Abstract
Bilateral traumatic carotid-cavernous fistulae (TCCFs) is rarely encountered neurovascular disease. For treatment of TCCF, detachable balloons have been widely used. Nowadays, transarterial and/or transvenous coil embolization with placement of covered stents is adopted as another treatment method. We experienced a patient with a bilateral TCCFs who was successfully treated with covered stents. However, cerebral hemorrhage occurred in the bed of previous infarction one day after treatment. Hyperperfusion syndrome was considered as a possible cause of the hemorrhage, so that barbiturate coma therapy was started and progression of hemorrhage was stopped. We emphasize that cerebral hyperperfusion hemorrhage can occur even after successful endovascular treatment of TCCF.
Keywords: Carotid-cavernous fistulae, Endovascular treatment, Cerebral hemorrhage
INTRODUCTION
Traumatic carotid-cavernous fistula (TCCF) is a high-flow communication between the internal carotid artery and the cavernous sinus. Depending on the size and venous drainage, it may cause neuro-opthalomolgic symptoms, cranial nerve palsy, or cerebral hemorrhage. Detachable balloons have been widely used for treatment of TCCF since the early 1970. But nowadays, other treatment options, such as transarterial and/or transvenous coil embolization and trapping of the ICA, are adopted on a case-by-case basis because detachable balloons are not always successful and not available everywhere6,7,13,16,18). Recently, covered stents have been emerged as a valid alternative to replace previous treatment methods2,4,11). However, the use of covered stents may involve some problems, for example, difficult navigation of the stent devices due to high stiffness, no availability of mounted covered stents >5 mm in diameter, and a risk of in-stent thrombosis or stenosis2,4,15). TCCF usually is single and unilateral, but unilateral double TCCFs and bilateral TCCFs are uncommon12,16,18-20). In addition, cerebral hemorrhage from hyperperfusion syndrome after the treatment has never been reported in the English literature. Herein, we report a severely injured patient developing cerebral hemorrhage, which would be because of hyperperfusion syndrome, after successful endovascular treatment of bilateral TCCFs with covered stents.
CASE REPORT
A 48-year-old man was involved in a motor vehicle accident and suffered from spleen laceration, multiple bilateral rib fractures, hemopneumothorax, basal skull fracture, and left frontotemporal epidural hemorrhage (EDH). On arrival in the emergency room, his Glasgow Coma Scale was rated as 4 and pupillary reflex was prompt to light. Vital signs indicated hypovolemic shock, which persisted despite fluid resuscitation and transfusion. An emergent splenectomy was performed because spleen laceration was the most likely to cause of hypovolemic shock. He was admitted to department of neurosurgery to treat traumatic head injury afterwards.
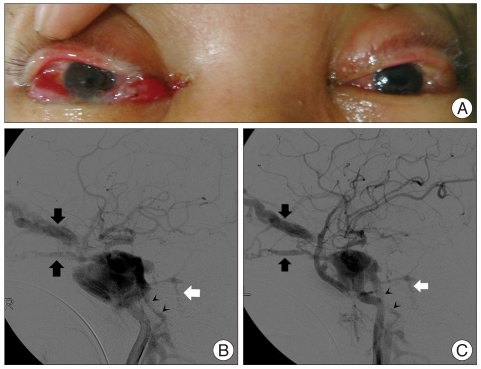
The day after admission, his pupil was dilated to 8 mm, and computerized tomography (CT) demonstrated increased amount of EDH and cerebral contusion. However, surgical treatment was impossible because of unstable vital signs at that time. EDH and cerebral contusion were progressively improved on serial CT scans. On the nineteenth day after admission, eyelid swelling, bilateral proptosis, and chemosis were found (Fig. 1A), and bruit was heard over the bilateral periorbital regions. CT images revealed engorged superior ophthalmic vein, pointing to TCCF. Catheter angiography confirmed the presence of bilateral large TCCFs, draining to the bilateral ophthalmic veins, bilateral inferior petrosal sinuses, left sylvian vein, and left petrosal vein (Fig. 1B, C).
Fig. 1.
Preprocedural findings of a patient with bilateral carotid-cavernous fistulae. Photograph shows bilateral proptosis, chemosis, and eyelid edema (A). Right (B) and left (C) carotid angiograms reveal bilateral carotid-cavernous fistulae with shunts to the ophthalmic vein (black arrows), inferior petrosal sinus (black arrowheads), and petrosal vein (white arrow).
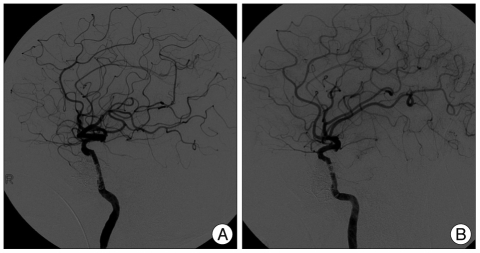
Endovascular treatment of TCCF was performed under general anesthesia. Percutaneous access was obtained via the right femoral artery. A bolus of 3,000 IU of heparin was administered via intravenous route after placement of a 7F femoral sheath, and an additional bolus of 1,000 IU was injected hourly. A 7F guiding catheter was placed in the right cervical ICA and a microcatheter over a microguidewire was introduced into the cavernous sinus through the fistula, and an attempt was made to close the fistula by balloon-protected transarterial embolization using Hyperglide™ occlusion balloon system for ICA protection and 8 detachable coils for occlusion of the fistula : 4 bare coils and 4 coated coils (HydroCoil™). However, this balloon-protected endovascular occlusion was incomplete and the number of coils applied already exceeded the cap put by the National Health Insurance System, so that a covered stent (Jostent GraftMaster™ 3.5×19 mm, Abott) was deployed to occlude the fistula. Eventually, right TCCF was completely treated (Fig. 2A). The same procedure was introduced to the left TCCF; balloon-protected coil embolization was attempted using the same Hyperglide™ occlusion balloon system and 5 coated coils. However, the fistular flow was not decreased, so that a covered stent (Jostent GraftMaster™ 3.5×16 mm, Abott) was additionally deployed (Fig. 2B).
Fig. 2.
Angiographic findings following endovascular treatment with covered stents. Completion angiogram shows complete occlusion of carotid-cavernous fistula on the right (A) and left (B).
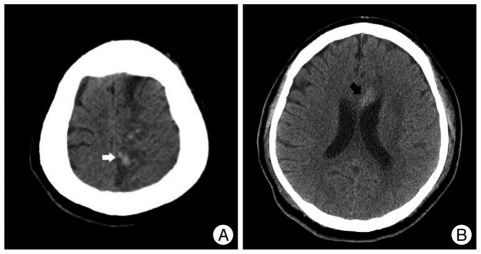
After the procedure, the patient was given 100 mg aspirin and 75 mg clopidogrel daily via nasogastric tube immediately, and was maintained at normotensive blood pressure in intensive care unit. The patient was semicomatous, same as in the preprocedural status, but periorbital bruit and ophthalmic manifestations were disappeared immediately after the procedure. Follow-up CT scans successively demonstrated hemorrhagic foci in the left parietal lobe at 1 day and the genu of the corpus callosum at 2 days (Fig. 3), both of which belonged to the region that was infracted previously of the left ACA territory from the initial subfalcine herniation. Assuming that the cerebral hemorrhages were associated with hyperperfusion syndrome after correction of the large shunts, we decided to induce barbiturate coma therapy for 6 days. New hemorrhages or any progression of the existing one were not identified, and follow-up CT scans showed decreased attenuation and size of the hemorrhages, consistent with resolving hemorrhage, on the ninth day. The patient was transferred to a rehabilitation hospital afterwards.
Fig. 3.
Follow-up CT images. One-day (A) and two-day (B) follow-up CT scans show two focal hemorrhagic foci in the left parietomedial region (white arrow) and the genu of the corpus callosum (black arrow), both of which occurred in the previously infarcted territory of the left anterior cerebral artery.
DISCUSSION
It is estimated that incidence of TCCF is 0.17-1.01% after head injury. It usually unilateral, but bilateral TCCFs have been rarely reported in the English literature3,9,13,16,18-20). Intracranial hemorrhage following treatment of bilateral TCCFs is found to be very rare, but several explanations are discussed : 1) occlusion of one CCF may change the hemodynamics of contralateral TCCF, in other words, sudden blockage of the contralateral venous drainage through intercavernous sinus may make venous hypertension worsen; 2) compromised flow in the ICA after therapeutic occlusion may increase intravascular pressure on the contralateral side to expand the shunt, particularly patient treated in different sessions. Treatment of bilateral TCCF in different sessions has shown to aggravate symptoms of contralateral lesion, or to cause ICH8,10,18). However in this case, bilateral TCCFs were treated simultaneously using covered stents after attempted balloon-protected coil embolization, while the ICA flow was preserved on both sides. Therefore, in this case, it is suggested that another explanation of cerebral hemorrhage after treatment of bilateral TCCFs is needed.
Hyperperfusion was theorized by Spetzler for the first time as a possible cause of intracranial hemorrhage following resection of arteriovenous malformation owing to loss of autoregulation in the surrounding brain parenchyma22). Numerous reports have supported this theory as hyperperfusion after cerebral revascularization techniques, including carotid endarterectomy (CEA) and carotid artery stenting (CAS)1,14,17,23). Basically, TCCF has carried blood flow from arterial to venous system, in which distal cerebral flow of the ICA territory is diminished or absent with the affected terriotory supplied by collaterals5). In this case, after TCCF was completely occluded by the use of covered stents, the cerebral flow would have abruptly increased and may have broken the autoregulation, and these are similar mechanisms of hemorrhage after CEA or CAS23). Like arterial clamping during CEA, balloon-protected coil embolization possibly produced oxygen-derived free radicals, which also may have a role in the development of hyperperfusion syndrome; these free radicals could damage to the cerebrovascular endothelium, resulting in endothelial dysfunction to promote the destruction of autoregulation23). As a result, hyperperfusion appeared to develop in the distal arteries that have less sympathetic innervations, and then ICH ensued21,23). Although two foci of cerebral hemorrhage occurred in the region of previous infarction of the left ACA territory, hemorrhagic transformation following acute infarction as a cause was excluded because the cerebral hemorrhage developed 21 days after the acute infarction. With the impression of hyperperfusion syndrome, we induced barbiturate coma therapy to avoid systemic hypertension and to reduce metabolic demand of the brain tissue, so that further events of hyperperfusion syndrome did not occur.
Other causes of intracranial hemorrhage following cerebral revascularization, including hypertension or the use of anticoagulants should be checked for the potential relations to the cerebral hemorrhage. In this case, the procedure was uncomplicated; the patient was maintained at the normotensive blood pressure through and after procedure; systemic heparinization was stopped at the end of the procedure; and even that bleeding occurred on the following day after procedure, so that these potential causes could be excluded. However, it is uncertain whether or how much antiplatelet medication for prevention of in-stent thrombosis played a role in that hemorrhage.
CONCLUSION
We emphasize that cerebral hyperperfusion hemorrhage can occur even after successful endovascular treatment of TCCF. However, its relations with bilaterality of TCCF or the amount of shunt, and its predilection for infarcted regions have yet to be determined.
Acknowledgements
The authors thank Bae Ju Kwon for his assistance for this study.
References
- 1.Andrews BT, Levy ML, Dillon W, Weinstein PR. Unilateral normal perfusion pressure breakthrough after carotid endarterectomy : case report. Neurosurgery. 1987;21:568–571. doi: 10.1227/00006123-198710000-00025. [DOI] [PubMed] [Google Scholar]
- 2.Archondakis E, Pero G, Valvassori L, Boccardi E, Scialfa G. Angiographic follow-up of traumatic carotid cavernous fistulas treated with endovascular stent graft placement. AJNR Am J Neuroradiol. 2007;28:342–347. [PMC free article] [PubMed] [Google Scholar]
- 3.Baudrillard JC, Lerais JM, Rousseaux P, Scherpereel B, Bazin A, Auquier F, et al. [Bilateral traumatic carotid-cavernous fistula treated using an inflatable and detachable balloon] J Radiol. 1987;68:143–150. [PubMed] [Google Scholar]
- 4.Choi BJ, Lee TH, Kim CW, Choi CH. Endovascular graft-stent placement for treatment of traumatic carotid cavernous fistulas. J Korean Neurosurg Soc. 2009;46:572–576. doi: 10.3340/jkns.2009.46.6.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costantino A, Vinters HV. A pathologic correlate of the 'steal' phenomenon in a patient with cerebral arteriovenous malformation. Stroke. 1986;17:103–106. doi: 10.1161/01.str.17.1.103. [DOI] [PubMed] [Google Scholar]
- 6.Debrun G, Lacour P, Caron JP, Hurth M, Comoy J, Keravel Y. Detachable balloon and calibrated-leak balloon techniques in the treatment of cerebral vascular lesions. J Neurosurg. 1978;49:635–649. doi: 10.3171/jns.1978.49.5.0635. [DOI] [PubMed] [Google Scholar]
- 7.Debrun GM, Viñuela F, Fox AJ, Davis KR, Ahn HS. Indications for treatment and classification of 132 carotid-cavernous fistulas. Neurosurgery. 1988;22:285–289. doi: 10.1227/00006123-198802000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Donnell MS, Larson SJ, Correa-Paz F, Worman LW. Traumatic bilateral carotid-cavernous sinus fistulas with progressive unilateral enlargement. Surg Neurol. 1978;10:115–118. [PubMed] [Google Scholar]
- 9.Fabian TS, Woody JD, Ciraulo DL, Lett ED, Phlegar RF, Barker DE, et al. Posttraumatic carotid cavernous fistula frequency analysis of signs, symptoms, and disability outcomes after angiographic embolization. J Trauma. 1999;47:275–281. doi: 10.1097/00005373-199908000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Gaston A, Combes C, Razavi F, Le Bras F, Tartière S, Marsault C. Bilateral post-traumatic carotid-cavernous fistula : anatomico-radiological correlations in a case treated by detachable balloon. J Neuroradiol. 1986;13:55–61. [PubMed] [Google Scholar]
- 11.Gomez F, Escobar W, Gomez AM, Gomez JF, Anaya CA. Treatment of carotid cavernous fistulas using covered stents : midterm results in seven patients. AJNR Am J Neuroradiol. 2007;28:1762–1768. doi: 10.3174/ajnr.A0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graziussi G, Granata F, Terracciano S. Bilateral carotid-cavernous fistula of traumatic origin. A case report. Acta Neurol (Napoli) 1977;32:347–353. [PubMed] [Google Scholar]
- 13.Kim JK, Seo JJ, Kim YH, Kang HK, Lee JH. Traumatic bilateral carotid-cavernous fistulas treated with detachable balloon. A case report. Acta Radiol. 1996;37:46–48. doi: 10.1177/02841851960371P109. [DOI] [PubMed] [Google Scholar]
- 14.Kumar S, Kato Y, Sano H, Imizu S, Nagahisa S, Kanno T. Normal perfusion pressure breakthrough in arteriovenous malformation surgery : the concept revisited with a case report. Neurol India. 2004;52:111–115. [PubMed] [Google Scholar]
- 15.Lee TH, Kim DH, Lee BH, Kim HJ, Choi CH, Park KP, et al. Preliminary results of endovascular stent-assisted angioplasty for symptomatic middle cerebral artery stenosis. AJNR Am J Neuroradiol. 2005;26:166–174. [PMC free article] [PubMed] [Google Scholar]
- 16.Liang W, Xiaofeng Y, Weiguo L, Desheng P, Gang S, Xuesheng Z, et al. Bilateral traumatic carotid cavernous fistula : the manifestations, transvascular embolization and prevention of the vascular complications aftreated ter therapeutic embolization. J Craniofac Surg. 2007;18:74–77. doi: 10.1097/01.scs.0000231629.18135.cd. [DOI] [PubMed] [Google Scholar]
- 17.Liu AY, Do HM, Albers GW, Lopez JR, Steinberg GK, Marks MP. Hyperperfusion syndrome with hemorrhage after angioplasty for middle cerebral artery stenosis. AJNR Am J Neuroradiol. 2001;22:1597–1601. [PMC free article] [PubMed] [Google Scholar]
- 18.Luo CB, Teng MM, Chang FC, Sheu MH, Guo WY, Chang CY. Bilateral traumatic carotid-cavernous fistulae : strategies for endovascular treatment. Acta Neurochir (Wien) 2007;149:675–680. doi: 10.1007/s00701-007-1229-7. discussion 680. [DOI] [PubMed] [Google Scholar]
- 19.Matsui Y, Yamada K, Hayakawa T, Wakayama A, Mitomo M, Kawai R, et al. [Bilateral traumatic carotid-cavernous fistulas. Case report] Neurol Med Chir (Tokyo) 1987;27:447–450. doi: 10.2176/nmc.27.447. [DOI] [PubMed] [Google Scholar]
- 20.Ng SH, Wan YL, Ko SF, Lee ST, Wong HF, Chen YL, et al. Bilateral traumatic carotid-cavernous fistulas successfully treated by detachable balloon technique. J Trauma. 1999;47:1156–1159. doi: 10.1097/00005373-199912000-00033. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz RB. Hyperperfusion encephalopathies : hypertensive encephalopathy and related conditions. Neurologist. 2002;8:22–34. doi: 10.1097/00127893-200201000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Spetzler RF, Wilson CB, Weinstein P, Mehdorn M, Townsend J, Telles D. Normal perfusion pressure breakthrough theory. Clin Neurosurg. 1978;25:651–672. doi: 10.1093/neurosurgery/25.cn_suppl_1.651. [DOI] [PubMed] [Google Scholar]
- 23.van Mook WN, Rennenberg RJ, Schurink GW, van Oostenbrugge RJ, Mess WH, Hofman PA, et al. Cerebral hyperperfusion syndrome. Lancet Neurol. 2005;4:877–888. doi: 10.1016/S1474-4422(05)70251-9. [DOI] [PubMed] [Google Scholar]