Abstract
Inward rectifier (Kir) potassium channels contribute to the control of electrical activity in excitable tissues and their activity is modulated by many biochemical factors, including protons. Heteromeric Kir4.1–Kir5.1 channels are highly pH sensitive within the physiological range of pH changes and are strongly expressed by the peripheral chemosensors as well as in the brainstem pH-sensitive areas which mediate respiratory responses to changes in blood and brain levels of  /[H+]. In the present study, Kir5.1 knockout mice (Kir5.1−/−) were used to determine the role of these channels in the chemosensory control of breathing. We found that Kir5.1−/− mice presented with persistent metabolic acidosis and a clear respiratory phenotype. Despite metabolic acidosis, ventilation at rest and in hyperoxic hypercapnia were similar in wild-type and Kir5.1−/− mice. Ventilatory responses to hypoxia and normoxic hypercapnia were significantly reduced in Kir5.1−/− mice; however, carotid body chemoafferent responses to hypoxia and CO2 were not affected. In the in situ brainstem–spinal cord preparations with denervated peripheral chemoreceptors, resting phrenic nerve activity and phrenic nerve responses to respiratory acidosis or isohydric hypercapnia were also similar in Kir5.1−/− and wild-type mice. In in situ preparations of Kir5.1−/− mice with intact peripheral chemoreceptors, application of CN− resulted in a significantly reduced phrenic nerve response, suggesting that the relay of peripheral chemosensory information to the CNS is compromised. We suggest that this compensatory modulation of the peripheral chemosensory inputs develops in Kir5.1−/− mice in order to counteract the effect of continuing metabolic acidosis on the activity of the peripheral chemoreceptors. These results therefore suggest that despite their intrinsic pH sensitivity, Kir4.1–Kir5.1 channels are dispensable for functional central and peripheral respiratory chemosensitivity.
/[H+]. In the present study, Kir5.1 knockout mice (Kir5.1−/−) were used to determine the role of these channels in the chemosensory control of breathing. We found that Kir5.1−/− mice presented with persistent metabolic acidosis and a clear respiratory phenotype. Despite metabolic acidosis, ventilation at rest and in hyperoxic hypercapnia were similar in wild-type and Kir5.1−/− mice. Ventilatory responses to hypoxia and normoxic hypercapnia were significantly reduced in Kir5.1−/− mice; however, carotid body chemoafferent responses to hypoxia and CO2 were not affected. In the in situ brainstem–spinal cord preparations with denervated peripheral chemoreceptors, resting phrenic nerve activity and phrenic nerve responses to respiratory acidosis or isohydric hypercapnia were also similar in Kir5.1−/− and wild-type mice. In in situ preparations of Kir5.1−/− mice with intact peripheral chemoreceptors, application of CN− resulted in a significantly reduced phrenic nerve response, suggesting that the relay of peripheral chemosensory information to the CNS is compromised. We suggest that this compensatory modulation of the peripheral chemosensory inputs develops in Kir5.1−/− mice in order to counteract the effect of continuing metabolic acidosis on the activity of the peripheral chemoreceptors. These results therefore suggest that despite their intrinsic pH sensitivity, Kir4.1–Kir5.1 channels are dispensable for functional central and peripheral respiratory chemosensitivity.
Arterial CO2 drives respiratory activity in mammals by its actions on the peripheral respiratory chemoreceptors within the carotid and aortic bodies and central chemoreceptors located within the medulla oblongata and pons. The latter are responsible for up to 80% of the overall ventilatory response to CO2 (Heeringa et al. 1979), which is mediated by several populations of putative central respiratory chemoreceptor neurones identified within anatomically distinct brainstem regions, including locus coeruleus (LC), nucleus of the solitary tract (NTS), medullary raphe, retrotrapezoid nucleus (RTN) and others (Nattie & Li, 2009).
There is evidence that depolarization of central pH-sensitive neurones, including RTN neurones, is associated with a decreased K+ conductance (Mulkey et al. 2004). Two-pore domain K+ channels (TASKs) are highly sensitive to changes in extracellular pH within the physiological range (7.4–7.2) and have long been considered as the favoured molecular candidates to underlie chemosensory responses of the brainstem neurones to changes in pH. However, in mice, TASK-2 deficiency results in a complex respiratory phenotype with hypersensitivity to low levels of CO2 (Gestreau et al. 2010), while TASK-1 and TASK-3 were found to be dispensable for central respiratory chemosensitivity and, therefore, eliminated as the possible transducing molecules (Mulkey et al. 2007;Trapp et al. 2008).
A potential alternative mechanism could involve pH-sensitive K+ channels of the inward rectifier (Kir) family (Hibino et al. 2010). These channels contribute to the control of cellular activity in excitable tissues and their activity is influenced by many factors, including [H+]. Although Kir1.1, Kir2.3 and Kir4.1 are pH sensitive, their sensitivity lays outside the physiological range of pH changes in the brain (pKa 6.8, 6.77 and 6.1, respectively) (Fakler et al. 1996; Zhu et al. 1999; Xu et al. 2000). Expression of Kir5.1 alone is unable to produce a functional channel (Bond et al. 1994; Pessia et al. 1996). However, coexpression of Kir5.1 with Kir4.1 produces channels that are inhibited by intracellular acidification with the pKa of 7.45 (Xu et al. 2000). In addition to Kir4.1, Kir5.1 can also coassemble with Kir4.2 (Pearson et al. 1999; Pessia et al. 2001), although little is known about the expression of Kir4.2 in the brain.
Both Kir4.1 and Kir5.1 genes are expressed within the chemoreceptor areas of the medulla oblongata and pons, including LC, NTS and regions near the ventral surface of the medulla (Wu et al. 2004). As heteromeric Kir4.1–Kir5.1 channels are highly pH sensitive within the physiological range of pH changes in the brain and strongly expressed in the brainstem, we examined the role of these channels in mediating respiratory responses to chemosensory challenges using transgenic animals. A global knockout of Kir4.1 showed extreme pathology, with survival of homozygotes limited to 3 weeks postnatal (Kofuji et al. 2000). In contrast, mice deficient in Kir5.1 (Kir5.1−/−) are overtly healthy as homozygotes and show no premature mortality. Furthermore, the pH sensitivity of chemosensitive LC neurons was found to be markedly reduced in Kir5.1−/− animals (D'Adamo et al. 2011). We therefore used adult Kir5.1−/− mice to determine the role of Kir5.1 in the mechanisms responsible for the development of adaptive respiratory responses to high CO2 (hypercapnia) and low O2 (hypoxia).
Methods
Animals
Generation of the Kcnj16 knockout strain (Kir5.1−/−) has been described elsewhere (D'Adamo et al. 2011), and the mice used in this study were obtained from the MRC Harwell stock (http://www.mousebook.org). These mice were on a C57Bl/6 background, were maintained as heterozygotes and were isogenic. For the experiments described here adult, (3–4 months) Kir5.1−/− mice and their respective wild-type (Kir5.1+/+) littermates were used. All genotypes were confirmed post mortem. All experimental procedures were approved and carried out in accordance with the UK Animals (Scientific Procedures) Act (1986) and associated guidelines.
Whole-body plethysmography
Respiratory rate (fR, in breaths per minute) and tidal volume (VT, in microlitres per gram) in conscious freely moving mice were assessed by whole-body plethysmography as described previously (Rong et al. 2003; Trapp et al. 2008). The animal was placed in a recording chamber (∼200 ml) which was flushed continuously with a humidified mixture of 79% nitrogen and 21% oxygen (unless otherwise required by the protocol) at a rate of ∼1 L min−1 (temperature 22–24°C). Levels of O2 and CO2 in the chamber were monitored online using a fast-response O2/CO2 analyser (Morgan Medical, Hertford, UK). The animals were allowed ∼30 min to acclimatize to the chamber at normoxia–normocapnia (21% O2, 79% N2 and <0.3% CO2) before measurements of baseline ventilation were taken. Hypoxia was induced by lowering the O2 concentration in the chamber down to 10% (with the balance being N2) for 5 min. Normoxic hypercapnia was induced by titrating CO2 into the chamber gas mixture up to a level of 3 and 6% (lowering N2 accordingly) for 5 min at each CO2 level. In a separate experiment, the animals were placed in a hyperoxic environment (>70% O2, with the balance being N2), and hypercapnia was induced 30 min later by an addition of CO2 up to the levels of 3 and 6% in accord with the above protocol. The measurements of the ventilatory variables were obtained during the last 2 min before exposure to the stimulus and during the 2 min period near the termination of each stimulus, when breathing had stabilized. Before and after each of the experiments, the plethysmograph was calibrated by repeated injections and withdrawal of air (0.05, 0.1 and 0.2 ml) from within the recording chamber. Changes in fR, VT and minute ventilation ( ; fR×VT; in millilitres per minute per gram) were averaged and expressed as means ±s.e.m.
; fR×VT; in millilitres per minute per gram) were averaged and expressed as means ±s.e.m.
In situ brainstem–spinal cord preparation
In situ brainstem–spinal cord preparations (Paton, 1996) with denervated peripheral chemoreceptors were used to study the contribution of Kir channels to central respiratory chemosensitivity. In brief, Kir5.1+/+ and Kir5.1−/− mice were injected with heparin (500 units, i.p.), deeply anaesthetized with isoflurane until loss of paw withdrawal reflex, bisected under the diaphragm, placed in cold (4°C) Ringer solution saturated with 95%O2–5% CO2, and decerebrated precollicularly. Preparations were then placed in a recording chamber and retrogradely perfused via the descending aorta with carbogenated (saturated with 95% O2–5% CO2) solution containing (mm): 124 NaCl, 26 NaHCO3, 3 KCl, 2 CaCl2, 1.25 MgSO4, 1.25 KH2PO4 and 10 dextrose ( 40 mmHg, pH 7.4, 32°C). To maintain oncotic pressure, Ficoll 70 (1.25%) was added. Vecuronium bromide (4 μg ml−1) was used to block neuromuscular transmission. When required by the experimental protocol, both vagi, aortic and carotid sinus nerves were sectioned to eliminate inputs from the peripheral chemoreceptors. Phrenic nerve activity was recorded using a suction electrode. Activity was amplified, filtered (0.1–3 kHz), rectified and integrated (50 ms time constant), relayed to a computer via a 1401 interface (CED, Cambridge, UK) and recorded using Spike2 software (CED, Cambridge, UK).
40 mmHg, pH 7.4, 32°C). To maintain oncotic pressure, Ficoll 70 (1.25%) was added. Vecuronium bromide (4 μg ml−1) was used to block neuromuscular transmission. When required by the experimental protocol, both vagi, aortic and carotid sinus nerves were sectioned to eliminate inputs from the peripheral chemoreceptors. Phrenic nerve activity was recorded using a suction electrode. Activity was amplified, filtered (0.1–3 kHz), rectified and integrated (50 ms time constant), relayed to a computer via a 1401 interface (CED, Cambridge, UK) and recorded using Spike2 software (CED, Cambridge, UK).
The following two stimulation protocols were used to assess respiratory responses in Kir5.1−/− mice evoked by activation of central chemoreceptors. (i) Respiratory acidosis – the amount of CO2 bubbled through the perfusion solution was first lowered to ∼3.5% (resulting in a solution with  35 mmHg and pH 7.5) and then increased to 8% (
35 mmHg and pH 7.5) and then increased to 8% ( 85 mmHg, pH 7.1). (ii) Isohydric hypercapnia – the preparations were perfused with a solution containing 64 mm HCO3− with the addition of extra CO2 to achieve the pH level of 7.5 and
85 mmHg, pH 7.1). (ii) Isohydric hypercapnia – the preparations were perfused with a solution containing 64 mm HCO3− with the addition of extra CO2 to achieve the pH level of 7.5 and  85 mmHg. In all the solutions,
85 mmHg. In all the solutions,  and pH values were measured using a Siemens Blood Gas Analyzer (Siemens Healthcare Diagnostics Ltd, Frimley, UK). In preparations with intact peripheral chemoreceptors, CN− (0.03% w/v; 50 μl bolus) was given to activate carotid body chemoreceptors.
and pH values were measured using a Siemens Blood Gas Analyzer (Siemens Healthcare Diagnostics Ltd, Frimley, UK). In preparations with intact peripheral chemoreceptors, CN− (0.03% w/v; 50 μl bolus) was given to activate carotid body chemoreceptors.
In vitro sinus nerve recording
The function of the peripheral chemoreceptors in Kir5.1−/− mice was assessed in superfused preparations of the carotid body and carotid sinus nerve as described previously (Rong et al. 2003; Trapp et al. 2008). Briefly, the animals were terminally anaesthetized with isoflurane, decapitated at the lower cervical level, and the carotid bifurcation containing the carotid body and the attached sinus nerve was dissected under a microscope and placed into a recording chamber (1 ml). The preparation was superfused with a solution containing (mm): 124 NaCl, 3 KCl, 2 CaCl2, 26 NaHCO3, 1.25 NaH2PO4, 1 MgSO4 and 10 d-glucose, saturated with 95% O2–5% CO2 ( 40 mmHg, pH 7.4, 37°C). Perfusion rate was 6 ml min−1. The sinus nerve was recorded using a suction electrode. The signal was amplified, filtered (0.2–3 kHz), relayed to a computer and recorded using 1401 interface and Spike2 software.
40 mmHg, pH 7.4, 37°C). Perfusion rate was 6 ml min−1. The sinus nerve was recorded using a suction electrode. The signal was amplified, filtered (0.2–3 kHz), relayed to a computer and recorded using 1401 interface and Spike2 software.
The following three stimulation protocols were used to assess carotid body chemosensory function. (i) Hypoxia – 3 min of superfusion of the preparation with the above solution in which O2 had been replaced by saturating it with 95% N2–5% CO2. (ii) Respiratory acidosis – 5 min of preparation superfusion with the solution in which extra CO2 had been added to increase  from its normal value of 40 mmHg to 80 mmHg, which is accompanied by a reduction in pH from 7.4 to 7.1. (iii) Isocapnic acidosis – the preparations were perfused with a solution containing 10 mm HCO3− saturated with 95% O2–5% CO2 to achieve the pH level of 7.0 at normal
from its normal value of 40 mmHg to 80 mmHg, which is accompanied by a reduction in pH from 7.4 to 7.1. (iii) Isocapnic acidosis – the preparations were perfused with a solution containing 10 mm HCO3− saturated with 95% O2–5% CO2 to achieve the pH level of 7.0 at normal 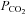 of 40 mmHg.
of 40 mmHg.
Measurements of blood gases and pH
Mice were terminally anaesthetized with a mixture of ketamine (100 mg kg−1; i.m.) and medetomidine (250 μg kg−1, i.m.). A sample of arterial blood was collected from the left ventricle of the heart, and the levels of  , [HCO3−] and pH were immediately determined using a Siemens Blood Gas Analyzer.
, [HCO3−] and pH were immediately determined using a Siemens Blood Gas Analyzer.
Data analysis
Recordings were processed using a 1401 interface and analysed using Spike2 software. All the data are reported as means ±s.e.m. Comparisons between experimental groups were made using unpaired Student's t test or ANOVA followed by the Tukey–Kramer post hoc test, as appropriate. A value of P < 0.05 was considered to be significant.
Results
Ventilatory responses to hypoxia and hypercapnia in Kir5.1−/− mice
In resting conditions (normoxia–normocapnia), ventilation was similar in Kir5.1−/− mice (1.51 ± 0.07 ml min−1 g−1, n = 6) and their wild-type counterparts (1.59 ± 0.04 ml min−1 g−1, n = 6; P = 0.37). When exposed to hypoxic conditions (10% O2 in the inspired gas mixture), both Kir5.1−/− and wild-type mice showed an increased rate (fR) and depth of breathing (VT) and, therefore, an increased minute ventilation ( ; Fig. 1A). However, the ventilatory response to hypoxia was significantly smaller in Kir5.1−/− animals (Fig. 1A). In 10% O2, Kir5.1−/− animals had a
; Fig. 1A). However, the ventilatory response to hypoxia was significantly smaller in Kir5.1−/− animals (Fig. 1A). In 10% O2, Kir5.1−/− animals had a  of 2.52 ± 0.12 ml min−1 g−1 (n = 6), whereas in the wild-type animals
of 2.52 ± 0.12 ml min−1 g−1 (n = 6), whereas in the wild-type animals  was 3.12 ± 0.15 ml min−1 g−1 (n = 6, P = 0.010; ANOVA). This difference arose from a significantly smaller VT (but not fR) increase in response to hypoxia in Kir5.1−/− animals (Fig. 1A)
was 3.12 ± 0.15 ml min−1 g−1 (n = 6, P = 0.010; ANOVA). This difference arose from a significantly smaller VT (but not fR) increase in response to hypoxia in Kir5.1−/− animals (Fig. 1A)
Figure 1.

Reduced ventilatory responses to hypoxia and normoxic hypercapnia in Kir5.1−/− miceA, ventilatory responses to hypoxia (10% O2 in the inspired air) in conscious Kir5.1-deficient mice (Kir5.1−/−) and their wild-type counterparts (Kir5.1+/+). B, ventilatory responses to varying levels of normoxic hypercapnia in Kir5.1−/− and Kir5.1+/+ mice. C, ventilatory responses to varying levels of hyperoxic (>70% O2 in the inspired air) hypercapnia in Kir5.1−/− and Kir5.1+/+ mice. Data are presented as means ±s.e.m. Numbers in parentheses indicate sample sizes. Abbreviations: fR, respiratory rate; VT, tidal volume; and  , minute ventilation (fR×VT). *Significantly different (P < 0.05) from Kir5.1+/+ mice.
, minute ventilation (fR×VT). *Significantly different (P < 0.05) from Kir5.1+/+ mice.
The Kir5.1−/− mice also displayed reduced ventilatory responses to increased levels of inspired CO2. Respiratory activities were similar in Kir5.1−/− and wild-type mice in an atmosphere containing 3% CO2 (Fig. 1B). However, with 6% CO2,  in Kir5.1−/− mice increased to 4.04 ± 0.15 ml min−1 g−1 (n = 6), while in the wild-type animals in the same conditions
in Kir5.1−/− mice increased to 4.04 ± 0.15 ml min−1 g−1 (n = 6), while in the wild-type animals in the same conditions  was elevated to 5.55 ± 0.43 ml min−1 g−1 (n = 6, P = 0.017; ANOVA; Fig. 1B). In contrast to the respiratory responses evoked by hypoxia, this difference in
was elevated to 5.55 ± 0.43 ml min−1 g−1 (n = 6, P = 0.017; ANOVA; Fig. 1B). In contrast to the respiratory responses evoked by hypoxia, this difference in  arose from a significantly smaller CO2-induced increase in respiratory rate (but not VT) in Kir5.1−/− mice (Fig. 1B). In hyperoxic conditions (>70% O2), the resting fR was significantly higher in wild-type mice (Fig. 1C); however, baseline
arose from a significantly smaller CO2-induced increase in respiratory rate (but not VT) in Kir5.1−/− mice (Fig. 1B). In hyperoxic conditions (>70% O2), the resting fR was significantly higher in wild-type mice (Fig. 1C); however, baseline  and the overall ventilatory response to hyperoxic hypercapnia was similar in Kir5.1−/− animals (n = 6) and their wild-type counterparts (n = 8; Fig. 1C).
and the overall ventilatory response to hyperoxic hypercapnia was similar in Kir5.1−/− animals (n = 6) and their wild-type counterparts (n = 8; Fig. 1C).
Carotid body function in Kir5.1−/− mice
The reduced hypoxic ventilatory response suggested that the function of the peripheral chemosensors may be compromised in Kir5.1−/− mice. In order to test this hypothesis, we recorded chemoafferent activity of the carotid sinus nerve in an in vitro superfused carotid body–carotid sinus nerve preparations taken from Kir5.1−/− mice and their wild-type counterparts. In baseline conditions, carotid sinus nerve chemoafferent discharge was similar in Kir5.1−/− and control mice (Fig. 2). As expected, hypoxic stimulation evoked marked activation of the carotid chemoreceptors (Fig. 2A and B). However, the magnitude of the hypoxia-evoked carotid sinus nerve chemoafferent responses was similar in Kir5.1−/− mice (increase in sinus nerve discharge from 76 ± 20 spikes s−1 to a peak of 295 ± 45 spikes s−1, n = 7) and control animals (increase from 64 ± 16 spikes s−1 to a peak of 307 ± 35 spikes s−1, n = 7, P = 0.8; Fig. 2A and B).
Figure 2.

Normal carotid body function in Kir5.1−/− miceA, representative raw data showing hypoxia-evoked increases in the carotid sinus nerve (CSN) chemoafferent discharge recorded in the in vitro superfused carotid body–carotid sinus nerve preparations taken from the Kir5.1-deficient mice (Kir5.1−/−), and their wild-type counterparts (Kir5.1+/+). ‘O2 trace’ depicts changes in oxygen concentration in the incubation chamber. B and C, summary data of the mean resting and peak hypoxia- and acidification-induced increases in the discharge frequency of the carotid sinus nerve in preparations taken from Kir5.1−/− and Kir5.1+/+ mice. Abbreviations: RA, respiratory acidosis stimulus; IA, isocapnic acidosis stimulus (see main text for details); and FF, discharge frequency. Data are presented as means ±s.e.m. Numbers in parentheses indicate sample sizes.
An in vitro analogue of respiratory acidosis (decrease in pH triggered by an increase in  at constant [HCO3−]) activated carotid body chemosensors, albeit to a lesser degree compared with that during hypoxia (Fig. 2C). Chemoafferent responses evoked by respiratory acidosis were identical in Kir5.1−/− (peak discharge 179 ± 40 spikes s−1, n = 7) and wild-type animals (peak discharge 164 ± 19 spikes s−1, n = 7, P = 0.7; Fig. 2C). Likewise, increases in the carotid sinus nerve discharge evoked by isocapnic acidosis (decrease in pH by lowering [HCO3−] at constant
at constant [HCO3−]) activated carotid body chemosensors, albeit to a lesser degree compared with that during hypoxia (Fig. 2C). Chemoafferent responses evoked by respiratory acidosis were identical in Kir5.1−/− (peak discharge 179 ± 40 spikes s−1, n = 7) and wild-type animals (peak discharge 164 ± 19 spikes s−1, n = 7, P = 0.7; Fig. 2C). Likewise, increases in the carotid sinus nerve discharge evoked by isocapnic acidosis (decrease in pH by lowering [HCO3−] at constant  ) were not affected by Kir5.1 deficiency (Fig. 2C). Interestingly, peak increases in chemoafferent discharges triggered in wild-type preparations by respiratory acidosis (
) were not affected by Kir5.1 deficiency (Fig. 2C). Interestingly, peak increases in chemoafferent discharges triggered in wild-type preparations by respiratory acidosis ( 80 mmHg, pH 7.1, [HCO3−] 26 mm) were similar to that evoked by isocapnic acidosis (
80 mmHg, pH 7.1, [HCO3−] 26 mm) were similar to that evoked by isocapnic acidosis ( 40 mmHg, pH 7.0, [HCO3−] 10 mm; Fig. 2C).
40 mmHg, pH 7.0, [HCO3−] 10 mm; Fig. 2C).
Central respiratory chemosensitivity in Kir5.1−/− mice
The data described in the previous subsection indicated that Kir5.1−/− deficiency does not appear to have an effect on the carotid body function. To examine the role of Kir5.1 in central respiratory chemosensitivity, the next series of experiments was conducted in the in situ brainstem–spinal cord preparation with denervated peripheral chemoreceptors. Phrenic nerve activity in baseline conditions was similar in the in situ brainstem–spinal cord preparations of Kir5.1−/− mice (n = 12) and their wild-type counterparts (n = 11, P = 0.1). Respiratory acidosis (decrease in pH triggered by an increase in  at constant [HCO3−], see Methods) evoked similar increases in respiratory activity in preparations of Kir5.1−/− and wild-type mice with denervated peripheral chemoreceptors (Fig. 3A and B). Respiratory responses to isohydric hypercapnia (increase in
at constant [HCO3−], see Methods) evoked similar increases in respiratory activity in preparations of Kir5.1−/− and wild-type mice with denervated peripheral chemoreceptors (Fig. 3A and B). Respiratory responses to isohydric hypercapnia (increase in  at constant pH and elevated [HCO3−]) were also unaffected by Kir5.1 deficiency (Fig. 3C).
at constant pH and elevated [HCO3−]) were also unaffected by Kir5.1 deficiency (Fig. 3C).
Figure 3.
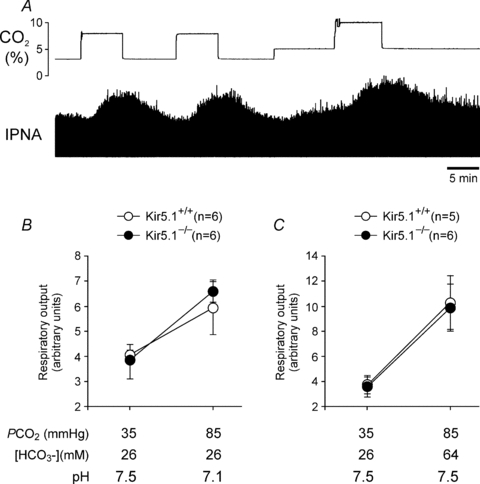
Normal central respiratory chemosensitivity in Kir5.1−/− miceA, raw data showing time-condensed record of hypercapnia-evoked changes in integrated phrenic nerve activity (IPNA) of a wild-type in situ brainstem–spinal cord preparation with denervated peripheral chemoreceptors. ‘CO2 trace’ depicts changes in CO2 concentration of the gas mixture used to saturate the perfusion solution, hence there is a lag between changes in CO2 and resultant respiratory responses. B and C, summary data of changes in minute respiratory output (phrenic amplitude × respiratory rate) in response to respiratory acidosis or isohydric hypercapnia, respectively, in preparations of Kir5.1-deficient mice (Kir5.1−/−) and their wild-type counterparts (Kir5.1+/+). Data are presented as means ±s.e.m. Numbers in parentheses indicate sample sizes.
Transmission of the peripheral chemosensory stimuli in Kir5.1−/− mice
Reduced ventilatory responses to hypoxia and normoxic hypercapnia and apparently normal peripheral and central respiratory chemoreceptor function suggested that the relay of the peripheral chemosensory stimuli is compromised in Kir5.1−/− mice. To test this hypothesis, in situ brainstem–spinal cord preparations with intact peripheral chemoreceptors were used. Activation of the carotid body chemoreceptors in preparations from Kir5.1−/− mice following bolus application of CN− resulted in a similar frequency response (Fig. 4B), but significantly smaller increases in phrenic nerve amplitude in comparison to that in wild-type preparations (increase by 47 versus 95%, P = 0.019, ANOVA; Fig. 4A and C)
Figure 4.
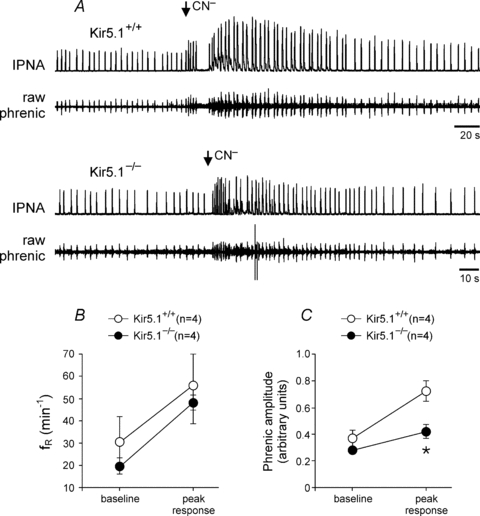
Compromised transmission of the peripheral chemosensory stimuli to the respiratory network in Kir5.1−/− miceA, representative raw data showing changes in phrenic nerve frequency and amplitude evoked by stimulation of the peripheral chemoreceptors with cyanide (0.03% w/v; 50 μl bolus) in the in situ brainstem–spinal cord preparations of Kir5.1-deficient mice (Kir5.1−/−) and their wild-type counterparts (Kir5.1+/+). Also shown are summary data of mean peak CN−-induced increases in respiratory frequency (fR; B) and phrenic amplitude (C) in preparations of Kir5.1−/− and Kir5.1+/+ mice. Data are presented as means ±s.e.m. Numbers in parentheses indicate sample sizes. *Significantly different (P < 0.05) from Kir5.1+/+ mice.
Arterial pH,  and [HCO3−] in Kir5.1−/− mice
and [HCO3−] in Kir5.1−/− mice
Kir5.1 has been found in the kidney (Tucker et al. 2000) and thus might influence the acid–base balance of the organism. In agreement with this hypothesis, adult Kir5.1−/−-deficient mice were found to have a profound metabolic acidosis, with arterial blood pH of 7.26 ± 0.03 (versus 7.38 ± 0.01 in the wild-type animals, P = 0.003, unpaired Student's t test),  of 41.6 ± 3.9 mmHg (versus 44.6 ± 2.6 mmHg in the wild-type animals, P = 0.68, unpaired Student's t test) and [HCO3−] of 18.1 ± 1.6 mm (versus 25.6 ± 0.2 mm in the wild-type animals, P = 0.005, n = 6, unpaired Student's t test).
of 41.6 ± 3.9 mmHg (versus 44.6 ± 2.6 mmHg in the wild-type animals, P = 0.68, unpaired Student's t test) and [HCO3−] of 18.1 ± 1.6 mm (versus 25.6 ± 0.2 mm in the wild-type animals, P = 0.005, n = 6, unpaired Student's t test).
Discussion
This study examined the potential contribution of Kir5.1 to the chemosensory control of breathing. Kir5.1 was an obvious target, because its coexpression with Kir4.1 produces K+ channels which are inhibited by decreases in intracellular pH within the physiological range (Xu et al. 2000). Although other members of the Kir family, including homomeric Kir4.1, are pH sensitive, their sensitivity lays well outside the physiological range of pH changes in the blood or brain. If pH-sensitive heteromeric Kir4.1–Kir5.1 channels are functionally important, then in Kir5.1 knockout animals the homomeric Kir4.1 channels will remain but the resultant Kir conductance will not be pH sensitive within the physiological range.
Reduced ventilatory responses to both hypoxia and normoxic hypercapnia and similar CO2-evoked increases in breathing in conditions of hyperoxia initially suggested that the function of the peripheral chemosensors may be compromised in Kir5.1−/− mice. Indeed, expression of both Kir4.1 and Kir5.1 by the peripheral chemoreceptors has been previously demonstrated (Yamamoto et al. 2008a). Chemosensitive glomus cells of the carotid body as well as some of the petrosal ganglion cells have been found to express Kir4.1 and Kir5.1 (Yamamoto et al. 2008a). However, our results suggest that these channels are likely to be dispensable for chemosensory function of the carotid body, since increases in the carotid sinus nerve chemoafferent discharge evoked by decreases in pH within the physiological range were not affected by deletion of Kir5.1. We also noted that the peak chemoafferent response triggered in wild-type as well as Kir5.1−/− carotid body–carotid sinus nerve preparations by respiratory acidosis were similar in amplitude to that evoked by isocapnic acidosis (Fig. 2C). Isocapnic acidosis would be expected to evoke smaller/delayed changes in intracellular pH, further weakening the case for Kir channels to be involved and intracellular pH to be the prime stimulus responsible for activation of the peripheral chemosensors in response to hypercapnia. Indeed, according to our previous observations the carotid body chemoafferent responses to increases in  /[H+] are almost entirely mediated by homomeric TASK-1 or heteromeric TASK-1–TASK-3 channels (Trapp et al. 2008), which are predominantly sensitive to changes in extracellular pH.
/[H+] are almost entirely mediated by homomeric TASK-1 or heteromeric TASK-1–TASK-3 channels (Trapp et al. 2008), which are predominantly sensitive to changes in extracellular pH.
The involvement of heteromeric Kir4.1–Kir5.1 channels in the central respiratory chemosensitivity to changes in  /[H+] has been suggested previously (Jiang et al. 2001) on the basis of their high sensitivity to changes in intracellular pH (Xu et al. 2000) and their abundant coexpression in the brainstem areas known to contain functional respiratory chemoreceptors (Wu et al. 2004). However, patterns of Kir4.1 and Kir5.1 expression in these regions are complex, with clear overlapping expression observed in some studies (Wu et al. 2004; Zhang et al. 2010), but not in the others (Yamamoto et al. 2008b). The study by Yamamoto et al. (2008b) also demonstrated strong expression of Kir4.1 in the brainstem glial cells. Intriguingly, Wenker et al. (2010) reported that inhibition of Kir4.1–Kir5.1-like channels may be responsible for pH-evoked depolarization of glial cells residing within the anatomical region of the RTN, which is one of the key central chemosensitive sites. However, our recent study of the role of astrocytes in central respiratory chemosensitivity (Gourine et al. 2010) argues against changes in astrocytic membrane potential as the key event in the central chemosensory transduction mechanism.
/[H+] has been suggested previously (Jiang et al. 2001) on the basis of their high sensitivity to changes in intracellular pH (Xu et al. 2000) and their abundant coexpression in the brainstem areas known to contain functional respiratory chemoreceptors (Wu et al. 2004). However, patterns of Kir4.1 and Kir5.1 expression in these regions are complex, with clear overlapping expression observed in some studies (Wu et al. 2004; Zhang et al. 2010), but not in the others (Yamamoto et al. 2008b). The study by Yamamoto et al. (2008b) also demonstrated strong expression of Kir4.1 in the brainstem glial cells. Intriguingly, Wenker et al. (2010) reported that inhibition of Kir4.1–Kir5.1-like channels may be responsible for pH-evoked depolarization of glial cells residing within the anatomical region of the RTN, which is one of the key central chemosensitive sites. However, our recent study of the role of astrocytes in central respiratory chemosensitivity (Gourine et al. 2010) argues against changes in astrocytic membrane potential as the key event in the central chemosensory transduction mechanism.
Regardless of any potential role for glial cells and the uncertainty of precisely which brainstem neurons may coexpress Kir4.1 and Kir5.1, we still observe a clear respiratory phenotype in Kir5.1−/− mice. However, the data obtained suggest that Kir channels are unlikely to be essential for functional central respiratory sensitivity to changes in  /[H+]. Interestingly, using the same Kir5.1−/− mice, D'Adamo et al. (2011) demonstrated that Kir5.1 contributes to pH-evoked responses of one functional population of central chemosensitive neurones in the LC. Together, these data and observations reported herein suggest that LC neuronal input may not be essential for the respiratory responses evoked by central actions of CO2 in adult mice.
/[H+]. Interestingly, using the same Kir5.1−/− mice, D'Adamo et al. (2011) demonstrated that Kir5.1 contributes to pH-evoked responses of one functional population of central chemosensitive neurones in the LC. Together, these data and observations reported herein suggest that LC neuronal input may not be essential for the respiratory responses evoked by central actions of CO2 in adult mice.
The metabolic acidosis observed in the Kir5.1−/− mice is consistent with the expression of Kir5.1 in the kidney (Tucker et al. 2000). Interestingly, development of metabolic acidosis was previously reported after genetic ablation of another notable kidney K+ channel, TASK-2 (Warth et al. 2004). Heteromeric Kir4.1–Kir5.1 channels have been identified in the basolateral membrane of distal tubular epithelia and are thought to be involved in the recycling of K+ across this membrane and H+/K+ homeostasis (Lourdel et al. 2002; Lachheb et al. 2008). Detailed investigation of renal function in Kir5.1-deficient mice is beyond the scope of the present study; however, the effect of persistent metabolic acidosis on the chemosensory control of breathing warrants further discussion.
The data obtained in the present study reveal markedly lower levels of the key homeostatic constants, pH and [HCO3−], in the arterial blood of adult Kir5.1−/− mice. This would be expected to have a stimulatory effect on central and peripheral chemoreceptors; however, despite profound metabolic acidosis the resting ventilation in Kir5.1−/− animals was similar to that in their wild-type counterparts (similar to observations by Gestreau et al. 2010 in TASK-2 knockout mice, which in conditions of metabolic acidosis also display normal respiratory activity at rest). This could be explained by compensatory reduction in peripheral chemoreceptor sensitivity, but the data obtained in our in vitro experiments demonstrate that at a given level of  , pH and [HCO3−] the Kir5.1−/− and Kir5.1+/+ preparations exhibit the same level of carotid sinus nerve chemoafferent discharge. Moreover, chemoafferent responses to isohydric hypercapnia (mimicking metabolic acidosis) are similar in Kir5.1−/− and wild-type animals. By extension, these data suggest that in conditions of continuing metabolic acidosis associated with Kir5.1 deficiency the resting carotid chemoafferent activity should be higher in Kir5.1−/− mice; however, this is not translated into facilitated ventilatory activity. Indeed, selective CN−-evoked activation of the carotid body chemoreceptors in the in situ preparations of Kir5.1−/− mice was found to result in a significantly smaller increase in phrenic nerve amplitude in comparison to that of wild-type preparations. This suggests that the relay of peripheral chemosensory information to the CNS is compromised by Kir5.1 deficiency; however, the exact nature of this compensatory mechanism as yet remains unknown.
, pH and [HCO3−] the Kir5.1−/− and Kir5.1+/+ preparations exhibit the same level of carotid sinus nerve chemoafferent discharge. Moreover, chemoafferent responses to isohydric hypercapnia (mimicking metabolic acidosis) are similar in Kir5.1−/− and wild-type animals. By extension, these data suggest that in conditions of continuing metabolic acidosis associated with Kir5.1 deficiency the resting carotid chemoafferent activity should be higher in Kir5.1−/− mice; however, this is not translated into facilitated ventilatory activity. Indeed, selective CN−-evoked activation of the carotid body chemoreceptors in the in situ preparations of Kir5.1−/− mice was found to result in a significantly smaller increase in phrenic nerve amplitude in comparison to that of wild-type preparations. This suggests that the relay of peripheral chemosensory information to the CNS is compromised by Kir5.1 deficiency; however, the exact nature of this compensatory mechanism as yet remains unknown.
The results obtained using a global gene knockout model have to be considered with caution, since the loss of a gene may be compensated for by increased expression of one or several other genes. In the latter case, this approach may not reveal the true functional role of a protein in question. A recent study by D'Adamo et al. (2011) demonstrated ablated pH-evoked responses of LC neurones in Kir5.1−/− animals and suggested that the loss of the Kir5.1 may not be fully compensated for, at least in the LC.
In summary, the data obtained in the present study demonstrate that although deletion of Kir5.1 in mice results in a clear respiratory phenotype, it appears that the loss of Kir5.1 does not directly affect the function of either central or peripheral respiratory chemoreceptors. Despite a profound metabolic acidosis, the resting ventilation in Kir5.1−/− mice is similar to that of the wild-type control animals. The ventilatory responses to systemic hypoxia and hypercapnia are reduced, as transmission of the signals from the peripheral chemoreceptors to the CNS appear to be compromised. We suggest that this compensatory modulation of the peripheral chemosensory inputs develops in order to counteract the effect of continuing metabolic acidosis on the activity of the peripheral chemoreceptors.
Acknowledgments
We thank the Medical Research Council (S.T., ref. G0600928), the Royal Society (S.J.T.) and the Wellcome Trust (A.V.G.) for financial support. We also thank the staff at the Mary Lyon Centre (MRC Harwell) for help with maintenance of the Kir5.1 knockout strain. A.V.G. is a Wellcome Trust Senior Research Fellow (ref. 079040).
References
- Bond CT, Pessia M, Xia XM, Lagrutta A, Kavanaugh MP, Adelman JP. Cloning and expression of a family of inward rectifier potassium channels. Receptors Channels. 1994;2:183–191. [PubMed] [Google Scholar]
- D'Adamo MC, Shang L, Imbrici P, Brown SD, Pessia M, Tucker SJ. Genetic inactivation of Kcnj16 identifies Kir5.1 as an important determinant of neuronal PCO2/pH sensitivity. J Biol Chem. 2011;286:192–198. doi: 10.1074/jbc.M110.189290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakler B, Schultz JH, Yang J, Schulte U, Brandle U, Zenner HP, Jan LY, Ruppersberg JP. Identification of a titratable lysine residue that determines sensitivity of kidney potassium channels (ROMK) to intracellular pH. EMBO J. 1996;15:4093–4099. [PMC free article] [PubMed] [Google Scholar]
- Gestreau C, Heitzmann D, Thomas J, Dubreuil V, Bandulik S, Reichold M, Bendahhou S, Pierson P, Sterner C, Peyronnet-Roux J, Benfriha C, Tegtmeier I, Ehnes H, Georgieff M, Lesage F, Brunet JF, Goridis C, Warth R, Barhanin J. TASK2 potassium channels set central respiratory CO2 and O2 sensitivity. Proc Natl Acad Sci USA. 2010;107:2325–2330. doi: 10.1073/pnas.0910059107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K, Kasparov S. Astrocytes control breathing through pH-dependent release of ATP. Science. 2010;329:571–575. doi: 10.1126/science.1190721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeringa J, Berkenbosch A, de Goede J, Olievier CN. Relative contribution of central and peripheral chemoreceptors to the ventilatory response to CO2 during hyperoxia. Respir Physiol. 1979;37:365–379. doi: 10.1016/0034-5687(79)90082-3. [DOI] [PubMed] [Google Scholar]
- Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, Kurachi Y. Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev. 2010;90:291–366. doi: 10.1152/physrev.00021.2009. [DOI] [PubMed] [Google Scholar]
- Jiang C, Xu H, Cui N, Wu J. An alternative approach to the identification of respiratory central chemoreceptors in the brainstem. Respir Physiol. 2001;129:141–157. doi: 10.1016/s0034-5687(01)00301-2. [DOI] [PubMed] [Google Scholar]
- Kofuji P, Ceelen P, Zahs KR, Surbeck LW, Lester HA, Newman EA. Genetic inactivation of an inwardly rectifying potassium channel (Kir4.1 subunit) in mice: phenotypic impact in retina. J Neurosci. 2000;20:5733–5740. doi: 10.1523/JNEUROSCI.20-15-05733.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachheb S, Cluzeaud F, Bens M, Genete M, Hibino H, Lourdel S, Kurachi Y, Vandewalle A, Teulon J, Paulais M. Kir4.1/Kir5.1 channel forms the major K+ channel in the basolateral membrane of mouse renal collecting duct principal cells. Am J Physiol Renal Physiol. 2008;294:F1398–F1407. doi: 10.1152/ajprenal.00288.2007. [DOI] [PubMed] [Google Scholar]
- Lourdel S, Paulais M, Cluzeaud F, Bens M, Tanemoto M, Kurachi Y, Vandewalle A, Teulon J. An inward rectifier K+ channel at the basolateral membrane of the mouse distal convoluted tubule: similarities with Kir4–Kir5.1 heteromeric channels. J Physiol. 2002;538:391–404. doi: 10.1113/jphysiol.2001.012961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nature Neurosci. 2004;7:1360–1369. doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]
- Mulkey DK, Talley EM, Stornetta RL, Siegel AR, West GH, Chen X, Sen N, Mistry AM, Guyenet PG, Bayliss DA. TASK channels determine pH sensitivity in select respiratory neurons but do not contribute to central respiratory chemosensitivity. J Neurosci. 2007;27:14049–14058. doi: 10.1523/JNEUROSCI.4254-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattie E, Li A. Central chemoreception is a complex system function that involves multiple brain stem sites. J Appl Physiol. 2009;106:1464–1466. doi: 10.1152/japplphysiol.00112.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton JFR. A working heart-brainstem preparation of the mouse. J Neurosci Methods. 1996;65:63–68. doi: 10.1016/0165-0270(95)00147-6. [DOI] [PubMed] [Google Scholar]
- Pearson WL, Dourado M, Schreiber M, Salkoff L, Nichols CG. Expression of a functional Kir4 family inward rectifier K+ channel from a gene cloned from mouse liver. J Physiol. 1999;514:639–653. doi: 10.1111/j.1469-7793.1999.639ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessia M, Imbrici P, D'Adamo MC, Salvatore L, Tucker SJ. Differential pH sensitivity of Kir4.1 and Kir4.2 potassium channels and their modulation by heteropolymerisation with Kir5.1. J Physiol. 2001;532:359–367. doi: 10.1111/j.1469-7793.2001.0359f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessia M, Tucker SJ, Lee K, Bond CT, Adelman JP. Subunit positional effects revealed by novel heteromeric inwardly rectifying K+ channels. EMBO J. 1996;15:2980–2987. [PMC free article] [PubMed] [Google Scholar]
- Rong W, Gourine AV, Cockayne DA, Xiang Z, Ford AP, Spyer KM, Burnstock G. Pivotal role of nucleotide P2X2 receptor subunit of the ATP-gated ion channel mediating ventilatory responses to hypoxia. J Neurosci. 2003;23:11315–11321. doi: 10.1523/JNEUROSCI.23-36-11315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp S, Aller MI, Wisden W, Gourine AV. A role for TASK-1 (KCNK3) channels in the chemosensory control of breathing. J Neurosci. 2008;28:8844–8850. doi: 10.1523/JNEUROSCI.1810-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker SJ, Imbrici P, Salvatore L, D'Adamo MC, Pessia M. pH dependence of the inwardly rectifying potassium channel, Kir5.1, and localization in renal tubular epithelia. J Biol Chem. 2000;275:16404–16407. doi: 10.1074/jbc.C000127200. [DOI] [PubMed] [Google Scholar]
- Warth R, Barriere H, Meneton P, Bloch M, Thomas J, Tauc M, Heitzmann D, Romeo E, Verrey F, Mengual R, Guy N, Bendahhou S, Lesage F, Poujeol P, Barhanin J. Proximal renal tubular acidosis in TASK2 K+ channel-deficient mice reveals a mechanism for stabilizing bicarbonate transport. Proc Natl Acad Sci USA. 2004;101:8215–8220. doi: 10.1073/pnas.0400081101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenker IC, Kréneisz O, Nishiyama A, Mulkey DK. Astrocytes in the retrotrapezoid nucleus sense H+ by inhibition of a Kir4.1-Kir5.1-like current and may contribute to chemoreception by a purinergic mechanism. J Neurophysiol. 2010;104:3042–3052. doi: 10.1152/jn.00544.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Xu H, Shen W, Jiang C. Expression and coexpression of CO2-sensitive Kir channels in brainstem neurons of rats. J Membr Biol. 2004;197:179–191. doi: 10.1007/s00232-004-0652-4. [DOI] [PubMed] [Google Scholar]
- Xu H, Cui N, Yang Z, Qu Z, Jiang C. Modulation of Kir4.1 and Kir5.1 by hypercapnia and intracellular acidosis. J Physiol. 2000;524:725–735. doi: 10.1111/j.1469-7793.2000.00725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Ishikawa R, Omoe K, Taniguchi K. Expression of inwardly rectifying K+ channels in the carotid body of rat. Histol Histopathol. 2008a;23:799–806. doi: 10.14670/HH-23.799. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Ishikawa R, Omoe K, Yoshikawa N, Yamaguchi-Yamada M, Taniguchi K. Immunohistochemical distribution of inwardly rectifying K+ channels in the medulla oblongata of the rat. J Vet Med Sci. 2008b;70:265–271. doi: 10.1292/jvms.70.265. [DOI] [PubMed] [Google Scholar]
- Zhang X, Cui N, Wu Z, Su J, Tadepalli JS, Sekizar S, Jiang C. Intrinsic membrane properties of locus coeruleus neurons in Mecp2-null mice. Am J Physiol Cell Physiol. 2010;298:C635–C646. doi: 10.1152/ajpcell.00442.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G, Chanchevalap S, Cui N, Jiang C. Effects of intra- and extracellular acidifications on single channel Kir2.3 currents. J Physiol. 1999;516:699–710. doi: 10.1111/j.1469-7793.1999.0699u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


