Abstract
Objective
Leukoaraiosis (LA) has been suggested to be related to the poor outcome or the occurrence of symptomatic intracerebral hemorrhage (sICH) after acute ischemic stroke. We retrospectively investigated the influences of LA on long-term outcome and the occurrence of sICH after thrombolysis in acute ischemic stroke (AIS).
Methods
In this study, we recruited 164 patients with AIS and magnetic resonance image (MRI)-detected thrombolysis. The presence and extent of LA were assessed using the Fazekas grading system. The National Institutes of Health Stroke Scale score was used to assess the baseline measure of neurologic severity, and the modified Rankin Scale score assessment was used up to 1 year after thrombolysis.
Results
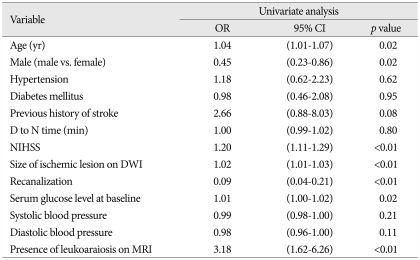
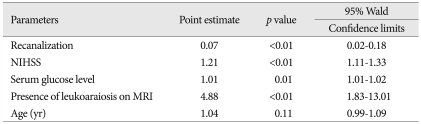
Of 164 subjects, 56 (34.2%) showed LA on MRI. Compared to the 108 patients without LA, the patients with LA were of much older age (p<0.01), had a higher prevalence of hypertension (p<0.01), and had a much poorer outcome at 90 days (p=0.05) and 1 yr (p=0.01) after thrombolysis. There were no significant differences in sICH between patients with and without LA on MRI. In univariate analysis for the occurrence of poor outcome at 90 days after thrombolysis, the size of ischemic lesion on diffusion weighted images (DWI), [odds ratio (OR), 1.03; 95% confidence interval (95% CI), 1.01-1.04; p<0.01], recanalization (OR, 0.03; 95% CI, 0.01-0.10; p<0.01), sICH (OR, 12.2; 95% CI, 1.54-95.8), neurologic severity (OR, 1.17; 95% CI, 1.09-1.25; p<0.01), blood glucose level (OR, 1.01; 95% CI, 1.00-1.02; p=0.03), and the presence of LA on MRI (OR, 2.01; 95% CI, 1.04-3.01; p=0.04) were statistically significant. In multivariate analysis, neurologic severity (OR, 1.14; 95% CI, 1.04-1.24; p<0.01), recanalization (OR, 0.03; 95% CI, 0.01-0.11; p<0.01), lesion size on DWI (OR, 1.02; 95% CI, 1.01-1.03; p=0.02), serum glucose level (OR, 1.01; 95% CI; 1.01-1.02; p=0.03), and the presence of LA on MRI (OR, 3.2; 95% CI, 1.22-8.48; p<0.01) showed statistically significant differences. These trends persisted up to 1 yr after thrombolysis.
Conclusion
In this study, we demonstrated that the presence of LA on MRI might be related to poor outcome after use of intravenous tissue plasminogen activator in AIS.
Keywords: Leukoaraiosis, Intracerebral hemorrhage, Thrombolysis, Acute ischemic stroke
INTRODUCTION
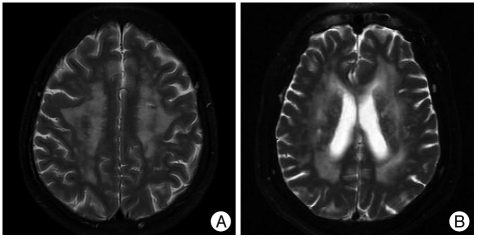
Leukoaraiosis (LA) is a term to describe the abnormal appearance of subcortical brain white matter in the elderly and which is most easily found on T2-weighted magnetic resonance imaging (MRI) images (Fig. 1)10,20). LA primarily involves small vessel disease-related brain lesions of variable severity that progress gradually over time with the accumulation of vascular risk factors and ultimately may result in extensive subcortical arteriosclerotic encephalopathy with concomitant cognitive decline3,16,29).
Fig. 1.
Leukoaraiosis is a term to describe the abnormal appearance of subcortical or periventricular brain white matter in elderly patient. Brain T2-weighted magnetic resonance images showing high signal intensity multiple patch lesions in deep white matter (A) and periventricular white matter (B).
Reports have shown that the presence of LA is related to the poor outcome after acute ischemic stroke (AIS)2,28). Several mechanisms in LA have participated in the worsening of its clinical course. Among them, the occurrence of bleeding complications plays a critical role in its poor outcome after acute ischemic stroke9,23). Recently, there have been several reports on the relationship between the occurrence of symptomatic intracerebral hemorrhage (sICH) and thrombolysis in LA. However, until now, it is undetermined whether LA is a potential risk for sICH after thrombolysis.
Meanwhile, the poor outcome after acute ischemic stroke in LA might be originated from its delayed recovery and higher prevalence of comorbidity as well as the occurrence of sICH after thrombolysis. Therefore, the poor outcome of LA might be related to its higher prevalence of comorbidity and higher susceptibility to complications after stroke rather than the thrombolysis itself. However, few studies have serially evaluated the long-term outcome (>1 yr) after thrombolysis in the presence of LA on MRI. Also, almost all of the earlier studies used brain CT or MRI images without diffusion weighted images (DWI). Therefore, they might have had limitations of lesser sensitivity to detect the presence of LA and to estimate the actual ischemic lesion size as an important confounding factor for the occurrence of sICH after thrombolysis. In this study, we evaluated the differences in long-term outcomes and occurrence of sICH after thrombolysis in AIS with and without LA based on MRI findings.
MATERIALS AND METHODS
We retrospectively reviewed the data of patients with acute ischemic stroke who were admitted to our hospital between January 2006 and August 2009 and selected the patients with thrombolysis. Only patients on whom an MRI was performed before or shortly after the initiation of treatment were included in this study.
Intravenous (IV) tissue plasminogen activator (t-PA) was given according to the National Institute of Neurological Disorders and Stroke (NINDS) criteria25). Patients with a sign of intracerebral hemorrhage on T2* images on MRI and those with large DWI lesions (>100 cm3) were excluded from the thromobolysis group. After the DWI and T2* images were collected on MRI, patients received intravenous t-PA at a dose of 0.9 mg/kg; 10% was given as a bolus, while the rest was infused over 1 h. Other images, including magnetic resonance angiogram (MRA) and perfusion weighted images (PWI), were collected after the infusion of IV t-PA. After use of IV t-PA, if a neuroradiologist was available, we transferred the patients to the intervention room. When 4-vessel angiography showed a persistent occlusion (grade 0) or trickle flow (grade 1) according to Thrombolysis in Cerebral Infarction grading, we attempted to recanalize the occluded vessels by using an intra-arterial approach. We performed intra-arterial thrombolysis based on the Prolyse in Acute Cerebral Tromboembolism II trial findings in cases arriving at the emergency room between 3 and 6 h after ischemic events4).
We determined the neurologic severity as an National Institutes of Health Stroke Scale (NIHSS) score at baseline, 24 h, and 5 days after using IV t-PA. Modified Rankin Score (mRS) was calculated at baseline, discharge, 90 days, and 1 yr after t-PA use. Major neurologic improvement was defined as an NIHSS score equal to 0 or 1 or an improvement >7 points compared to baseline at 24 h and 5 days after thrombolysis. We defined the poor outcome as an mRS of >2 at 90 days after t-PA use. We compared the prevalence of major neurologic improvement at 24 h and 5 days after thrombolysis and the rate of the poor outcome at 90 days and 1 yr after it.
After thrombolysis, brain CT was performed to check for intracranial hemorrhage. DWI and MRA were evaluated to determine the final infarct size and whether the obstructed vessel was reopened within 24 h. We determined recanalization if MRA showed complete visualization of branch arteries distal to the occluded vessels with residual stenosis of <50%. Symptomatic intracerebral hemorrhage (sICH) was defined as any signs of hemorrhage on follow-up CT or MRI associated with clinical deterioration of 4 points on the NIHSS11). Our institutional ethics review board approved this study.
MRI protocol
All subjects underwent MRI (1.5T, Signa Echospeed Superconducting Imaging System; General Electric Medical Systems, Milwaukee, WI, USA). Images included T1, axial fluid-attenuated inversion recovery, 3-dimensional time-of-flight MR angiography, axial DWI, and axial PWI. DWI was performed by use of echo planar imaging techniques. DWI lesion volumes were determined by manually tracing the edge of the hyperintense signal on each slice of the trace DWI scans. T2-weighted MRI images were scored according to a semiquantitative rating scale devised by Fazekas et al.7). Areas of signal hyperintensity were classified as either deep white matter (subcortical) or periventricular and were scored on a 4-point scale, with scores defined as follows : for deep white-matter areas of hyperintensity, absent (grade 0), punctate (grade 1), nearlycoalescent (grade 2), or confluent (grade 3); and for periventricular areas of hyperintensity, absent (grade 0), pencil-thin lines (grade 1), caps or bands (grade 2), or confluent (grade 3). Three trained neurologists and 2 neuroradiologists, each of whom was blinded to patient data and stroke subtype, graded the LA by consensus. If 1 or both sides of the brain were focally abnormal, estimates were based on the uninvolved side with assumed symmetry.
Statistical analysis
Patient and clinical characteristics were summarized as a whole and described specifically for subgroups by descriptive statistics. A Fisher's exact 2test was used to compare categorical variables, while t-tests were used to compare continuous variables between groups.
The odds ratio (OR) for comparison of the 2 groups was summarized with its 95% confidence interval (95% CI) and p value using logistic regression. The multivariate model was created using a backward elimination method, and the probability was set at 0.05 for removal. ORs were also adjusted for factors affecting the response variable.
To determine the independent factors related to the occurrence of poor outcome after the use of t-PA, we performed multiple logistic regression analysis by using a backward elimination method and set the probability at 0.10 for removal.
p values less than 0.05 were considered statistically significant. This study is explorative in nature; therefore, no adjustment for α was applied. All statistical analyses were carried out using SAS version 9.1 statistical software.
RESULTS
From January 2006 to August 2009, 172 patients with AIS and thrombolysis were admitted to our stroke center. MRI images were collected around 24 h of thrombolysis in 164 of these patients. In >90% of them, MRI was performed before the thrombolysis occurred; in the remaining patients, MRI was evaluated during or shortly after treatment (after CT had been performed to rule out hemorrhage). Among them, 111 patients were given IV t-PA only, 41 were given intra-arterial thrombolysis, and 12 were given t-PA plus IA thrombolysis.
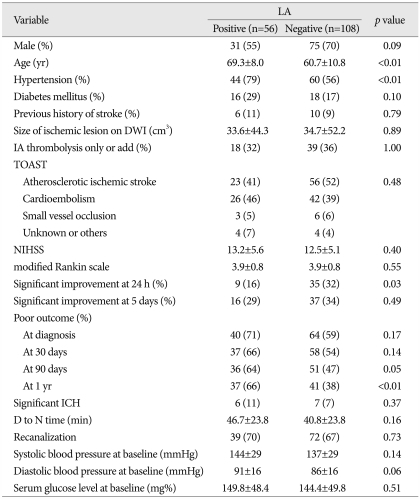
Of 164 subjects, 56 (34.1%) showed LA on MRI. Patients with LA were significantly older and had a higher prevalence of previous hypertension than patients without LA. Regarding the outcome after thrombolysis between patients with and without LA, the rate of major neurologic improvement at 24 h of thrombolysis was significantly higher in the non-LA group than in the LA group. However, its difference disappeared between the two groups at 5 days after thrombolysis. The in-hospital mortality at 7 days was 6.7% (n=11) : 2 (3.6%) patients with LA versus 9 (9%) patients without LA (p=0.43). The prevalence of poor functional outcome at 90 days of thrombolysis was significantly higher in the LA group than in the non-LA group, and this pattern was more prominent after 1 yr of thrombolysis. In total, 13 (7.9%) patients had sICH. There was no difference in occurrence between the 2 groups. Table 1 summarizes the difference in clinical and radiologic findings between the two groups.
Table 1.
Comparisons of clinical and radiologic findings in patients with and without leukoaraiosis (LA) after thrombolysis
DWI : diffusion weighted images, TOAST : Trial of ORG 10172 in Acute Stroke Treatment, NIHSS : National Institutes of Health Stroke Scale, ICH : intracerebral hemorrhage, D to N time : door to needle time
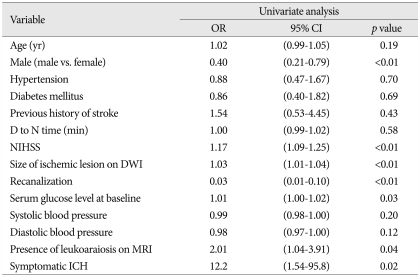
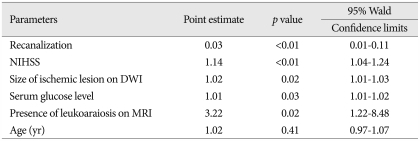
After 90 days of thrombolysis, 84 (53.0%) patients had poor functional outcome estimated by mRS (Table 2). In univariate analysis, sex (OR, 0.4; 95% CI 0.21-0.79; p<0.01), neurologic severity (NIHSS; OR, 1.17; 95% CI, 1.09-1.25; p<0.01), size of ischemic lesions on DWI (OR, 1.03; 95% CI, 1.01-1.04; p<0.01), recanalization (OR, 0.03; 95% CI, 0.01-0.10; p<0.01), serum glucose level (OR, 1.01; 95% CI, 1.00-1.02; p=0.03), symptomatic ICH (OR 12.2; 95% CI, 1.54-95.8; p=0.02), and the presence of LA on MRI (OR, 2.01; 95% CI, 1.04-3.91, p=0.04) were significantly related to the poor outcome. In multivariate analysis, neurologic severity (OR, 1.14; 95% CI, 1.04-1.24; p<0.01), size of ischemic lesion on DWI (OR, 1.02; 95% CI, 1.01-1.03; p=0.02), recanalization (OR, 0.03; 95% CI, 0.01-0.11; p<0.01), serum glucose level (OR, 1.01; 95% CI, 1.01-1.02; p=0.03), and the presence of LA on MRI (OR, 3.22; 95% CI, 1.22-8.48; p=0.02) had independent significance for poor outcome after 90 days of thrombolysis (Table 3). After 1 yr of thrombolysis, the influence of LA on its functional outcome persisted and had more significance at 1 yr after thrombolysis (Table 4, 5).
Table 2.
Univariate analysis of the occurrence of poor outcome 90 days after thrombolysis
D to N time : door to needle time, NIHSS : National Institutes of Health Stroke Scale, ICH : intracerebral hemorrhage, OR : odds ratio, 95% CI : 95% confidence interval, DWI : diffusion weighted images
Table 3.
Multivariate analysis of the occurrence of poor outcome 90 days after thrombolysis
NIHSS : National Institutes of Health Stroke Scale, DWI : diffusion weighted images, MRI : magnetic resonance imaging
Table 4.
Univariate analysis for the occurrence of poor outcome 1 yr after thrombolysis
D to N time : door to needle time, NIHSS : National Institutes of Health Stroke Scale, MRI : magnetic resonance imaging, OR : odds ratio, 95% CI : 95% confidence interval, DWI : diffusion weighted images
Table 5.
Multivariate analysis of the occurrence of poor outcome 1 year after thrombolysis
NIHSS : National Institutes of Health Stroke Scale, MRI : magnetic resonance imaging
DISCUSSION
LA is frequently found in elderly individuals and has been related to hypertension13), increased stroke risk27), and increased mortality after stroke26). In line with this notion, our results also demonstrated that patients with LA were much older and had a much higher prevalence of hypertension than those without it.
In this study, we showed that the presence of LA was an independent predictive factor of poor functional outcome after thrombolysis but not for the occurrence of sICH. It has been reported that LA greatly influences on outcome, length of hospital stay, or mortality after AIS13). Among them, LA is known to be a potential risk of bleeding complications after AIS9,23). Therefore, the possibility of increased sICH after thrombolysis in LA on brain images has been suggested. A retrospective study showed that it is a potential risk for sICH after thrombolysis17). The risk of sICH after thrombolysis may be explained by preexisting chronic damage of the endothelium and weakening of the blood-brain barrier5). Also, old age24) is thought to be a factor associated with LA and may be a potential risk for ICH. The present study also showed that patients with LA were significantly much older than those without it. However, the relationship between its presence and the risk of sICH after thrombolysis is still under debate. Post hoc analyses of the NINDS6) demonstrated that there was no significant relationship of the occurrence of sICH between t-PA treatment and LA. In this study, there was also no significant difference in sICH occurrence between patients with and without LA. The conflicting results regarding sICH after thrombolysis might be related to the use of different imaging methods to evaluate the presence of LA. Almost all studies using brain CT1,6) had relatively lower sensitivity than MRI for detecting the presence of LA. As a result, its incidence was only 24% in a CT-based thrombolysis trial. In contrast, 34.1% of subjects had sICH on follow-up images. Therefore, we suggest that the difference in a method to detect it might have been a contributing factor to the different results. Although brain CT remains the standard modality for detection of thrombolysis, MRI has several advantages over it. Among them, MRI can easily estimate the actual size of an ischemic lesion before thrombolysis. In particular, in contrast to studies using brain CT or MRI without DWI before thrombolysis, recent published MRI-based data analyzing the risk of sICH after thrombolysis showed that diffusion image size is a potential risk factor21). Our study enrolled >90% of cases by using diffusion MRI to select the proper patients before it. Therefore, we believe that our MRI-based selection of patients for thrombolysis might have affected the results regarding the occurrence of sICH in LA after thrombolysis. Finally, the definition of LA was originally defined based on radiologic findings. However, it has been reported that its pathologic mechanism is heterogeneous and too diverse to define in a single clinical course22). For example, LA is considered a hallmark of small-vessel diseases in brain. Recently, however, several studies demonstrated that LA is much more prevalent in large-artery atherosclerosis rather than other types of stroke in Asia15). Therefore, it might be possible that the different mechanisms of LA may have different responses to the occurrence of sICH after thrombolysis. In many recent studies, the presence of microbleeds on brain MRI was a potential surrogate marker for sICH after acute ischemic stroke and was thought to be significantly related to LA on brain images14). A recent study showed that the occurrence of sICH was much more correlated with the presence of microbleeds on MRI than the presence of LA14). However, another study showed that LA cannot be used as a surrogate for the presence of microbleeds because a high proportion of patients without LA have microbleeds and many patients with LA do not8). The above findings suggest that the concomitant presence of microbleeds with LA on brain images might be a potential risk factor for the occurrence of sICH after thrombolysis. We believe that these studies focused on only the presence of LA without considering microbleeds, which would have caused the conflicting results regarding the occurrence of sICH after thrombolysis. We also believe that more detailed studies are required to consider the presence of microbleeds on brain MRI, in order to precisely identify the relationship between the presence of LA and sICH.
Our study demonstrated that the presence of LA on MRI is an independent predictive factor of long-term poor outcome after thrombolysis. To date, several studies have investigated the issue but have shown conflicting results. In this study, there was no significant difference in early mortality between patients with and without LA, which means that the poor functional outcome at 3 months in patients with LA treated with t-PA is not related to the thrombolytic therapy itself. Also, in this study, the rate of early major neurologic improvement was much lower in the LA group than in the non-LA group. Furthermore, LA is significantly related to the occurrence of poor functional outcome at 90 days, a trend that was more prominent at 1 yr after thrombolysis in this study. In multivariate analysis, LA itself was a strong independent factor for poor functional outcome after thrombolysis. The above findings suggest that the poor outcome after thrombolysis in LA might have been related to the higher prevalence of comorbidity and delayed recovery.
Several mechanisms related to poor clinical outcome after acute stroke with LA have been suggested. Resting blood flow is reduced by up to 30% in brains with LA19). Additionally, LA is associated with platelet hyperactivation and hypercoagulability12), which might complicate tissue perfusion after thrombolysis. Furthermore, the presence of a dysfunctional neuronal network in LA might be partially responsible for the poor outcome after AIS18). The intact connection system of the brain is a critical factor for recovery from ischemic damage after AIS. Many studies have shown the presence of reduced neuronal networks in LA patients18,30), which is most likely due to demyelination, loss of axons and oligodendrocytes, and astrocytic gliosis. Due to the above-mentioned mechanisms, LA could be related to delayed recovery as well as impaired plasticity after thrombolysis in AIS.
The main limitation of this study is a retrospective one, which made it impossible to exclude the possibility of bias. Also, our sample size was small that the validity of our conclusions could not be confirmed.
CONCLUSION
Taken together, we demonstrated that the presence of LA on MRI might be a potential risk for a poor functional outcome after thrombolysis. Our data also confirmed that there is no need to exclude patients with LA for thrombolysis due to fear of a higher occurrence of sICH.
Acknowledgements
This work was supported by the Dong-A University Research Fund.
References
- 1.Ariës MJ, Uyttenboogaart M, Vroomen PC, De Keyser J, Luijckx GJ. tPA treatment for acute ischaemic stroke in patients with leukoaraiosis. Eur J Neurol. 2010;17:866–870. doi: 10.1111/j.1468-1331.2010.02963.x. [DOI] [PubMed] [Google Scholar]
- 2.Arsava EM, Rahman R, Rosand J, Lu J, Smith EE, Rost NS, et al. Severity of leukoaraiosis correlates with clinical outcome after ischemic stroke. Neurology. 2009;72:1403–1410. doi: 10.1212/WNL.0b013e3181a18823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Leeuw FE, de Groot JC, Achten E, Oudkerk M, Ramos LM, Heijboer R, et al. Prevalence of cerebral white matter lesions in elderly people : a population based magnetic resonance imaging study. The Rotterdam Scan Study. J Neurol Neurosurg Psychiatry. 2001;70:9–14. doi: 10.1136/jnnp.70.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.del Zoppo GJ, Higashida RT, Furlan AJ, Pessin MS, Rowley HA, Gent M. PROACT : a phase II randomized trial of recombinant pro-urokinase by direct arterial delivery in acute middle cerebral artery stroke. PROACT Investigators. Prolyse in Acute Cerebral Thromboembolism. Stroke. 1998;29:4–11. doi: 10.1161/01.str.29.1.4. [DOI] [PubMed] [Google Scholar]
- 5.del Zoppo GJ, von Kummer R, Hamann GF. Ischaemic damage of brain microvessels : inherent risks for thrombolytic treatment in stroke. J Neurol Neurosurg Psychiatry. 1998;65:1–9. doi: 10.1136/jnnp.65.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Demchuk AM, Khan F, Hill MD, Barber PA, Silver B, Patel S, et al. Importance of leukoaraiosis on CT for tissue plasminogen activator decision making : evaluation of the NINDS rt-PA Stroke Study. Cerebrovasc Dis. 2008;26:120–125. doi: 10.1159/000139658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol. 1987;149:351–356. doi: 10.2214/ajr.149.2.351. [DOI] [PubMed] [Google Scholar]
- 8.Görner A, Lemmens R, Schrooten M, Thijs V. is leukoaraiosis on CT an accurate surrogate marker for the presence of microbleeds in acute stroke patients? J Neurol. 2007;254:284–289. doi: 10.1007/s00415-006-0311-z. [DOI] [PubMed] [Google Scholar]
- 9.Gorter JW. Major bleeding during anticoagulation after cerebral ischemia : patterns and risk factors. Stroke Prevention In Reversible Ischemia Trial (SPIRIT). European Atrial Fibrillation Trial (EAFT) study groups. Neurology. 1999;53:1319–1327. doi: 10.1212/wnl.53.6.1319. [DOI] [PubMed] [Google Scholar]
- 10.Hachinski VC, Potter P, Merskey H. Leuko-araiosis. Arch Neurol. 1987;44:21–23. doi: 10.1001/archneur.1987.00520130013009. [DOI] [PubMed] [Google Scholar]
- 11.Hacke W, Kaste M, Fieschi C, von Kummer R, Davalos A, Meier D, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet. 1998;352:1245–1251. doi: 10.1016/s0140-6736(98)08020-9. [DOI] [PubMed] [Google Scholar]
- 12.Iwamoto T, Kubo H, Takasaki M. Platelet activation in the cerebral circulation in different subtypes of ischemic stroke and Binswanger's disease. Stroke. 1995;26:52–56. doi: 10.1161/01.str.26.1.52. [DOI] [PubMed] [Google Scholar]
- 13.Jørgensen HS, Nakayama H, Raaschou HO, Olsen TS. Leukoaraiosis in stroke patients. The Copenhagen Stroke Study. Stroke. 1995;26:588–592. doi: 10.1161/01.str.26.4.588. [DOI] [PubMed] [Google Scholar]
- 14.Koennecke HC. Cerebral microbleeds on MRI : prevalence, associations, and potential clinical implications. Neurology. 2006;66:165–171. doi: 10.1212/01.wnl.0000194266.55694.1e. [DOI] [PubMed] [Google Scholar]
- 15.Lee SJ, Kim JS, Lee KS, An JY, Kim W, Kim YI, et al. The leukoaraiosis is more prevalent in the large artery atherosclerosis stroke subtype among Korean patients with ischemic stroke. BMC Neurol. 2008;8:31. doi: 10.1186/1471-2377-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Longstreth WT, Jr, Bernick C, Manolio TA, Bryan N, Jungreis CA, Price TR. Lacunar infarcts defined by magnetic resonance imaging of 3660 elderly people : the Cardiovascular Health Study. Arch Neurol. 1998;55:1217–1225. doi: 10.1001/archneur.55.9.1217. [DOI] [PubMed] [Google Scholar]
- 17.Neumann-Haefelin T, Hoelig S, Berkefeld J, Fiehler J, Gass A, Humpich M, et al. Leukoaraiosis is a risk factor for symptomatic intracerebral hemorrhage after thrombolysis for acute stroke. Stroke. 2006;37:2463–2466. doi: 10.1161/01.STR.0000239321.53203.ea. [DOI] [PubMed] [Google Scholar]
- 18.Nordahl CW, Ranganath C, Yonelinas AP, Decarli C, Fletcher E, Jagust WJ. White matter changes compromise prefrontal cortex function in healthy elderly individuals. J Cogn Neurosci. 2006;18:418–429. doi: 10.1162/089892906775990552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Sullivan M, Lythgoe DJ, Pereira AC, Summers PE, Jarosz JM, Williams SC, et al. Patterns of cerebral blood flow reduction in patients with ischemic leukoaraiosis. Neurology. 2002;59:321–326. doi: 10.1212/wnl.59.3.321. [DOI] [PubMed] [Google Scholar]
- 20.Pantoni L, Garcia JH. Pathogenesis of leukoaraiosis : a review. Stroke. 1997;28:652–659. doi: 10.1161/01.str.28.3.652. [DOI] [PubMed] [Google Scholar]
- 21.Singer OC, Humpich MC, Fiehler J, Albers GW, Lansberg MG, Kastrup A, et al. Risk for symptomatic intracerebral hemorrhage after thrombolysis assessed by diffusion-weighted magnetic resonance imaging. Ann Neurol. 2008;63:52–60. doi: 10.1002/ana.21222. [DOI] [PubMed] [Google Scholar]
- 22.Smith EE. Leukoaraiosis and stroke. Stroke. 2010;41:S139–S143. doi: 10.1161/STROKEAHA.110.596056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith EE, Rosand J, Knudsen KA, Hylek EM, Greenberg SM. Leukoaraiosis is associated with warfarin-related hemorrhage following ischemic stroke. Neurology. 2002;59:193–197. doi: 10.1212/wnl.59.2.193. [DOI] [PubMed] [Google Scholar]
- 24.Tanne D, Kasner SE, Demchuk AM, Koren-Morag N, Hanson S, Grond M, et al. Markers of increased risk of intracerebral hemorrhage after intravenous recombinant tissue plasminogen activator therapy for acute ischemic stroke in clinical practice : the Multicenter rt-PA Stroke Survey. Circulation. 2002;105:1679–1685. doi: 10.1161/01.cir.0000012747.53592.6a. [DOI] [PubMed] [Google Scholar]
- 25.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 26.Thein SS, Hamidon BB, Teh HS, Raymond AA. Leukoaraiosis as a predictor for mortality and morbidity after an acute ischaemic stroke. Singapore Med J. 2007;48:396–399. [PubMed] [Google Scholar]
- 27.van Swieten JC, Kappelle LJ, Algra A, van Latum JC, Koudstaal PJ, van Gijn J. Hypodensity of the cerebral white matter in patients with transient ischemic attack or minor stroke : influence on the rate of subsequent stroke. Dutch TIA Trial Study Group. Ann Neurol. 1992;32:177–183. doi: 10.1002/ana.410320209. [DOI] [PubMed] [Google Scholar]
- 28.Weimar C, König IR, Kraywinkel K, Ziegler A, Diener HC. Age and National Institutes of Health Stroke Scale Score within 6 hours after onset are accurate predictors of outcome after cerebral ischemia : development and external validation of prognostic models. Stroke. 2004;35:158–162. doi: 10.1161/01.STR.0000106761.94985.8B. [DOI] [PubMed] [Google Scholar]
- 29.Whitman GT, Tang Y, Lin A, Baloh RW. A prospective study of cerebral white matter abnormalities in older people with gait dysfunction. Neurology. 2001;57:990–994. doi: 10.1212/wnl.57.6.990. [DOI] [PubMed] [Google Scholar]
- 30.Yamanouchi H, Sugiura S, Tomonaga M. Decrease in nerve fibres in cerebral white matter in progressive subcortical vascular encephalopathy of Binswanger type. An electron microscopic study. J Neurol. 1989;236:382–387. doi: 10.1007/BF00314894. [DOI] [PubMed] [Google Scholar]