Abstract
Objective
The purpose of this retrospective study was to evaluate the outcome of gamma knife radiosurgery (GKRS) and/or whole brain radiation therapy (WBRT) for the treatment of small cell lung carcinoma (SCLC) metastasis to the brain.
Methods
From 2000 to 2010, 50 patients underwent GKRS for metastatic brain lesions originating from SCLC. Among these patients, 11 received prophylactic cranial irradiation (PCI) before the development of metastatic lesions (PCI group), and GKRS was performed as an initial treatment for newly diagnosed lesions in 12 patients who had not received PCI (primary GKRS group). In addition, GKRS was performed as a salvage treatment for progressive lesions after WBRT in 27 patients (salvage GKRS group). The medical records and imaging data of all patients were retrospectively analyzed.
Results
The overall survival of the 50 patients was 20.8 months (range 1-53) after the diagnosis of primary tumor and 12.0 months (range 1-47) after the development of cerebral metastasis. Median survival after GKRS was 4.8 months (range 1-15) in the PCI group, 4.6 months (range 0-18) in the primary GKRS group, and 7.6 months (range 0-33) in the salvage GKRS group. Further treatment for progressive lesions after GKRS was necessary in 15 patients, after a mean interval of 3.8 months. Causes of death were systemic organ failure in 15 patients, deterioration of neurological state in 13 patients, and unknown or combined causes in 16 patients. The local control rate of the lesions treated with GKRS was 76.4% (decreased in 13 patients and stable in 16 patients at the final imaging follow-up (mean 5.60 months).
Conclusion
GKRS is an effective local treatment for brain metastasis from SCLC both as an initial treatment for newly diagnosed lesions after PCI and as a salvage treatment for recurrent or progressive lesions. However, the survival benefit is not significant because most patients die of systemic multi-organ failure with a short life expectancy.
Keywords: Small cell lung carcinoma, Radiosurgery, Gamma knife, Metastasis
INTRODUCTION
Small cell lung carcinoma (SCLC) is characterized by rapid growth and metastasis, and brain metastases (BM) are frequent in SCLC. The overall incidence of this type of BM is greater than 50% over two years12). Therefore, the standard treatment for SCLC remains appropriate systemic chemotherapy followed by prophylactic cranial irradiation (PCI) for prevention of brain metastasis because SCLC is typically radiosensitive5,11). The prognosis of patients with BM is generally poor. Nearly one-half of those who develop BM die of central nervous system progression13). The aim of treating symptomatic BM from SCLC is to improve the survival and quality of life in these patients. However, treatment modalities for BM of SCLC have not been established. After the diagnosis of SCLC with BMs, the median survival is 4.5 months, despite treatment with high-dose cranial irradiation11). In this retrospective study, we evaluated the effectiveness of gamma knife radiosurgery (GKRS) for the treatment of SCLC metastasis to the brain.
MATERIALS AND METHODS
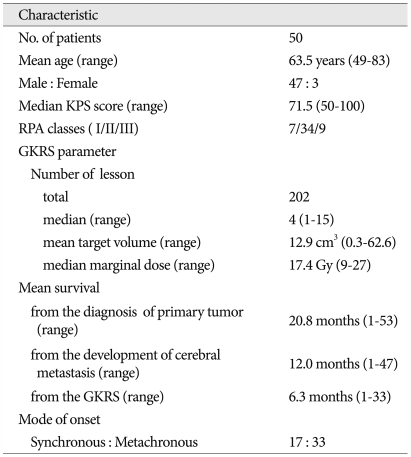
From 2000 to 2010, 50 patients underwent GKRS for metastatic brain lesions originating from SCLC, and their medical records and imaging data were retrospectively analyzed. GKRS was performed as an initial treatment for newly diagnosed lesions in 12 patients with no previous treatment (primary GKRS group) and 11 patients with previous PCI (PCI group). In addition, GKRS was performed as a salvage treatment for progressive lesions after WBRT in 27 patients (salvage GKRS group). The patient group included 3 females and 47 males with a mean age of 63.5 years (range 49-83). The median Karnofsky Performance Scale (KPS) score was mean 71.5 (range 50-100) and the recursive partitioning analysis (RPA) classes I/II/III were 7/34/9. Leksell Gamma Knife types B or C (Elekta Instruments, Atlanta, GA) were used for radiosurgery. Baseline characteristics of the 50 patients are summarized in Table 1. The median number of tumors treated with GKRS was four (range 1-15) per patient, and a total of 202 lesions were treated with GKRS. The mean target volume was 12.9 cm3 (range 0.3-62.6). The median marginal dose was 17.4 Gy (range 9-27) and the median marginal isodose was 50% (45-80). The mean follow-up duration was 20.8 months (range 1-53) from diagnosis of SCLC. Overall survival (from the diagnosis of primary tumor and cerebral metastasis), survival after GKRS, survival without further treatment after GKRS and cause of death were investigated. The Kaplan-Meier method was used to estimate the overall survival rates. Statistical analysis was performed using the SPSS software package (version 18.0, SPSS Inc., Chicago, IL, USA).
Table 1.
Summary of patient characteristics (n=50)
KPS : Karnofsky Performance Scale, RPA : recursive partitioning analysis, GKRS : gamma knife radiosurgery
RESULTS
The mean overall survival of 50 patients was 20.8 months (range 1-53) after the diagnosis of primary tumor, 12.0 months (range 1-47) after the development of cerebral metastasis and 6.3 months (range 1-33) after radiosurgery. The median survival after GKRS was 4.8 months (range 1-15) in the PCI group, 4.6 months (range 0-18) in the primary GKRS group, and 7.6 months (range 0-33) in the salvage GKRS group. Further treatment for progressive lesions after GKRS was necessary in 15 patients, after a mean interval of 3.8 months. The characteristics of patients according to treatment modality at the time of brain metastasis are summarized in Table 2.
Table 2.
Characteristics of patients according to treatment modality at the time of brain metastasis
GKRS : gamma knife radiosurgery, WBRT : whole brain radiation therapy, PCI : prophylactic cranial irradiation, SCLC : small cell lung carcinoma, KPS : Karnofsky Performance Scale, RPA : recursive partitioning analysis
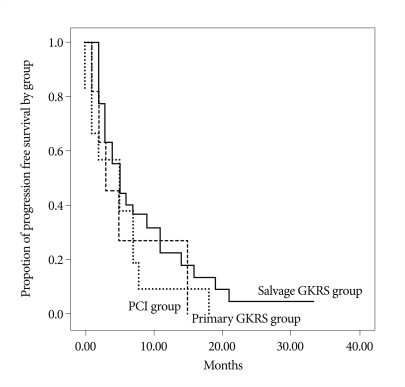
Four patients underwent subsequent craniotomy and tumor resection for a tumor refractory to radiosurgery and radiation therapy. Causes of death were systemic organ failure in 15 patients, deterioration of neurological state in 13 patients, and unknown or combined causes in 16 patients. Follow-up images were checked for total 154 lesions in 37 patients. The local control rate of the lesions treated with GKRS was 70.3% (decreased in 14 patients and stable in 12 patients) at the final imaging follow-up (mean 5.6 months after GKRS). However newly developed BM and increased lesions after GKRS were found in 11 patients (29.7%). Thirteen patients were unable to undergo follow-up imaging due to poor general conditions. Survival rate after GKRS was not significantly different among each group (p=0.346) (Fig. 1). However, survival after BM was significantly better with subsequent GKRS after WBRT or PCI (p=0.05) (Fig. 1).
Fig. 1.
Kaplan-Meier curves shows survival rates of patients after radiosurgery according to each group (p=0.346). GKRS : gamma knife radiosurgery, PCI : prophylactic cranial irradiation.
DISCUSSION
SCLC accounts for 20% of all lung cancers10), and approximately 14-24% of patients have BM at the time of diagnosis. BM occurrs in up to 40% of these patients during the course of their disease15). The prognoses of these patients are generally poor, with the median survival measured in months3). Current studies have focused on the evaluation of outcome after GKRS for these patients.
PCI is recommended for the patients with remission after chemotherapy. For newly diagnosed BM, PCI improves both overall and disease-free survival in patients in complete remission5). WBRT and/or chemotherapy are considered to be the standard treatment modalities for metastatic brain tumors originating from SCLC, because the tumors grow rapidly and metastasize, even when only a single tumor is present5,7,8,11). WBRT after diagnosis of metastasis is a typical choice because SCLC tends to be radiosensitive. The mean survival period is 4.5 months when the WBRT dose is 30 to 40 Gy fractionated into 10 to 20 segments4). However, it is accompanied with complications concerned similarly in WBRT for BM of other pathology. Particularly, additional WBRT in the patients treated with previous PCI carries higher risk. Systemic chemotherapy for asymptomatic lesions shows a 27% response rate11), and that of salvage chemotherapy for recurrent lesions with prior irradiation is 33-43%4). However chemotherapy alone had a limitation in treatment of patients with BM because of anatomic barriers (blood-brain barrier and blood-tumor barrier).
Recently, radiosurgery has become a popular treatment for metastatic or recurrent brain lesions of SCLC. Although GKRS is a representative method of radiosurgery, it is not yet established as an effective treatment for the management of metastatic or recurrent brain lesions of SCLC. In previous reports, GKRS for SCLC was shown to be effective for tumor control and improved survival. The tumor control rate is 81% in patients treated with GKRS for recurrent SCLC9). The overall mean survival period for brain metastasis is 18 months and the overall mean survival period after GKRS is 4.5 months9). In our series, the mean overall survival period for brain metastasis was 12.0 months and the mean overall survival period after GKRS was 6.3 months, similar to results from a previous report9).
Prognostic factors can be used to help select the most appropriate treatment for individual patients with brain metastases due to SCLC. In a previous study, age, KPS, and lack of extracerebral metastases were identified as predictors of overall survival in patients with brain metastases6). The RPA classification has also been demonstrated to be valid for the prediction of overall survival in SCLC patients16). In addition, Sundstrom et al. found the absence of extracerebral metastases and a good performance status to be associated with improved survival in a series of 75 patients11). The number of brain metastases has been previously described as a significant predictor of overall survival in a randomized trial that compared WBRT alone to WBRT plus a stereotactic boost for the treatment of patients with 1-3 brain metastases1).
Patients with a relatively favorable survival prognosis and a limited number of brain metastases may be considered candidates for more intensive therapies such as radiosurgery1). However, in our study, improved overall survival was not significantly associated with younger age, better KPS, <4 brain metastases or RPA class 1. Only early onset brain metastasis significantly increased overall survival, and this may be due to the earlier initiation of treatment in these cases (p=0.019).
After WBRT, residual tumor foci may be present. These patients appear to benefit from an additional stereotactic boost, performed either with linac-based radiosurgery or gamma knife radiosurgery. In the randomized trial of Andrews et al.1), additional radiosurgery resulted in better survival in patients with only one cerebral lesion (6.5 months versus 4.9 months, p=0.039). Thus, radiosurgery in addition to WBRT may be recommended for patients with a relatively favorable survival prognosis such as controlled extracerebral disease and only one lesion. However, the question may arise whether radiosurgery alone is sufficient for these patients or if it should be supplemented with WBRT.
Sneed et al.14) compared radiosurgery alone to radiosurgery plus WBRT in a retrospective series of 105 patients. The intracerebral control rates at one year were 28% after radiosurgery alone and 69% after the combined approach (p=0.03). However, the median survivals were similar (11.3 months versus 11.1 months). A randomized trial of 132 patients with 1-4 brain metastases demonstrated better one-year intracerebral control with radiosurgery plus WBRT compared to that of radiosurgery alone (53% versus 24%, respectively, p<0.001)2). However, survival was not significantly improved (39% versus 28%, p=0.42). Similar findings were observed in our own study of 50 patients. PFS was not significantly different among WBRT plus GKRS, GKRS only and PCI plus GKRS (p=0.346). Though survival was significantly better with subsequent GKRS after WBRT or PCI (p=0.05) (Fig. 2) in the presented data, it seems to be caused by several defects in this study. Our study was limited by the fact that it was a retrospective non-randomized analysis of a small number of cases. Despite those limitations, the results of this study show that overall survival of the patients with BM from SCLC may not significantly improve with GKRS but at least GKRS is effective for local control of BM from SCLC as well as BM from other pathology.
Fig. 2.
Kaplan-Meier curves shows significantly better survival of the patients treated with subsequent GKRS after WBRT or PCI than those with GKRS as an initial treatment (p=0.05). GKRS : gamma knife radiosurgery, PCI : prophylactic cranial irradiation, WBRT : whole brain radiation therapy.
CONCLUSION
GKRS is an effective salvage treatment for BM originating from SCLC, as well as for non-SCLC. GKRS for BM from SCLC affords effective local tumor control though overall survival of these patients is still disappointingly short. Because GKRS is easily applicable in combination with any other systemic or local treatment modalities, the early detection of brain lesions and GKRS combined with other treatment modality may improve quality of life and reduce mortality by deterioration of neurological state even though there is only modest survival benefit.
Acknowledgements
This study was supported by a grant of the Korea Healthcare technology R&D project, Ministry for Health & Welfare Affairs, Republic of Korea (A092255).
References
- 1.Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases : phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363:1665–1672. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- 2.Aoyama H, Shirato H, Tago M, Nakagawa K, Toyoda T, Hatano K, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases : a randomized controlled trial. JAMA. 2006;295:2483–2491. doi: 10.1001/jama.295.21.2483. [DOI] [PubMed] [Google Scholar]
- 3.Arnold SM, Patchell RA. Diagnosis and management of brain metastases. Hematol Oncol Clin North Am. 2001;15:1085–1107. vii. doi: 10.1016/s0889-8588(05)70269-0. [DOI] [PubMed] [Google Scholar]
- 4.Arriagada R, Le Chevalier T, Borie F, Rivière A, Chomy P, Monnet I, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. J Natl Cancer Inst. 1995;87:183–190. doi: 10.1093/jnci/87.3.183. [DOI] [PubMed] [Google Scholar]
- 5.Aupérin A, Arriagada R, Pignon JP, Le Péchoux C, Gregor A, Stephens RJ, et al. Prophylactic Cranial Irradiation Overview Collaborative Group. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. N Engl J Med. 1999;341:476–484. doi: 10.1056/NEJM199908123410703. [DOI] [PubMed] [Google Scholar]
- 6.Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37:745–751. doi: 10.1016/s0360-3016(96)00619-0. [DOI] [PubMed] [Google Scholar]
- 7.Jett JR. Current treatment of unresectable lung cancer. Mayo Clin Proc. 1993;68:603–611. doi: 10.1016/s0025-6196(12)60376-0. [DOI] [PubMed] [Google Scholar]
- 8.Lee JS, Murphy WK, Glisson BS, Dhingra HM, Holoye PY, Hong WK. Primary chemotherapy of brain metastasis in small-cell lung cancer. J Clin Oncol. 1989;7:916–922. doi: 10.1200/JCO.1989.7.7.916. [DOI] [PubMed] [Google Scholar]
- 9.Pan HC, Sheehan J, Stroila M, Steiner M, Steiner L. Gamma knife surgery for brain metastases from lung cancer. J Neurosurg. 2005;102(Suppl):128–133. doi: 10.3171/jns.2005.102.s_supplement.0128. [DOI] [PubMed] [Google Scholar]
- 10.Quan AL, Videtic GM, Suh JH. Brain metastases in small cell lung cancer. Oncology. 2004;18:961–972. discussion 974, 979-980, 987. [PubMed] [Google Scholar]
- 11.Seute T, Leffers P, Wilmink JT, ten Velde GP, Twijnstra A. Response of asymptomatic brain metastases from small-cell lung cancer to systemic first-line chemotherapy. J Clin Oncol. 2006;24:2079–2083. doi: 10.1200/JCO.2005.03.2946. [DOI] [PubMed] [Google Scholar]
- 12.Sheehan J, Kondziolka D, Flickinger J, Lunsford LD. Radiosurgery for patients with recurrent small cell lung carcinoma metastatic to the brain : outcomes and prognostic factors. J Neurosurg. 2005;102(Suppl):247–254. doi: 10.3171/jns.2005.102.s_supplement.0247. [DOI] [PubMed] [Google Scholar]
- 13.Sheehan RG. Small cell lung cancer : a problem of tumor heterogeneity. Am J Med Sci. 1986;292:241–256. doi: 10.1097/00000441-198610000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Sneed PK, Lamborn KR, Forstner JM, McDermott MW, Chang S, Park E, et al. Radiosurgery for brain metastases : is whole brain radiotherapy necessary? Int J Radiat Oncol Biol Phys. 1999;43:549–558. doi: 10.1016/s0360-3016(98)00447-7. [DOI] [PubMed] [Google Scholar]
- 15.Sundström JT, Minn H, Lertola KK, Nordman E. Prognosis of patients treated for intracranial metastases with whole-brain irradiation. Ann Med. 1998;30:296–299. doi: 10.3109/07853899809005858. [DOI] [PubMed] [Google Scholar]
- 16.Videtic GM, Adelstein DJ, Mekhail TM, Rice TW, Stevens GH, Lee SY, et al. Validation of the RTOG recursive partitioning analysis (RPA) classification for small-cell lung cancer-only brain metastases. Int J Radiat Oncol Biol Phys. 2007;67:240–243. doi: 10.1016/j.ijrobp.2006.08.019. [DOI] [PubMed] [Google Scholar]