Abstract
Background
How to allocate limited vaccine supplies in the event of an influenza pandemic is currently under debate. Conventional vaccination strategies focus on those at highest risk for severe outcomes, including seniors, but do not consider (1) the signature pandemic pattern in which mortality risk is shifted to younger ages, (2) likely reduced vaccine response in seniors, and (3) differences in remaining years of life with age.
Methods
We integrated these factors to project the age-specific years of life lost (YLL) and saved in a future pandemic, on the basis of mortality patterns from 3 historical pandemics, age-specific vaccine efficacy, and the 2000 US population structure.
Results
For a 1918-like scenario, the absolute mortality risk is highest in people <45 years old; in contrast, seniors (those ⩾65 years old) have the highest mortality risk in the 1957 and 1968 scenarios. The greatest YLL savings would be achieved by targeting different age groups in each scenario; people <45 years old in the 1918 scenario, people 45–64 years old in the 1968 scenario, and people >45 years old in the 1957 scenario.
Conclusions
Our findings shift the focus of pandemic vaccination strategies onto younger populations and illustrate the need for real-time surveillance of mortality patterns in a future pandemic. Flexible setting of vaccination priority is essential to minimize mortality.
The Centers for Disease Control and Prevention (CDC) has estimated that the population of the United States at increased risk for influenza is 91 million, whereas the total population targeted for vaccination is ~218 million [1], which will lead to a clear resource-allocation dilemma in the event of a pandemic. Pandemic influenza preparedness strategies focus on the prevention of severe outcomes as the guiding principle for the utilization of vaccines and therapeutics. The US pandemic preparedness draft plan provides guidance for vaccine allocation, prioritizing key government and health care workers as well as emergency responders. In the general population, seniors (those ⩾65 years of age) are prioritized over younger healthy adults in all 3 of the pandemic scenarios considered, which range from mild to severe [2]. Government officials are seeking input from the public to refine this proposed prioritization strategy. In the meantime, several bioethicists and scientists have questioned the prioritization of seniors over younger persons and sparked a recent policy debate centered around the question of whom should be vaccinated first [3–8]. Essentially, ethicists criticize the inherent axiom that any life lost has the same value, regardless of whether the deceased was 5 or 99 years old.
When considering the best approach to mitigate the impact of mortality in a pandemic, the age-specific historical experience of mortality risk must be taken into consideration. Mortality during influenza pandemics has ranged from relatively mild (1968) to catastrophic (1918) [9]. Although severe influenza predominantly affects the elderly and infirm in interpandemic seasons, mortality shifts toward younger adults in pandemic years [10]. The 1918 pandemic is an extreme case, in which young adults were at highest risk of death, whereas seniors were essentially spared relative to the immediately preceding influenza seasons [11]; a similar phenomenon was seen in the 1968 pandemic [9, 12]. Decisions on vaccine allocation should also incorporate information on the decline in vaccine response with age due to immune senescence [13], an issue that has been overlooked in current pandemic plans. A recent literature review of randomized, placebo-controlled vaccine response found that influenza vaccine efficacy is reduced by 25%–50% in seniors, compared with that in younger adults [14]. Incorporating the phenomenon of declining response with age in a priority setting will also intuitively drive the priority groups toward younger ages.
In this article, we propose the integration of these concepts and explore a quantitative approach to be used to set priorities for vaccination in pandemic planning. Measurement of the potential years of life lost (YLL) during influenza pandemics is a quantitative tool that integrates mortality risks in various age groups with age differences in life expectancy. The YLL measure implies a differential between the prevention of death in younger and older persons [15] and is commonly used as a metric in public health decision making, particularly for vaccination recommendations [16, 17]. Intuitively, the use of YLL instead of the customary number of deaths would generate a very different weighting of age group priorities, simply because YLL “values” the prevention of the death of a younger person more highly than that of an older individual. Although YLL measures were recently applied to the estimation of seasonal influenza economic costs [18], this important metric has not been incorporated into the current debate about optimal control of pandemic influenza thus far.
Health economics analyses conventionally consider complex health outcome metrics such as quality-adjusted life years and disability-adjusted life years (DALYs) [19], which incorporates both YLL and years of life spent in poor health or with disability after a disease episode. These metrics are useful for evaluating the burdens of chronic diseases or injuries [20] but do not add much to the assessment of influenza vaccine strategies, given that influenza is an acute viral infection from which survivors usually fully recover in a week or two with no life-long sequelae. Furthermore, when death and life with disability are integrated in a single measure of DALYs, different weights on these health outcomes must be set a priori, a sometimes difficult and debated approach [21]. In addition, because the YLL metric does not put different weights on remaining years of life based on disability or societal preferences related to the value of life at different ages, it tends to overestimate disease burden in seniors compared with the DALY metric [22]. Hence, in this work, we focus on YLL, a conservative metric that captures the major health burden of pandemic influenza with no need to set arbitrary values on quality of life.
Although the importance of assessing YLL has been suggested as an important consideration for vaccine allocation in pandemic situations [3, 6], here we provide the first quantitative estimates of this metric. We project the age-specific YLL and the benefits of vaccination in a future pandemic, integrating historical mortality rates, life expectancy, and age-specific vaccine efficacy. The purpose of this analysis is to provide an alternative quantitative tool to help guide pandemic vaccine priority setting and achieve the greatest possible population impact, by preventing the loss of as many years of life as possible.
METHODS
We estimated influenza-related deaths by age for the 1918, 1957, and 1968 pandemics. For the earliest 2 pandemics, we relied on age-specific mortality estimates available in the literature, based on New York City data for the 1918 pandemic [9, 11] and national US data for the 1957 pandemic [23, 24]. We used data from New York City for the 1918 pandemic because the overall death rate there for the main pandemic wave in 1918–1919 was equal to that in the entire United States (53 deaths per 10,000 population), and reliable age-specific mortality estimates were available only for New York City [11]. Data were available for 6 age groups for the 1918 pandemic (<5, 5–14, 15–24, 25–44, 45–64, and ⩾65 years) and for 7 age groups for the 1957 pandemic (<1, 1–14, 15–24, 25–44, 45–64, 65–74, and ⩾75 years). For the 1968 pandemic, we used monthly national vital statistics by age (<1, 1–4, 5–19, 20–44, 45–64, 65–74, and ⩾75 years) [25]. Estimates of excess deaths for all 3 pandemics were based on the same Serfling-like seasonal regression approach long used by the CDC and others [11, 24, 25]. We applied this approach to all-cause mortality to capture the overall mortality burden of past influenza pandemics [9].
Next, to project deaths and YLL in a future pandemic, we modeled scenarios in which pandemics with mortality patterns similar to those of the 1918, 1957, or 1968 pandemics occur in a contemporary setting. We calculated “period expected” YLL [19] by applying historical age-specific death rates for the 1918, 1957, and 1968 pandemics to the age structure of the US population in 2000 and standard life expectancy at age of death in 2000 from the midpoint of each age category for the 6 (1918 pandemic) or 7 (1957 and 1968 pandemics) age categories available [26]. For example, in the 1918-like scenario, projections of YLL numbers among people <45 years old were calculated as follows:
where DR1918,i is the historical age-specific death rate in the 1918 pandemic for age subgroup i; Pop2000,i is the 2000 population size of group i; LE2000,i is the 2000 life expectancy of an individual at age i; and i represents the 4 age subgroups <45 years old available in the historical 1918 mortality data. We then derived projected YLL rates in people <45 years old for the 1918-like scenario, as follows:
Our algorithm follows a standard approach [27] in that we do not use a social discount rate that favors life saved in the near future; nor do we use age-specific coefficients to weight deaths in young adults more heavily than those in children or seniors. Such a weighting and discounting system can be used in DALYs [19] but relies on disputed value judgments based on economic considerations [21].
To account for differences in population sizes by age group, it is important to show excess mortality and YLL as incidence rates for the 3 pandemic scenarios. For each scenario, we also present the relative risk (RR) of mortality and YLL for those aged <45 years and 45–64 years, compared with the reference group of persons aged ⩾65 years (the age group currently prioritized for vaccination). These 3 age groups were the only ones compatible with comparisons across all 3 scenarios, given the limited availability of historical age-specific mortality data.
To incorporate the expected age-specific benefits of a pandemic vaccine, we used estimates of seasonal influenza vaccine immunogenicity stratified by age groups. A recent study indicated that the antibody response in seniors was only approximately one-fourth to one-half as rigorous as that in adults <65 years old [14]. Assuming that the vaccine efficacy in terms of preventing deaths is between 70% and 90% of deaths in persons <65 years old [28, 29], this result suggests that the corresponding vaccine efficacy for seniors is between 17% and 53% [14]. Using this range of relative immunogenicity, we estimated the direct effects of vaccination in the different age groups, measured as YLL and deaths prevented per dose of administered vaccine.
RESULTS
Aside from the sheer magnitude of deaths in the 1918 pandemic, the disproportionate age pattern of deaths is of note; deaths occurred predominantly in young adults, and seniors were spared [9]. The mortality rate of 560 deaths per 100,000 population in persons <45 years old was 3.75 times greater than that in persons ⩾65 years old (table 1). By contrast, in the moderate 1957 pandemic, the overall mortality rate was 6.4 per 100,000 in persons <45 years old, only 0.02 times that in persons ⩾65 years old. Similarly, in the mild 1968 pandemic, seniors experienced the highest mortality rate, although the differential with younger age groups was less pronounced than for the 1957 pandemic.
Table 1.
Mortality per 100,000 population, estimated no. of prevented deaths, and years of life lost prevented per vaccine dose, by age group and relative risk.
| Influenza pandemic scenario, age group |
Deaths | Years of life lost | |||
|---|---|---|---|---|---|
| Rate per 100,000 population |
Prevented per 100,000 vaccine doses, range |
Rate per 10,000 population |
Prevented per 10,000 vaccine doses, range |
Relative riska | |
| 1918 | |||||
| <45 years | 563 | 394–507 | 3209 | 2246–2888 | 28–68 |
| 45–64 years | 210 | 147–189 | 992 | 694–893 | 8.8–21 |
| ⩾65 years | 150 | 26–80 | 192 | 33–102 | Reference |
| All ages | 434 | 294–383 | 2344 | 1628–2102 | … |
| 1957 | |||||
| <45 years | 6.4 | 4.5–5.8 | 36 | 25–32 | 0.2–0.4 |
| 45–64 years | 45 | 32–41 | 131 | 92–118 | 0.6–1.6 |
| ⩾65 years | 315 | 54–167 | 347 | 59–184 | Reference |
| All ages | 53 | 17–33 | 96 | 44–70 | … |
| 1968 | |||||
| <45 years | 4.2 | 2.9–3.8 | 23 | 16–21 | 0.2–0.6 |
| 45–64 years | 41 | 29–37 | 110 | 77–99 | 1.1–2.8 |
| ⩾65 years | 151 | 26–80 | 167 | 28–89 | Reference |
| All ages | 30 | 11–21 | 60 | 32–48 | … |
NOTE. Scenarios are based on the 1918 experience in New York City and the 1957 and 1968 pandemic experiences in the United States [11, 23, 25]. Vaccination was assumed to prevent 70%–90% of deaths in persons <65 years old and 17%–53% of deaths in persons ⩾65 years old [14]. Results for age groups with the highest vaccine benefits are shown in boldface. See also the sensitivity analysis in table 2.
The relative risk was calculated as the ratio of the prevented no. of years of life lost per vaccine dose in younger age groups to that in seniors.
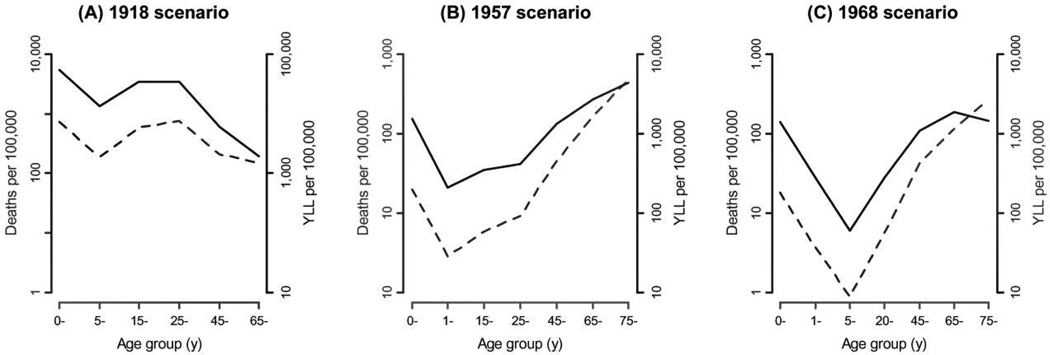
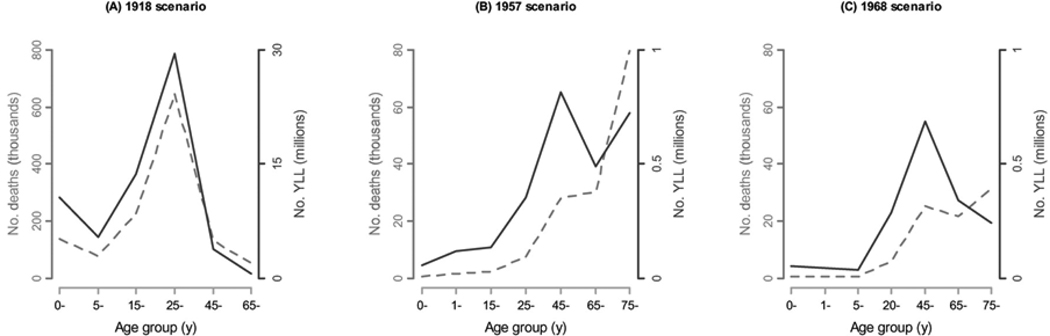
When YLL are considered rather than simply deaths, the impact of pandemics is more pronounced in younger populations. Figure 1 compares mortality and YLL rates by age in the 1918, 1957, and 1968 pandemic scenarios, where projections are based on the 2000 US population. With the exception of the 1918 pandemic, persons ⩾65 years old have higher rates of influenza-related deaths than those <65 years old. However, when YLL rates are considered, the projected burden shifts toward younger populations. For the 1957 and 1968 pandemic scenarios in particular, the YLL metric reduces the differences in rates between seniors and younger people. We also note that 56%–99% of YLL would occur in persons <65 years old, depending on the scenario considered (see figure 2 for numbers of deaths and YLL).
Figure 1.
Comparison of projected rates of influenza-related deaths and years of life lost (YLL) for 3 pandemic scenarios. Solid lines show YLL, and dashed lines show deaths (both per 100,000 population). Estimates are based on historical age-specific influenza-related mortality rates from the 1918 pandemic (A), the 1957 pandemic (B), and the 1968 pandemic (C), projected onto the 2000 US population structure and life expectancies. The age groups studied vary between the pandemics due to the availability of historical data [11, 23, 25].
Figure 2.
Comparison of projected nos. of influenza-related deaths and years of life lost (YLL) for 3 pandemic scenarios.
Taking into account differential vaccine efficacy with age produces an even greater shift in vaccine benefits toward younger age groups (table 1). Using the 3 age groups for which we have mortality data from all 3 historic pandemics (<45, 45–64, and ⩾65 years old), we can define the optimal age group for targeted influenza vaccination as the one that maximizes the YLL prevented per vaccine dose. The optimal group comprises people <45 years old for the 1918-like scenario and people 45–64 years old for the 1968-like scenario (RR > 1 in table 1). For the 1957-like scenario, the range of YLL prevented per dose in people 45–64 years old overlaps with that in people ⩾65 years old; we cannot resolve whether vaccinating the middle-age group would be better than vaccinating seniors, and we therefore conclude that these age groups would be equally good choices.
DISCUSSION
Some years ago, a CDC health economics study of pandemic influenza noted that nearly opposite age- and high-risk-group priorities would be generated if, instead of death counts, prioritization was based on a more complex measure of health outcomes that had characteristics similar to our proposed YLL measure [30]. However, the important finding—that priority setting critically depends on what outcomes we want to prevent—did not get the attention it deserved until recent commentaries were made by bioethicists [3, 6, 8].
Focusing on vaccination of seniors in preference to younger populations fails to account for 4 important factors in pandemic situations: (1) the differential in YLL for deaths in younger versus older persons; (2) reduced vaccine immunogenicity in seniors; (3) the observed shift in mortality to younger groups during pandemics; and (4) the indirect (herd immunity) effects of vaccination. In this article, we offer a comprehensive quantitative study that seeks to account for the first 3 of these factors and project the potential impact per vaccine dose in different age groups on the basis of past pandemic mortality patterns extrapolated to the current US population structure. This is particularly relevant given the history of inadequate vaccine supply [31] and the need to prioritize vaccination to maximize health benefits per administered vaccine dose [16, 17]. For 2 of 3 pandemic scenarios studied, our new integrated approach to project the likely benefits of a pandemic vaccination effort prioritizes persons <65 years old over seniors and differs from the US pandemic preparedness draft plan [2] as well as 14 of 28 national plans that specifically prioritized vaccine [32]. Importantly, if vaccine resources were limited, the greatest YLL mortality benefits would be achieved by targeting different age groups in each pandemic scenario; people <45 years old in the 1918 scenario, people 45–64 years old in the 1968 scenario, and people >45 years old in the 1957 scenario. Unfortunately, the US draft plan [2] currently considers young, healthy adults to be the lowest priority for vaccination in all 3 scenarios, even though historical events suggest that mortality could be disproportionately high in this age group.
Pandemics differ from influenza epidemics not necessarily in causing more deaths but in causing proportionally more deaths in younger age groups [10]. Pandemic impact is not always evident if one simply compares the total number of influenza-related deaths between pandemic and interpandemic seasons, especially in the case of the mild 1968 pandemic [9, 25]. Although the greatest mortality and YLL burden during interpandemic influenza occur in seniors (data not shown), in a future pandemic situation the majority of YLL (56%–99%) are expected to occur in persons <65 years old, regardless of which of the 3 scenarios reflecting our past experience with pandemic influenza is considered. The heavier YLL burden in younger age groups is truly a characteristic of pandemic influenza and is not found for seasonal influenza, thus confirming that the YLL metric is sensitive and specific to the mortality age pattern in influenza pandemics.
The mortality age shift observed during past pandemics may result from the recycling of influenza viral antigens over time [33], thus conferring protection against severe disease in the most senior age groups [11, 12], possibly combined with a shift in disease transmission patterns [34]. In particular, recent analyses of monthly data from the 1918 pandemic in New York City and Denmark document little excess mortality in seniors [11, 35], suggesting a sparing of the elderly. This pattern challenges the classically described W-shaped age-specific mortality curve, which was based on crude annual mortality data rather than monthly excess mortality [36]. In addition, for the 1918 pandemic, the curious age pattern of young adults having the highest mortality risk may be further explained by an unusual immune pathology that affected young adults [37]. Because the contributions of the 2 possibilities—recycling and immune pathology—cannot be resolved for the 1918 pandemic [37], projections of a future pandemic impact cannot unequivocally evaluate the full range of possible age patterns. We note that recycling is not an issue for a contemporary pandemic threat such as A/H5N1, yet the age distribution of human cases and deaths so far is reminiscent of that of the 1918 pandemic, with the highest impact being among the young [38]. Clearly, more research is warranted to help us fully understand and potentially predict the age distribution of deaths for pandemic influenza.
We note some limitations of our study. We assigned YLL for life tables from age groups of the total US population and did not stratify by underlying medical conditions or socioeconomic risk factors, which may affect life expectancy. However, although seasonal influenza generally kills exclusively “high-risk” persons with underlying predisposing medical conditions, deaths in historic influenza pandemics occurred predominantly among previously healthy individuals [11, 12], so this is unlikely to be a serious limitation. Furthermore, to estimate the influenza mortality burden, we exclusively studied all-cause excess mortality, a measure that is thought to best reflect the total burden of influenza but is imprecise relative to other outcomes, such as excess pneumonia and influenza mortality [9]. However, applying the same methodology to deaths from pneumonia and influenza leads to the same qualitative conclusions in terms of optimal age groups for vaccination (data not shown).
We also limited our analysis to New York City data and assumed that there was little regional geographical variation in the age pattern of pandemic influenza deaths across the United States. Although considerable variability in the overall impact of the 1918 flu was reported across US cities [39, 40], there is no evidence that the general age pattern of deaths differed. Furthermore, unusually high mortality among young adults in 1918 has been consistently found in locales as diverse as the United States, the United Kingdom, Scandinavia, and Australia [11, 41–43]. In particular, a sensitivity analysis based on historical data from Copenhagen [35] suggested that the death and YLL burden in people <45 years old was similar in Copenhagen and New York City. By contrast, older age groups experienced a lower burden in Copenhagen than in New York City, such that targeting younger age groups for vaccination is even more dramatically beneficial in the Copenhagen-based 1918 scenario (table 2). We also recognize that the overall pandemic mortality impact has historically played out in several waves in the first 3–5 years [9], but we have considered only the primary pandemic wave in our scenarios. However, it is in the first year of a pandemic that vaccine resources are likely to be most scarce.
Table 2.
Sensitivity analysis for the 1918 scenario, using excess mortality rates derived from historical data from Copenhagen [35].
Mortality per 100,000 population, estimated no. of prevented deaths, and years of life lost prevented per vaccine dose, by age group and relative risk.
| Influenza pandemic scenario, age group |
Deaths | Years of life lost | |||
|---|---|---|---|---|---|
| Rate per 100,000 population |
Prevented per 100,000 vaccine doses, range |
Rate per 10,000 population |
Prevented per 10,000 vaccine doses, range |
Relative riska | |
| 1918 | |||||
| <45 years | 563 | 394–507 | 3209 | 2246–2888 | 28–68 |
| 45–64 years | 210 | 147–189 | 992 | 694–893 | 8.8–21 |
| ⩾65 years | 150 | 26–80 | 192 | 33–102 | Reference |
| All ages | 434 | 294–383 | 2344 | 1628–2102 | … |
| 1957 | |||||
| <45 years | 6.4 | 4.5–5.8 | 36 | 25–32 | 0.2–0.4 |
| 45–64 years | 45 | 32–41 | 131 | 92–118 | 0.6–1.6 |
| ⩾65 years | 315 | 54–167 | 347 | 59–184 | Reference |
| All ages | 53 | 17–33 | 96 | 44–70 | … |
| 1968 | |||||
| <45 years | 4.2 | 2.9–3.8 | 23 | 16–21 | 0.2–0.6 |
| 45–64 years | 41 | 29–37 | 110 | 77–99 | 1.1–2.8 |
| ⩾65 years | 151 | 26–80 | 167 | 28–89 | Reference |
| All ages | 30 | 11–21 | 60 | 32–48 | … |
NOTE. Scenarios are based on the 1918 experience in New York City and the 1957 and 1968 pandemic experience in the United States [11, 23, 25]. Vaccination was assumed to prevent 70%–90% of deaths in persons <65 years old and 17%–53% pf deaths in persons ⩾65 years old [14]. Results for age groups with the highest vaccine benefits are shown in boldface. See also the sensitivity analysis in table 2.
The relative risk was calculated as the ratio of the prevented no. of years of life lost per vaccine dose in younger age groups to that in seniors.
Finally, our analysis considers only the direct benefits of vaccination. Previous research suggests that community-wide protection against influenza may be best achieved by vaccinating those who are responsible for most transmission, particularly children [44–46]. A priori, incorporating the indirect effect of vaccination (herd immunity) through an age-structured transmission model can only shift the optimal vaccination policy further away from seniors, because epidemics are driven by transmission in younger age groups. Recent mathematical modeling efforts have highlighted the possibility that vaccination of high-transmitter groups could indirectly mitigate the overall pandemic disease burden [47–50]. However, these studies focused on the optimal strategy for reducing morbidity rather than mortality [48–50], and they did not differentiate between deaths in younger and older persons in terms of life expectancy [3, 47]. In such modeling exercises, the optimal strategy critically depends on whether available vaccine resources are sufficient to achieve herd immunity by vaccinating high-transmitter groups. In turn, model results are highly sensitive to the level of mixing between the different age groups [48], viral transmissibility [47, 48], and age-specific case fatality rates, the values of which need to be fixed a priori but remain unclear for pandemic influenza. Irrespective of the feasibility of achieving indirect protection, we have shown that simply considering the direct benefits of vaccination in terms of YLL is enough to refocus current priority groups toward younger ages. Hence, our quantitative study rooted in analysis of historical mortality data adds complementary elements to the current debate on who should be prioritized for pandemic influenza vaccination; these new considerations [47–50] all shift attention away from seniors and toward either younger adults or children as more cost-effective targets.
Although projections of past pandemic experience to the present-day population are naturally fraught with uncertainties, they provide guidance for future policies and highlight the most relevant issues. Our estimation is not an endorsement of any particular policy but highlights how the choice of health outcome metrics such as YLL can influence the prioritization of age groups to vaccinate in pandemic settings. It also shows that the vaccine priority scheme for seasonal influenza is not optimized to mitigate the impact of pandemic influenza. Finally, although our analysis of mortality patterns for pandemic influenza is restricted to only 3 historic scenarios for which age-stratified data are available, each scenario prioritizes a different age group. These results suggest the need for pandemic plans to have an element of flexibility that allows the prioritization of age groups for immunization at the start of a pandemic to be modified as age-specific epidemiological data on the novel virus become available in real time. This is a challenge in both developed and developing countries, because conventional mortality reporting often lags by 2 or 3 years, and new systems for near real-time reporting of mortality need to be designed, implemented, and tested before a pandemic occurs. Equally important, the question of who should be vaccinated first needs to be debated and reasoned through now, before the onset of a public health emergency, while we have the time to reflect on which decision-making metric is the most appropriate.
Acknowledgment
Financial support: National Institutes of Health; Fogarty International Center; National Institute of Allergy and Infectious Disease.
We thank Robert Taylor for his role in editing the manuscript.
Footnotes
Potential conflicts of interest: none reported.
Presented in part: Options for the Control of Influenza VI, Toronto, 16–23 June 2007 (abstract O104); Ninth Annual Conference on Vaccine Research, Baltimore, 8 –10 May 2006 (presentation S6).
This article reflects the opinions of the authors and in no way reflects current policies of the Department of Health and Human Services of the United States government.
References
- 1.US Centers for Disease Control and Prevention. [Accessed 11 December 2006];Estimates of influenza vaccination target population sizes in 2006 and recent vaccine uptake levels. Available at: http://www.cdc.gov/flu/professionals/vaccination/pdf/targetpopchart.pdf.
- 2.US Department of Health and Human Services. [Accessed 27 October 2007];Draft guidance on allocating and targeting pandemic influenza vaccine. Available at: http://www.pandemicflu.gov/vaccine/prioritization.html.
- 3.Emanuel EJ, Wertheimer A. Public health: who should get influenza vaccine when not all can? Science. 2006;312:854–855. doi: 10.1126/science.1125347. [DOI] [PubMed] [Google Scholar]
- 4.Frey HS. The ethics of influenza vaccination. Science. 2006;313:758–760. author reply 758–60. [PubMed] [Google Scholar]
- 5.Galvani AP, Medlock J, Chapman GB. The ethics of influenza vaccination. Science. 2006;313:758–760. author reply 758–60. [PubMed] [Google Scholar]
- 6.Gostin LO. Medical countermeasures for pandemic influenza: ethics and the law. JAMA. 2006;295:554–556. doi: 10.1001/jama.295.5.554. [DOI] [PubMed] [Google Scholar]
- 7.Silverstein RP. The ethics of influenza vaccination. Science. 2006;313:758–760. doi: 10.1126/science.313.5788.758b. author reply 758–60. [DOI] [PubMed] [Google Scholar]
- 8.Zimmerman RK. Rationing of influenza vaccine during a pandemic: ethical analyses. Vaccine. 2007;25:2019–2026. doi: 10.1016/j.vaccine.2006.11.045. [DOI] [PubMed] [Google Scholar]
- 9.Simonsen L, Olson D, Viboud C, Miller M. Forum on microbial threats. Pandemic influenza: assessing capabilities for prevention and response. Washington, DC: Institute of Medicine, National Academy of Sciences; 2004. Pandemic influenza and mortality: past evidence and projections for the future. [Google Scholar]
- 10.Simonsen L, Clarke MJ, Schonberger LB, Arden NH, Cox NJ, Fukuda K. Pandemic versus epidemic influenza mortality: a pattern of changing age distribution. J Infect Dis. 1998;178:53–60. doi: 10.1086/515616. [DOI] [PubMed] [Google Scholar]
- 11.Olson DR, Simonsen L, Edelson PJ, Morse SS. Epidemiological evidence of an early wave of the 1918 influenza pandemic in New York City. Proc Natl Acad Sci USA. 2005;102:11059–11063. doi: 10.1073/pnas.0408290102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simonsen L, Reichert TA, Miller M. Options for the control of influenza V. International Congress Series 1263. Okinawa, Japan: Elsevier; 2003. The virtues of antigenic sin: consequences of pandemic recycling on influenza-associated mortality. [Google Scholar]
- 13.Vallejo AN. Immune remodeling: lessons from repertoire alterations during chronological aging and in immune-mediated disease. Trends Mol Med. 2007;13:94–102. doi: 10.1016/j.molmed.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine. 2006;24:1159–1169. doi: 10.1016/j.vaccine.2005.08.105. [DOI] [PubMed] [Google Scholar]
- 15.Techniques to measure the impact of mortality: years of potential life lost. Epidemiol Bull. 2003;24:1–4. [PubMed] [Google Scholar]
- 16.Edmunds WJ, Brisson M, Rose JD. The epidemiology of herpes zoster and potential cost-effectiveness of vaccination in England and Wales. Vaccine. 2001;19:3076–3090. doi: 10.1016/s0264-410x(01)00044-5. [DOI] [PubMed] [Google Scholar]
- 17.Lieu TA, Ray GT, Black SB, et al. Projected cost-effectiveness of pneumococcal conjugate vaccination of healthy infants and young children. JAMA. 2000;283:1460–1468. doi: 10.1001/jama.283.11.1460. [DOI] [PubMed] [Google Scholar]
- 18.Molinari NA, Ortega-Sanchez IR, Messonnier ML, et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine. 2007;25:5086–5096. doi: 10.1016/j.vaccine.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 19.Murray CJ. Quantifying the burden of disease: the technical basis for disability-adjusted life years. Bull World Health Organ. 1994;72:429–445. [PMC free article] [PubMed] [Google Scholar]
- 20.McKenna MT, Michaud CM, Murray CJ, Marks JS. Assessing the burden of disease in the United States using disability-adjusted life years. Am J Prev Med. 2005;28:415–423. doi: 10.1016/j.amepre.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 21.Cohen J. The Global Burden of Disease Study: a useful projection of future global health? J Public Health Med. 2000;22:518–524. doi: 10.1093/pubmed/22.4.518. [DOI] [PubMed] [Google Scholar]
- 22.Mathers CD, Salomon JA, Ezzati M, Begg S, Vander Hoorn S, Lopez AD. Sensitivity and uncertainty analyses for burden of disease and risk factor estimates. In: Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ, editors. Global burden of disease and risk factors. New York: Oxford University Press; 2006. pp. 399–426. [Google Scholar]
- 23.Housworth WJ, Spoon MM. The age distribution of excess mortality during A2 Hong Kong influenza epidemics compared with earlier A2 outbreaks. Am J Epidemiol. 1971;94:348–350. doi: 10.1093/oxfordjournals.aje.a121329. [DOI] [PubMed] [Google Scholar]
- 24.Serfling RE, Sherman IL, Houseworth WJ. Excess pneumonia-influenza mortality by age and sex in three major influenza A2 epidemics, United States, 1957–58, 1960 and 1963. Am J Epidemiol. 1967;86:433–441. doi: 10.1093/oxfordjournals.aje.a120753. [DOI] [PubMed] [Google Scholar]
- 25.Viboud C, Grais RF, Lafont BAP, et al. Multinational impact of the 1968 Hong Kong influenza pandemic: evidence for a smoldering pandemic. J Infect Dis. 2005;192:233–248. doi: 10.1086/431150. [DOI] [PubMed] [Google Scholar]
- 26.US National Center for Health Statistics. [Accessed 11 December 2006];United States life tables, 1999–2003. Available at: http://www.cdc.gov/nchs/datawh/statab/unpubd/mortabs/lewk3_10.htm.
- 27.Premature mortality in the United States: public health issues in the use of years of potential life lost. MMWR Morb Mortal Wkly Rep. 1986;35:1S–11S. [PubMed] [Google Scholar]
- 28.Demicheli V, Rivetti D, Deeks JJ, Jefferson TO. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev. 2004:CD001269. doi: 10.1002/14651858.CD001269.pub2. [DOI] [PubMed] [Google Scholar]
- 29.Harper SA, Fukuda K, Uyeki TM, Cox NJ, Bridges CB. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2004;53:1–40. [PubMed] [Google Scholar]
- 30.Meltzer MI, Cox NJ, Fukuda K. The economic impact of pandemic influenza in the United States: priorities for intervention. Emerg Infect Dis. 1999;5:659–671. doi: 10.3201/eid0505.990507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wynia MK. Ethics and public health emergencies: rationing vaccines. Am J Bioeth. 2006;6:4–7. doi: 10.1080/15265160601021256. [DOI] [PubMed] [Google Scholar]
- 32.Uscher-Pines L, Omer SB, Barnett DJ, Burke TA, Balicer RD. Priority setting for pandemic influenza: an analysis of national preparedness plans. PLoS Med. 2006;3:e436. doi: 10.1371/journal.pmed.0030436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dowdle WR. Influenza A virus recycling revisited. Bull World Health Organ. 1999;77:820–828. [PMC free article] [PubMed] [Google Scholar]
- 34.Epstein SL. Prior H1N1 influenza infection and susceptibility of Cleveland Family Study participants during the H2N2 pandemic of 1957: an experiment of nature. J Infect Dis. 2006;193:49–53. doi: 10.1086/498980. [DOI] [PubMed] [Google Scholar]
- 35.Andreasen V, Viboud C, Simonsen L. Epidemiologic characterization of the 1918 influenza pandemic summer wave in Copenhagen: implications for pandemic control strategies. J Infect Dis. 2008;197:270–278. doi: 10.1086/524065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dauer C, Serfling R. Mortality from influenza, 1957–1958 and 1959–1960. Am Rev Respir Dis. 1961;83:15–26. [Google Scholar]
- 37.Palese P, Tumpey TM, Garcia-Sastre A. What can we learn from reconstructing the extinct 1918 pandemic influenza virus? Immunity. 2006;24:121–124. doi: 10.1016/j.immuni.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 38.Smallman-Raynor M, Cliff AD. Avian influenza A (H5N1) age distribution in humans. Emerg Infect Dis. 2007;13:510–512. doi: 10.3201/eid1303.060849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bootsma MC, Ferguson NM. The effect of public health measures on the 1918 influenza pandemic in U.S. cities. Proc Natl Acad Sci USA. 2007;104:7588–7593. doi: 10.1073/pnas.0611071104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hatchett RJ, Mecher CE, Lipsitch M. Public health interventions and epidemic intensity during the 1918 influenza pandemic. Proc Natl Acad Sci USA. 2007;104:7582–7587. doi: 10.1073/pnas.0610941104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Curson P, McCracken K. An Australian perspective of the 1918–1919 influenza pandemic. NSW Public Health Bull. 2006;17:103–107. [PubMed] [Google Scholar]
- 42.Langford C. The age pattern of mortality in the 1918–19 influenza pandemic: an attempted explanation based on data for England and Wales. Med Hist. 2002;46:1–20. [PMC free article] [PubMed] [Google Scholar]
- 43.Mamelund SE. A socially neutral disease? Individual social class, household wealth and mortality from Spanish influenza in two socially contrasting parishes in Kristiania 1918–19. Soc Sci Med. 2006;62:923–940. doi: 10.1016/j.socscimed.2005.06.051. [DOI] [PubMed] [Google Scholar]
- 44.Brownstein JS, Kleinman KP, Mandl KD. Identifying pediatric age groups for influenza vaccination using a real-time regional surveillance system. Am J Epidemiol. 2005;162:686–693. doi: 10.1093/aje/kwi257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Halloran ME, Longini IM., Jr Public health: community studies for vaccinating schoolchildren against influenza. Science. 2006;311:615–616. doi: 10.1126/science.1122143. [DOI] [PubMed] [Google Scholar]
- 46.Olson DR, Heffernan RT, Paladini M, Konty K, Weiss D, Mostashari F. Monitoring the impact of influenza by age: emergency department fever and respiratory complaint surveillance in New York City. PLoS Med. 2007;4:e247. doi: 10.1371/journal.pmed.0040247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bansal S, Pourbohloul B, Meyers LA. A comparative analysis of influenza vaccination programs. PLoS Med. 2006;3:e387. doi: 10.1371/journal.pmed.0030387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dushoff J, Plotkin JB, Viboud C, et al. Vaccinating to protect a vulnerable subpopulation. PLoS Med. 2007;4:e174. doi: 10.1371/journal.pmed.0040174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ferguson NM, Cummings DA, Fraser C, Cajka JC, Cooley PC, Burke DS. Strategies for mitigating an influenza pandemic. Nature. 2006;442:448–452. doi: 10.1038/nature04795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Germann TC, Kadau K, Longini IM, Jr, Macken CA. Mitigation strategies for pandemic influenza in the United States. Proc Natl Acad Sci USA. 2006;103:5935–5940. doi: 10.1073/pnas.0601266103. [DOI] [PMC free article] [PubMed] [Google Scholar]