.CAN-07-5997. Provides evidence in xenograft LNCaP tumors that are castrate resistant that [14C]-actetate can be incorporated into 5α-DHT and that [3H]-progesterone is incorporated into pregnane derivatives in the backdoor pathway to 5α-DHT under ex vivo conditions. [DOI] [PubMed] [Google Scholar]
- 53.Stanbrough M, Bubley GJ, Ross K, et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66:2815–25. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- 54••.Attard G, Reid AH, Yap TA, et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol. 2008;26:4563–4571. doi: 10.1200/JCO.2007.15.9749. Phase 1 clinical trial to report efficacy of abiraterone acetate in suppressing castrate resistant prostate cancer. Suppression of circulating T and PSA levels and a reduction in bone metastases was reported. [DOI] [PubMed] [Google Scholar]
- 55••.Attard G, Reid AH, A’Hern R, et al. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J Clin Oncol. 2009;27:3742–3748. doi: 10.1200/JCO.2008.20.0642. Phase 1/11 clinical trial of abiraterone acetate. A decline in PSA of > or = 50% was observed in 28 (67%) of 42 phase II patients, and declines of > or = 90% were observed in eight (19%) of 42 patients. Independent radiologic evaluation reported partial responses in nine (37.5%) of 24 phase II patients with measurable disease. These data support the concept that CRPC is androgen dependent. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56••.Leon CG, Locke JA, Adomat HH, et al. Alteration in cholesterol regulation contribute to the production of intra-tumoral androgens during progession to castration-resistant prostate cancer in a mouse xenograft model. Prostate. 2009 doi: 10.1002/pros.21072. This study shows that in xenograft LNCaP models of CRPC that there is an adaptive response to mobilize cholesterol for steroidogenesis within the tumor ex vivo. [DOI] [PubMed] [Google Scholar]
- 57.Locke JA, Nelson CC, Adomat HH, et al. Steroidogenesis inhibitors alter but do not eliminate androgen synthesis mechanisms during progression to castration-resistance in LNCaP prostate xenografts. J Steroid Biochem Mol Biol. 2009;115:126–136. doi: 10.1016/j.jsbmb.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 58.Locke J, Guns EST, Lehman ML, et al. Arachidonic acid activation of intratumoral steroid synthesis during prostate cancer progression to castration resistance. Prostate. 2009;70:239–51. doi: 10.1002/pros.21057. [DOI] [PubMed] [Google Scholar]
- 59.Hille UE, Hu Q, Vock C, et al. Novel CYP17 inhibitors: synthesis, biological evaluation, structure-activity relationships and modelling of methoxy- and hydroxy-substituted methyleneimidazolyl biphenyls. Eur J Med Chem. 2009;44:2765–75. doi: 10.1016/j.ejmech.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 60••.Vasaitis T, Belosay A, Schayowitz A, et al. Androgen receptor inactivation contributes to antitumor efficacy of 17α-hydroxylase/17,20-lyase inhibitor 3β-hydroxy-17-(1H-benzimidazole-1-yl)androsta-5,16-diene in prostate cancer. Mol Cancer Ther. 2008;7:2348–2357. doi: 10.1158/1535-7163.MCT-08-0230. This study describes the evaluation of a second generation CYP17α-hydroxylase/17,20-lyase inhibitor that also binds to the AR and reduces receptor half-life. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61••.Byrns MC, Steckelbroeck S, Penning TM. An indomethacin analogue, N-(4-chlorobenzoyl)-melatonin, is a selective inhibitor of aldo-keto reductase 1C3 (type 2 3α-HSD, type 5 17β-HSD, and prostaglandin F synthase), a potential target for the treatment of hormone dependent and hormone independent malignancies. Biochem Pharmacol. 2008;75:484–93. doi: 10.1016/j.bcp.2007.09.008. This study describes the first selective inhibitors of AKR1C3 based on indomethacin analogs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bauman DR, Rudnick S, Szewczuk L, et al. Development of nonsteroidal anti-inflammatory drug analogs and steroid carboxylates selective for human aldo-keto reductase isoforms: potential antineoplastic agents that work independently of cyclooxygenase isozymes. Mol Pharmacol. 2005;67:60–68. doi: 10.1124/mol.104.006569. [DOI] [PubMed] [Google Scholar]
- 63.Féau C, Arnold L, Kosinski A, et al. Novel flufenamic acid analogues as inhibitors of androgen receptor mediated transcription. ACS Chem Biol. 2009;4:834–43. doi: 10.1021/cb900143a. [DOI] [PMC free article] [PubMed] [Google Scholar]