Abstract
Objective
There are few studies comparing small and large craniotomies for the initial treatment of chronic subdural hematoma (CSDH) which had non-liquefied hematoma, multilayer intrahematomal loculations, or organization/calcification on computed tomography and magnetic resonance imaging. These procedures were compared to determine which would produce superior postoperative results.
Methods
Between 2001 and 2009, 317 consecutive patients were surgically treated for CSDH at our institution. Of these, 16 patients underwent a small craniotomy with partial membranectomy and 42 patients underwent a large craniotomy with extended membranectomy as the initial treatment. A retrospective review was performed to compare the postoperative outcomes of these two techniques, focusing on improvement of neurological status, complications, reoperation rate, and days of post-operative hospitalization.
Results
The mean ages were 69.4±12.1 and 55.6±9.3 years in the small and large craniotomy groups, respectively. The recurrence of hematomas requiring reoperation occurred in 50% and 10% of the small and large craniotomy patients, respectively (p<0.001). There were no significant differences in postoperative neurological status, complications, or days of hospital stay between these two groups.
Conclusion
Among the cases of CSDH initially requiring craniotomy, the large craniotomy with extended membranectomy technique reduced the reoperation rate, compared to that of the small craniotomy with partial membranectomy technique.
Keywords: Chronic subdural hematoma, Small craniotomy, Large craniotomy, Reoperation
INTRODUCTION
Chronic subdural hematoma (CSDH) represents one of the most frequent intracranial hemorrhages encountered in neurosurgical department, with elderly citizens being more frequently affected. The reasons why this type of hematoma occurs frequently among the elderly include an increase in antithrombotic medications, venous fragility, augmentation of the subdural space, and an increased exposure to traumatic injury resulting from frequent falls1). For the initial management of CSDH, numerous surgical treatments have been proposed5,14,15,18,21). However, the extent of surgical treatment required for CSDH is still controversial2,24), and the optimal treatment for CSDH is not well defined16). The choice of surgical technique for CSDH must be dictated by the degrees of organization of the hematoma. Burr-hole with drainage is mandatory for non-septated and mostly liquefied CSDH. Conversely, craniotomy is generally accepted as the optimal approach for reaccumulation of a CSDH, existence of a solid hematoma, failure of brain reexpansion, or marked swelling subjacent to the hematoma5,14). The purpose of this study was to analyze the efficacy of small or large craniotomy with membranectomy as the initial treatment for CSDH.
MATERIALS AND METHODS
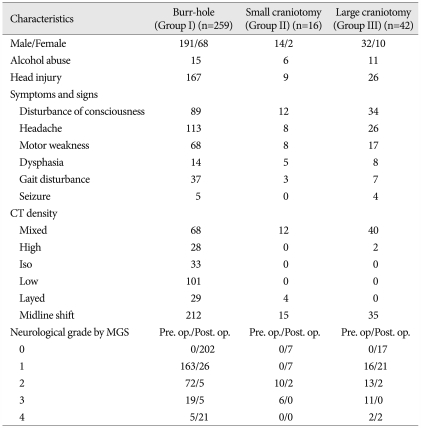
A retrospective study was performed using the medical records retained by our hospital. computed tomography (CT) scans were the primary imaging method for the evaluation of CSDH in all patients. The technique of a burr-hole with closed system drainage for 24 to 72 hours was chosen for cases of non-septated and mostly liquefied CSDH. However, on the basis of CT and/or magnetic resonance imaging (MRI) findings, cases of CSDH demonstrating either mixed density or hyperdense lesions (Fig. 1), intrahematomal membranes or web-like structures (Fig. 2), or an organized/calcified CSDHs (Fig. 3) were selected for craniotomy. We classified 317 patients with CSDH into three groups according to the extent of surgery required : group I, burr hole with drainage (n=259); group II, small craniotomy (approximately 3-4 cm diameter) with partial membranectomy and subdural drainage (n=16) (Fig. 1B); group III, large craniotomy (approximately the diameter of the hematoma) with extended membranectomy (n=42) (Fig. 2B, 3B). In the cases requiring craniotomy, the choice of a small or large craniotomy was dependent on surgeon's judgment; the factors influencing this judgment were CT/MRI findings, age, and neurological status of the patients.
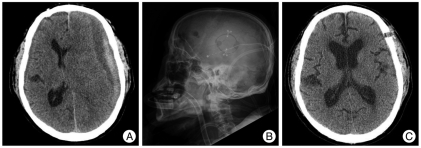
Fig. 1.
A : Preoperative computed tomography (CT) showing mixed density chronic subdural hematoma (CSDH) having non-liquefied hematoma. B : Postoperative lateral skull X-ray film showing small craniotomy on left hematoma site. C : After postoperative 31 days, CT scan showing nearly complete cure of the CSDH.
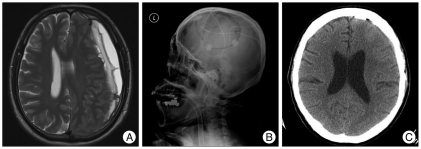
Fig. 2.
A : Preoperative T2-wighted axial magnetic resonance imaging (MRI) showing multilayer intrahematomal loculations within CSDH. B : Postoperative lateral skull X-ray film showing large craniotomy on the left hematoma site. C : After postoperative 2 months, computed tomography (CT) showing complete removal of CSDH. CSDH : chronic subdural hematoma.
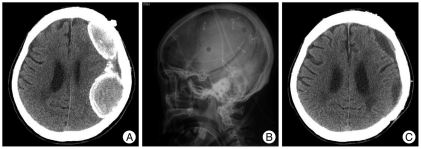
Fig. 3.
A : Preoperative computed tomography (CT) showing double CSDH with thickened calcified membrane. B : Postoperative lateral skull X-ray film showing large craniotomy on the left hematoma site. C : Postoperative CT showing total removal of hematoma with small residual subdural fluid collection. CSDH : chronic subdural hematoma.
Neurological performances of patients were preoperatively and postoperatively evaluated with the "Markwalder's Neurological Grading System", the most commonly used neurological grading system for CSDH13).
Follow-up information was obtained for the 56 surviving patients. The average duration of follow-up was six months, with a range from 1 to 17 months.
Statistical analysis
SPSS software (version 18.0 for Windows, SPSS Inc., Chicago, IL, USA) was used for statistical analysis. The mean values±standard deviations among the three groups were analyzed with ANOVA, and those between two groups were evaluated using Student's t-test. We also performed a multivariable statistical analysis to evaluate the relationship between the factors and groups using the X2 test with a logistic regression model. Statistical significance was assumed if p<0.05.
RESULTS
Clinical presentation
Of these 317 patients, 259 (82%) received one or two burr-holes with drainage, including 191 males and 68 females. The average age of these burr-hole patients was 63.7±16.9 years, and 167 cases had experienced minor head trauma. The leading symptoms were headache (n=113), disturbance of consciousness (n=89), motor weakness (n=68), and gait disturbance (n=37). Fifteen of these patients were chronic alcoholics. Reoperation was performed in 23 (8.9%) cases.
Of the remaining 58 patients undergoing craniotomy, there were 46 men and 12 women, and the mean ages was 59.4 years (69.4±12.1, and 55.6±9.3 years, groups II and III, respectively), with a range of 40 to 83 years. Thirty-five patients had a history of minor head trauma approximately three weeks prior to admission. The major symptoms at presentation were disturbance of consciousness (n=46), headache (n=34), motor deficits (n=25), and dysphasia (n=13). Seventeen of these patients were chronic alcoholics. Of the hematomas, 56 were heterogeneous, and two were homogeneous on CT imaging. In 50 (86%) patients, CT scans demonstrated a marked midline shift (Table 1, 2).
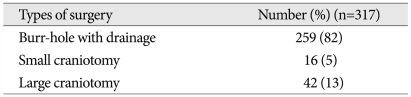
Table 1.
Surgical types in 317 patients with CSDH
CSDH : chronic subdural hematoma
Table 2.
Clinical data and CT findings in 317 patients with CSDH
CT : computed tomography, CSDH : chronic subdural hematoma, MGS : Markwalder's grade scale
Patient selection for craniotomy in groups II and, III
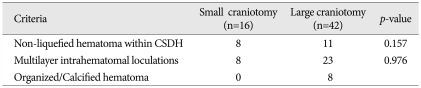
Based on CT and/or MRI findings, non-liquefied hematomas within CSDH (n=19), multilayer intrahematomal loculations (n=31), or thickened calcified membranes with heterogeneous structures in the hematoma cavity (n=8) were selected for small or large craniotomies (Table 3).
Table 3.
Criteria of patient's selection for craniotomy in CSDH
CSDH : chronic subdural hematoma
Operative methods
In the craniotomy groups, under general anesthesia, the dura was reflected and the outer membrane of a solid hematoma was excised following craniotomy. The removal of the hematoma was carried out under direct visualization. Non-liquefied hematomas or multilayer loculations were only be partially removed with a small craniotomy, though these were completely removed with a large craniotomy. If neovascularization existed in the hematoma capsule, it was coagulated. The inner membrane was removed as much as possible.
Outcomes
The postoperative percentages of the patients who achieved a grade 0 or 1 (no or only mild neurologic deficits) were 88% in group I, 88% in group II, and 90% in group III.
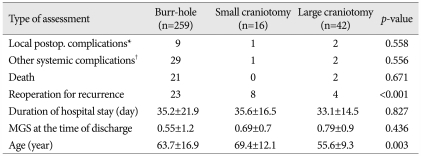
In group I, local postoperative complications, such as surgical wound infection, tension pneumocephalus, epidural hematoma, and seizure, occurred in nine patients. Systemic complications, including pneumonia and sepsis, occurred in 29 patients. An SDH recurred in 23 patients (8.9%), who required reoperation. The operative mortality rate in this group, defined as death within 30 days after surgery, was 8.1% (n=21).
In groups II and III, one patient had an intracerebral hemorrhage and two patients experienced seizures. Systemic complications comprised three cases of pneumonia. Reoperation, due to a recurrent SDH, was done in 12 patients. The operative mortality rate was 3.5% (n=2) in group III; no death was seen in group II. The mean hospital stay following the initial operation were 35.2±21.9 days (group I), 35.6±16.5 days (group II) and 33.1±14.5 days (group III). The p-values for postoperative neurologic outcome, duration of hospital stay, local complication rate, and systemic complication rate were 0.436, 0.827, 0.558 and 0.556, respectively. These data suggest no significant differences for these characteristics among these three groups (Table 4).
Table 4.
Outcome assessment in 317 patients with CSDH
*Cerebral hemorrhage, seizure, infection, epidural hematoma, †Pneumonia, sepsis. CSDH : chronic subdural hematoma, MGS : Markwalder's Grade Scale
Reoperation
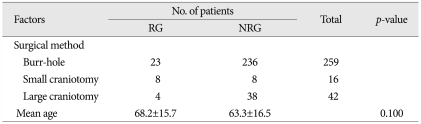
The reoperation rate among the 317 patients with CSDH was 11.0% (n=35). The mean ages of reoperation (n=35) and non-reoperation groups (n=282) were 68.2±15.7, and 63.3±16.5 years, respectively, with no significant difference (p=0.100) (Table 5).
Table 5.
Relation between age and reoperation in 317 patients with CSDH
CSDH : chronic subdural hematoma, RG : recurrence group, NRG : non-recurrence group
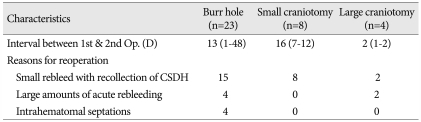
The average elapsed days before reoperation were 13 (group I), 16 (group II) and two (group III). The causes for reoperation after burr-hole with drainage (n=23) were acute rebleeding (n=4), an increased volume of residual subdural fluid within the hematoma cavity (n=15), and failure of brain re-expansion due to intrahematomal septations (n=4). Repeated burr-holes with drainage were performed in 15 patients, and a large craniotomy was required in eight patients. The causes for reoperation in the small craniotomy group (n=8) were recollection of subdural fluid (n=4), and a small rebleed with recollection of the CSDH (n=4). The causes for reoperation in the large craniotomy group (n=4) were recollection of CSDH with a considerable amount of rebleeding (n=2), and significant rebleeding in the subdural space (n=2). Among the cases of reoperation in groups II and III, ten patients received burr-holes with drainage, and two patients (group III) underwent a large craniectomy due to significant rebleeding in the subdural space (Table 6).
Table 6.
Reasons for reoperation in 35 patients with CSDH
CSDH : chronic subdural hematoma, D : days
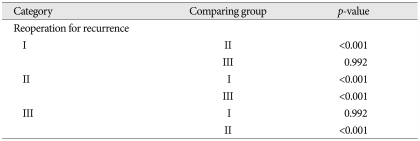
In the craniotomy groups, the reoperation rate was 50% (n=8) in group II, and 10% (n=4) in group III. The p-value for the reoperation rate comparison between the small and large craniotomy groups was <0.001, indicating that the large craniotomy group had a lower reoperation rate than the small craniotomy group (Table 7). CT imaging studies were performed in 56 patients in groups II and III prior to discharge. In 34 (61%) of these patients, subdural fluid remained. CT scans were performed in 51 of these patients during follow-up, and a complete resolution of the subdural fluid collection occurred in 43 patients (84%). The remaining eight patients had minimal subdural fluid collections, but none required subsequent surgery.
Table 7.
Statistical comparison study for reoperation in three groups
Group I : burr-hole with drainage (n=259), Group II : small craniotomy (n=16), Group III : large craniotomy (n=42)
DISCUSSION
Although CSDH is well known as a curable disease in the elderly and can be adequately managed with burr-holes with drainage, the initial treatment of CSDH can be ineffective; this results in a reoperation rate between 3 and 37%2,10,13,16,17). The causes of treatment failure include the presence of non-liquefied hematomas with various bleeding foci14); multilayer loculations within the hematoma, which produce non-communicating compartments22); and excessive formation of a solid membranes5).
Occasionally, advanced age has been considered to be a risk factor for recurrence and reoperation19,20). However, most studies have demonstrated no relationship between recurrence rate and age8,9,11,17,23). In the current study of 317 surgically treated patients, the mean ages of patients in the reoperation and non-reoperation groups were not significantly different, implying no relationship between age and reoperation rate. However, many authors have reported that older patients may require a prolonged recovery time to restore the brain2,3,12,23), and that unnecessary reoperation have been performed secondary to prolonged reaccumulations of blood within the hematoma cavity2,23). Jeong et al.7) suggested that if complete brain re-expansion is not observed immediately after operation in elderly patients, a six-week trace-observation should be performed, and that reoperation should be performed only if neurologic symptoms reappeared or if the cerebral sulci were diffusely effaced by recurrence2,17).
MRI is not frequently used for the diagnosis of CSDH, but its superiority over CT has been well documented. MRI provides precise evidence for the extension of SDH, facilitates the detection of CT - isodense SDH, as well as detection of small clots near the skull base and vertex, and often provides an accurate estimation of the age of the SDH4). Furthermore, contrast-enhanced MRI may demonstrate connective tissue reactions occurring during the maturation of SDH4). Rocchi et al.21) recommended that preoperative MRI should always be performed in the following cases : 1) CSDH with an unusual appearances on CT scans, such as the presence of heterogeneous areas with high-density margins, multiple compartments, septations, and various bleeding foci, 2) cases of recurrent CSDH, and 3) enhancement of some portions of the hematoma and its membranes after contrast enhanced CT. Furthermore, they insisted that craniotomy should be performed primarily in these cases, without attempting other approaches.
In our craniotomy groups, MRI scans were performed in 29 cases. Of these, 12 cases showed irregular web-like structures within the CSDH, 11 demonstrated non-liquefied hematomas with various bleeding foci, and six had thickened, calcified inner membranes with heterogeneous structures within the hematoma cavity. In the past, when a CSDH displayed these radiologic features, we performed craniotomy with membranectomy to remove the hematoma only after one attempt of evacuation with burr-hole. Recently, we adopted craniotomy with membranectomy as the initial treatment.
Tanikawa et al.22) reported that a multilayer intrahematomal structure led to a high recurrence rate and that these structures were well visualized on T2-weighted MRI. They found that a significant number of these cases did not achieve complete recovery using initial burr-hole surgery. Therefore, they initially performed small craniotomies, and achieved more favorable results and a significantly shortened hospital stay. Lee et al.10) reported a reoperation rate of 6.7% in 30 cases of small craniotomies used as initial treatment. Rocchi et al.21) reported that MRI detection of thick and extensive membranes or solid clots with mass effect required an immediate large craniotomy to remove the CSDH. These authors emphasized that using a saline solution jet at the reflection zone of the hematoma avoids injury to the underlying arachnoid surface and newly formed capillaries during removal of the membranes. Mohamed14) reported 39 cases of CSDH treated by large craniotomy with extended membranectomy. These patients had mixed density or hyperdense lesions on CT, and intrahematomal membranes or web-like structures on MRI. The incidence of recollection of the CSDH after a large craniotomy was 5%, an acceptable rate for surgical treatment of CSDH. They treated cases of recollection with percutaneous tapping.
In the current study, the rate of reoperation in the small craniotomy group was 50%, significantly higher than that of the large craniotomy group, most likely due to the limited surgical view associated with small craniotomy with partial membranectomy. It was difficult to coagulate the neovascularized vessels and remove the inner and outer membranes using a small craniotomy. This technically tedious procedure frequently resulted in rebleeding and recollection of subdural fluid. In the four cases of reoperation in the large craniotomy group, the craniotomy was not extended over the entire hematoma, making it difficult to coagulate the fragile, new vessels, contributing to rebleeding. We performed burr-holes with drainage and craniectomy in two cases each on the second postoperative day. There was no recurrence in the burr-hole cases following reoperation, but the two cases of craniectomy resulted in patient death; these patients were preoperatively comatose and had signs of brain stem dysfunction. In these patients, evacuation of the CSDHs was performed, but a fixed subdural space remained due to failure of brain re-expansion and was filled with blood. In the current study, the rate of reoperation were significantly different between the two craniotomy groups (p<0.001), indicating that the extent of craniotomy was an important factor affecting reoperation rate. Large craniotomies provided a superior and safer opportunity to adequately deal with the hematoma, its membranes, and occasional troublesome bleeding.
Isobe et al.6) reviewed six patients diagnosed with an organized CSDH, five of whom had a history of burr-hole surgery. These patients collectively underwent four small craniotomies and two enlarged craniotomies. The authors emphasized that it was important to remove the organized CSDH and the outer membrane in proportion to the hematoma expansion. Imaizumi et al.5) reported five cases of an organized CSDH, and proposed that a large craniotomy was the best treatment for calcified or organized CSDH associated with progressive symptoms. In our eight cases of organized/calcified CSDH, we performed large craniotomy with extended membranectomy; these cases required no reoperation and postoperative results were excellent.
In our craniotomy cases, the choice of a small or large craniotomy for CSDH treatment depended on the surgeon's judgment. The mean age of patients undergoing a small craniotomy was older than that of large craniotomy patients. The p-value for non-liquefied hematomas between the large and small craniotomy groups was 0.157 and that for multilayer intrahematomal loculations was 0.976. These results indicate that non-liquefied hematomas and multilayer intrahematomal loculations are not important factors when deciding between either a small or large craniotomy. A large craniotomy was the treatment choice for calcified or organized CSDHs.
CONCLUSION
Among the 58 patients requiring craniotomy with membranectomy as the initial treatment, 16 underwent a small craniotomy (group II) and 42 patients underwent a large craniotomy (group III). There were no significant differences in the postoperative neurological status, complications, or days of hospital stay between these two groups. However, the large craniotomy with extended membranectomy group had superior results in terms of rate of reoperation. Until a systemic evaluation of these techniques is undertaken, we feel that a large craniotomy remains the acceptable, safe, and efficacious alternative compared with a small craniotomy in selected cases of CSDH.
References
- 1.Asghar M, Adhiyaman V, Greenway MW, Bhowmick BK, Bates A. Chronic subdural hematoma in the elderly-a north wales experience. J R Soc Med. 2002;95:290–292. doi: 10.1258/jrsm.95.6.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ernestus R-I, Beldzinski P, Lanfermann H, Klug N. Chronic subdural hematoma : surgical treatment and outcome in 104 patients. Surg Neurol. 1997;48:220–225. doi: 10.1016/s0090-3019(97)80031-6. [DOI] [PubMed] [Google Scholar]
- 3.Fukuhara T, Gotoh M, Asari S, Ohmoto T, Akioka T. The relationship between brain surface elastance and brain re-expansion after evacuation of chronic subdural hematoma. Surg Neurol. 1996;45:570–574. doi: 10.1016/0090-3019(95)00471-8. [DOI] [PubMed] [Google Scholar]
- 4.Hosoda K, Tamaki N, Masumura M, Matsumoto S, Maeda F. Magnetic resonance images of chronic subdural hematomas. J Neurosurg. 1987;67:677–683. doi: 10.3171/jns.1987.67.5.0677. [DOI] [PubMed] [Google Scholar]
- 5.Imaizumi S, Onuma T, Kameyama M, Naganuma H. Organized chronic subdural hematoma requiring craniotomy--five case reports. Neurol Med Chir (Tokyo) 2001;41:19–24. doi: 10.2176/nmc.41.19. [DOI] [PubMed] [Google Scholar]
- 6.Isobe N, Sato H, Murakami T, Kurokawa Y, Seyama G, Oki S. [Six cases of organized chronic subdural hematomas.] No Shinkei Geka. 2008;36:1115–1120. [PubMed] [Google Scholar]
- 7.Jeong CA, Kim TW, Park KH, Chi MP, Kim JC. Retrospective analysis of re-operated patients after chronic subdural hematoma surgery. J Korean Neurosurg Soc. 2005;38:116–120. [Google Scholar]
- 8.Kang MS, Koh HS, Kwon HJ, Choi SW, Kim SH, Youm JY. Factors influencing chronic subdural hematoma after surgery. J Korean Neurosurg Soc. 2007;41:11–15. [Google Scholar]
- 9.Ko BS, Lee JK, Seo BR, Moon SJ, Kim JH, KIM SH. Clinical analysis of risk factors related to recurrent chronic subdural hematoma. J Korean Neurosurg Soc. 2008;43:11–15. doi: 10.3340/jkns.2008.43.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JK, Choi JH, Kim CH, Lee HK, Moon JG. Chronic subdural hematomas : a comparative study of three types of operative procedures. J Korean Neurosurg Soc. 2009;46:210–214. doi: 10.3340/jkns.2009.46.3.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee JY, Ebel H, Ernestus RI, Klug N. Various surgical treatment of chronic subdural hematoma and outcome in 172 patients : is membranectomy necessary? Surg Neurol. 2004;61:523–527. doi: 10.1016/j.surneu.2003.10.026. discussion 527-528. [DOI] [PubMed] [Google Scholar]
- 12.Lee SC, Kang JK, Jung HT, Dho JO. Factors affecting brain re-expansion after simple burr hole drainage in chronic subdural hematoma. J Korean Neurosurg Soc. 1998;27:757–762. [Google Scholar]
- 13.Markwalder TM, Steinsiepe KF, Rohner M, Reichenbach W, Markwalder H. The course of chronic subdural hematomas after burr-hole craniostomy and closed-system drainage. J Neurosurg. 1981;55:390–396. doi: 10.3171/jns.1981.55.3.0390. [DOI] [PubMed] [Google Scholar]
- 14.Mohamed EE. Chronic subdural hematoma treated by craniotomy, durectomy, outer membranectomy and subgaleal suction drainage. Personal experience in 39 patients. Br J Neurosurg. 2003;17:244–247. doi: 10.1080/0268869031000153134. [DOI] [PubMed] [Google Scholar]
- 15.Mondorf Y, Abu-Owaimer M, Gaab MR, Oertel JM. Chronic subdural hematoma-craniotomy versus burr hole trephination. Br J Neurosurg. 2009;23:612–616. doi: 10.3109/02688690903370297. [DOI] [PubMed] [Google Scholar]
- 16.Muzii VF, Bistazzoni S, Zalaffi A, Carangelo B, Mariottini A, Palma L. Chronic subdural hematoma : comparison of two surgical techniques. Preliminary results of a prospective randomized study. J Neurosurg Sci. 2005;49:41–46. discussion 46-47. [PubMed] [Google Scholar]
- 17.Nakaguchi H, Tanishima T, Yoshimasu N. Factors in the natural history of chronic subdural hematomas that influence their postoperative recurrence. J Neurosurg. 2001;95:256–262. doi: 10.3171/jns.2001.95.2.0256. [DOI] [PubMed] [Google Scholar]
- 18.Okada Y, Akai T, Okamoto K, Iida T, Takata H, Iizuka H. A comparative study of the treatment of chronic subdural hematoma-burr hole drainage versus burr hole irrigation. Surg Neurol. 2002;57:405–409. doi: 10.1016/s0090-3019(02)00720-6. discussion 410. [DOI] [PubMed] [Google Scholar]
- 19.Probst C. Peritoneal drainage of the chronic subdural hematoma in older patients. J Neurosurg. 1988;68:908–911. doi: 10.3171/jns.1988.68.6.0908. [DOI] [PubMed] [Google Scholar]
- 20.Robinson RG. Chronic subdural hematoma : surgical management in 133 patients. J Neurosurg. 1984;61:263–268. doi: 10.3171/jns.1984.61.2.0263. [DOI] [PubMed] [Google Scholar]
- 21.Rocchi G, Caroli E, Salvati M, Delfini R. Membranectomy in organized chronic subdural hematomas : indications and technical notes. Surg Neurol. 2007;67:374–380. doi: 10.1016/j.surneu.2006.08.066. discussion 380. [DOI] [PubMed] [Google Scholar]
- 22.Tanikawa M, Mase M, Yamada K, Yamashita N, Matsumoto T, Banno T, et al. Surgical treatment of chronic subdural hematoma based on intrahematomal membrane structure on MRI. Acta Neurochir (Wien) 2001;143:613–618. doi: 10.1007/s007010170067. discussion 618-619. [DOI] [PubMed] [Google Scholar]
- 23.Tsutsumi K, Maeda K, Iijima A, Usui M, Okada Y, Kirino T. The relationship of preoperative magnetic resonance imaging findings and closed system drainage in the recurrence of chronic subdural hematoma. J Neurosurg. 1997;87:870–875. doi: 10.3171/jns.1997.87.6.0870. [DOI] [PubMed] [Google Scholar]
- 24.Tyson G, Strachan WE, Newman P, Winn Hr, Butler A, Jane J. The role of craniectomy in the treatment of chronic subdural hematomas. J Neurosurg. 1980;52:776–781. doi: 10.3171/jns.1980.52.6.0776. [DOI] [PubMed] [Google Scholar]