Abstract
Over the past decade, a large body of evidence has emerged demonstrating an integration of metabolic and immune response pathways. It is now clear that obesity and associated disorders such as insulin resistance and type 2 diabetes are associated with a metabolically driven, low-grade, chronic inflammatory state, referred to as “metaflammation.” Several inflammatory cytokines as well as lipids and metabolic stress pathways can activate metaflammation, which targets metabolically critical organs and tissues including adipocytes and macrophages to adversely affect systemic homeostasis. On the other hand, inside the cell, fatty acid-binding proteins (FABPs), a family of lipid chaperones, as well as endoplasmic reticulum (ER) stress, and reactive oxygen species derived from mitochondria play significant roles in promotion of metabolically triggered inflammation. Here, we discuss the molecular and cellular basis of the roles of FABPs, especially FABP4 and FABP5, in metaflammation and related diseases including obesity, diabetes, and atherosclerosis.
1. Introduction
Inflammation is classically characterized as heat (calor), pain (dolor), redness (rubor), and swelling (tumor) [1]. The short-term adaptive response of inflammation is crucial for integration of injury response and repair in cells and tissues. However, the long-term consequences of prolonged inflammation are often not beneficial. It has recently been shown that low-grade and chronic features of inflammation are observed in metabolic diseases including obesity, insulin resistance, type 2 diabetes, and cardiovascular disease [2, 3]. This atypical immune response emerging from metabolic tissues is referred to as metabolically triggered inflammation, “metaflammation,” which is principally triggered by nutrients and metabolic surplus, resulting in the engagement of at least a subset of molecules and signaling pathways involved in classical and canonical inflammation [2].
A number of hormones, cytokines, and bioactive lipids function in both metabolic and immune responses. Metabolic and immune systems regulate each other by the same cellular machinery. In metabolically active cells such as adipocytes and macrophages, metaflammatory pathways can be initiated by not only extracellular mediators such as cytokines and lipids, particularly saturated fatty acids, but also by intracellular stresses such as endoplasmic reticulum stress and excess production of reactive oxygen species derived from mitochondria. Signals from all of these mediators converge on inflammatory signaling pathways, including signaling kinases: c-Jun N-terminal kinase (JNK), inhibitor of nuclear kappa B kinase (IKK), protein kinase R (PKR), and others. These pathways lead to the inhibition of insulin signaling [4–6] and a vicious spiral of additional production of inflammatory mediators through transcriptional regulation using activating protein-1 (AP-1) and nuclear factor-kappa B (NF-κB).
In this paper, we will focus on metabolically active cell-derived fatty acid-binding proteins (FABPs), which have been shown to regulate inflammatory and metabolic responses mainly in adipocytes and macrophages, and also discuss molecular and cellular links between FABPs and metaflammation, particularly in the context of metabolic diseases such as obesity, diabetes, and atherosclerosis.
2. Fatty Acid-Binding Protein (FABP) as a Lipid Chaperone
FABPs are a family of 14-15-kDa proteins that coordinate lipid trafficking and responses in cells [7]. FABPs can reversibly bind to hydrophobic ligands, such as saturated and unsaturated long-chain fatty acids, eicosanoids, and other lipids, with high affinity and broad selectivity. To date, at least 9 different FABP isoforms have been identified. Different members of the FABP family are expressed most abundantly in tissues involved in active lipid metabolism. The family contains liver (L-FABP/FABP1), intestinal (I-FABP/FABP2), heart (H-FABP/FABP3), adipocyte (A-FABP/FABP4/aP2), epidermal (E-FABP/FABP5/mal1), ileal (Il-FABP/FABP6), brain (B-FABP/FABP7), myelin (M-FABP/FABP8), and testis (T-FABP/FABP9) isoforms. FABPs have been proposed to facilitate the transport of lipids to specific compartments in the cell, such as to the mitochondrion or peroxisome for oxidation, to the nucleus for lipid-mediated transcriptional regulation, to the endoplasmic reticulum for signaling, trafficking, and membrane synthesis, to cytoplasmic enzymes for activity regulation, and to the cytoplasm for storage as lipid droplets. However, regulatory mechanisms of tissue-specific expression and function of various FABPs are still poorly understood. Specific contribution of each type of FABP to cell biology, physiology, and lipid metabolism had not been demonstrated until FABP-deficient mice models were created.
3. Adipocyte/Macrophage FABPs
Among the FABPs, FABP4, known as adipocyte FABP (A-FABP) or adipocyte P2 (aP2), is one of best-characterized isoforms (Table 1). FABP4 is highly expressed in adipocytes, making up about 1% of all soluble proteins in adipose tissue [8]. FABP5, another FABP known as epidermal FABP (E-FABP) or mal1, is expressed most abundantly in epidermal cells of the skin but is also present in several other tissues and cells including adipocytes [7] (Table 1). FABP5 constitutes a minor fraction of FABPs in adipocytes, the amount being about 100-fold smaller than that of FABP4 in adipocytes [9]. These two proteins, FABP4 and FABP5, have 52% amino acid similarity and bind to a variety of fatty acids with similar selectivity and affinity [10]. Interestingly, both FABP4 and FABP5 are also expressed in macrophages and dendritic cells [11, 12]. The stochiometry of these two proteins appears to be approximately equal in macrophages under physiological conditions [11]. The content of FABP4 in adipocytes is about 10,000-fold higher than that in macrophages [13]. In a state of germline FABP4 deficiency, FABP5 expression exhibits a strong compensatory increase in adipose tissue but not in macrophages or dendritic cells [11, 12, 14]. It has been demonstrated that both FABP4 and FABP5 play important roles in the regulation of insulin sensitivity and the development of atherosclerosis and that their impacts differentially involve adipocytes or macrophages [11, 14–22].
Table 1.
Features of FABP4 and FABP5 in metaflammation and related diseases.
| Expression | Regulation and function | Connection to diseases | Reference | |
|---|---|---|---|---|
| FABP4 | Adipocyte | Induction by fatty acids, PPARγ agonists, dexamethazone, and insulin | [23–27] | |
| Lipolysis (interaction with HSL) | [28–30] | |||
| Regulation of insulin secretion during lipolysis | [29] | |||
| Fatty acid sensor (interaction with JAK2) | [31] | |||
| Regulation of lipid metabolism and differentiation (interaction with PTEN) | [32] | |||
| Protection from insulin resistance and diabetes in deficient mice | Insulin resistance, diabetes | [14, 15, 18, 19, 21] | ||
| Protection from insulin resistance and diabetes by a FABP4 inhibitor | Insulin resistance, diabetes | [33] | ||
| Macrophage | Induction by PMA, LPS, PPARγ agonists, ox-LDL, and AGE/RAGE | [11, 34–38] | ||
| Reduction by atorvastatin and metformin | [39, 40] | |||
| Activation of IKK-NF-κB pathway | [41] | |||
| Activation of JNK-AP-1 pathway | [42] | |||
| Inhibition of PPARγ-LXRα-ABCA1 pathway | [41] | |||
| FOXO1-mediated transcription | [40] | |||
| Association with ER stress | [22] | |||
| Protection from insulin resistance and diabetes in double-deficient mice* | Insulin resistance, diabetes | [21] | ||
| Protection from atherosclerosis in deficient mice | Atherosclerosis | [11, 16, 20] | ||
| Protection from insulin resistance and atherosclerosis by a FABP4 inhibitor | Insulin resistance, atherosclerosis | [33] | ||
| Dendritic cell | Activation of IKK-NF-κB pathway | [12] | ||
| T-cell priming | [12] | |||
| Endothelial cell | Expression in capillary and small vein but not in artery | [43] | ||
| Regulation by VEGF-A/VEGFR2 and bFGF | [43] | |||
| Induction in regenerated endothelial cells after balloon denudation of artery | [44] | |||
| Induction by intermittent hypoxia | [45] | |||
| FOXO1-mediated transcription inhibited by angiopoietin-1 | [46] | |||
| Expression in aortic endothelium of old ApoE-deficient mice | [47] | |||
| Improvement of dysfunction in aortic endothelium by a FABP4 inhibitor | Endothelial dysfunction | [47] | ||
| Association with oxidative stress and activation of NF-κB and P53 pathways | Cellular senescence | [48, 49] | ||
| Bronchial epithelial cell | Induction by Th2 cytokines IL-4 and IL-13 | [13] | ||
| Suppression by Th1 cytokine interferon γ | [13] | |||
| Noninduction by PPARγ agonists | [13] | |||
| Protection from asthma in deficient mice | Asthma | [13] | ||
| Lung | Detection in lung lavage cells obtained from patients | Bronchopulmonary dysplasia | [50] | |
| Detection in lung lavage cells obtained from patients | Sarcoidosis | [51] | ||
| Ovary | Expression in granulosa cells inside atretic antral follicles | [52] | ||
| Association with FABP4 gene polymorphisms | Polycystic ovary syndrome | [53] | ||
| Spleen | Induction by dexamethazone | [54] | ||
| T cell | Induction by dexamethazone | [54] | ||
| Keratinocyte | Induction in PTEN-deficient keratinocytes | [55] | ||
| Tumor | Detection in tumor | Lipoblastoma, liposarcoma | [56] | |
| Detection in tumor | Urothelial carcinoma | [57] | ||
| FABP5 | Adipocyte | Lipolysis | [58] | |
| Protection from insulin resistance and diabetes in deficient mice | Insulin resistance, diabetes | [17–19, 21] | ||
| Induction of insulin resistance in adipose-specific transgenic mice | Insulin resistance, diabetes | [17] | ||
| Macrophage | Regulation by TLR agonists: LPS (TLR4) and zymosan (TLR2) | [59] | ||
| Induction of inflammatory genes, COX2 and IL-6 | [60] | |||
| Protection from insulin resistance and diabetes in double-deficient mice* | Insulin resistance, diabetes | [21] | ||
| Protection from atherosclerosis in deficient mice | Atherosclerosis | [20, 60] | ||
| Liver | Induction by a high-cholesterol diet feeding in LDL-receptor-deficient mice | [61] | ||
| Others | Expression in skin, dendritic cell, tongue, mammary gland, brain, intestine, kidney, lung, heart, skeletal muscle, testis, retina, lens, and spleen | [7] |
ABCA1: ATP-binding cassette A1; AGE: advanced glycation end products; AP-1: activating protein-1; ApoE: apolipoprotein E; bFGF: basic fibroblast growth factor; COX2: cyclooxygenase-2; ER: endoplasmic reticulum; FOXO1: forkhead box protein O1; HSL: hormone-sensitive lipase; IKK: inhibitor of nuclear kappa B kinase; IL: interleukin; JAK2: Janus kinase 2; JNK: c-Jun N-terminal kinase; LDL: low-density lipoprotein; LPS: lipopolysaccharide; LXR: liver X receptor; NF-κB: nuclear factor-kappa B; ox-LDL: oxidized LDL; PMA: phorbol 12-myristate 13-acetate; PPAR: peroxisome proliferator-activated receptor; PTEN: phosphatase and tensin homolog on chromosome 10; RAGE: receptor for AGE; TLR: Toll-like receptor; VEGF-A: vascular endothelial growth factor-A; VEGFR2: VEGF-receptor-2.
*FABP4−/−FABP5−/− mice.
3.1. FABP4 (A-FABP/aP2)
Expression of FABP4 in adipocytes is highly regulated during differentiation of adipocytes and is transcriptionally controlled by fatty acids, peroxisome proliferator-activated receptor (PPAR) γ agonists, dexamethasone, and insulin [23–27]. Potential functional domains of FABP4 have been reported to include a nuclear localization signal, its regulation site, and a nuclear export signal [7, 62, 63]. The primary sequence of FABP4 does not demonstrate a readily identifiable nuclear localization signal or nuclear export signal. However, the signals could be found in the tertiary structure of FABP4. It has also been shown that there is a protein-protein interaction between FABP4 and hormone-sensitive lipase [28]. In this model, it has been postulated that FABP4 binds to and activates hormone-sensitive lipase in adipocytes, resulting in regulation of lipolysis. Adipocytes in FABP4-deficient mice exhibited reduced efficiency of lipolysis [29, 30]. Interestingly, during experimentally induced lipolysis, FABP4-deficient mice also revealed reduction in insulin secretion [29]. As another protein-protein interaction, ligand-bound FABP4 has been reported to bind to Janus kinase 2 (JAK2) and attenuate its signaling, indicating a new role for FABP4 as a fatty acid sensor affecting cellular metabolism [31]. It has also been reported that phosphatase and tensin homolog on chromosome 10 (PTEN), which negatively regulates the phosphoinositide 3-kinase pathway, interacts with FABP4, possibly regulating of lipid metabolism and adipocyte differentiation [32]. Interestingly, PTEN-null keratinocytes showed an elevated expression of FABP4, suggesting that PTEN plays a role in the transcriptional regulation of FABP4 expression [55].
In the whole body metabolic phenotype, FABP4-deficient mice exhibited an increase in body weight but reduced insulin resistance in the context of both dietary and genetic obesity [14, 15]. RNA interference-mediated Fabp4 germline knockdown in mice on a high fat diet also increased body weight and fat mass but did not significantly affect glucose and lipid homeostasis [64], which is similar to phenotype of the diet-induced obesity in FABP4 heterozygous knockout mice [14] and indicates that residual FABP4 protein sustains some elements of its function in metabolic control.
In human and mouse monocyte cell lines, FABP4 expression is induced during differentiation from monocytes and by treatment with phorbol 12-myristate 13-acetate, lipopolysaccharide (LPS), PPARγ agonists, and oxidized low-density lipoprotein (ox-LDL) [11, 34–37]. FABP4 expression in macrophages was also elevated by advanced glycation end products (AGE) via engagement of the receptor for AGE (RAGE) [38]. Conversely, a cholesterol-lowering statin, atorvastatin, has been shown to suppress FABP4 expression in macrophages in vitro [39]. It has also been reported that metformin, an antidiabetic drug, inhibits forkhead box protein O1- (FOXO1-) mediated transcription of FABP4, leading to reduced lipid accumulation in macrophages [40].
In macrophages, FABP4 modulates cholesterol ester accumulation and foam cell formation via inhibition of the PPARγ-liver X receptor α (LXRα)-ATP-binding cassette A1 (ABCA1) pathway and induces inflammatory responses through activation of the IKK-NF-κB and JNK-AP-1 pathways [41, 42]. Deficiency of FABP4 protected against atherosclerosis in apolipoprotein E- (ApoE-) deficient mice with or without high-cholesterol-containing western diets [11, 16]. Bone marrow transplantation studies demonstrated that the protective effect of FABP4 deficiency on atherosclerosis is predominantly related to actions in macrophages rather than in adipocytes [11]. FABP4 in dendritic cells has been shown to regulate the IKK-NF-κB pathway and T-cell priming [12], which might contribute to the development of atherosclerosis since there is clear evidence for the involvement of both dendritic and T cells in the pathogenesis of atherosclerosis [65]. Involvement of FABP4 in atherosclerosis has also been indicated by clinical studies. In human endarterectomy samples of carotid stenosis, expression of FABP4 by macrophages was increased in unstable carotid plaques [66].
3.2. FABP5 (E-FABP/mal1)
Transgenic mice with adipose tissue-specific overexpression of FABP5 exhibited enhanced basal and hormone-stimulated lipolysis and a decrease in insulin sensitivity in a high-fat diet model [17, 58]. Deletion of FABP5 resulted in a mild increase in systemic insulin sensitivity in genetic and dietary obesity mouse models [17]. Adipocytes in FABP5-deficient mice showed an increased capacity for insulin-dependent glucose transport. Except for increased FABP3 (H-FABP) in the liver [67], there was no compensatory increase in the expression of FABPs in tissues including adipose tissue in FABP5-deficient mice [17]. Interestingly, feeding a western-type high-cholesterol diet increased the expression of FABP5, but not that of FABP1 (L-FABP), in liver parenchymal cells of atherosclerotic LDL-receptor- (LDLR-) deficient mice together with an increase in plasma levels of atherogenic lipoproteins, VLDL and LDL [61]. These observations indicate a specific role of FABP5 in atherogenesis.
FABP5 expression in macrophages was increased by treatment with Toll-like receptor (TLR) agonists: LPS, a TLR4 agonist, and zymosan, a fungal product that activates TLR2 [59]. A recent study showed that macrophage FABP5 deficiency suppressed atherosclerosis in LDLR-deficient mice on a western-style diet through a reduction of the expression of inflammatory genes, cyclooxygenase-2 and interleukin 6, and macrophage recruitment in atherosclerotic lesions due to decreased CC chemokine receptor 2 expression [60].
3.3. Combined Deficiency of FABP4 and FABP5
Mice with combined deficiency of FABP4 and FABP5 (Fabp4−/−Fabp5−/−) on a high-fat diet or in a genetic obesity model exhibit remarkably improved insulin resistance and protection against type 2 diabetes and fatty liver disease more than did FABP4- or FABP5-deficient mice [18, 19]. Furthermore, Fabp4−/−Fabp5−/− mice intercrossed into an ApoE-deficient atherosclerosis model developed dramatically less atherosclerosis than that in FABP4-deficient or wild-type mice on the same background [20]. Interestingly, Fabp4−/−Fabp5−/−Apoe−/− mice on a western-type hypercholesterolemic diet also had a significantly higher survival rate than that of Apoe−/− mice, presumably due to better plaque stability and good overall metabolic health [20].
It has recently been suggested that macrophage infiltration and accumulation in adipose tissue is an important feature of metaflammation triggered by obesity [68, 69]. Although the impact of FABP4/FABP5 on atherosclerosis was shown to be mainly due to actions in macrophages [11, 60], cell-based coculture experiments with adipocytes and macrophages and bone marrow transplantation using wild-type and Fabp4−/−Fabp5−/− mice showed that FABP actions in both adipocytes and macrophages have distinct roles in modulation of insulin sensitivity through inflammatory and metabolic responses as shown in Figure 1 [21]. In this setting, the predominant action was related to adipocyte FABPs with a more modest contribution from macrophages.
Figure 1.
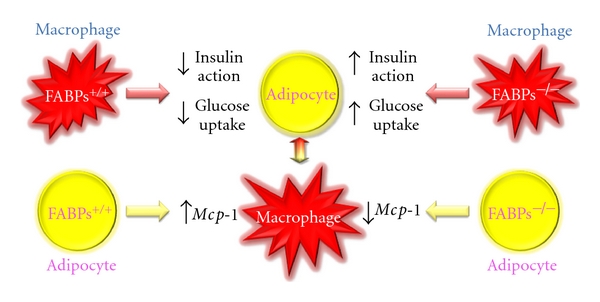
Interaction of adipocytes and macrophages. FABPs, FABP4, and FABP5, in adipocytes and macrophages, are critical for regulating inflammatory and metabolic responses in each type of cells and also interaction of the two types of cells.
4. Therapeutic Target for Diabetes and Atherosclerosis
Since FABP4 and FABP5 act at the interface of metabolic and inflammatory pathways and play a significant role in the development of insulin resistance, type 2 diabetes, and atherosclerosis, it is expected that modification of the function of these FABPs may provide a new class of multi-indication therapeutic agents. In fact, several series of FABP4 inhibitors have recently been identified [70–75]. We previously demonstrated that chemical inhibition of FABP4 could be a therapeutic strategy against insulin resistance, diabetes mellitus, fatty liver disease, and atherosclerosis in experimental models using one of the specific FABP4 inhibitors, BMS309403 [33]. This compound is an orally active small molecule and interacts with the fatty acid-binding pocket within the interior of FABP4 to inhibit binding of endogenous fatty acids [7, 33, 72] (Figure 2). X-ray crystallographic studies identified the specific interactions of BMS309403 with key residues, such as Ser53, Arg106, Arg126, and Tyr128, within the fatty-acid-binding pocket as the basis of its high in vitro binding affinity and selectivity for FABP4 over other FABPs [72].
Figure 2.
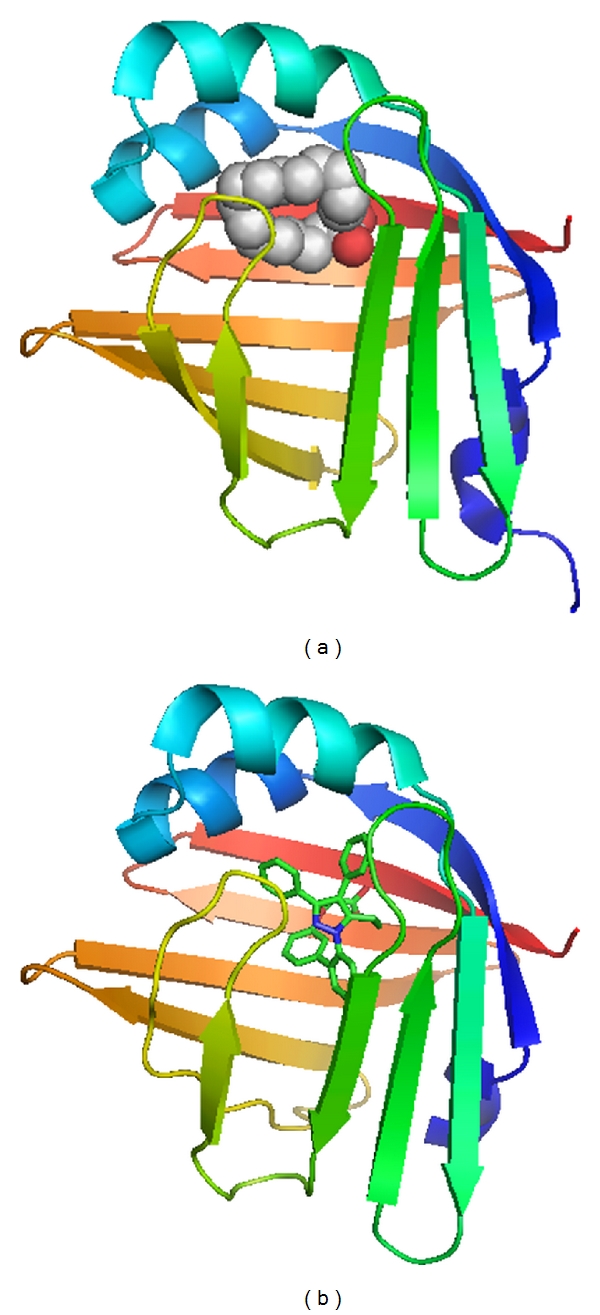
FABP4 bound with a fatty acid or a small molecule inhibitor. (a) Human FABP4 binds to an endogenous fatty acid, palmitic acid, as a twisted U-shaped entity in the binding pocket (PDB code: 2hnx). (b) Human FABP4 crystallized in complex with BMS309403, a synthetic FABP4 inhibitor, is shown (PDB code: 2nnq). The molecule occupies the internal binding pocket of FABP4 and competitively inhibits binding of endogenous fatty acids. The figures were created using PyMOL.
The FABP4 inhibitor, BMS309403, improved glucose metabolism and enhanced insulin sensitivity in both dietary (high fat-fed) and genetic (ob/ob) mouse models of obesity and diabetes [33]. Involvement of FABP4 inhibition in those beneficial effects was confirmed in vivo using wild-type and Fabp4−/−Fabp5−/− mice. Although Fabp4−/− mice were not protected against fatty liver disease, inhibition of FABP4 suppressed fatty liver infiltration, similar to the liver phenotype of Fabp4−/−Fabp5−/− mice. One possible explanation for the different effects between genetic deficiency of FABP4 and chemical inhibition of FABP4 is that there was no compensatory increase in FABPs in the adipose tissue of FABP4-inhibitor-treated mice. Furthermore, the FABP4 inhibitor markedly reduced the extent of atherosclerotic lesions in ApoE-deficient mice [33]. Cell-based studies showed that BMS309403 reduced macrophage foam cell formation with decreased cholesterol ester accumulation, increased cholesterol efflux, and decreased production of several inflammatory mediators in a target tissue-specific manner [33].
In high fat-diet-induced obesity models beginning at 4 weeks of age, treatment with the FABP4 inhibitor for 4 weeks improved insulin sensitivity in 24-week-old mice [33], which had severe macrophage infiltration in adipose tissue, but not in 20-week-old mice, which had much less macrophage accumulation in adipose tissue (Furuhashi M and Hotamisligil GS. unpublished data 2007). Recently, a similar pattern was also found in another study in which a different inhibitor was not effective in increasing insulin sensitivity [75]. It is difficult to completely inhibit whole FABP4 in adipocytes because the amounts of FABP4 in adipose tissue and adipocytes are very large [8], and these observations therefore raise the possibility that small molecules developed so far against FABP4 may be more effective in macrophages and hence their effects in vivo may be related to the extent of macrophage involvement with the disease process at the stage that these molecules are tested. Undoubtedly, future studies and alternative strategies to modulate FABP action, alone or in combination, in disease models should address these outstanding issues. Further studies are also needed to determine whether chemical or other modes of inhibition of FABP4 can be safely used in humans and to demonstrate their efficacy for metabolic diseases.
5. Ectopic Expression of FABP4
There is accumulating evidence to indicate that FABP4 is expressed in several cells other than adipocytes and macrophages under both special and physiological conditions (Table 1). For example, FABP4 expression was observed in endothelial cells of capillaries and small veins in several mouse and human tissues, including the heart and kidney [43]. FABP4 was significantly induced by treatment with vascular endothelial growth factor-A (VEGF-A) via VEGF-receptor-2 (VEGFR2) and by treatment with basic fibroblast growth factor (bFGF) in endothelial cells [43]. Conversely, knockdown of FABP4 in endothelial cells reduced proliferation both under baseline conditions and in response to VEGF-A and bFGF, suggesting that FABP4 is a target of the VEGF-A/VEGFR2 pathway and a positive regulator of cell proliferation in endothelial cells.
Interesting observations have been reported for roles of FABP4 in vascular injury. FABP4 was markedly upregulated in regenerated endothelial cells obtained after endothelial balloon denudation in vivo [44]. In human aortic endothelial cells, intermittent hypoxia increased FABP4 expression [45]. Anigiopoietin-1, which participates in blood vessel stabilization and remodeling together with angiopoietin-2, inhibited FOXO1-mediated expression of genes including FABP4 in endothelial cells [46]. FABP4 was expressed in the aortic endothelium of 12-week-old ApoE-deficient mice showing endothelial dysfunction, whereas FABP4 was not detected at the aortic endothelium in 8-week-old ApoE-deficient mice or in wild-type mice [47]. Chronic administration of BMS309403, a small molecule FABP4 inhibitor, significantly improved endothelial dysfunction in ApoE-deficient mice [47]. Notably, recent studies have shown possible involvement of FABP4/FABP5 in senescence of endothelial cells [48, 49]. These observations support the notion that pathological induction, but not physiological expression, of FABP4 in the endothelium significantly contributes to pathogenesis of atherosclerosis and other types of vascular injury.
Evidence is also accumulating as for involvement of FABP4 in respiratory diseases. Recently, FABP4 has been reported to be detected in lungs and bronchoalveolar samples from patients with bronchopulmonary dysplasia (BPD) [50]. Density of FABP4-positive endothelial cells was increased in peribronchial blood vessels, and FABP4 was also localized in a subset of macrophages in lung tissues. Several studies using lung lavage cells suggested that FABP4 gene expression is responsible for pathogenesis of sarcoidosis [51]. It is notable that expression of FABP4 in human bronchial epithelial cells is under regulation of cytokines. FABP4 expression in bronchial epithelial cells was enhanced by the Th2 cytokines IL-4 and IL-13, which are involved in development of asthma, and was suppressed by the Th1 cytokine interferon γ [13]. Interestingly, FABP4-deficient mice were protected from airway inflammation independently of bone marrow-derived elements, indicating possible protection against asthma through FABP action in stromal cells [13]. However, it should be noted that there are possible differences in response of FABP4 to stimuli depending on cell types. FABP4 expression in bronchial epithelial cells was significantly lower than that in adipocytes and macrophages, even after stimulation. In contrast to its effects in adipocytes and macrophages, PPARγ agonists could not induce FABP4 expression in bronchial epithelial cells. Such tissue-specific roles and response of FABP4 need to be taken into account for FABP4 modulating therapy.
In atretic antral follicles of the mouse ovary, FABP4 was detected in apoptotic granulosa cells [52], suggesting a possible relevance to polycystic ovary syndrome (PCOS), which often coexists with insulin resistance. Interestingly, association between FABP4 gene polymorphisms and the development of PCOS has been reported [53]. Additionally, dexamethasone treatment induced FABP4 in mouse spleen and in cultured T lymphocytes, and its distinct nuclear localization occurred with the dexamethasone-induced apoptosis process [54].
FABP4 expression was also detected in lipoblasts in lipoblastoma and liposarcoma but not in other benign adipose tissue or malignant connective tissue or in epithelial tumors [56]. Moreover, FABP4 expression has been linked to human urothelial carcinomas [57]. The significance of these associations remains to be elucidated but points to potential utility of FABP-based strategies to explore metabolic mechanisms related to tumorigenesis and related therapeutic possibilities.
6. Secretion and Circulating Concentrations of FABPs
In recent years, numerous studies have shown the presence of FABPs in circulation. Since these cytoplasmic proteins lack a secretory signal sequence, the presence of FABPs in serum is considered to be a biochemical marker of tissue injury in related cells that produce FABP proteins: FABP3 (H-FABP) for acute myocardial infarction and ongoing myocardial damage in heart failure, FABP7 (B-FABP) for brain injury, and FABP2 (I-FABP) for intestinal damage [76–78]. It has recently been reported that FABP4 is detected in serum and cultured adipocyte supernatants [79] and that the serum concentration of FABP4 is associated with obesity, type 2 diabetes, and cardiovascular diseases [79–82]. Similar findings have also been reported for FABP5 [83, 84]. Proteomics analysis using differentiated THP-1 macrophages revealed the presence of FABP4 and FABP5 in cell supernatants derived from macrophages [85]. However, the mechanisms and biological correlates of extracellular FABP4 and FABP5 remain unknown.
Serum levels of FABP4 were significantly increased in overweight and obese subjects compared to the level in lean controls and were positively correlated with waist circumference, blood pressure, and insulin resistance [79]. Similar to FABP4, circulating FABP5 levels were detected at the level of about one tenth or less of FABP4 concentrations and were associated with metabolic syndrome components [83, 84]. High serum levels of FABP4 at baseline independently predicted the development of metabolic syndrome during a 5-year follow-up period in a Chinese population [80]. A 10-year prospective study showed that high FABP4 concentration at baseline was a biomarker predicting development of type 2 diabetes, which was independent of obesity and insulin resistance [81]. Furthermore, it has also been reported that serum FABP4 levels are positively correlated with carotid intima-media thickness as an index of atherosclerosis [82]. These findings support the notion that FABP4 is a biomarker of ongoing atherosclerosis. Interestingly, serum levels of FABP4 could also represent noncardiovascular pathologic processes as well. A recent study has shown that FABP4 levels could be a novel and obesity-independent prognostic factor in patients with breast cancer [86].
Several drugs have been reported to modify FABP4 levels in blood. Atorvastatin, a HMG-CoA reductase inhibitor, and olmesartan, an angiotensin II receptor blocker, reduced circulating FABP4 levels [87, 88], whereas pioglitazone, an insulin-sensitizing thiazolidinedione (a PPARγ agonist), increased FABP4 concentrations [89], which could be explained through direct activation of PPARγ since the PPAR response element is present in the FABP4 gene promoter [90]. As general information for circulating FABPs, the concentrations of FABPs are influenced by renal clearance [91–93], and it might be necessary to evaluate the role of renal dysfunction in regulation of FABP level. Future studies should provide further insights into these phenomenon and how they contribute to disease progression in related FABP isoforms.
7. Lipokine
Meticulous lipidomic analyses using several samples including adipose tissue, liver, skeletal muscle, and blood from Fabp4−/−Fabp5−/− and wild-type mice showed markedly increased de novo lipogenesis in adipose tissue resulting from activation/induction of fatty acid synthase (FAS) and stearoyl-CoA desaturase-1 (SCD-1) [94]. Consequently, an unsaturated free fatty acid, palmitoleate (C16:1n7), was identified as an adipose tissue-derived lipid hormone, referred to as “lipokine,” that strongly suppresses hepatosteatosis and stimulates glucose transport in skeletal muscle [94]. That study revealed a lipid-mediated endocrine network of tissues/organs, in which adipose tissue uses lipokines such as palmitoleate to communicate with distant organs, regulating systemic metabolic homeostasis. Absence of FABP4 in macrophages also resulted in an activation of de novo lipogenesis pathways particularly through LXRα-mediated activation of SCD-1 [22]. This enhanced lipogenesis induced production of bioactive lipids including palmitoleate and resistance to ER stress. These changes also translate into protection against atherosclerosis in mouse models [22]. Conversely, unsaturated fatty acids including palmitoleate repressed basal and LPS-induced FABP4 expression in macrophages via the modulation of histone deacetylation [95].
After results of animal studies on a lipokine were reported [94], palmitoleate in humans was examined in several studies in the context of metabolic disease, particularly in determining the risk for insulin resistance and type 2 diabetes. In a study that recruited 100 Caucasian subjects, circulating palmitoleate was positively correlated with insulin sensitivity assessed by euglycemic-hyperinsulinemic clamp studies, independent of age, gender, and adiposity [96]. Another study using 3630 subjects in the US showed that high concentrations of circulating cis isomer palmitoleate, which is primarily produced by the liver in humans, were associated with adiposity, carbohydrate consumption, and alcohol use [97]. However, the associations between circulating cis palmitoleate and metabolic risk factors were complex, perhaps related to divergent lifestyle determinants or tissue sources of endogenous palmitoleate synthesis from liver and adipose tissue: high fat- and carbohydrate-containing diet and fatty liver would confound or modify the ability to detect its metabolic effects [97]. Interestingly, it has recently been reported that circulating trans isomer of palmitoleate, an exogenous source of C16:1n7, is associated with markedly lower insulin resistance, higher HDL-cholesterol level, and lower incidence of diabetes, suggesting metabolic benefits of dairy product consumption [98]. Since this isoform is not related to endogenous production, the relation to reduced metabolic disease points to possibilities of the utilization of the trans isomer of palmitoleate as a potential strategy for intervention in human diseases.
8. Concluding Remarks
FABPs, especially FABP4 and FABP5, play significant roles in the regulation of glucose and lipid metabolism linked to inflammatory and metabolic processes through modulating critical lipid-sensitive pathways in target cells, adipocytes, and macrophages. There was no compromised phenotype of FABP4- or FABP5-deficient mice under normal physiologic conditions [14, 17]. However, the mice in the context of dietary or genetic obesity were protected from systemic pathologic stresses such as metaflammation, suggesting that the adipocyte/macrophage FABP genes may represent an example of the “thrifty” gene hypothesis [99]. FABPs have been evolutionarily preserved from invertebrates (lower eukaryotes) to vertebrates including humans [100], indicating that a close and conserved link between inflammatory and metabolic responses underlies the conservation of FABP function. The presence of these FABPs may have been beneficial for ensuring a strong macrophage immune response under pressure with pathogens or for maintaining adipose tissue energy stores as part of the “thrifty” phenotype to survive in famine. Under contemporary life-style accompanied by excessive caloric intake and decreased energy expenditure, presence or induction of adipocyte/macrophage FABPs may be rather disadvantageous for maintaining inflammatory or metabolic homeostasis. FABPs appear to be responsible for the development of obesity, diabetes, dyslipidemia, and atherosclerosis, and targeting the adipocyte/macrophage FABPs, particularly FABP4, offers highly attractive therapeutic opportunities for intervening metabolic derangements as an evolutionary bottleneck in humans. Much work is still needed to elucidate the precise biological functions of different forms of FABPs and to establish strategies to target these proteins for therapeutic purposes.
Acknowledgments
In relation to this paper, M. Furuhashi has been supported by grants from Grant-in-Aid for Scientific Research from The Ministry of Education, Culture, Sports, Science and Technology, Uehara Memorial Foundation, Mitsubishi Pharma Research Foundation, Natio Foundation Natural Science Scholarship, Takeda Science Foundation, Mochida Memorial Foundation for Medical and Pharmaceutical Research, Kanae Foundation for the Promotion of Medical Science, Cardiovascular Research Foundation, Suzuken Memorial Foundation, Sumitomo Foundation, Tokyo Biochemical Research Foundation, Japan Diabetes Foundation, Ono Medical Research Foundation, Novartis Foundation (Japan) for the Promotion of Science, Akiyama Life Science Foundation, Japan Foundation for Applied Enzymology, and Ichiro Kanehara Foundation. The authors would like to acknowledge Dr. Gökhan S. Hotamisligil (Harvard School of Public Health) for invaluable advice and discussion. They are grateful to group members of their department, IZAYOI (Boston, Mass), and G-PUC (Sapporo, Japan) for their scientific inputs and contribution. They also regret the inadvertent omission of many important references owing to space limitations.
References
- 1.Larsen GL, Henson PM. Mediators of inflammation. Annual Review of Immunology. 1983;1:335–359. doi: 10.1146/annurev.iy.01.040183.002003. [DOI] [PubMed] [Google Scholar]
- 2.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 3.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annual Review of Immunology. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 4.Hirosumi J, Tuncman G, Chang L, et al. A central, role for JNK in obesity and insulin resistance. Nature. 2002;420(6913):333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 5.Yuan M, Konstantopoulos N, Lee J, et al. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkβ . Science. 2001;293(5535):1673–1677. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- 6.Nakamura T, Furuhashi M, Li P, et al. Double-stranded RNA-dependent protein kinase links pathogen sensing with stress and metabolic homeostasis. Cell. 2010;140(3):338–348. doi: 10.1016/j.cell.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Furuhashi M, Hotamisligil GS. Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nature Reviews Drug Discovery. 2008;7(6):489–503. doi: 10.1038/nrd2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baxa CA, Sha RS, Buelt MK, et al. Human adipocyte lipid-binding protein: purification of the protein and cloning of its complementary DNA. Biochemistry. 1989;28(22):8683–8690. doi: 10.1021/bi00448a003. [DOI] [PubMed] [Google Scholar]
- 9.Simpson MA, LiCata VJ, Coe NR, Bernlohr DA. Biochemical and biophysical analysis of the intracellular lipid binding proteins of adipocytes. Molecular and Cellular Biochemistry. 1999;192(1-2):33–40. [PubMed] [Google Scholar]
- 10.Haunerland NH, Spener F. Fatty acid-binding proteins—insights from genetic manipulations. Progress in Lipid Research. 2004;43(4):328–349. doi: 10.1016/j.plipres.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Makowski L, Boord JB, Maeda K, et al. Lack of macrophage fatty-acid-binding protein aP2 protects mice deficient in apolipoprotein E against atherosclerosis. Nature Medicine. 2001;7(6):699–705. doi: 10.1038/89076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rolph MS, Young TR, Shum BOV, et al. Regulation of dendritic cell function and T cell priming by the fatty acid-binding protein aP2. The Journal of Immunology. 2006;177(11):7794–7801. doi: 10.4049/jimmunol.177.11.7794. [DOI] [PubMed] [Google Scholar]
- 13.Shum BOV, Mackay CR, Gorgun CZ, et al. The adipocyte fatty acid-binding protein aP2 is required in allergic airway inflammation. The Journal of Clinical Investigation. 2006;116(8):2183–2192. doi: 10.1172/JCI24767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hotamisligil GS, Johnson RS, Distel RJ, Ellis R, Papaioannou VE, Spiegelman BM. Uncoupling of obesity from insulin resistance through a targeted mutation in aP2, the adipocyte fatty acid binding protein. Science. 1996;274(5291):1377–1379. doi: 10.1126/science.274.5291.1377. [DOI] [PubMed] [Google Scholar]
- 15.Uysal KT, Scheja L, Wiesbrock SM, Bonner-Weir S, Hotamisligil GS. Improved glucose and lipid metabolism in genetically obese mice lacking aP2. Endocrinology. 2000;141(9):3388–3396. doi: 10.1210/endo.141.9.7637. [DOI] [PubMed] [Google Scholar]
- 16.Boord JB, Maeda K, Makowski L, et al. Adipocyte fatty acid-binding protein, aP2, alters late atherosclerotic lesion formation in severe hypercholesterolemia. Arteriosclerosis, Thrombosis, and Vascular Biology. 2002;22(10):1686–1691. doi: 10.1161/01.atv.0000033090.81345.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maeda K, Uysal KT, Makowski L, et al. Role of the fatty acid binding protein mal1 in obesity and insulin resistance. Diabetes. 2003;52(2):300–307. doi: 10.2337/diabetes.52.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maeda K, Cao H, Kono K, et al. Adipocyte/macrophage fatty acid binding proteins control integrated metabolic responses in obesity and diabetes. Cell Metabolism. 2005;1(2):107–119. doi: 10.1016/j.cmet.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Cao H, Maeda K, Gorgun CZ, et al. Regulation of metabolic responses by adipocyte/macrophage fatty acid-binding proteins in leptin-deficient mice. Diabetes. 2006;55(7):1915–1922. doi: 10.2337/db05-1496. [DOI] [PubMed] [Google Scholar]
- 20.Boord JB, Maeda K, Makowski L, et al. Combined adipocyte-macrophage fatty acid-binding protein deficiency improves metabolism, atherosclerosis, and survival in apolipoprotein E-deficient mice. Circulation. 2004;110(11):1492–1498. doi: 10.1161/01.CIR.0000141735.13202.B6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Furuhashi M, Fucho R, Görgün CZ, Tuncman G, Cao H, Hotamisligil GS. Adipocyte/macrophage fatty acid-binding proteins contribute to metabolic deterioration through actions in both macrophages and adipocytes in mice. The Journal of Clinical Investigation. 2008;118(7):2640–2650. doi: 10.1172/JCI34750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erbay E, Babaev VR, Mayers JR, et al. Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nature Medicine. 2009;15(12):1383–1391. doi: 10.1038/nm.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amri EZ, Bertrand B, Ailhaud G, Grimaldi P. Regulation of adipose cell differentiation. I. Fatty acids are inducers of the aP2 gene expression. Journal of Lipid Research. 1991;32(9):1449–1456. [PubMed] [Google Scholar]
- 24.Distel RJ, Robinson GS, Spiegelman BM. Fatty acid regulation of gene expression. Transcriptional and post- transcriptional mechanisms. The Journal of Biological Chemistry. 1992;267(9):5937–5941. [PubMed] [Google Scholar]
- 25.Kletzien RF, Foellmi LA, Harris PKW, Wyse BM, Clarke SD. Adipocyte fatty acid-binding protein: regulation of gene expression in vivo and in vitro by an insulin-sensitizing agent. Molecular Pharmacology. 1992;42(4):558–562. [PubMed] [Google Scholar]
- 26.Cook JS, Lucas JJ, Sibley E, et al. Expression of the differentiation-induced gene for fatty acid-binding protein is activated by glucocorticoid and cAMP. Proceedings of the National Academy of Sciences of the United States of America. 1988;85(9):2949–2953. doi: 10.1073/pnas.85.9.2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Melki SA, Abumrad NA. Expression of the adipocyte fatty acid-binding protein in streptozotocin- diabetes: effects of insulin deficiency and supplementation. Journal of Lipid Research. 1993;34(9):1527–1534. [PubMed] [Google Scholar]
- 28.Shen WJ, Sridhar K, Bernlohr DA, Kraemer FB. Interaction of rat hormone-sensitive lipase with adipocyte lipid-binding protein. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(10):5528–5532. doi: 10.1073/pnas.96.10.5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scheja L, Makowski L, Uysal KT, et al. Altered insulin secretion associated with reduced lipolytic efficiency in aP2−/− mice. Diabetes. 1999;48(10):1987–1994. doi: 10.2337/diabetes.48.10.1987. [DOI] [PubMed] [Google Scholar]
- 30.Coe NR, Simpson MA, Bernlohr DA. Targeted disruption of the adipocyte lipid-binding protein (aP2 protein) gene impairs fat cell lipolysis and increases cellular fatty acid levels. Journal of Lipid Research. 1999;40(5):967–972. [PubMed] [Google Scholar]
- 31.Thompson BR, Mazurkiewicz-Muñoz AM, Suttles J, Carter-Su C, Bernlohr DA. Interaction of adipocyte fatty acid-binding protein (AFABP) and JAK2: AFABP/aP2 as a regulator of JAK2 signaling. The Journal of Biological Chemistry. 2009;284(20):13473–13480. doi: 10.1074/jbc.M900075200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gorbenko O, Panayotou G, Zhyvoloup A, Volkova D, Gout I, Filonenko V. Identification of novel PTEN-binding partners: PTEN interaction with fatty acid binding protein FABP4. Molecular and Cellular Biochemistry. 2010;337(1-2):299–305. doi: 10.1007/s11010-009-0312-1. [DOI] [PubMed] [Google Scholar]
- 33.Furuhashi M, Tuncman G, Görgün CZ, et al. Treatment of diabetes and atherosclerosis by inhibiting fatty-acid-binding protein aP2. Nature. 2007;447(7147):959–965. doi: 10.1038/nature05844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fu Y, Luo N, Lopes-Virella MF, Garvey WT. The adipocyte lipid binding protein (ALBP/aP2) gene facilitates foam cell formation in human THP-1 macrophages. Atherosclerosis. 2002;165(2):259–269. doi: 10.1016/s0021-9150(02)00305-2. [DOI] [PubMed] [Google Scholar]
- 35.Kazemi MR, McDonald CM, Shigenaga JK, Grunfeld C, Feingold KR. Adipocyte fatty acid-binding protein expression and lipid accumulation are increased during activation of murine macrophages by toll-like receptor agonists. Arteriosclerosis, Thrombosis, and Vascular Biology. 2005;25(6):1220–1224. doi: 10.1161/01.ATV.0000159163.52632.1b. [DOI] [PubMed] [Google Scholar]
- 36.Pelton PD, Zhou L, Demarest KT, Burris TP. PPARγ activation induces the expression of the adipocyte fatty acid binding protein gene in human monocytes. Biochemical and Biophysical Research Communications. 1999;261(2):456–458. doi: 10.1006/bbrc.1999.1071. [DOI] [PubMed] [Google Scholar]
- 37.Fu Y, Luo N, Lopes-Virella MF. Oxidized LDL induces the expression of ALBP/aP2 mRNA and protein in human THP-1 macrophages. Journal of Lipid Research. 2000;41(12):2017–2023. [PubMed] [Google Scholar]
- 38.Wang XQ, Yang K, He YS, Lu L, Shen WF. Receptor mediated elevation in FABP4 levels by advanced glycation end products induces cholesterol and triacylglycerol accumulation in THP-1 macrophages. Lipids. 2011;46(6):479–486. doi: 10.1007/s11745-011-3542-4. [DOI] [PubMed] [Google Scholar]
- 39.Llaverias G, Noé V, Peñuelas S, et al. Atorvastatin reduces CD68, FABP4, and HBP expression in oxLDL-treated human macrophages. Biochemical and Biophysical Research Communications. 2004;318(1):265–274. doi: 10.1016/j.bbrc.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 40.Song J, Ren P, Zhang L, Wang XL, Chen L, Shen YH. Metformin reduces lipid accumulation in macrophages by inhibiting FOXO1-mediated transcription of fatty acid-binding protein 4. Biochemical and Biophysical Research Communications. 2010;393(1):89–94. doi: 10.1016/j.bbrc.2010.01.086. [DOI] [PubMed] [Google Scholar]
- 41.Makowski L, Brittingham KC, Reynolds JM, Suttles J, Hotamisligil GS. The fatty acid-binding protein, aP2, coordinates macrophage cholesterol trafficking and inflammatory activity: macrophage expression of aP2 impacts peroxisome proliferator-activated receptor γ and IκB kinase activities. The Journal of Biological Chemistry. 2005;280(13):12888–12895. doi: 10.1074/jbc.M413788200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hui X, Li H, Zhou Z, et al. Adipocyte fatty acid-binding protein modulates inflammatory responses in macrophages through a positive feedback loop involving c-Jun NH 2-terminal kinases and activator protein-1. The Journal of Biological Chemistry. 2010;285(14):10273–10280. doi: 10.1074/jbc.M109.097907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elmasri H, Karaaslan C, Teper Y, et al. Fatty acid binding protein 4 is a target of VEGF and a regulator of cell proliferation in endothelial cells. The FASEB Journal. 2009;23(11):3865–3873. doi: 10.1096/fj.09-134882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee MYK, Tse HF, Siu CW, Zhu SG, Man RYK, Vanhoutte PM. Genomic changes in regenerated porcine coronary arterial endothelial cells. Arteriosclerosis, Thrombosis, and Vascular Biology. 2007;27(11):2443–2449. doi: 10.1161/ATVBAHA.107.141705. [DOI] [PubMed] [Google Scholar]
- 45.Han Q, Yeung SC, Ip MSM, Mak JCW. Effects of intermittent hypoxia on A-/E-FABP expression in human aortic endothelial cells. International Journal of Cardiology. 2010;145(2):396–398. doi: 10.1016/j.ijcard.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 46.Daly C, Wong V, Burova E, et al. Angiopoietin-1 modulates endothelial cell function and gene expression via the transcription factor FKHR (FOXO1) Genes and Development. 2004;18(9):1060–1071. doi: 10.1101/gad.1189704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee MYK, Li H, Xiao Y, Zhou Z, Xu A, Vanhoutte PM. Chronic administration of BMS309403 improves endothelial function in apolipoprotein E-deficient mice and in cultured human endothelial cells. British Journal of Pharmacology. 2011;162(7):1564–1576. doi: 10.1111/j.1476-5381.2010.01158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ha MK, Cho JS, Baik OR, Hoon LK, Koo HS, Chung KY. Caenorhabditis elegans as a screening tool for the endothelial cell-derived putative aging-related proteins detected by proteomic analysis. Proteomics. 2006;6(11):3339–3351. doi: 10.1002/pmic.200500395. [DOI] [PubMed] [Google Scholar]
- 49.Lee MYK, Wang Y, Vanhoutte PM. Senescence of cultured porcine coronary arterial endothelial cells is associated with accelerated oxidative stress and activation of nfκB. Journal of Vascular Research. 2010;47(4):287–298. doi: 10.1159/000265563. [DOI] [PubMed] [Google Scholar]
- 50.Ghelfi E, Karaaslan C, Berkelhamer S, et al. Fatty acid binding proteins and peribronchial angiogenesis in bronchopulmonary dysplasia. American Journal of Respiratory Cell and Molecular Biology. 2011;45(3):550–556. doi: 10.1165/rcmb.2010-0376OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maver A, Medica I, Peterlin B. Search for sarcoidosis candidate genes by integration of data from genomic, transcriptomic and proteomic studies. Medical Science Monitor. 2009;15(12):SR22–SR28. [PubMed] [Google Scholar]
- 52.Nourani MR, Owada Y, Kitanaka N, et al. Occurrence of immunoreactivity for adipocyte-type fatty acid binding protein in degenerating granulosa cells in atretic antral follicles of mouse ovary. Journal of Molecular Histology. 2005;36(8-9):491–497. doi: 10.1007/s10735-006-9024-y. [DOI] [PubMed] [Google Scholar]
- 53.Wang J, Tang J, Wang B, et al. FABP4: a novel candidate gene for polycystic ovary syndrome. Endocrine. 2009;36(3):392–396. doi: 10.1007/s12020-009-9228-5. [DOI] [PubMed] [Google Scholar]
- 54.Abdelwahab SA, Owada Y, Kitanaka N, et al. Enhanced expression of adipocyte-type fatty acid binding protein in murine lymphocytes in response to dexamethasone treatment. Molecular and Cellular Biochemistry. 2007;299(1-2):99–107. doi: 10.1007/s11010-005-9050-1. [DOI] [PubMed] [Google Scholar]
- 55.Tsuda M, Inoue-Narita T, Suzuki A, Itami S, Blumenberg M, Manabe M. Induction of gene encoding FABP4 in Pten-null keratinocytes. FEBS Letters. 2009;583(8):1319–1322. doi: 10.1016/j.febslet.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 56.Bennett JH, Shousha S, Puddle B, Athanasou NA. Immunohistochemical identification of tumours of adipocytic differentiation using an antibody to aP2 protein. Journal of Clinical Pathology. 1995;48(10):950–954. doi: 10.1136/jcp.48.10.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ohlsson G, Moreira JMA, Gromov P, Sauter G, Celis JE. Loss of expression of the adipocyte-type fatty acid-binding protein (A-FABP) is associated with progression of human urothelial carcinomas. Molecular and Cellular Proteomics. 2005;4(4):570–581. doi: 10.1074/mcp.M500017-MCP200. [DOI] [PubMed] [Google Scholar]
- 58.Vogel Hertzel A, Bennaars-Eiden A, Bernlohr DA. Increased lipolysis in transgenic animals overexpressing the epithelial fatty acid binding protein in adipose cells. Journal of Lipid Research. 2002;43(12):2105–2111. doi: 10.1194/jlr.m200227-jlr200. [DOI] [PubMed] [Google Scholar]
- 59.Feingold KR, Kazemi MR, Magra AL, et al. ADRP/ADFP and Mal1 expression are increased in macrophages treated with TLR agonists. Atherosclerosis. 2010;209(1):81–88. doi: 10.1016/j.atherosclerosis.2009.08.042. [DOI] [PubMed] [Google Scholar]
- 60.Babaev VR, Runner RP, Fan D, et al. Macrophage mal1 deficiency suppresses atherosclerosis in low-density lipoprotein receptor-null mice by activating peroxisome proliferator-activated receptor-γ-regulated genes. Arteriosclerosis, Thrombosis, and Vascular Biology. 2011;31(6):1283–1290. doi: 10.1161/ATVBAHA.111.225839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hoekstra M, Stitzinger M, Van Wanrooij EJA, et al. Microarray analysis indicates an important role for FABP5 and putative novel FABPs on a Western-type diet. Journal of Lipid Research. 2006;47(10):2198–2207. doi: 10.1194/jlr.M600095-JLR200. [DOI] [PubMed] [Google Scholar]
- 62.Avers SD, Nedrow KL, Gillilan RE, Noy N. Continuous nucleocytoplasmic shuttling underlies transcriptional activation of PPARγ by FABP4. Biochemistry. 2007;46(23):6744–6752. doi: 10.1021/bi700047a. [DOI] [PubMed] [Google Scholar]
- 63.Gillilan RE, Ayers SD, Noy N. Structural basis for activation of fatty acid-binding protein 4. Journal of Molecular Biology. 2007;372(5):1246–1260. doi: 10.1016/j.jmb.2007.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang R, Castriota G, Chen Y, et al. RNAi-mediated germline knockdown of FABP4 increases body weight but does not improve the deranged nutrient metabolism of diet-induced obese mice. International Journal of Obesity. 2010;35(2):217–225. doi: 10.1038/ijo.2010.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nature Immunology. 2011;12(3):204–212. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 66.Agardh HE, Folkersen L, Ekstrand J, et al. Expression of fatty acid-binding protein 4/aP2 is correlated with plaque instability in carotid atherosclerosis. Journal of Internal Medicine. 2011;269(2):200–210. doi: 10.1111/j.1365-2796.2010.02304.x. [DOI] [PubMed] [Google Scholar]
- 67.Owada Y, Suzuki I, Noda T, Kondo H. Analysis on the phenotype of E-FABP-gene knockout mice. Molecular and Cellular Biochemistry. 2002;239(1-2):83–86. [PubMed] [Google Scholar]
- 68.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. The Journal of Clinical Investigation. 2003;112(12):1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. The Journal of Clinical Investigation. 2003;112(12):1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lehmann F, Haile S, Axen E, et al. Discovery of inhibitors of human adipocyte fatty acid-binding protein, a potential type 2 diabetes target. Bioorganic and Medicinal Chemistry Letters. 2004;14(17):4445–4448. doi: 10.1016/j.bmcl.2004.06.057. [DOI] [PubMed] [Google Scholar]
- 71.Ringom R, Axen E, Uppenberg J, Lundbäck T, Rondahl L, Barf T. Substituted benzylamino-6-(trifluoromethyl)pyrimidin-4(1H)-ones: a novel class of selective human A-FABP inhibitors. Bioorganic and Medicinal Chemistry Letters. 2004;14(17):4449–4452. doi: 10.1016/j.bmcl.2004.06.058. [DOI] [PubMed] [Google Scholar]
- 72.Sulsky R, Magnin DR, Huang Y, et al. Potent and selective biphenyl azole inhibitors of adipocyte fatty acid binding protein (aFABP) Bioorganic and Medicinal Chemistry Letters. 2007;17(12):3511–3515. doi: 10.1016/j.bmcl.2006.12.044. [DOI] [PubMed] [Google Scholar]
- 73.Hertzel AV, Hellberg K, Reynolds JM, et al. Identification and characterization of a small molecule inhibitor of fatty acid binding proteins. Journal of Medicinal Chemistry. 2009;52(19):6024–6031. doi: 10.1021/jm900720m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barf T, Lehmann F, Hammer K, et al. N-Benzyl-indolo carboxylic acids: design and synthesis of potent and selective adipocyte fatty-acid binding protein (A-FABP) inhibitors. Bioorganic and Medicinal Chemistry Letters. 2009;19(6):1745–1748. doi: 10.1016/j.bmcl.2009.01.084. [DOI] [PubMed] [Google Scholar]
- 75.Lan H, Cheng CC, Kowalski TJ, et al. Small-molecule inhibitors of FABP4/5 ameliorate dyslipidemia but not insulin resistance in mice with diet-induced obesity. Journal of Lipid Research. 2011;52(4):646–656. doi: 10.1194/jlr.M012757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tanaka T, Hirota Y, Sohmiya KI, Nishimura S, Kawamura K. Serum and urinary human heart fatty acid-binding protein in acute myocardial infarction. Clinical Biochemistry. 1991;24(2):195–201. doi: 10.1016/0009-9120(91)90571-u. [DOI] [PubMed] [Google Scholar]
- 77.Setsuta K, Seino Y, Ogawa T, Arao M, Miyatake Y, Takano T. Use of cytosolic and myofibril markers in the detection of ongoing myocardial damage in patients with chronic heart failure. American Journal of Medicine. 2002;113(9):717–722. doi: 10.1016/s0002-9343(02)01394-3. [DOI] [PubMed] [Google Scholar]
- 78.Pelsers MMAL, Hermens WT, Glatz JFC. Fatty acid-binding proteins as plasma markers of tissue injury. Clinica Chimica Acta. 2005;352(1-2):15–35. doi: 10.1016/j.cccn.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 79.Xu A, Wang Y, Xu JY, et al. Adipocyte fatty acid-binding protein is a plasma biomarker closely associated with obesity and metabolic syndrome. Clinical Chemistry. 2006;52(3):405–413. doi: 10.1373/clinchem.2005.062463. [DOI] [PubMed] [Google Scholar]
- 80.Xu A, Tso AWK, Cheung BMY, et al. Circulating adipocyte-fatty acid binding protein levels predict the development of the metabolic syndrome: a 5-year prospective study. Circulation. 2007;115(12):1537–1543. doi: 10.1161/CIRCULATIONAHA.106.647503. [DOI] [PubMed] [Google Scholar]
- 81.Tso AWK, Xu A, Sham PC, et al. Serum adipocyte fatty acid-binding protein as a new biomarker predicting the development of type 2 diabetes: a 10-year prospective study in a Chinese cohort. Diabetes Care. 2007;30(10):2667–2672. doi: 10.2337/dc07-0413. [DOI] [PubMed] [Google Scholar]
- 82.Yeung DCY, Xu A, Cheung CWS, et al. Serum adipocyte fatty acid-binding protein levels were independently associated with carotid atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2007;27(8):1796–1802. doi: 10.1161/ATVBAHA.107.146274. [DOI] [PubMed] [Google Scholar]
- 83.Yeung DCY, Wang Y, Xu A, et al. Epidermal fatty-acid-binding protein: a new circulating biomarker associated with cardio-metabolic risk factors and carotid atherosclerosis. European Heart Journal. 2008;29(17):2156–2163. doi: 10.1093/eurheartj/ehn295. [DOI] [PubMed] [Google Scholar]
- 84.Bagheri R, Qasim AN, Mehta NN, et al. Relation of plasma fatty acid binding proteins 4 and 5 with the metabolic syndrome, inflammation and coronary calcium in patients with type-2 diabetes mellitus. American Journal of Cardiology. 2010;106(8):1118–1123. doi: 10.1016/j.amjcard.2010.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fach EM, Garulacan LA, Gao J, et al. In vitro biomarker discovery for atherosclerosis by proteomics. Molecular and Cellular Proteomics. 2004;3(12):1200–1210. doi: 10.1074/mcp.M400160-MCP200. [DOI] [PubMed] [Google Scholar]
- 86.Hancke K, Grubeck D, Hauser N, Kreienberg R, Weiss JM. Adipocyte fatty acid-binding protein as a novel prognostic factor in obese breast cancer patients. Breast Cancer Research and Treatment. 2010;119(2):367–377. doi: 10.1007/s10549-009-0577-9. [DOI] [PubMed] [Google Scholar]
- 87.Karpisek M, Stejskal D, Kotolova H, et al. Treatment with atorvastatin reduces serum adipocyte-fatty acid binding protein value in patients with hyperlipidaemia. European Journal of Clinical Investigation. 2007;37(8):637–642. doi: 10.1111/j.1365-2362.2007.01835.x. [DOI] [PubMed] [Google Scholar]
- 88.Miyoshi T, Doi M, Hirohata S, et al. Olmesartan reduces arterial stiffness and serum adipocyte fatty acid-binding protein in hypertensive patients. Heart and Vessels. 2010;26(4):408–413. doi: 10.1007/s00380-010-0060-x. [DOI] [PubMed] [Google Scholar]
- 89.Cabré A, Lázaro I, Girona J, et al. Fatty acid binding protein 4 is increased in metabolic syndrome and with thiazolidinedione treatment in diabetic patients. Atherosclerosis. 2007;195(1):e150–e158. doi: 10.1016/j.atherosclerosis.2007.04.045. [DOI] [PubMed] [Google Scholar]
- 90.Schachtrup C, Emmler T, Bleck B, Sandqvist A, Spener F. Functional analysis of peroxisome-proliferator-responsive element motifs in genes of fatty acid-binding proteins. Biochemical Journal. 2004;382(1):239–245. doi: 10.1042/BJ20031340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yeung DCY, Xu A, Tso AWK, et al. Circulating levels of adipocyte and epidermal fatty acid-binding proteins in relation to nephropathy staging and macrovascular complications in type 2 diabetic patients. Diabetes Care. 2009;32(1):132–134. doi: 10.2337/dc08-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sommer G, Ziegelmeier M, Bachmann A, et al. Serum levels of adipocyte fatty acid-binding protein (AFABP) are increased in chronic haemodialysis (CD) Clinical Endocrinology. 2008;69(6):901–905. doi: 10.1111/j.1365-2265.2008.03277.x. [DOI] [PubMed] [Google Scholar]
- 93.Furuhashi M, Ura N, Hasegawa K, et al. Serum ratio of heart-type fatty acid-binding protein to myoglobin. A novel marker of cardiac damage and volume overload in hemodialysis patients. Nephron. 2003;93(2):C69–C74. doi: 10.1159/000068520. [DOI] [PubMed] [Google Scholar]
- 94.Cao H, Gerhold K, Mayers JR, Wiest MM, Watkins SM, Hotamisligil GS. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell. 2008;134(6):933–944. doi: 10.1016/j.cell.2008.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Coleman SL, Park Y-K, Lee J-Y. Unsaturated fatty acids repress the expression of adipocyte fatty acid binding protein via the modulation of histone deacetylation in RAW 264.7 macrophages. European Journal of Clinical Nutrition. 2011;50(5):323–330. doi: 10.1007/s00394-010-0140-9. [DOI] [PubMed] [Google Scholar]
- 96.Stefan N, Kantartzis K, Celebi N, et al. Circulating palmitoleate strongly and independently predicts insulin sensitivity in humans. Diabetes Care. 2010;33(2):405–407. doi: 10.2337/dc09-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mozaffarian D, Cao H, King IB, et al. Circulating palmitoleic acid and risk of metabolic abnormalities and new-onset diabetes. American Journal of Clinical Nutrition. 2010;92(6):1350–1358. doi: 10.3945/ajcn.110.003970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mozaffarian D, Cao H, King IB, et al. Trans-palmitoleic acid, metabolic risk factors, and new-onset diabetes in U.S. adults: a cohort study. Annals of Internal Medicine. 2010;153(12):790–799. doi: 10.1059/0003-4819-153-12-201012210-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Auwerx J. PPARγ, the ultimate thrifty gene. Diabetologia. 1999;42(9):1033–1049. doi: 10.1007/s001250051268. [DOI] [PubMed] [Google Scholar]
- 100.Esteves A, Ehrlich R. Invertebrate intracellular fatty acid binding proteins. Comparative Biochemistry and Physiology—C Toxicology and Pharmacology. 2006;142(3-4):262–274. doi: 10.1016/j.cbpc.2005.11.006. [DOI] [PubMed] [Google Scholar]


