Abstract
We report a case of cerebral actinomycosis in a 69-year-old immunocompetent woman. The patient showed a progressive worsened mental status for one week. MRI examination showed an increased size of multiple enhancing nodular lesions associated with mild perilesional edema. We performed an open biopsy for the right frontal enhancing lesion. The intraoperative finding showed a yellowish friable lesion that was not demarcated with normal tissue. Pathologically, an actinomycotic lesion with sulfur granules and inflammatory cells was diagnosed. We report an unusual case of diffuse involvement of cerebral actinomycosis. The presence of the uncapsulated friable lesion that consisted mainly of foamy macrophages and lymphocytes could explain the unusual radiological features.
Keywords: Actinomycosis, Cerebral abscess, MR imaging
INTRODUCTION
Actinomycosis is an indolent, slowly progressive infectious disease caused by an organism of the Actinomyces species1). Actinomycosis of the central nervous system (CNS) is usually secondary to hematogenous spread from a primary infection in the lung, abdomen or pelvis or by an extension of oral-cervico-facial disease14). In cerebral actinomycosis, the clinical presentation and conventional radiological findings are similar to typical findings in pyogenic abscesses15). For immunocompromised patients, local or disseminated actinomycotic abscesses may develop.
We report a case of cerebral actinomycosis in a 69-year-old immunocompetent woman. This patient had rapidly progressive symptoms and unusual radiological findings as compared with previously reported cases of brain actinomycosis.
CASE REPORT
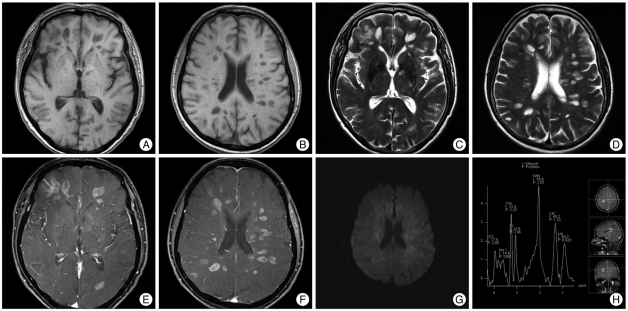
A 69-year-old woman with no significant medical history was admitted to our institution with a confused mental status. An initial brain MRI scan showed the presence of multiple small round or ovoid lesions in the periventricular white matter of the cerebral hemispheres. These lesions were hypointense as seen on T1-weighted images and hyperintense as seen on T2 weighted images. No associated edema and diffusion restriction were evident. Subsequently, the patient had a rapidly progressive worsened mental state for one week, with the patient in a semi-coma. A follow-up brain MRI examination performed one week later showed an increased size of the lesions and mild perilesional edema (Fig. 1A-D). As seen on contrast enhanced T1-weighted images, the lesions showed ring or nodular enhancement and there was no diffusion restriction (Fig. 1E, F, G). 1H-MR spectroscopy showed an increased choline level and elevated lactate and lipid peaks, which could be seen in both tumefactive demyelinating lesions and a high grade tumor (Fig. 1H). The provisional diagnosis was multiple sclerosis, neurocyticercosis or metastases. There was no metabolic evidence of a malignancy based on torso PET-CT findings. Cerebrospinal fluid analysis showed a WBC count of 3 cells/mL, protein level of 36 mg/dL and a glucose level of 98 mg/dL. The opening pressure was 13 cmH2O. An enzyme linked immunosorbent assay was negative for the diagnosis of neurocysticercosis.
Fig. 1.
Radiological findings (pretreatment). These lesions are hypointense as seen on T1-weighted images and hyperintense as seen on T2 weighted images with mild perilesional edema (A-D). The lesions show ring or nodular enhancement and there is no diffusion restriction (E, F and G). 1H-MR spectroscopy shows an increased choline level, decreased N-acetylaspartate level and elevated lactate and lipid peaks (H).
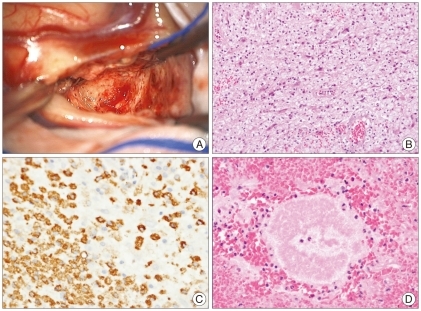
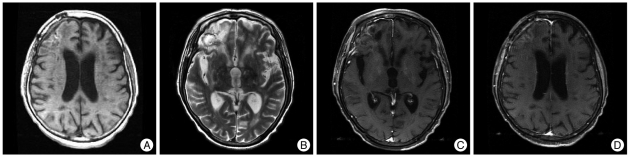
We performed an open biopsy for the right frontal enhancing mass among them. The intraoperative finding showed a yellowish friable uncapsulated lesion that was not well demarcated with normal tissue and there was no necrotic pus within the mass (Fig. 2A). Pathologically, the enhancing lesion was mostly composed of foamy macrophages that were immunopositive for CD68 (Fig. 2B, C). Infiltration of mature lymphocytes was found adjacent to the vessels. Sulfur granules were present, a typical finding associated with actinomycosis (Fig. 2D). There was no infective focus on the systemic evaluation. The patient was treated with intravenous ampicillin/sulbactam. Three months later, MRI showed resolution of the enhancing lesions and multifocal encephalomalacias in periventricular white matter were shown on follow-up MRI exam (Fig. 3). Despite the radiological improvement, the patient did not obey by commands.
Fig. 2.
Intraoperative and pathological findings. The intraoperative finding is a yellowish friable uncapsulated lesion that is not well demarcated with normal tissue and there was no necrotic pus (A). Pathologically, the enhancing lesion is mostly composed of foamy macrophages that were immunopositive for CD68 (B and C). Sulfur granules are present, a typical finding associated with actinomycosis (D).
Fig. 3.
Radiological findings (posttreatment). Multifocal encephalomalacias in periventricular white matter are shown (A and B) with disappearance of enhancing lesions (C and D).
DISCUSSION
Actinomycosis is a noncontagious, slow suppurative bacterial infection caused by Actinomices israelii2,4). These organisms are classified as gram-positive, non-acid fast branching filamentous bacteria with anaerobic or microaerophilic requirements. Actinomycosis of the CNS may present as a brain abscess, meningitis or meningoencephalitis, a subdural empyema, an actinomycoma and a spinal and cranial epidural abscess6,8,10,12,16). Actinomycosis of the CNS is usually secondary to hematogenous spread from a primary infection in the lung, abdomen or pelvis. However, extension from foci of an infection in the ears, paranasal sinuses and cervicofacial regions may proceed along connective tissue planes or through foramina located at the base of the skull, causing focal infection of the CNS or diffuse basilar meningitis.
Clinical symptoms are usually mild and nonspecific as the interval from the onset of symptoms to diagnosis is typically long comparing to a typical pyogenic brain abscess5,13). The clinical features are indistinguishable from features of pyogenic infections on intracranial and spinal structures. For a nonmeningitic infection, the signs and symptoms are typically of a space-occupying lesion, with focal neurological defects and symptoms of increasing intracranial pressure that dominate the clinical picture. The patient in this case had only multiple brain parenchymal lesions without meningitis. However, the patient had rapidly progressive symptoms within one week. Therefore, the main cause of symptoms might be related with the diffuse involvement of the brain.
Actinomycotic cerebral abscesses are usually singular but may be multiple, unilocular or multilocular; encapsulated or, less frequently, unencapsulated14). There is a predilection for involvement of the temporal and frontal lobes13). Diffusion-weighted images (DWI) is more sensitive in distinguishing between brain abscesses and cystic tumors than conventional MRI. Restricted diffusion for a pyogenic brain abscess has been reported previously and restricted diffusion may facilitate differentiation from cystic brain tumors7,9). A pus structure has been demonstrated as being responsible for low apparent diffusion coefficient (ADC) values observed and the heavily impeded water mobility of pus might be related to high cellularity and viscosity because of the presence of inflammatory cells, bacteria, and macromolecules15), whereas most necrotic or cystic brain tumors show an elevated ADC values. The capsule of the abscess showed a high ADC value, probably due to an increase of the amount of extracellular fluid in the capsular wall from inflammation. In our case, multiple nodular enhancing lesions showed a high ADC value and exhibited hypointensity on DWI. These were not typical findings of a pyogenic abscess. However, we could explain these findings as compared to surgical and pathological findings. Intraoperatively, the mass was an uncapsulated friable lesion that was not well demarcated with the normal tissue and there was no necrotic portion. Pathological findings showed that the mass consisted mainly of foamy macrophages and lymphocytes. There was no pus material and only inflammatory cells caused a high ADC value and hypointensity on diffusion-weighted images. Brain abscesses have distinct spectroscopic findings that allow differentiation of the lesions from other entities. Typical MR spectroscopic features of abscesses include elevated peaks of amino acids, lactate, alanine, acetate, pyruvate and succinate and absent signals of N-acetylaspartate (NAA), creatine and choline3,15). In our case, there was an increased choline level, normal NAA level and elevated lactate and lipid peaks in contrast to the spectroscopic features for brain abscesses. Typical proton MR spectroscopic features for tumefactive demyelinating lesions include an elevated choline peak and reduced NAA signal. A fulminant tumefactive demyelinating lesion may show high choline and low NAA signals as well as the presence of lactate, similar to the findings for the present case11).
We report a case of diffuse involvement of cerebral actinomycosis in an immunocompetent patient. Based on the imaging findings, it was difficult to distinguish cerebral actinomycosis from other causes. The presence of the uncapsulated friable lesion that consisted mainly of foamy macrophages and lymphocytes could explain the unusual radiological features.
CONCLUSION
In this case, it was difficult to make a preoperative diagnosis because rapidly progressive symptom and unusual radiological findings compared with previously reported actinomycosis cases. We report a rare case of cerebral actinomycosis that presented unusul clinical and radiological finding, with special interest on its neuroradiologic features.
References
- 1.Adeyemi OA, Gottardi-Littell N, Muro K, Kane K, Flaherty JP. Multiple brain abscesses due to Actinomyces species. Clin Neurol Neurosurg. 2008;110:847–849. doi: 10.1016/j.clineuro.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 2.Alday R, Lopez-Ferro MO, Fernandez-Guerrero M, Ruiz-Barnés P. Spinal intrarhecal empyema due to Actinomyces israeli. Acta Neurochir (Wien) 1989;101:159–162. doi: 10.1007/BF01410532. [DOI] [PubMed] [Google Scholar]
- 3.Al-Okaili RN, Krejza J, Wang S, Woo JH, Melhern ER. Advanced MR imaging techniques in the diagnosis of intraaxial brain tumors in adults. Radiographics. 2006;26(Suppl 1):S173–S189. doi: 10.1148/rg.26si065513. [DOI] [PubMed] [Google Scholar]
- 4.Bébrová E, Lochmann O, Tichý M, Nyc O. [Actinomyces viscosus in subdural empyema] Cesk Epidemiol Mikrobiol Imunol. 1994;43:21–22. [PubMed] [Google Scholar]
- 5.Brown JR. Human actinomycosis. A study of 181 subjects. Hum Pathol. 1973;4:319–330. doi: 10.1016/s0046-8177(73)80097-8. [DOI] [PubMed] [Google Scholar]
- 6.Brunon J, Pialat J, Brun Y, Sindou M, Fischer C, Perrin G. Actinomycotic brain abscess. Neurochirurgie. 1980;26:31–38. [PubMed] [Google Scholar]
- 7.Ebisu T, Tanaka C, Umeda M, Kitamura M, Naruse S, Higuchi T, et al. Discrimination of brain abscess from necrotic or cystic tumors by diffusion-weighted echo planar imaging. Magn Reson Imaging. 1996;14:1113–1116. doi: 10.1016/s0730-725x(96)00237-8. [DOI] [PubMed] [Google Scholar]
- 8.Edwards C, Elliott WA, Randall KJ. Spinal meningitis due to Actinomyces bovis treated with penicillin and streptomycin. J Neurol Neurosurg Psychiatry. 1951;14:134–136. doi: 10.1136/jnnp.14.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guzman R, Barth A, Lövblad KO, El-Koussy M, Weis J, Schroth G, et al. Use of diffusion-weighted magnetic resonance imaging in differentiating purulent brain processes from cystic brain tumors. J Neurosurg. 2002;97:1101–1107. doi: 10.3171/jns.2002.97.5.1101. [DOI] [PubMed] [Google Scholar]
- 10.Powers JM, Dodds HM. Primary actinomycoma of the third ventricle -- the colloid cyst. A histochemical and ultrastructural study. Acta Neuropathol. 1977;37:21–26. doi: 10.1007/BF00684535. [DOI] [PubMed] [Google Scholar]
- 11.Saindane AM, Cha S, Law M, Xue X, Knopp EA, Zagzag D. Proton MR spectroscopy of tumefactive demyelinating lesions. AJNR Am J Neuroradiol. 2002;23:1378–1386. [PMC free article] [PubMed] [Google Scholar]
- 12.Schwartz DG, Christoff N. Actinomycosis with cerebral and probable endocardial involvement. J Mt Sinai Hosp N Y. 1960;27:23–27. [PubMed] [Google Scholar]
- 13.Smego RA., Jr Actinomycosis of the central nervous system. Rev Infect Dis. 1987;9:855–865. doi: 10.1093/clinids/9.5.855. [DOI] [PubMed] [Google Scholar]
- 14.Smego RA, Jr, Foglia G. Actinomycosis. Clin Infect Dis. 1998;26:1255–1261. doi: 10.1086/516337. [DOI] [PubMed] [Google Scholar]
- 15.Wang S, Wolf RL, Woo JH, Wang J, O'Rourke DM, Roy S, et al. Actinomycotic brain infection : registered diffusion, perfusion MR imaging and MR spectroscopy. Neuroradiology. 2006;48:346–350. doi: 10.1007/s00234-006-0067-2. [DOI] [PubMed] [Google Scholar]
- 16.Wickbom GI, Davidson AJ. Angiographic findings in intracranial actinomycosis. A case report and consideration of pathogenesis. Radiology. 1967;88:536–537. doi: 10.1148/88.3.536. [DOI] [PubMed] [Google Scholar]