Abstract
Background
HMG-CoA reductase inhibitors (statins) have been shown to reduce sympathetic nervous system (SNS) activation in experimental heart failure (HF). However, this potential mechanism of action of statins in HF has not been well studied in humans.
Methods and Results
Twenty-six patients with non-ischemic, systolic HF (left ventricular ejection fraction [LVEF] ≤ 35%) were randomized to atorvastatin (10mg) vs. placebo for 3 months. Pre- and post- treatment testing included echocardiography, laboratories, quality of life (QOL) questionnaires, and peroneal nerve muscle sympathetic nerve activity (MSNA) via microneurography. Eighteen subjects had technically adequate pre- and post- MSNA tracings. The cohort was 65% male, 81% NYHA II, LVEF 26 ± 6%, and LDL cholesterol 108 ± 26 mg/dl. Baseline MSNA was 41 ± 2 bursts / minute. LDL significantly decreased in the atorvastatin (−36.8%) vs. placebo (−0.1%) group (p<0.0001). However, there was no significant change in MSNA (−16.2% vs. – 2.5%), LVEF, B-type natriuretic peptide, or QOL score in the atorvastatin compared to placebo group.
Conclusions
Short-term statin therapy in patients with non-ischemic HF does not result in a significant decrease in SNS activation as measured by MSNA. These findings are consistent with the neutral outcomes of large clinical trials of statins in HF.
Keywords: heart failure, lipids, nervous system, sympathetic
Introduction
Heart failure (HF) is a national public health problem with an overall prevalence in the United States of approximately 6 million. One out of five patients newly diagnosed with HF will die within one year, despite advances in medical and device therapies for HF over the past decades.1, 2 Numerous observational, experimental, and small clinical studies of HMG-CoA reductase inhibitor (statin) therapy in HF suggested that statins would have beneficial effects on outcomes in HF of both ischemic and non-ischemic etiology;3–9 however, initial encouraging findings were not translated into survival benefit in two large randomized controlled trials of statins powered for clinical outcomes (Controlled Rosuvastatin Multinational Trial in Heart Failure [CORONA] and the GISSI-HF trial).10, 11
Several pleiotrophic effects of statins of theoretic benefit in non-ischemic HF have been demonstrated experimentally, including anti-inflammatory actions, anti-remodeling effects, and improvement in endothelial function.12 Statins have also been shown in experimental models to substantially decrease the excess sympathetic nervous system (SNS) activation characteristic of HF.13–15 This study aimed to assess the effect of statin therapy on SNS activation, as measured by muscle sympathetic nerve activity (MSNA) in humans with non-ischemic, systolic dysfunction HF.
Methods
Participants
We studied 26 subjects with non-ischemic cardiomyopathy and HF followed at a single university center. Eligibility criteria included age ≥ 18 years, NYHA II–III HF or NYHA I HF with HF symptoms in past year, and left ventricular ejection fraction (LVEF) ≤ 35%. LVEF was documented by 2D echocardiography within 3 months of study enrollment. Those with coronary artery disease (CAD) – defined as angiographic evidence of ≥ 50% lesion in 1 or more of the 3 major epicardial vessels, history of myocardial infarction, history of revascularization procedure, or evidence of significant perfusion defect in the setting of ischemic symptoms – were excluded from our study. Additional exclusion criteria included other clinical indication for statin treatment such as peripheral vascular disease or cerebrovascular disease, major cardiovascular event or surgical procedure within past 8 weeks (including implantable cardioverter defibrillator placement), low density lipoprotein (LDL) cholesterol < 70 mg/dL, and HF secondary to congenital heart disease or uncorrected valvular disease. Patients were excluded if they were already on treatment with a statin, had been treated with a statin in the prior two months, had statin intolerance, moderate – severe liver disease, liver enzymes > 3× upper limit of normal, or known peripheral or autonomic neuropathy.
Study Protocol
Subjects were randomized in a double-blinded fashion (1:1) to either atorvastatin (10 mg orally per day) or matching placebo for a treatment period of 3 months. This dose and duration of therapy were chosen based on a prior randomized trial in patients with HF which showed a significant effect of atorvastatin 10 mg in improving heart rate variability, an alternative index of autonomic tone 16. Study visits were conducted at baseline (pre-treatment), 6 weeks (mid-treatment monitoring visit), and 3 months (final study visit). Patients were required to be on stable, optimal doses of standard HF therapy, including stable dose of beta-blocker for 8 weeks, angiotensin converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB) for 4 weeks, and aldosterone antagonist (AA, NYHA III subjects) for 4 weeks. Throughout the study period, patients were maintained on baseline doses of beta-blockers, ACEI / ARB, aldosterone antagonists, and diuretics, with the exception of two patients who needed adjustment in their diuretic doses due to fluid retention. The primary endpoint of the study was change in muscle sympathetic nerve activity (MSNA, bursts/minute). Secondary endpoints included change in LVEF by transthoracic echocardiography, health-related quality of life (QOL), and cardiac biomarkers, including B-type natriuretic peptide (BNP), cardiac troponin I (cTnI), and high sensitivity C-reactive protein (hs-CRP). The study protocol was approved by the UCLA Medical Institutional Review Board (Medical IRB −1), overseen by the UCLA Office for Human Research Protection Program and accredited by the Association for Accreditation of Human Research Protection Programs, Inc.
MSNA was recorded directly from the peroneal nerve using the technique of microneurography, as previously described 17. The location of the nerve was first identified with transcutaneous stimulation using a pencil-shaped electrode. A tungsten microelectrode (tip diameter 5–15 um) (Bioengineering, University of Iowa) was then inserted into the nerve, and a reference electrode is inserted subcutaneously 1–2 cm from the recording electrode. The microelectrodes were connected to a preamplifier (gain 1000), and an amplifier (gain 50–100); nerve signals were bandpass filtered (700–2000 Hz)(Nerve Traffic Analyzer, Model 662C-3, University of Iowa, Bioengineering). For recording and analysis, nerve activity was rectified and integrated (time constant 0.1 sec) to obtain a mean voltage display of sympathetic nerve activity that is recorded on paper. Blood pressure was recording during MSNA recording using automated pressure cuff on the upper arm and heart rate was monitored continuously from electrocardiogram patch electrodes. Muscle sympathetic bursts were identified by visual inspection by a single blinded investigator (TBH) and expressed as burst frequency (bursts/min).
2D echocardiography was performed by experienced technicians using Acuson Sequoia Echocardiography System (Siemens Medical, Inc.) at baseline and final study visit. LVEF was quantified by the modified Simpson’s biplane method using harmonic imaging. 18 Left ventricular systolic and diastolic dimensions and degree of regurgitation were also assessed. Echoes were interpreted by physician blinded to treatment. Blood was collected and processed by UCLA Clinical Laboratory and Pathology Services; blood samples were sent to the lab immediately after phlebotomy and analyzed within one hour. Biomarkers – BNP, cTnI, and hsCRP – were analyzed by using industry-standard analytical platforms (Stratus CS STAT from Dade Behring for cTnI and Triage, Biosite, for BNP, and nephelometry for hs-CRP).
HRQL was measured using the Minnesota Living with Heart Failure Questionnaire (MLHFQ), a 21-item disease-specific measure of QOL.19 The MLHFQ was self-administered during the baseline and final study visits. NYHA functional class was also assessed at baseline and final study visits by physician blinded to treatment. A subject global assessment, in which subjects describe their condition as categories of better, unchanged, or worse, was also performed at the final study visit.20
Statistical Analysis
Power analysis was based on previous study of MSNA in HF subjects treated with carvedilol vs. placebo; 21 a sample size of 7 / group afforded 95% power to detect a mean difference of 13 bursts / minute in the treatment group with a standard deviation of 8 / group and based on a 2-sided 5% significance level. All data was analyzed on an intention to treat basis. The independent sample t test and Mann-Whitney U tests were used for comparison of parametric and non-parametric continuous variables, respectively. Chi-square test was used to compare categorical variables. Two-way Analyses of Variance (ANOVA were used to compare mean difference of change between active therapy and placebo groups. As a secondary analysis, paired samples t-test were also compare baseline to post-treatment variables within groups. Statistical comparisons were performed using two-sided significance tests, and a p value of < 0.05 was considered statistically significant. Calculations were performed with PASW Statistics version 18.0.1 (IBM, Somers, NY).
Results
Study Population
Twenty-six patients were enrolled in the study, with 14 randomized to atorvastatin and 12 randomized to placebo. The subjects were predominantly male and NYHA II, with a mean age of 48 ±15 years. Mean LVEF at baseline was 26 ± 6 % and mean peak oxygen uptake by cardiopulmonary exercise testing was 16.0 ± 4.5 ml/kg/min. Ninety-six percent (n=25) subjects completed the study; one subject randomized to placebo could not complete the study because he unexpectedly underwent status II heart transplantation. The remainder of the patients had excellent compliance with therapy, as assessed by pill counts (data not shown). There were no significant differences between the active treatment and placebo groups in terms of baseline age, LVEF, lipid levels, or cardiac biomarkers(Table 1). The only significant differences between the groups were slightly more males and a higher mean creatinine value in the placebo group. In the cohort who had baseline and 3-month MSNA recordings (microneurography cohort, n=18), the atorvastatin and placebo groups were also similar in terms of baseline patient characteristics; the only significant difference between the groups was more males in the placebo group (Table 1).
Table 1.
Baseline Characteristics of the Total Study Cohort and the Microneurography Cohort
| Total Cohort (n=26) | Microneurography Cohort (n=18) |
|||
|---|---|---|---|---|
| Atorvastatin Group (n= 14) |
Placebo Group (n=12) |
Atorvastatin Group (n = 9) |
Placebo Group (n = 9) |
|
| Age, years | 47 ± 14 | 49 ± 17 | 47 ± 15 | 46 ± 19 |
| Male, % | 42* | 86 | 44* | 89 |
| Systolic Blood Pressure, mmHg | 100 ± 13 | 101 ± 13 | 96 ± 11 | 98 ± 9 |
| Diastolic Blood Pressure, mmHg | 65 ± 9 | 61 ± 8 | 66 ± 11 | 60 ± 10 |
| Heart Rate, bpm | 74 ± 11 | 71 ± 8 | 73 ± 8 | 71 ± 8 |
| Body Mass Index, kg/m2 | 31 ± 7 | 31 ± 6 | 29 ± 4 | 33 ± 6 |
| NYHA I/II/III, % | 7/71/21 | 0/92/8 | 11/67/22 | 0/89/11 |
| LVEF, % | 24 ± 6 | 28 ± 7 | 24 ± 6 | 29 ± 6 |
| Left ventricular end diastolic dimension, mm | 65 ± 12 | 66 ± 11 | 65 ± 13 | 66 ± 8 |
| Peak oxygen uptake, ml/kg/min | 15.6 ± 4.1 | 16.6 ± 5.2 | 16.0 ± 3.6 | 15.9 ± 5.8 |
| Laboratory Values | ||||
| Total cholesterol, mg/dL | 185 ± 34 | 187 ± 39 | 182 ± 37 | 188 ± 44 |
| LDL cholesterol, mg/dL | 106 ± 24 | 110 ± 29 | 101 ± 23 | 108 ± 28 |
| HDL cholesterol, mg/dL | 49 ± 13 | 41 ± 10 | 49 ± 14 | 42 ± 12 |
| Triglycerides, mg/dL† | 151 (66 – 235) | 177 (87 – 226) | 187 (66–245) | 193 (89–267) |
| High sensitivity C-reactive protein, mg/dL† | 1.6 (0.6 – 4.4) | 1.9 (1.0 – 3.0) | 1.7 (0.8 – 3.7) | 2.1 (0.8 – 4.2) |
| B-type natriuretic peptide, pg/mL† | 176 (36 – 472) | 55 (29 – 154) | 185 (53 – 482) | 66 (32–115) |
| Creatinine, mg/dL | 0.9 ± 0.2* | 1.2 ± 0.4 | 1.0 ± 0.2 | 1.1 ± 0.3 |
| Alanine transferase (ALT), U/L | 22 ± 10 | 26 ± 12 | 24 ± 10 | 29 ± 13 |
| Aspartate transferase (AST), U/L | 22 ± 7 | 24 ± 7 | 24 ± 7 | 24 ± 9 |
| Medications | ||||
| Beta-blocker, % | 100 | 100 | 100 | 100 |
| Angiotensin Converting Enzyme Inhibitor or Angiotensin Receptor Blocker, % | 100 | 100 | 100 | 100 |
| Aldosterone Antagonist, % | 83 | 71 | 88 | 88 |
| Loop diuretic, % | 66 | 79 | 67 | 88 |
| Digoxin, % | 17 | 43 | 11 | 44 |
p<0.05 atorvastatin vs. placebo groups
Median and interquartile range reported
Effect of Atorvastatin on Lipid Parameters
At the end of the 12-week study period, there was a significant difference between atorvastatin and placebo groups in terms of changes in total cholesterol and LDL cholesterol levels. There was a 36.8% reduction in LDL cholesterol in the atorvastatin group and no significant reduction in the placebo group. There were no significant changes in HDL cholesterol or triglycerides in either of the treatment groups (Table 2).
Table 2.
Changes in Serum Cholesterol Levels with Treatment
| Baseline | Final† | % Change | ||||||
|---|---|---|---|---|---|---|---|---|
| Statin | Placebo | P value* | Statin | Placebo | P value | Statin | Placebo | |
| Total cholesterol, mg/dL | 185 ± 34 | 187 ± 39 | 0.881 | 137 ± 7 | 183 ± 43 | 0.0001 | −25.9% | −2.2% |
| LDL cholesterol, mg/dL | 106 ± 24 | 110 ± 29 | 0.710 | 67 ± 17 | 110 ± 33 | 0.001 | −36.8% | −0.1% |
| HDL cholesterol, mg/dL | 49 ± 13 | 41 ± 10 | 0.130 | 46 ± 14 | 40 ± 10 | 0.638 | −6.1% | −2.4% |
| Triglycerides, mg/dL‡ | 151 (66 – 235) | 177 (87 – 226) | 0.775 | 96 (86 – 178) | 143 (90 – 224) | 0.265 | −36.4% | −19.2% |
Baseline p values were determined by an unpaired t test.
Changes between the two groups from baseline were determined by two-way analysis of variance and non-parametric test for triglycerides.
Median (interquartile range) reported.
Statin therapy and MSNA
Baseline MSNA in the total cohort was 41 ± 2 (standard error of the mean [SEM]) bursts / minute and 60 ± 4 bursts / 100 heart beats. Systolic blood pressure at time of MSNA recording was higher in the placebo group compared to atorvastatin group at baseline; however, there was no significant difference between the groups in terms of change in systolic blood pressure by the end of the study. Baseline and changes in diastolic blood pressure and heart rate at time of MSNA recording were similar between the groups (Table 3).
Table 3.
Baseline and Follow-up Muscle Sympathetic Nerve Activity (MSNA) and Hemodynamics in Treatment and Placebo Groups
| Baseline (n=18) | Final (n=18) | % Change | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Statin (n=9) |
Placebo (n=9) |
P value* | Statin (n=9) |
Placebo (n=9) |
P value† | Statin | Placebo | ||
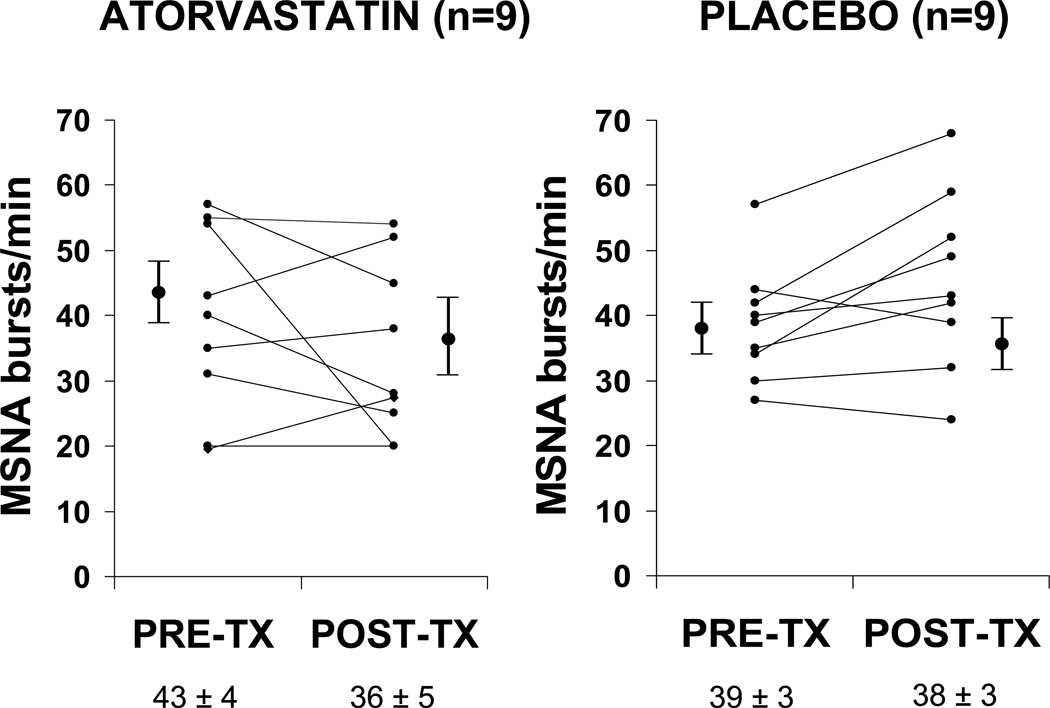
| MSNA, bursts/minute | 43 ± 3 | 39 ± 3 | 0.875 | 36 ± 5‡ | 38 ± 3 | 0.269 | −16.2% | −2.5% | |
| MSNA, bursts/100 heart beats | 62 ± 6 | 57 ± 5 | 0.949 | 52 ± 6 | 55 ± 5 | 0.308 | −16.1% | −3.5% | |
| Systolic Blood Pressure, mm Hg | 107 ± 4 | 118 ± 3 | 0.051 | 102 ± 6 | 116 ± 4 | 0.619 | −4.6% | −1.6% | |
| Diastolic Blood Pressure, mm Hg | 63 ± 5 | 62 ± 3 | 0.783 | 62 ± 5 | 66 ± 4 | 0.322 | −1.5% | +6.5% | |
| Heart Rate, bpm | 70 ± 3 | 69 ± 3 | 0.721 | 69 ± 3 | 70 ± 4 | 0.459 | −1.4% | +1.4% | |
Values reported as mean ± S.E.M.
Baseline p values were determined by an unpaired t test.
Changes between the two groups from baseline were determined by two-way analysis of variance.
P values as determined by paired t-test were also not statistically significant (p=0.117 and p=0.443 for atorvastatin and placebo groups, respectively)
Eighteen subjects had baseline and final sympathetic microneurographic tracings technically adequate for analysis. Reasons for inadequate microneurographic tracing were 1) inability of investigators to locate sympathetic nerve on baseline or final study, or 2) inability of patient to tolerate discomfort of the procedure. In the 18 subjects with baseline and final MSNA, There was no significant effect of atorvastatin (n=9) compared to placebo (n=9) on the primary endpoint of change in MSNA in this cohort of non-ischemic systolic HF patients, as shown in Table 3 and Figure 1. Muscle sympathetic nerve activity showed a non-significant 16.2% decrease in the atorvastatin group and 2.5% decrease in the placebo group. There was also no difference in the percentage of patients with a 20% or more decrease in MSNA when treated with atorvastatin compared to placebo.
Figure 1.
Graph of MSNA (bursts / minute) at baseline and after three months of therapy in the active treatment (atorvastatin) and placebo groups. Pre-tx = pre-treatment; post-tx = post treatment.
Cardiac Biomarkers
There was no baseline difference in the cardiac biomarkers BNP, hs-CRP, or cTnI between the atorvastatin and placebo treatment groups. Furthermore, there was no significant change in any biomarkers with statin treatment compared to placebo by the end of the study period (Table 4).
Table 4.
Changes in Cardiac Biomarker Levels with Treatment
| Baseline | Final | % Change | ||||||
|---|---|---|---|---|---|---|---|---|
| Statin | Placebo | P value* |
Statin | Placebo | P value† | Statin | Placebo | |
| B-type natriuretic peptide, pg/mL | 175 (36 – 472) | 66 (29 – 154) | 0.227 | 107 (34 – 173) | 67 (21 – 299) | 0.605 | −38.9% | +1.5% |
| High sensitivity C-reactive protein, mg/L | 1.6 (0.6 – 4.4) | 1.9 (1.0 – 3.0) | 0.700 | 1.9 (0.5 – 5.7) | 3.4 (1.1 – 5.9) | 0.307 | +18.8% | −78.9% |
| Troponin I ≥ 0.04 ng/mL, % | 8 | 21 | 0.356 | 0 | 15 | 0.511 | - | - |
Baseline p values were determined by Mann-Whitney U test, and chi-square for Troponin I.
Changes between the two groups from baseline were determined by Mann-Whitney U test, and Chi-Square for Troponin I.
Cardiac Structure and Function, Clinical Assessments and Quality of Life
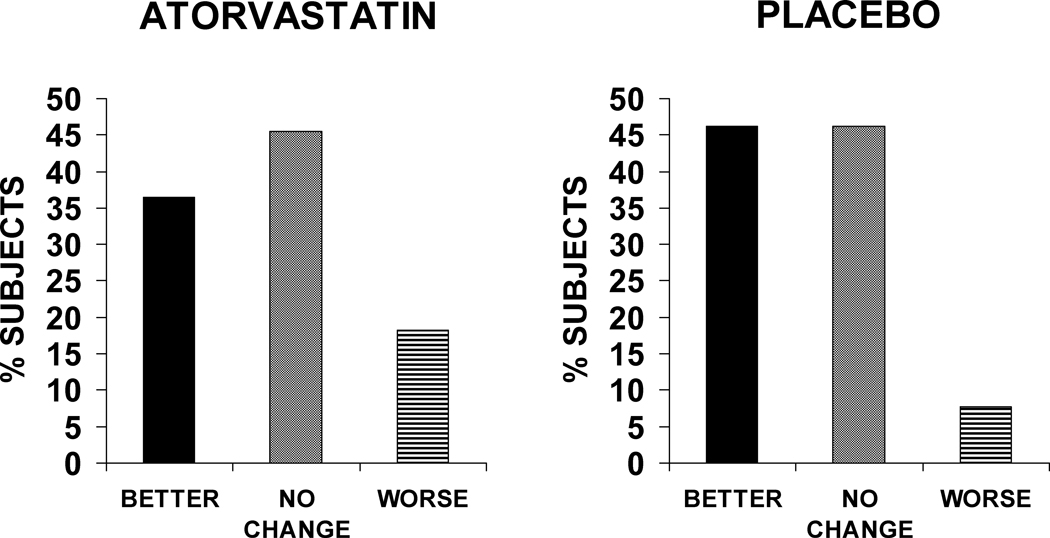
Statin therapy had no significant effect on changes in cardiac size, systolic, or diastolic function, although there was a trend in terms of change in LVEF in the placebo group (decreased at follow-up) compared to the atorvastatin group (stable at follow-up) (Table 5). At the end of the study, most patients (46%) reported no significant change in their overall health status, while 42% felt better and 12% felt worse; this global assessment at the end of the study was similar between the two groups (Figure 2). There was no significant difference between the atorvastatin and placebo groups in terms of baseline MLHFQ score (41±21 vs. 38±28) or change in MLHFQ score at follow-up (−3±12 vs. −4±20).
Table 5.
Echocardiographic Variables in Atorvastatin and Placebo Groups
| Baseline | Final | % Change | ||||||
|---|---|---|---|---|---|---|---|---|
| Statin | Placebo | P value* | Statin | Placebo | P value† | Statin | Placebo | |
| Left ventricular ejection fraction, % | 24 ± 6 | 28 ± 7 | 0.173 | 25 ± 6 | 24 ± 7‡ | 0.102 | +4.2% | −14.3% |
| Left ventricular end diastolic dimension, mm | 65 ± 12 | 67 ± 11 | 0.811 | 65 ± 11 | 64 ± 11 | 0.217 | 0% | −4.5% |
| E (cm/sec) | 75 ± 7 | 83 ± 10 | 0.524 | 84 ± 9 | 75 ± 11 | 0.102 | +12.0% | −9.6% |
| E/A ratio | 1.2 ± 0.2 | 1.5 ± 0.3 | 0.530 | 1.6 ± 0.3 | 1.2 ± 0.2 | 0.199 | +33.3% | −20% |
| E/E’ ratio | 8 ± 2 | 6 ± 1 | 0.680 | 9 ± 2 | 7 ± 2 | 0.994 | +12.8% | +16.7% |
Baseline p values were determined by an unpaired t test.
Changes between the two groups from baseline were determined by two-way analysis of variance.
P value for change in LVEF at 3 months as determined by paired t-test was significant for the placebo group (p=0.025) but not the atorvastatin group. E, E/A ratio, and E/E’ ratio are presented as mean ± standard error of the mean (SEM).
Figure 2.
Subject global assessment for atorvastatin and placebo at end of study.
Discussion
Statins have been reported and shown experimentally to have a number of mechanisms of action which may be particularly beneficial for HF, including anti-inflammatory actions, prevention of remodeling, and SNS inhibition. The current randomized, controlled, double-blinded study of humans with non-ischemic HF patients optimized on standard HF therapies failed to show a significant effect of atorvastatin 10 mg orally a day on the primary endpoint of MSNA, an index of SNS activation in HF, in addition to the secondary endpoints of LVEF, cardiac biomarkers, or QOL. This is the first study to evaluate the effect of statins on MSNA in humans with non-ischemic HF measured by sympathetic microneurography.
The central role of SNS activation in HF pathophysiology and progression has been recognized for over a quarter century, when plasma norepinephrine levels were found to be strong predictors of mortality risk in HF.22 More recently, changes in the autonomic nervous system in HF have been characterized by a complex interplay of cardiac, central, and systemic factors; this “new model” of the SNS activation in HF describes mechanisms including impaired vagal regulation of heart rate, an adjusted set-point for central sympathetic outflow, blunted ventricular baroreceptor reflex control of MSNA, and early increase in cardiac norepinephrine spillover. 23 Although SNS activity is difficult to evaluate in the clinical setting, two techniques thought to best quantify SNS activity in humans are radiotracer measurements of regional norepinephrine spillover and sympathetic microneurography, which uses a microelectrode to directly measures post-ganglionic sympathetic nerve activity.24
MSNA, at rest and during stress, correlates well with cardiac sympathetic nerve activity in humans, as measured by cardiac norepinephrine spillover.25 Furthermore, MSNA quantified by sympathetic microneurography at the peroneal nerve has been validated as a tool to study sympathetic nervous system activation in humans with HF,26, 27 as well as in a variety of other disease processes, including hypertension and obesity.28 MSNA at rest has consistently been found to be elevated in HF patients when compared to normal controls, and furthermore, life-prolonging therapies for HF patients, including ACEIs, ARBs, and beta-blockers have also been shown to decrease MSNA over time in humans with HF.21, 29, 30
Improvements in autonomic nervous system function with statin treatment have been demonstrated in animal models of HF. Pliquett et al. studied autonomic function in rabbits with pacing-induced HF.14 HF rabbits fed simvastatin for three weeks had higher heart rate variability (HRV) than HF rabbits not treated with simvastatin, indicating an improvement in autonomic function; the HF rabbits fed the highest dose (3mg/kg/day) had HRV similar to non-HF controls. Pliquett et al. also investigated the effects of statin therapy on baroreceptor sensitivity, renal sympathetic nerve activity (RSNA), and plasma norepinephrine levels in rabbits with pacing-induced HF.13 Norepinephrine levels and RSNA were significantly lower in HF rabbits who received moderate to high dose simvastatin compared to non-statin treated HF animals. Furthermore, baroreflex responses in terms of heart rate and RSNA were depressed in HF rabbits treated with vehicle but restored to near-normal in HF rabbits treated with simvastatin. Cholesterol levels were unchanged by simvastatin in both studies, suggesting a cholesterol-independent, or pleiotrophic, effect of statins on autonomic function. This group subsequently linked autonomic improvement to an effect of simvastatin on inhibition of central angiotensin II and the superoxide pathway. In this study of pacing-induced HF in rabbits, the heightened blood pressure and RSNA responses to intracerebral angiotensin II injection seen in HF animals was abolished by simvastatin therapy.15
This human study, which fails to demonstrate an appreciable effect of atorvastatin for three months on MSNA in subjects with non-ischemic HF, is consistent with neutral findings from larger clinical trials. In the GISSI-HF trial, in which > 50% of patients enrolled had HF of a non-ischemic etiology, rosuvastatin 10 mg orally per day compared to placebo had no significant effect on the co-primary outcomes of death and death / cardiovascular hospitalization in 4631 randomized patients and followed for a median of 3.9 years.11 Krum et al, in a study of 85 exclusively non-ischemic, systolic HF patients also found rosuvastatin compared to placebo therapy for 6 months had no appreciable effect on LVEF, neurohormonal parameters, or other clinical outcomes.20
One recently published study assessed MSNA in 7 patients with prior myocardial infarction and HF at three timepoints: during statin therapy, 8 weeks after discontinuation of statin, and 4 weeks after restarting statin (differing doses of simvastatin and atorvastatin). MSNA was higher (42 ± 3 bursts/min) 8 weeks after discontinuation of statin compared to during statin therapy (32 ± 3 and 28 ± 3 bursts/min, respectively).31 This study differs from ours significantly in that there is no blinding or placebo control, and that all patients have coronary artery disease and prior myocardial infarction. In the CORONA trial, in which 5011 systolic HF patients with exclusively ischemic heart disease were enrolled, rosuvastatin 10 mg a day compared to placebo had no significant effect on the primary outcome of death from cardiovascular causes, nonfatal myocardial infarction, and nonfatal stroke or the secondary outcome of all cause mortality. However, there was a modest but significantly lower rate of hospitalizations for cardiovascular causes in the statin (n = 2193) vs. placebo (n = 2564) groups.10
Although in the general population, higher cholesterol levels are clearly associated with increased risk of cardiovascular events, a reversal of traditional epidemiology has been demonstrated in advanced HF populations; lower cholesterol levels are associated with worsened outcomes in both ischemic and non-ischemic HF.32–34 Likewise, despite the clear-cut evidence for cholesterol-lowering with statins in patients with coronary artery disease, cholesterol-lowering has not been shown to improve survival in symptomatic HF patients with coronary artery disease.10, 35 Low cholesterol levels in HF may be associated with poor prognosis in HF because they reflect malnutrition and/or inflammation. It has also been hypothesized that lipoproteins in HF play a cardioprotective role neutralizing excess circulating endotoxin.36 Thus, lowering cholesterol levels with statins or other agents in HF may activate inflammatory, neurohormonal, and sympathetic pathways, thus diminishing other beneficial mechanisms of action of statins. Additional investigations are needed to help explain why traditional epidemiology and traditional therapeutic targets in the general population cannot be applied to patients with advanced HF.
Limitations
The presence of obstructive or central sleep apnea, which can profoundly affect MSNA, was not assessed as part of this study.23 We do not have data on cardiac or systemic norepinephrine levels, other indicators of sympathetic status in patients with HF. This study was powered to detect a 13 burst/minute or greater difference in MSNA but based on the sample size cannot exclude statins may have a smaller magnitude of effect on MSNA. In particular, those patients with low MSNA at baseline (Figure 1), may not have been plausibly expected to have a further decrease MSNA. This cohort was comprised primarily of NYHA II patients with relatively low BNP levels and results cannot be extrapolated to sicker HF populations; however, a post-hoc analysis of the CORONA trial suggested that statin therapy might be beneficial in HF patients who are “less sick”, with the lowest levels of natriuretric peptides 37. Our population was predominantly male; however, one recent study suggests that MSNA and changes in MSNA in response to an intervention (exercise) are similar in men and women 38. We only tested one dose of atorvastatin and did not compare this dose to a higher dose; a higher dose may have had a significant effect. A different statin, such as simvastatin as was used in animal models, may have had a different effect on MSNA. It is also possible that statins or atorvastatin do have sympathoinhibitory effects in HF, but that these effects are not detectable on top of optimal medical therapy with beta-blockers, ACEIs, and aldosterone antagonists; however, the objective of our study was to investigate whether statins could exert potentially beneficial mechanisms of action in HF patients already on standard, life-prolonging HF therapies.
Conclusions
Short-term atorvastatin treatment in humans with systolic, non-ischemic HF on optimal medical therapy does not significantly lower sympathetic activation, as quantified by MSNA (bursts/min). To date, therapies which definitively improve survival in humans with HF – including ACEIs, ARBs, beta-blockers, aldosterone antagonists, as well as cardiac resynchronization therapy, have also been shown to diminish SNS excitation in translational studies.21, 29, 30, 39, 40 Thus, our findings are consistent with the results of large randomized controlled trials which showed no mortality benefit in a wide range of HF patients of ischemic and non-ischemic etiology treated with statins.
Acknowledgments
Funding Sources:
Dr. Horwich was supported by NHLBI / NIH 1K23HL085097 and Heart Failure Society of America Research Fellowship. Dr. Middlekauff was supported by NIH-RO1 HL084525. This work was also supported by UCLA GCRC NIH-MO1-RR00865.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, et al. Heart Disease and Stroke Statistics--2011 Update: A Report From the American Heart Association. Circulation. 123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jessup M, Brozena S. Heart failure. N Engl J Med. 2003;348:2007–2018. doi: 10.1056/NEJMra021498. [DOI] [PubMed] [Google Scholar]
- 3.Horwich TB, MacLellan WR, Fonarow GC. Statin therapy is associated with improved survival in ischemic and non-ischemic heart failure. J Am Coll Cardiol. 2004;43:642–648. doi: 10.1016/j.jacc.2003.07.049. [DOI] [PubMed] [Google Scholar]
- 4.Go AS, Lee WY, Yang J, Lo JC, Gurwitz JH. Statin Therapy and Risks for Death and Hospitalization in Chronic Heart Failure. JAMA. 2006;296:2105–2111. doi: 10.1001/jama.296.17.2105. [DOI] [PubMed] [Google Scholar]
- 5.Foody JM, Shah R, Galusha D, Masoudi FA, Havranek EP, Krumholz HM. Statins and mortality among elderly patients hospitalized with heart failure. Circulation. 2006;113:1086–1092. doi: 10.1161/CIRCULATIONAHA.105.591446. [DOI] [PubMed] [Google Scholar]
- 6.Patel R, Nagueh SF, Tsybouleva N, Abdellatif M, Lutucuta S, Kopelen HA, et al. Simvastatin induces regression of cardiac hypertrophy and fibrosis and improves cardiac function in a transgenic rabbit model of human hypertrophic cardiomyopathy. Circulation. 2001;104:317–324. doi: 10.1161/hc2801.094031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Indolfi C, Di Lorenzo E, Perrino C, Stingone AM, Curcio A, Torella D, et al. Hydroxymethylglutaryl coenzyme A reductase inhibitor simvastatin prevents cardiac hypertrophy induced by pressure overload and inhibits p21ras activation. Circulation. 2002;106:2118–2124. doi: 10.1161/01.cir.0000034047.70205.97. [DOI] [PubMed] [Google Scholar]
- 8.Node K, Fujita M, Kitakaze M, Hori M, Liao JK. Short-term statin therapy improves cardiac function and symptoms in patients with idiopathic dilated cardiomyopathy. Circulation. 2003;108:839–843. doi: 10.1161/01.CIR.0000084539.58092.DE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sola S, Mir MQS, Lerakis S, Tandon N, Khan BV. Atorvastatin Improves Left Ventricular Systolic Function and Serum Markers of Inflammation in Nonischemic Heart Failure. Journal of the American College of Cardiology. 2006;47:332. doi: 10.1016/j.jacc.2005.06.088. [DOI] [PubMed] [Google Scholar]
- 10.Kjekshus J, Apetrei E, Barrios V, Bohm M, Cleland JG, Cornel JH, et al. Rosuvastatin in older patients with systolic heart failure. N Engl J Med. 2007;357:2248–2261. doi: 10.1056/NEJMoa0706201. [DOI] [PubMed] [Google Scholar]
- 11.Tavazzi L, Maggioni AP, Marchioli R, Barlera S, Franzosi MG, Latini R, et al. Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1231–1239. doi: 10.1016/S0140-6736(08)61240-4. [DOI] [PubMed] [Google Scholar]
- 12.Khush KK, Waters DD. Effects of Statin Therapy on the Development and Progression of Heart Failure: Mechanisms and Clinical Trials. Journal of Cardiac Failure. 2006;12:664–674. doi: 10.1016/j.cardfail.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Pliquett RU, Cornish KG, Peuler JD, Zucker IH. Simvastatin normalizes autonomic neural control in experimental heart failure. Circulation. 2003;107:2493–2498. doi: 10.1161/01.CIR.0000065606.63163.B9. [DOI] [PubMed] [Google Scholar]
- 14.Pliquett RU, Cornish KG, Zucker IH. Statin therapy restores sympathovagal balance in experimental heart failure. J Appl Physiol. 2003;95:700–704. doi: 10.1152/japplphysiol.00265.2003. [DOI] [PubMed] [Google Scholar]
- 15.Gao L, Wang W, Li YL, Schultz HD, Liu D, Cornish KG, et al. Simvastatin Therapy Normalizes Sympathetic Neural Control in Experimental Heart Failure. Roles of Angiotensin II Type 1 Receptors and NAD(P)H Oxidase. Circulation. 2005 doi: 10.1161/CIRCULATIONAHA.105.552174. [DOI] [PubMed] [Google Scholar]
- 16.Vrtovec B, Okrajsek R, Golicnik A, Ferjan M, Starc V, Radovancevic B. Atorvastatin Therapy Increases Heart Rate Variability, Decreases QT Variability, and Shortens QTc Interval Duration in Patients With Advanced Chronic Heart Failure. Journal of Cardiac Failure. 2005;11:684. doi: 10.1016/j.cardfail.2005.06.439. [DOI] [PubMed] [Google Scholar]
- 17.Middlekauff HR, Hui K, Yu JL, Hamilton MA, Fonarow GC, Moriguchi J, et al. Acupuncture inhibits sympathetic activation during mental stress in advanced heart failure patients. J Card Fail. 2002;8:399–406. doi: 10.1054/jcaf.2002.129656. [DOI] [PubMed] [Google Scholar]
- 18.Nahar T, Croft L, Shapiro R, Fruchtman S, Diamond J, Henzlova M, et al. Comparison of four echocardiographic techniques for measuring left ventricular ejection fraction. The American Journal of Cardiology. 2000;86:1358–1362. doi: 10.1016/s0002-9149(00)01243-1. [DOI] [PubMed] [Google Scholar]
- 19.Rector TS, Kubo SH, Cohn JN. Validity of the minnesota living with heart failure questionnaire as a measure of therapeutic response to enalapril or placebo. The American Journal of Cardiology. 1993;71:1106–1107. doi: 10.1016/0002-9149(93)90582-w. [DOI] [PubMed] [Google Scholar]
- 20.Krum H, Ashton E, Reid C, Kalff V, Rogers J, Amarena J, et al. Double-Blind, Randomized, Placebo-Controlled Study of High-Dose HMG CoA Reductase Inhibitor Therapy on Ventricular Remodeling, Pro-Inflammatory Cytokines and Neurohormonal Parameters in Patients With Chronic Systolic Heart Failure. Journal of Cardiac Failure. 2007;13:1–7. doi: 10.1016/j.cardfail.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 21.De Matos LD, Gardenghi G, Rondon MU, Soufen HN, Tirone AP, Barretto AC, et al. Impact of 6 months of therapy with carvedilol on muscle sympathetic nerve activity in heart failure patients. J Card Fail. 2004;10:496–502. doi: 10.1016/j.cardfail.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 22.Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, Francis GS, et al. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med. 1984;311:819–823. doi: 10.1056/NEJM198409273111303. [DOI] [PubMed] [Google Scholar]
- 23.Floras JS. Sympathetic nervous system activation in human heart failure: clinical implications of an updated model. J Am Coll Cardiol. 2009;54:375–385. doi: 10.1016/j.jacc.2009.03.061. [DOI] [PubMed] [Google Scholar]
- 24.Triposkiadis F, Karayannis G, Gqiamouzis G, Skoularigis J, Louridas G, Butler J. The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. J Am Coll Cardiol. 2009;54:1747–1762. doi: 10.1016/j.jacc.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 25.Wallin BG, Esler M, Dorward P, Eisenhofer G, Ferrier C, Westerman R, et al. Simultaneous measurements of cardiac noradrenaline spillover and sympathetic outflow to skeletal muscle in humans. J Physiol. 1992;453:45–58. doi: 10.1113/jphysiol.1992.sp019217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leimbach WN, Jr, Wallin BG, Victor RG, Aylward PE, Sundlof G, Mark AL. Direct evidence from intraneural recordings for increased central sympathetic outflow in patients with heart failure. Circulation. 1986;73:913–919. doi: 10.1161/01.cir.73.5.913. [DOI] [PubMed] [Google Scholar]
- 27.Middlekauff HR, Hamilton MA, Stevenson LW, Mark AL. Independent control of skin and muscle sympathetic nerve activity in patients with heart failure. Circulation. 1994;90:1794–1798. doi: 10.1161/01.cir.90.4.1794. [DOI] [PubMed] [Google Scholar]
- 28.Grassi G, Seravalle G, Quarti-Trevano F, Dell'Oro R, Bolla G, Mancia G. Effects of hypertension and obesity on the sympathetic activation of heart failure patients. Hypertension. 2003;42:873–877. doi: 10.1161/01.HYP.0000098660.26184.63. [DOI] [PubMed] [Google Scholar]
- 29.Grassi G, Cattaneo BM, Seravalle G, Lanfranchi A, Pozzi M, Morganti A, et al. Effects of chronic ACE inhibition on sympathetic nerve traffic and baroreflex control of circulation in heart failure. Circulation. 1997;96:1173–1179. doi: 10.1161/01.cir.96.4.1173. [DOI] [PubMed] [Google Scholar]
- 30.Hikosaka M, Yuasa F, Yuyama R, Mimura J, Kawamura A, Motohiro M, et al. Candesartan and arterial baroreflex sensitivity and sympathetic nerve activity in patients with mild heart failure. J Cardiovasc Pharmacol. 2002;40:875–880. doi: 10.1097/00005344-200212000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Gomes M, Lenders J, Bellersen L, Verheugt F, Smits P, Tack C. Sympathoinhibitory effect of statins in chronic heart failure. Clinical Autonomic Research. 20:73–78. doi: 10.1007/s10286-009-0041-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pekkanen J, Linn S, Heiss G, Suchindran CM, Leon A, Rifkind BM, et al. Ten-year mortality from cardiovascular disease in relation to cholesterol level among men with and without preexisting cardiovascular disease. N Engl J Med. 1990;322:1700–1707. doi: 10.1056/NEJM199006143222403. [DOI] [PubMed] [Google Scholar]
- 33.Horwich TB, Hamilton MA, Maclellan WR, Fonarow GC. Low serum total cholesterol is associated with marked increase in mortality in advanced heart failure. J Card Fail. 2002;8:216–224. doi: 10.1054/jcaf.2002.0804216. [DOI] [PubMed] [Google Scholar]
- 34.Afsarmanesh N, Horwich TB, Fonarow GC. Total cholesterol levels and mortality risk in nonischemic systolic heart failure. Am Heart J. 2006;152:1077–1083. doi: 10.1016/j.ahj.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 35.Smith SC, Jr, Allen J, Blair SN, Bonow RO, Brass LM, Fonarow GC, et al. AHA/ACC Guidelines for Secondary Prevention for Patients With Coronary and Other Atherosclerotic Vascular Disease: 2006 Update: Endorsed by the National Heart, Lung, and Blood Institute. Circulation. 2006;113:2363–2372. doi: 10.1161/CIRCULATIONAHA.106.174516. [DOI] [PubMed] [Google Scholar]
- 36.Anker SD, Clark AL, Winkler R, Zugck C, Cicoira M, Ponikowski P, et al. Statin use and survival in patients with chronic heart failure -- results from two observational studies with 5200 patients. International Journal of Cardiology. 2006;112:234. doi: 10.1016/j.ijcard.2006.03.057. [DOI] [PubMed] [Google Scholar]
- 37.Cleland JGF, McMurray JJV, Kjekshus J, Cornel JH, Dunselman P, Fonseca C, et al. Plasma Concentration of Amino-Terminal Pro-Brain Natriuretic Peptide in Chronic Heart Failure: Prediction of Cardiovascular Events and Interaction With the Effects of Rosuvastatin: A Report From CORONA (Controlled Rosuvastatin Multinational Trial in Heart Failure) Journal of the American College of Cardiology. 2009;54:1850–1859. doi: 10.1016/j.jacc.2009.06.041. [DOI] [PubMed] [Google Scholar]
- 38.Antunes-Correa LM, Melo RC, Nobre TS, Ueno LM, Franco FGM, Braga AMW, et al. Impact of gender on benefits of exercise training on sympathetic nerve activity and muscle blood flow in heart failure. European Journal of Heart Failure. 12:58–65. doi: 10.1093/eurjhf/hfp168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kasama S, Toyama T, Kumakura H, Takayama Y, Ichikawa S, Suzuki T, et al. Effect of spironolactone on cardiac sympathetic nerve activity and left ventricular remodeling in patients with dilated cardiomyopathy. J Am Coll Cardiol. 2003;41:574–581. doi: 10.1016/s0735-1097(02)02855-3. [DOI] [PubMed] [Google Scholar]
- 40.Adamson PB, Kleckner KJ, VanHout WL, Srinivasan S, Abraham WT. Cardiac resynchronization therapy improves heart rate variability in patients with symptomatic heart failure. Circulation. 2003;108:266–269. doi: 10.1161/01.CIR.0000083368.75831.7A. [DOI] [PubMed] [Google Scholar]