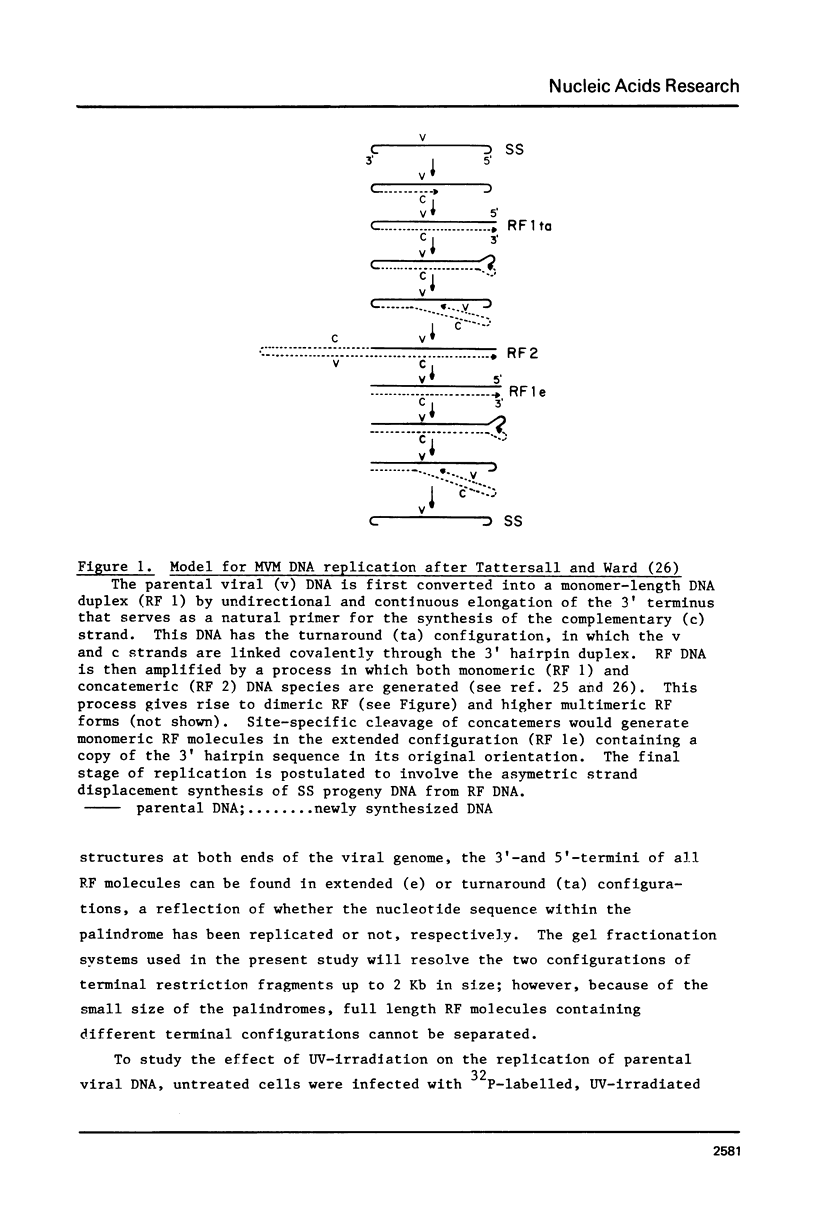
Abstract
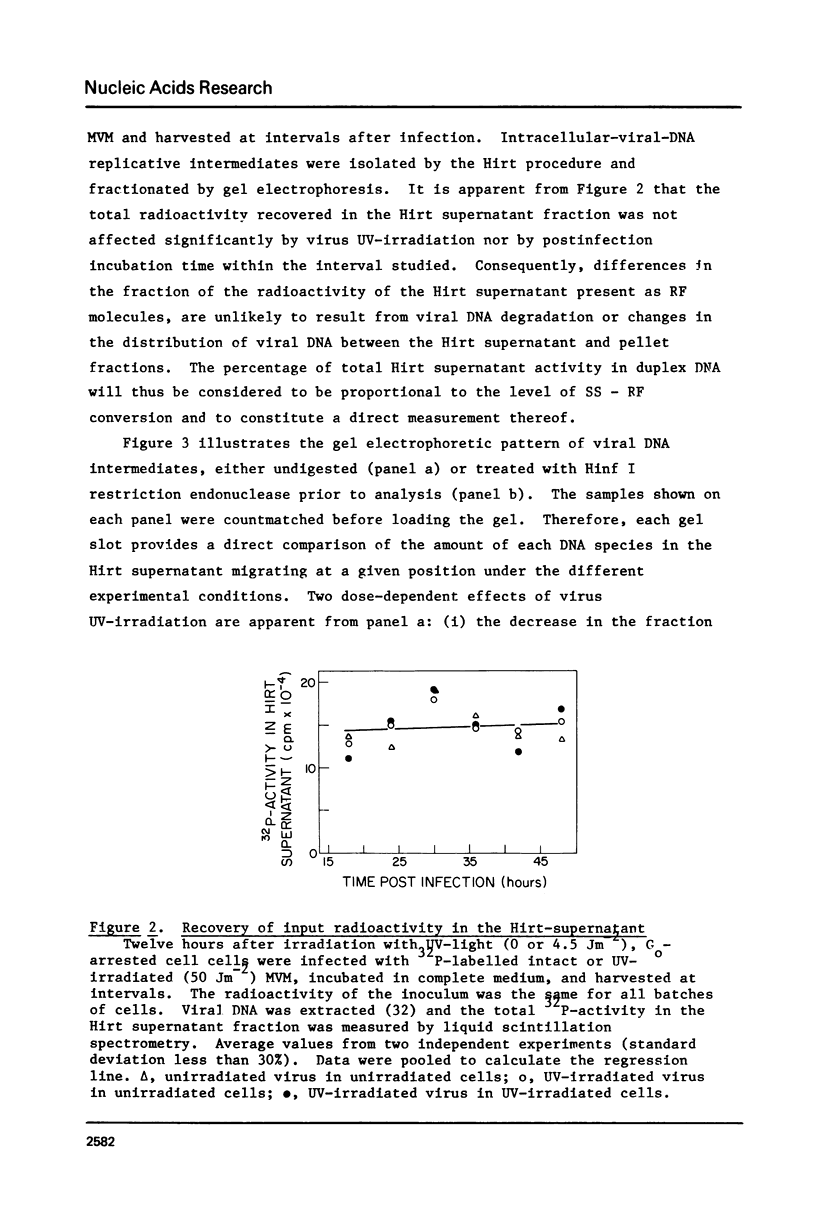
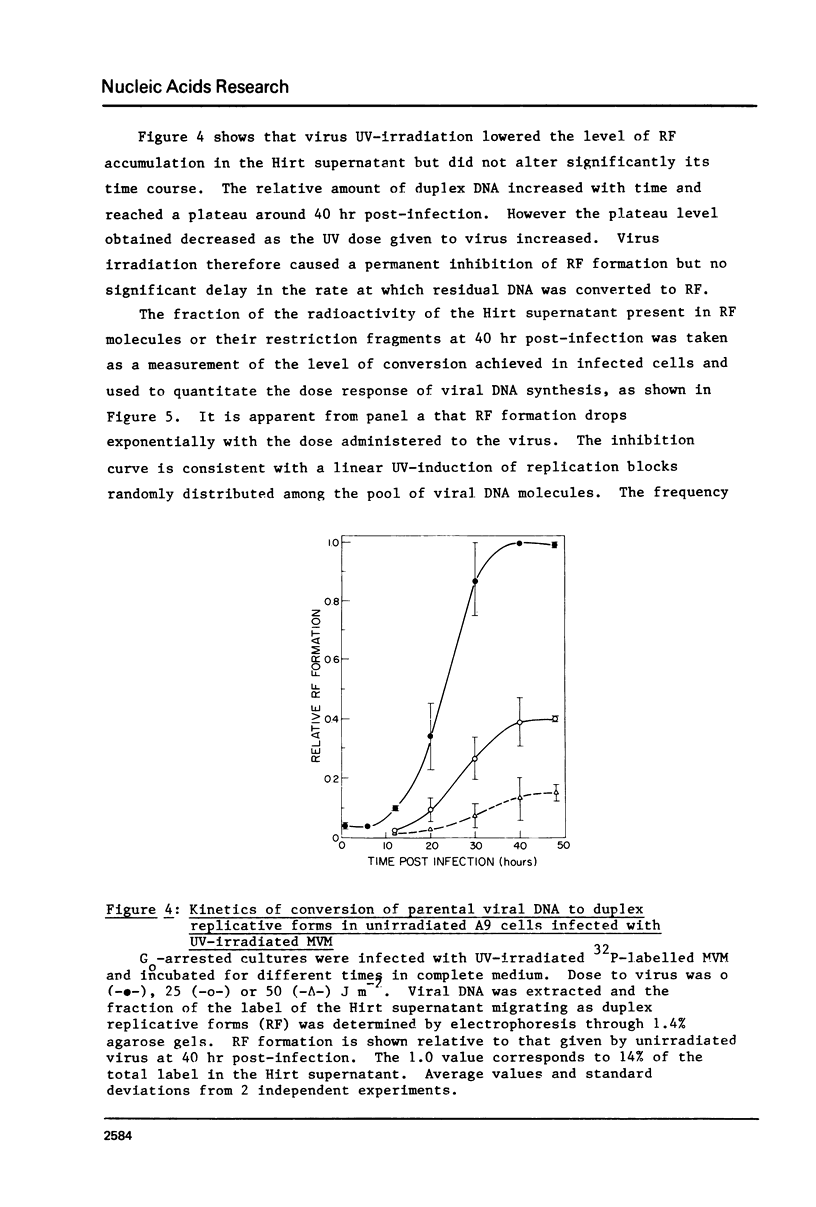
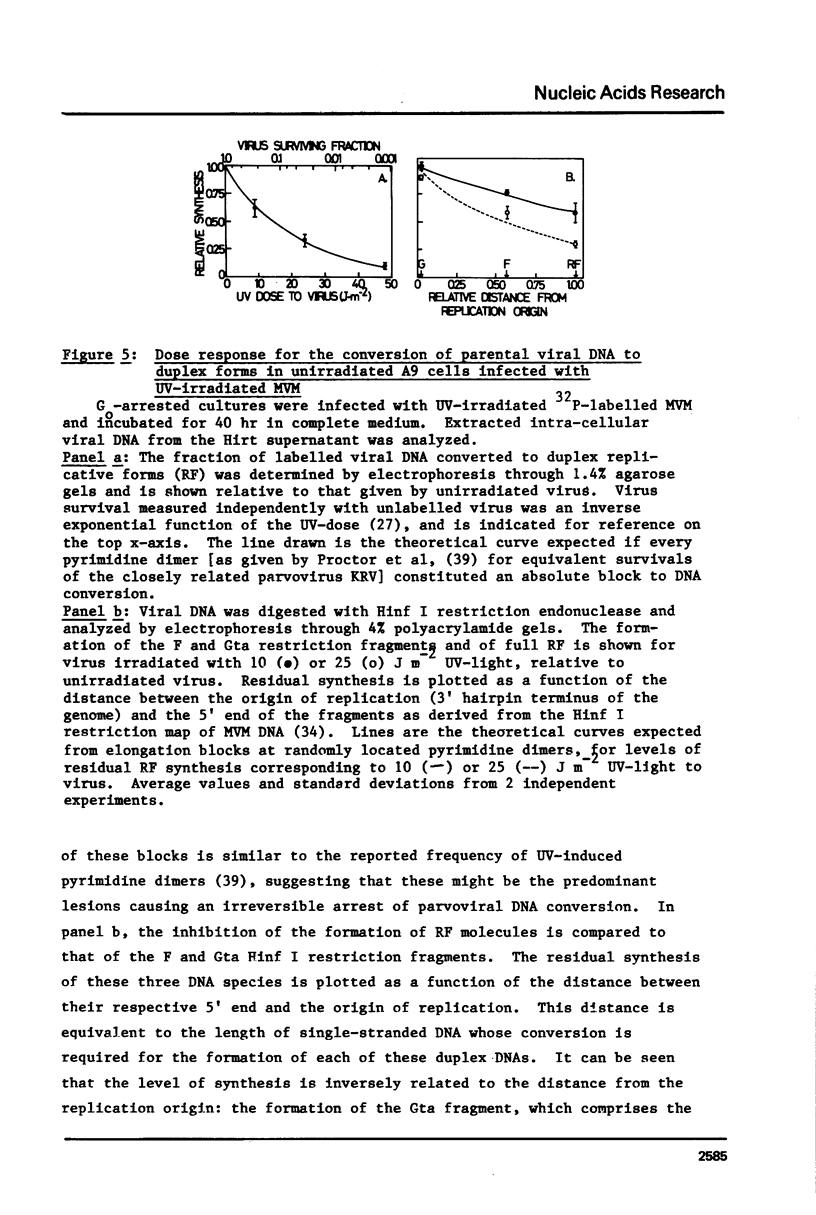
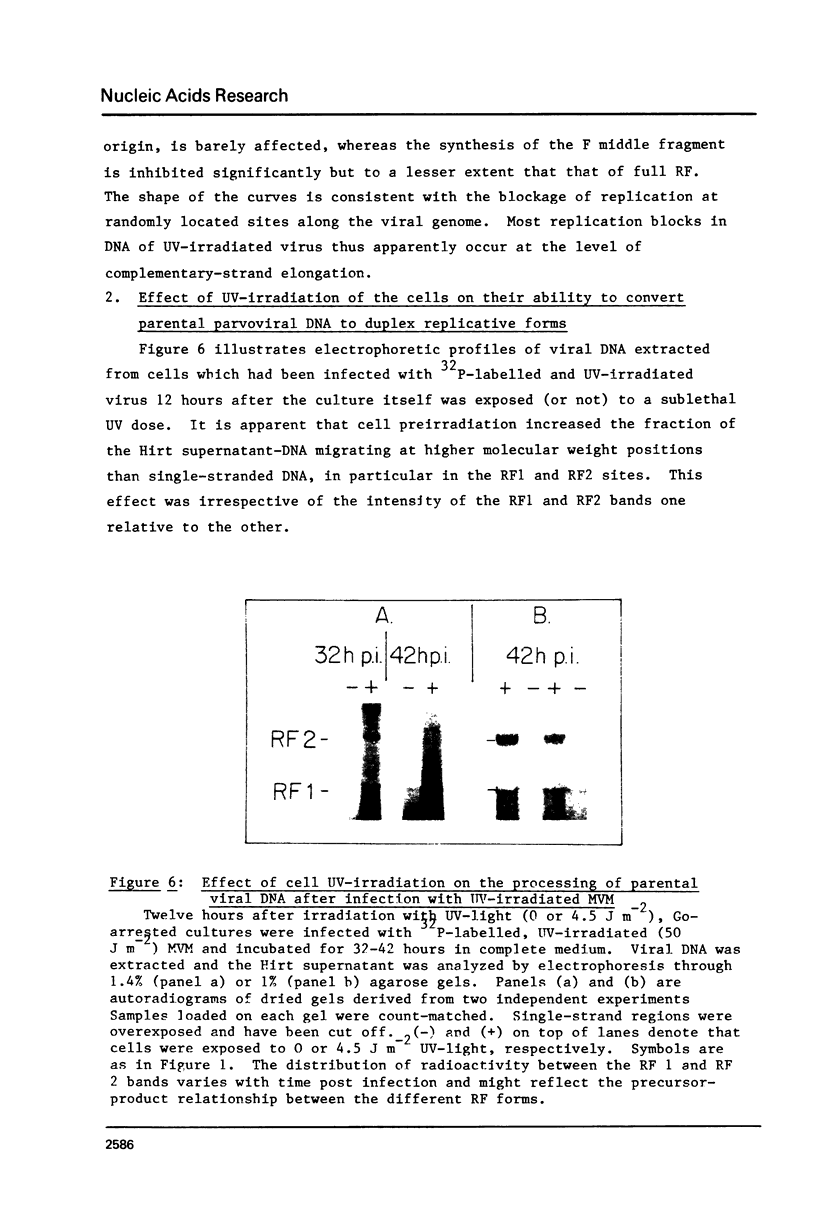
The effect of UV-irradiation on the conversion of the single-stranded DNA of the parvovirus Minute-Virus-of-Mice (MVM) to duplex Replicative Forms (RF) was studied after infection of mouse A9 fibroblasts. UV-irradiation of the virus prior to infection of unirradiated cells resulted in a dose-dependent, single-hit, inhibition of RF formation. Restriction fragment analysis indicated that this inhibition could be ascribed to the introduction of absolute blocks which prevent elongation of the newly synthesized complementary strand. Cell exposure to UV-light prior to infection with UV-irradiated MVM enhanced the fraction of input viral DNA which was converted to RF. This enhancement required de novo protein synthesis during the interval between cell irradiation and virus infection. These results suggest that DNA replication constitutes a target in the viral life cycle that leads to the UV-enhanced Reactivation of virus survival, however, they do not permit us to identify the step of RF formation which is enhanced in UV-pretreated cells.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bockstahler L. E., Lytle C. D. Radiation enhanced reactivation of nuclear replicating mammalian viruses. Photochem Photobiol. 1977 May;25(5):477–482. doi: 10.1111/j.1751-1097.1977.tb09173.x. [DOI] [PubMed] [Google Scholar]
- Bourguignon G. J., Tattersall P. J., Ward D. C. DNA of minute virus of mice: self-priming, nonpermuted, single-stranded genome with a 5'-terminal hairpin duplex. J Virol. 1976 Oct;20(1):290–306. doi: 10.1128/jvi.20.1.290-306.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhl S. N., Setlow R. B., Regan J. D. Recovery of the ability to synthesize DNA in segments of normal size at long times after ultraviolet irradiation of human cells. Biophys J. 1973 Dec;13(12):1265–1275. doi: 10.1016/S0006-3495(73)86061-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhl S. N., Setlow R. B., Regan J. D. Steps in DNA chain elongation and joining after ultra-violet irradiation of human cells. Int J Radiat Biol Relat Stud Phys Chem Med. 1972 Nov;22(5):417–424. doi: 10.1080/09553007214551301. [DOI] [PubMed] [Google Scholar]
- Caillet-Fauquet P., Defais M., Radman M. Molecular mechanisms of induced mutagenesis. Replication in vivo of bacteriophage phiX174 single-stranded, ultraviolet light-irradiated DNA in intact and irradiated host cells. J Mol Biol. 1977 Nov 25;117(1):95–110. doi: 10.1016/0022-2836(77)90025-0. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E. Investigations into the effects of ultraviolet light on the rate of deoxyribonucleic acid synthesis in mammalian cells. Biochim Biophys Acta. 1965 Sep 6;108(1):42–52. doi: 10.1016/0005-2787(65)90106-1. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E., Thomas G. H., Park S. D. Xeroderma pigmentosum variants have a slow recovery of DNA synthesis after irradiation with ultraviolet light. Biochim Biophys Acta. 1979 Aug 29;564(1):122–131. doi: 10.1016/0005-2787(79)90193-x. [DOI] [PubMed] [Google Scholar]
- Cordeiro-Stone M., Schumacher R. I., Meneghini R. Structure of the replication fork in ultraviolet light-irradiated human cells. Biophys J. 1979 Aug;27(2):287–300. doi: 10.1016/S0006-3495(79)85218-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahle D., Griffiths T. D., Carpenter J. G. Inhibition and recovery of DNA synthesis in UV-irradiated Chinese hamster V-79 cells. Photochem Photobiol. 1980 Aug;32(2):157–165. doi: 10.1111/j.1751-1097.1980.tb04003.x. [DOI] [PubMed] [Google Scholar]
- Doniger J. DNA replication in ultraviolet light irradiated Chinese hamster cells: the nature of replicon inhibition and post-replication repair. J Mol Biol. 1978 Apr 15;120(3):433–446. doi: 10.1016/0022-2836(78)90429-1. [DOI] [PubMed] [Google Scholar]
- Doniger J., DiPaolo J. A. The early and late modes of DNA replication in ultraviolet irradiated Syrian hamster embryo cells. Biophys J. 1980 Aug;31(2):247–254. doi: 10.1016/S0006-3495(80)85054-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenberg H. J. Inhibition of DNA replication by ultraviolet light. Biophys J. 1976 Aug;16(8):849–860. doi: 10.1016/S0006-3495(76)85735-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust E. A., Ward D. C. Incomplete genomes of the parvovirus minute virus of mice: selective conservation of genome termini, including the origin for DNA replication. J Virol. 1979 Oct;32(1):276–292. doi: 10.1128/jvi.32.1.276-292.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeppesen P. G. A method for separating DNA fragments by electrophoresis in polyacrylamide concentration gradient slab gels. Anal Biochem. 1974 Mar;58(1):195–207. doi: 10.1016/0003-2697(74)90458-8. [DOI] [PubMed] [Google Scholar]
- Kaufmann W. K., Cleaver J. E. Mechanisms of inhibition of DNA replication by ultraviolet light in normal human and xeroderma pigmentosum fibroblasts. J Mol Biol. 1981 Jun 25;149(2):171–187. doi: 10.1016/0022-2836(81)90297-7. [DOI] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. THREE DEGREES OF GUANYLIC ACID--INOSINIC ACID PYROPHOSPHORYLASE DEFICIENCY IN MOUSE FIBROBLASTS. Nature. 1964 Sep 12;203:1142–1144. doi: 10.1038/2031142a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lehmann A. R., Kirk-Bell S. Pyrimidine dimer sites associated with the daughter DNA strands in UV-irradiated human fibroblasts. Photochem Photobiol. 1978 Mar;27(3):297–307. doi: 10.1111/j.1751-1097.1978.tb07604.x. [DOI] [PubMed] [Google Scholar]
- Lehmann A. R. Postreplication repair of DNA in ultraviolet-irradiated mammalian cells. J Mol Biol. 1972 May 28;66(3):319–337. doi: 10.1016/0022-2836(72)90418-4. [DOI] [PubMed] [Google Scholar]
- Lehmann A. R. The relationship between pyrimidine dimers and replicating DNA in UV-irradiated human fibroblasts. Nucleic Acids Res. 1979 Dec 11;7(7):1901–1912. doi: 10.1093/nar/7.7.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lytle C. D. Radiation-enhanced virus reactivation in mammalian cells. Natl Cancer Inst Monogr. 1978 Dec;(50):145–149. [PubMed] [Google Scholar]
- McMaster G. K., Beard P., Engers H. D., Hirt B. Characterization of an immunosuppressive parvovirus related to the minute virus of mice. J Virol. 1981 Apr;38(1):317–326. doi: 10.1128/jvi.38.1.317-326.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meneghini R., Cordeiro-Stone M., Schumacher R. I. Size and frequency of gaps in newly synthesized DNA of xeroderma pigmentosum human cells irradiated with ultraviolet light. Biophys J. 1981 Jan;33(1):81–92. doi: 10.1016/S0006-3495(81)84873-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meneghini R., Hanawalt P. T4-endonuclease V-sensitive sites in DNA from ultraviolet-irradiated human cells. Biochim Biophys Acta. 1976 Apr 2;425(4):428–437. doi: 10.1016/0005-2787(76)90007-1. [DOI] [PubMed] [Google Scholar]
- Meyn R. E., Hewitt R. R., Thomas L. F., Humphrey R. M. Effects of ultraviolet irradiation on the rate and sequence of DNA replication in synchronized Chinese hamster cells. Biophys J. 1976 May;16(5):517–525. doi: 10.1016/S0006-3495(76)85706-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyn R. E., Kasschau M. R., Hewitt R. R. The recovery of normal DNA replication kinetics in ultraviolet-irradiated Chinese hamster cells. Mutat Res. 1977 Jul;44(1):129–138. doi: 10.1016/0027-5107(77)90121-x. [DOI] [PubMed] [Google Scholar]
- Moore P. D., Bose K. K., Rabkin S. D., Strauss B. S. Sites of termination of in vitro DNA synthesis on ultraviolet- and N-acetylaminofluorene-treated phi X174 templates by prokaryotic and eukaryotic DNA polymerases. Proc Natl Acad Sci U S A. 1981 Jan;78(1):110–114. doi: 10.1073/pnas.78.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S. D., Cleaver J. E. Postreplication repair: questions of its definition and possible alteration in xeroderma pigmentosum cell strains. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3927–3931. doi: 10.1073/pnas.76.8.3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S. D., Cleaver J. E. Recovery of DNA synthesis after ultraviolet irradiation of xeroderma pigmentosum cells depends on excision repair and is blocked by caffeine. Nucleic Acids Res. 1979 Mar;6(3):1151–1159. doi: 10.1093/nar/6.3.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor W. R., Cook J. S., Tennant R. W. Ultraviolet photobiology of Kilham rat virus and the absolute ultraviolet photosensitivities of other animal viruses: influence of DNA strandedness, molecular weight, and host-cell repair. Virology. 1972 Aug;49(2):368–378. doi: 10.1016/0042-6822(72)90489-8. [DOI] [PubMed] [Google Scholar]
- Rommelaere J., Miller-Faurès A. Detection by density equilibrium centrifugation of recombinant-like DNA molecules in somatic mammalian cells. J Mol Biol. 1975 Oct 15;98(1):195–218. doi: 10.1016/s0022-2836(75)80109-4. [DOI] [PubMed] [Google Scholar]
- Rommelaere J., Vos J. M., Cornelis J. J., Ward D. C. UV-enhanced reactivation of minute-virus-of-mice: stimulation of a late step in the viral life cycle. Photochem Photobiol. 1981 Jun;33(6):845–854. doi: 10.1111/j.1751-1097.1981.tb05502.x. [DOI] [PubMed] [Google Scholar]
- Sarasin A. R., Hanawalt P. C. Replication of ultraviolet-irradiated simian virus 40 in monkey kidney cells. J Mol Biol. 1980 Apr;138(2):299–319. doi: 10.1016/0022-2836(80)90288-0. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Su Z. Z., Cornelis J. J., Rommelaere J. Mutagenesis of intact parvovirus H-1 is expressed co-ordinately with enhanced reactivation of ultraviolet irradiated virus in human and rat cells treated with 2-nitronaphthofurans. Carcinogenesis. 1981;2(10):1039–1043. doi: 10.1093/carcin/2.10.1039. [DOI] [PubMed] [Google Scholar]
- Tatsumi K., Strauss B. Production of DNA bifilarly substituted with bromodeoxyuridine in the first round of synthesis: branch migration during isolation of cellular DNA. Nucleic Acids Res. 1978 Feb;5(2):331–347. doi: 10.1093/nar/5.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattersall P., Crawford L. V., Shatkin A. J. Replication of the parvovirus MVM. II. Isolation and characterization of intermediates in the replication of the viral deoxyribonucleic acid. J Virol. 1973 Dec;12(6):1446–1456. doi: 10.1128/jvi.12.6.1446-1456.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattersall P. Replication of the parvovirus MVM. I. Dependence of virus multiplication and plaque formation on cell growth. J Virol. 1972 Oct;10(4):586–590. doi: 10.1128/jvi.10.4.586-590.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattersall P., Ward D. C. Rolling hairpin model for replication of parvovirus and linear chromosomal DNA. Nature. 1976 Sep 9;263(5573):106–109. doi: 10.1038/263106a0. [DOI] [PubMed] [Google Scholar]