Abstract
A one-pot condensation of isotryptamines and aldehydes that affords enantiomerically enriched 4-substituted tetrahydro-γ-carbolines is reported. The reaction is induced by a chiral thiourea/benzoic acid dual catalyst system. Purification of the N-Boc-protected products by trituration or crystallization provides the optically pure tetrahydro-γ-carboline derivatives in a scalable and highly practical procedure.
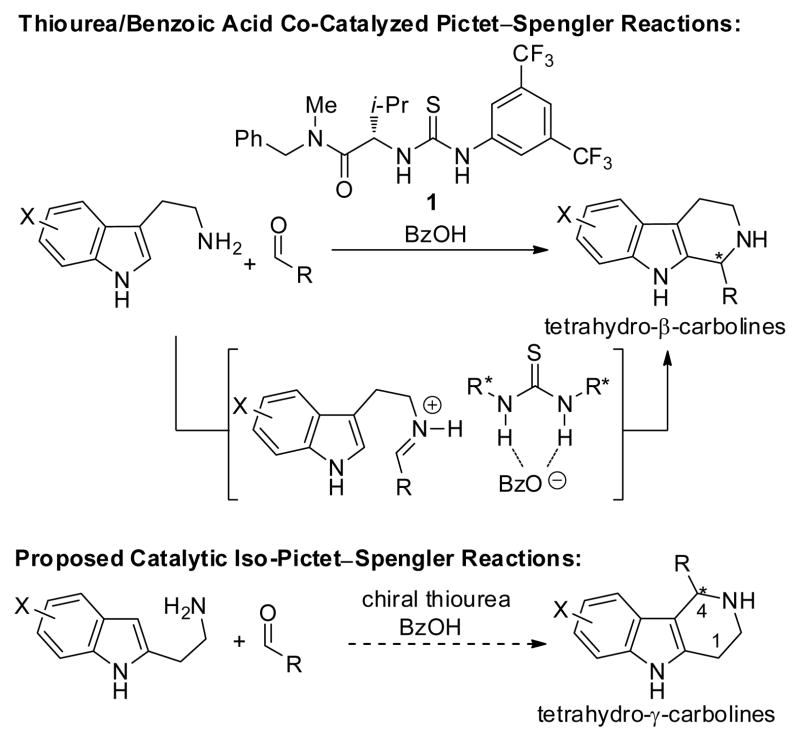
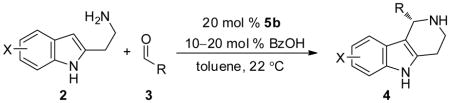
Natural and synthetic compounds containing the tetrahydro-β-carboline heterocyclic framework are endowed with an extraordinary range of important biological activities. 1 The closely related tetrahydro-γ-carboline framework is unknown in natural product structures, but also holds considerable potential as a template for drug discovery. 2 In contrast to the rich assortment of known synthetic routes to chiral tetrahydro-β-carboline derivatives,3, 4 few methods have been indentified for the direct preparation of optically enriched tetrahydro-γ-carbolines. Reported strategies to the latter class of compounds include classical resolution, 5 diastereoselective cyclization of chiral, substituted precursors, 6 and Pd-catalyzed enantioselective intramolecular allylic alkylation. 7 We describe here a straightforward and direct route to enantiomerically enriched 4-substituted tetrahydro-γ-carbolines through an enantioselective, catalytic “iso-Pictet–Spengler reaction”, the one-pot condensation/cyclization of 2-substituted indolylethylamines (isotryptamines) and aldehydes (Scheme 1).8
Scheme 1.
Synthesis of tetrahydro-β-carbolines and proposed route to tetrahydro-γ-carbolines co-catalyzed by chiral thioureas and BzOH
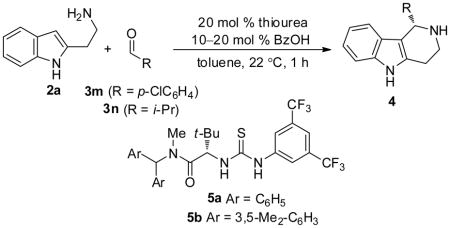
Our approach drew directly on the recent discovery that the combination of chiral thioureas and carboxylic acid derivatives can serve as a highly effective co-catalyst system for enantioselective one-pot Pictet–Spengler reactions of tryptamines and aldehydes (Scheme 1).4a Under the conditions optimized for that reaction (thiourea 1/benzoic acid, 20 mol%), the model iso-Pictet–Spengler reaction between unsubstituted isotryptamine 2a and 4-chlorobenzaldehyde 3m was found to proceed efficiently to the desired tetrahydro-γ-carboline 4am (>98% conversion within 1 h), and with 66% ee (entry 1, Table 1). Chiral thiourea 5a, which was identified previously as an effective catalyst for Strecker reactions,9 was found to be more effective, affording 4am in 79% ee (entry 2). Substantially higher enantioselectivity was observed in the reaction of 2a and isobutyraldehyde 3n (89% ee). In all cases, it was found that the thiourea needed to be present at a concentration equal to or greater than that of the achiral carboxylic acid in order to prevent diminished enantioselectivities due to an acid-catalyzed racemic background reaction. 10 This proved to a particular concern with iso-Pictet–Spengler reactions with aliphatic aldehydes, which may contain detectable levels of the corresponding aliphatic acids as received from commercial suppliers. Accordingly, for some aliphatic substrates, it was found that reducing the loading of BzOH led to measurable improvements in product ee (e.g., 91% ee vs. 89% ee for 4an, entries 3 and 4). Further optimization of the thiourea catalyst structure revealed that the highly sterically demanding derivative 5b bearing the 3,5-dimethylbenzhydryl group on the amide component afforded tetrahydro-γ-carboline 4an in 97% yield and 95% ee (entry 5).
Table 1.
Optimization of the Enantioselective Iso-Pictet–Spengler Reaction
 | ||||||
|---|---|---|---|---|---|---|
| entry | aldehyde | thiourea | BzOH (mol %) | product | yield (%)a | ee (%)b |
| 1 | 3m | 1 | 20 | 4am | ndc | 66 |
| 2 | 3m | 5a | 20 | 4am | 95 | 79 |
| 3 | 3n | 5a | 20 | 4an | 98 | 89 |
| 4 | 3n | 5a | 10 | 4an | 97 | 91 |
| 5 | 3n | 5b | 10 | 4an | 97 | 95 |
Isolated yield after purification; >98% conversion in all cases.
Determined by HPLC analysis of the N-Boc derivative.
nd = not determined.
The thiourea/benzoic acid co-catalyzed iso-Pictet–Spengler reaction was applied successfully to a variety of isotryptamine-aldehyde combinations, as illustrated in Table 2. In particular, high enantioselectivities were obtained in the cyclization of sterically demanding aliphatic or aromatic aldehydes with both electron-rich and electron deficient isotryptamine derivatives using thiourea 5b (entries 2–18). In contrast, aldehydes lacking branching at the α position proved less effective as substrates (entry 1, and discussion below).
Table 2.
Substrate Scope of the Thiourea-/BzOH-Co-Catalyzed Enantioselective Iso-Pictet–Spengler Reaction Catalyzed by 5b
 | |||||||
|---|---|---|---|---|---|---|---|
| entry | isotryptamine (X) | aldehyde (R) | BzOH(mol %) | time (h) | product | yield (%)b | ee (%)a |
| 1 | 2a (H) | 3o (i-Bu) | 20 | 1 | 4ao | 96 | 79 |
| 2 | 2a | 3p (c-Hex) | 20 | 1 | 4ap | 96 | 91 |
| 3 | 2a | 3q (CH(Et)2) | 20 | 3 | 4aq | 97 | 95 |
| 4 | 2a | 3r (t-Bu) | 20 | 7 d | 4ar | 83c | 87 |
| 5 | 2a | 3s (o-MeC6H4) | 20 | 1 | 4as | 99 | 92 |
| 6 | 2a | 3t (1-naph) | 20 | 2 | 4at | 96 | 86 |
| 7 | 2b (5-F) | 3n | 10 | 1 | 4bn | 98 | 94 |
| 8 | 2c (6-F) | 3n | 10 | 1 | 4cn | 97 | 94 |
| 9 | 2d (5-MeO) | 3n | 10 | 1 | 4dn | 96 | 94 |
| 10 | 2e (6-MeO) | 3n | 10 | 1 | 4en | 94 | 94 |
| 11 | 2f (5-Me) | 3n | 10 | 1 | 4fn | 95 | 95 |
| 12 | 2g (5-vinyl) | 3n | 10 | 1 | 4gn | 97 | 95 |
| 13 | 2d | 3p | 20 | 1 | 4dp | 98 | 91 |
| 14 | 2f | 3p | 20 | 1 | 4fp | 93 | 93 |
| 15 | 2b | 3p | 20 | 1 | 4bp | 99 | 90 |
| 16 | 2c | 3q | 20 | 5 | 4cq | 96 | 93 |
| 17 | 2b | 3s | 20 | 2 | 4bs | 96 | 88 |
| 18 | 2d | 3s | 20 | 1 | 4ds | 93 | 91 |
Isolated yield after purification.
Determined by HPLC analysis of the N-Boc derivative.
90% conversion.
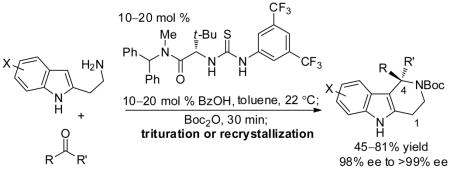
Any variability in the enantioselectivity of the thiourea-catalyzed iso-Pictet–Spengler reaction proved to be of only minor consequence, however, thanks to the identification of a remarkably straightforward protocol for upgrading the ee of the tetrahydro-γ-carboline products. As illustrated in Table 3, the crude products of the cyclization reaction were subjected to reaction with Boc2O at ambient temperature, and the enantiomeric composition of the resulting N-Boc tetrahydro-γ-carboline 6 could be upgraded to >99% ee in nearly all cases by direct crystallization or trituration.11 This allowed use of reduced loadings of the less enantioselective but commercially available catalyst 5a,12 and extension of the method to substrates that undergo reaction with only moderate intrinsic enantioselectivity, such as unhindered aliphatic (entries 1–2) and aromatic (entries 6–9) aldehydes. This one-pot synthetic procedure for preparing enantiopure N-Boc tetrahydro-γ-carboline derivatives required no chromatographic purification steps and should be readily adaptable to preparative scale.
Table 3.
One-Pot Method for the Preparation of Optically Pure Tetrahydro-γ-Carboline Derivatives
 | ||||||
|---|---|---|---|---|---|---|
| entry | isotryptamine | aldehyde (R) | time (h) | product | ee (%)a | yield (%)b |
| 1 | 2a | 3u (n-pent) | 1 | 6au | >99 (61) | 45 |
| 2 | 2a | 3o (i-Bu)c | 1 | 6ao | >99 (74) | 55 |
| 3 | 2a | 3n (i-Pr) | 1 | 6an | >99 (88) | 81 |
| 4 | 2a | 3p (c-Hex)c | 1 | 6ap | 99 (91) | 65 |
| 5 | 2a | 3q (CH(Et)2) | 5 | 6aq | >99 (90) | 79 |
| 6 | 2a | 3v (C6H5) | 1 | 6av | >99 (70) | 52 |
| 7 | 2a | 3w (p-FC6H4) | 1 | 6aw | >99 (74) | 64 |
| 8 | 2a | 3m (p-ClC6H4) | 1 | 6am | >99 (76) | 63 |
| 9 | 2a | 3x (p-BrC6H4) | 1 | 6ax | >99 (75) | 56 |
| 10 | 2a | 3s (o-MeC6H4 | 2 | 6as | >99 (86) | 77 |
| 11 | 2b | 3n | 1 | 6bn | >99 (88) | 78 |
| 12 | 2c | 3n | 1 | 6cn | >99 (89) | 77 |
| 13 | 2d | 3n | 1 | 6dn | 98 (85) | 78 |
| 14 | 2g | 3nc | 1 | 6gn | >99 (92) | 67 |
| 15 | 2d | 3p | 1 | 6dp | >99 (92) | 81 |
| 16 | 2b | 3s | 2 | 6bs | >99 (79) | 66 |
| 17 | 2d | 3s | 2 | 6ds | 99 (87) | 74 |
Determined by HPLC analysis. Numbers in parentheses correspond to the ee of 6 before trituration or recrystallization. For details on the trituration or recrystallization procedures, see the Supporting Information.
Isolated yield of product with upgraded ee.
20 mol % 5a used.
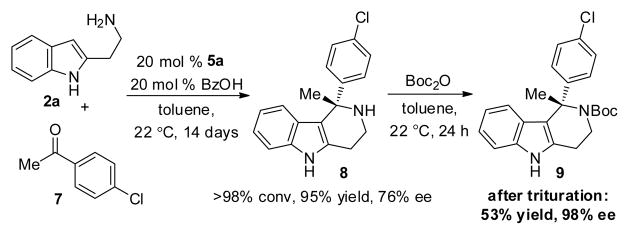
Ketone substrates were also applied successfully to the iso-Pictet–Spengler protocol. As depicted in Scheme 2, the ketoimine generated in situ from 2a and ketone 7 underwent enantioselective cyclization in the presence of 5a and benzoic acid to afford the tetrahydro-γ-carboline 8 in 95% yield and 76% ee. After N-Boc protection and trituration, 9 was isolated in 98% ee.
Scheme 2.
Enantioselective Iso-Pictet–Spengler Reaction of 2a and Ketone 7
Tetrahydro-β-carboline derivatives are the common biosynthetic precursors of the monoterpenoid indole alkaloid natural product family, which includes rearranged examples such as morphine.13 We explored whether the tetrahydro-γ-carboline framework might undergo analogous transformations into structurally and stereochemically complex alkaloid scaffolds. In particular, we targeted the synthesis of a spirocyclic oxindole, a frequently observed structural motif in biologically active compounds.14, 15 Through the treatment of tetrahydro-γ-carboline 6ao with NBS under acidic conditions, optically active spiro indoxyl 10 was isolated in 48% yield (Scheme 3).16 Oxidative rearrangement product 10 features contiguous nitrogen-bearing stereogenic centers, one of which is fully substituted.
Scheme 3.
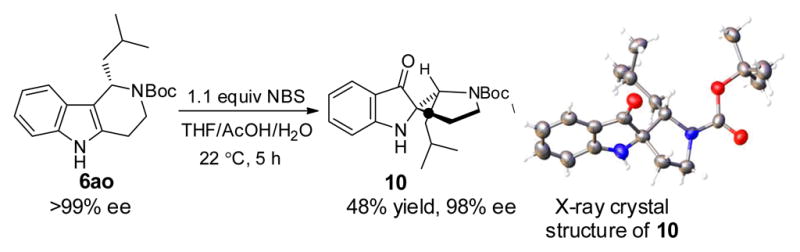
Synthesis of Spiro Indoxyl Derivative 10
In summary, we have developed an efficient method for the catalytic enantioselective synthesis of 4-substituted tetrahydro-γ-carbolines using a readily available chiral thiourea and BzOH. Optically pure products (98% to >99% ee) were obtained through a one-pot protocol of condensation, enantioselective cyclization, and Boc-protection, followed by trituration or recrystallization. The application of this methodology to new alkaloid-like scaffolds such as 10 is the subject of ongoing investigation.
Supplementary Material
Acknowledgments
This work was supported by the NIGMS (PO1 GM-69721). We thank Dr. Shao-Liang Zheng for the X-ray data collection and structural determination.
Footnotes
Supporting Information Available: Experimental procedures and characterization data for products and X-ray crystallographic data of 6ax and 10. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Cordell GA, editor. Introduction to Alkaloids: A Biogenetic Approach. Wiley; New York: 1981. [Google Scholar]; (b) Cane DE, Barton DHR, Nakanishi K, Meth-Cohn O, Kelly JW, editors. Comprehensive Natural Products Chemistry. Elsevier; New York: 1999. [Google Scholar]; (c) Karolina P. Curr Opin Drug Disc Dev. 2010;13:669–684. [PubMed] [Google Scholar]
- 2.(a) Doody RS, Gavrilova SI, Sano M, Thomas RG, Aisen PS, Bachurin SO, Seely L, Hung D. Lancet. 2008;372:207–215. doi: 10.1016/S0140-6736(08)61074-0. [DOI] [PubMed] [Google Scholar]; (b) Bridoux A, Millet R, Pommery J, Pommery N, Henichart JP. Bioorg Med Chem. 2010;18:3910–3924. doi: 10.1016/j.bmc.2010.04.034. [DOI] [PubMed] [Google Scholar]; (c) Butler KV, Kalin J, Brochier C, Vistoli G, Langley B, Kozikowski AP. J Am Chem Soc. 2010;132:10842–10846. doi: 10.1021/ja102758v. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Harbart CA, Plattner JJ, Welch WM. J Med Chem. 1980;23:635–643. doi: 10.1021/jm00180a011. [DOI] [PubMed] [Google Scholar]; (e) Gharbia-Abou M, Patel UR, Webb MB, Moyer JA, Andree TH, Muth EA. J Med Chem. 1987;30:1818–1823. doi: 10.1021/jm00393a023. [DOI] [PubMed] [Google Scholar]; (f) Khorana N, Smith C, Herrick-Davis K, Purohit A, Teitler M, Grella B, Dukat M, Glennon RA. J Med Chem. 2003;46:3930–3937. doi: 10.1021/jm030080s. [DOI] [PubMed] [Google Scholar]; (g) Bachurin S, Bukatina E, Lermontova N, Tkachenko S, Afanasiev A, Grigoriev V, Grigorieva I, Ivanov YU, Sablin S, Zefirov N. Ann NY Acad Sci. 2001;939:425–435. doi: 10.1111/j.1749-6632.2001.tb03654.x. [DOI] [PubMed] [Google Scholar]
- 3.For reviews of Pictet–Spengler reactions, see: Cox ED, Cook JM. Chem Rev. 1995;95:1797–1842.Lorenz M, van Linn ML, Cook JM. Curr Org Synth. 2010;7:189–223.Stockigt J, Antonchick AP, Wu F, Waldmann H. Angew Chem Int Ed. 2011;50:8538–8564. doi: 10.1002/anie.201008071.For reductive approaches to enantioenriched tetrahydro-β-carbolines, see: Itoh T, Yokoya M, Miyauchi K, Nagata K, Ohsawa A. Org Lett. 2003;5:4301–4304. doi: 10.1021/ol030103x.Martin SF. Acc Chem Res. 2002;35:895–904. doi: 10.1021/ar950230w.Li C, Xiao J. J Am Chem Soc. 2008;130:13208–13209. doi: 10.1021/ja8050958.Evanno L, Ormala J, Pihko PM. Chem Eur J. 2009;15:12963–12969. doi: 10.1002/chem.200902289.Uematsu N, Fujii A, Hashiguchi S, Ikariya T, Noyori R. J Am Chem Soc. 1996;118:4916–4917.Nugent TC, El-Shazly M. Adv Synth Catal. 2010;352:753–819.
- 4.For catalytic enantioselective Pictet–Spengler reactions, see: Klausen RS, Jacobsen EN. Org Lett. 2009;11:887–890. doi: 10.1021/ol802887h.Sewgobind NV, Wanner MJ, Ingemann S, de Gelder R, van Maarseveen JH, Hiemstra H. J Org Chem. 2008;73:6405–6408. doi: 10.1021/jo8010478.Wanner MJ, vad der Haas RNS, de Cuba KR, van Maarseveen JH, Hiemstra H. Angew Chem Int Ed. 2007;46:7485–7487. doi: 10.1002/anie.200701808.Raheem IT, Thiara PS, Peterson EA, Jacobsen EN. J Am, Chem Soc. 2007;129:13404–13405. doi: 10.1021/ja076179w.Seayad J, Seayad AM, List B. J Am Chem Soc. 2006;128:1086–1087. doi: 10.1021/ja057444l.Taylor MS, Jacobsen EN. J Am Chem Soc. 2004;126:10558–10559. doi: 10.1021/ja046259p.
- 5.Vecchietti V, Clarke GD, Colle R, Giardina G, Petrone G, Sbacchi M. J Med Chem. 1991;34:2624–2633. doi: 10.1021/jm00112a042. [DOI] [PubMed] [Google Scholar]
- 6.Sheng YF, Li GQ, Kang Q, Zhang AJ, You SL. Chem Eur J. 2009;15:3351–3354. doi: 10.1002/chem.200900033. [DOI] [PubMed] [Google Scholar]
- 7.Bandini M, Melloni A, Piccinelli F, Sinisi R, Tommasi S, Umani-Ronchi A. J Am Chem Soc. 2006;128:1424–1425. doi: 10.1021/ja054109o. [DOI] [PubMed] [Google Scholar]
- 8.For the racemic variant, see: Molina P, Alcántara J, López-Leonardo C. Tetrahedron. 1996;52:5833–5844.
- 9.(a) Zuend SJ, Coughlin MP, Lalonde MP, Jacobsen EN. Nature. 2009;461:968–971. doi: 10.1038/nature08484. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Zuend SJ, Jacobsen EN. J Am Chem Soc. 2009;131:15358–15374. doi: 10.1021/ja9058958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.In the absence of thiourea, 20 mol % BzOH catalyzes iso-Pictet–Spengler reaction of 2a with 3n to 30% conversion in 0.5 h.
- 11.The absolute configuration of the products was assigned by X-ray crystallographic analysis of compound 6ax. See Supporting Information for details.
- 12.Strem Chemicals. Newburyport; MA, USA: [Google Scholar]
- 13.O’Connor SE, Maresh JJ. Nat Prod Rep. 2006;23:532–547. doi: 10.1039/b512615k. [DOI] [PubMed] [Google Scholar]
- 14.(a) Baran PS, Corey EJ. J Am Chem Soc. 2002;124:7904–7905. doi: 10.1021/ja026663t. [DOI] [PubMed] [Google Scholar]; (b) Takayama H, Ishikawa H, Kurihara M, Kitajima M, Aimi N, Ponglux D, Koyama F, Matsumoto K, Moriyama T, Yamamoto LT, Watanabe K, Murayama T, Horie S. J Med Chem. 2002;45:1949–1956. doi: 10.1021/jm010576e. [DOI] [PubMed] [Google Scholar]; (c) Williams RM. Chem Pharm Bull. 2002;50:711–740. doi: 10.1248/cpb.50.711. [DOI] [PubMed] [Google Scholar]
- 15.Badillo JJ, Hanhan NV, Franz AK. Curr Opin Drug Disc Dev. 2010;13:758–776. [PubMed] [Google Scholar]
- 16.(a) Trost BM, Brennan MK. Synthesis. 2009:3003–3025. [Google Scholar]; (b) Marti C, Carreira EM. Eur J Org Chem. 2003:2209–2219. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.