Data show that 24-hour acylated ghrelin levels predict overall glucose control and that sleep, but not time of day, impacts post-prandial total ghrelin and acylated ghrelin responses.
Abstract
Context:
The acylation of ghrelin is essential for its stimulatory effects on GH release and appetite. Most of the physiology of ghrelin has been defined based on the assay of total ghrelin (TG), which mainly reflects levels of unacylated ghrelin. Whether levels of acylated ghrelin (AG) are influenced by circadian time and sleep and impact glucose regulation under physiologic conditions is not known.
Methods:
Blood was sampled at 10- to 30-min intervals for 24 h in 14 healthy young lean men under controlled conditions of activity, light-dark cycle, and sleep-wake schedule. The subjects ingested three identical carbohydrate-rich meals at 5-h intervals. Sleep was polygraphically monitored. Levels of TG and AG were measured by RIA. The 24-h profiles of glucose and insulin levels were assessed simultaneously.
Results:
Postprandial glucose concentrations were positively correlated with mean levels of AG but not TG, independently of insulin. Postprandial suppression and rebound of AG and TG occurred in parallel and were not impacted by time of day. The nocturnal elevation of AG and TG reflects the postdinner rebound curbed by an inhibitory effect of sleep. The ratio of AG to TG was lower during sleep than during wake, consistent with a reduction of orexigenic signal.
Conclusions:
Individual differences in AG levels may be an important predictor of overall glucose control under physiological conditions. Sleep, but not time of day, impacts postprandial TG and AG responses. The inhibitory effect of sleep on ghrelin release and acylation is consistent with the association between sleeping and fasting.
Ghrelin was identified in 1999 as a natural ligand of the GH secretagogue receptor type 1a (1) but has since been recognized as an appetite-stimulating hormone and a key component of the regulation of energy balance (2–5). Ghrelin acylation, under the control of the enzyme ghrelin-O-acyl-transferase, is essential for its binding to the GH secretagogue receptor type 1a and for its orexigenic effects (2, 5). Therefore, until recently, acylated ghrelin (AG) and unacylated ghrelin (UAG) were commonly referred to as active and inactive ghrelin, respectively. Evidence has since accumulated to indicate that UAG, which circulates in concentrations 3- to 4-fold higher than those of AG, has pleiotropic nonendocrine actions including cardiovascular and metabolic effects (2–4).
Most of the physiology of human ghrelin has been based on the assay of peripheral levels of total ghrelin (TG), which mainly reflect UAG levels. TG levels decrease after food ingestion in proportion to caloric load and rise before initiation of the next meal, paralleling the increase in hunger (6, 7). Despite the fact that the ghrelin-releasing stomach cells function as food-entrainable circadian oscillators (8), it is not known whether postprandial ghrelin suppression and rebound vary according to time of day. As early as 2001 (9), studies involving frequent sampling across the 24-h cycle observed a nocturnal elevation of TG levels with peak values during the sleep period and declining levels toward the morning. To date, the significance of this rise and fall of TG during the overnight fast remains unknown. The only human study that directly examined the role of sleep on nocturnal ghrelin release found that TG levels were inhibited by sleep deprivation, contradicting a large body of evidence from animal models linking the promotion of feeding with the maintenance of wakefulness (10).
Only a few studies have attempted to characterize the postprandial and/or overnight profiles of AG in relation to UAG (11–14). Lucidi et al. (11) first reported that meal intake similarly reduced levels of AG and TG as assessed by RIA. Using a two-site sandwich assay of higher specificity, Liu et al. (13) showed that in the fed state, the dynamics of AG mirror closely those of UAG but that following 1.5 d of fasting, AG levels are suppressed by 60–70%, whereas UAG remains at peak premeal values. These observations extended an emerging body of evidence supporting distinct and sometimes opposing interactions of AG vs. UAG with glucose regulation (2–4). Whether overall levels of AG and/or TG impact glucose control under normal physiological conditions is not known.
The present study was designed to elucidate the roles of sleep and time of day on postprandial profiles of AG and TG and their relationship to concomitant variations of glucose and insulin using frequent sampling across the 24-h period, identical and equally spaced high-carbohydrate meals during the day and polygraphic sleep recording.
Subjects and Methods
Subjects
The study included 14 healthy men [age(mean ± sd): 24 ± 3 yr; body mass index (mean ± sd): 22 ± 2 kg/m2]. Subjects with a history of psychiatric or endocrine illness, sleep disorders, shift workers, subjects having recently crossed time zones, and subjects taking any drugs were excluded. Only subjects with regular lifestyles and habitual bedtimes from 2300–2400 until 0700–0800 h were included. The protocol was approved by the Institutional Review Board of the University of Chicago. Written informed consent was obtained from all participants.
Experimental protocol
During the week preceding the study, the participants were asked to comply with a standardized schedule of bedtimes (2300 h ± 30 min) and wake-up times (0700 h ± 30 min). Compliance was verified by examination of wrist activity recordings (Actiwatch; Respironics, Bend, OR). The subjects were admitted to the Clinical Research Center on the evening before the study. They received a standardized dinner at 1900 h. On the following day, blood sampling for 24 h was initiated. Sampling interval was 10 min in the postprandial periods, and at 20–30 min at all other times. An iv sterile heparin-lock catheter was inserted in the forearm at least 2 h before the beginning of sampling. The line was kept patent with a slow drip of heparinized saline. Food intake was restricted to three identical and equally spaced meals (30% fat, 60% carbohydrates, 10% protein; 2400 kcal per 24 h) served at 0900, 1400, and 1900 and consumed within 20 min. Snacks and beverages other than water were not allowed. During waking, the subjects remained at bed rest with the head of the bed tilted at a 45° angle. Naps were not allowed. Approximately 2 h before bedtime, electrodes for sleep recordings were attached. Lights were turned off from 2300 to 0700 h. Sleep was recorded via a digital EEG acquisition system (Neurofax EEG-1100A; Nihon Kohden, Foothill Ranch, CA). During bedtimes, the catheter was connected to plastic tubing extending to an adjacent room to sample distally. For AG, 1.5 ml of blood was collected in chilled EDTA tubes kept on ice and containing 20 μl of 0.75% phenylmethanesulfonyl fluoride and 50 μl of 1 m HCl. Each blood sample was immediately centrifuged at 4 C. For TG, blood was collected at room temperature in nonchilled tubes that did not contain inhibitors. Plasma and serum samples were frozen at −80 C until assay.
Assays
All plasma samples for assessing AG levels were thawed and aliquoted on ice. Both TG and AG levels were measured using commercially available RIA procedures (Linco Research, St. Charles, MO). For TG, the limit of sensitivity was 93 pg/ml; the intraassay coefficient of variation (CV) was less than 3%. AG sensitivity limit was 7.8 pg/ml; the intraassay CV was less than 3%. Each assay was validated by adding three doses of synthetic acyl-ghrelin or des-acyl ghrelin (Phoenix Pharmaceuticals, Belmont CA) to aliquots of a plasma pool. Recovery of des-acyl ghrelin in the TG assay averaged 97%. Recovery of acyl-ghrelin in the AG assay ranged from 70 to 75%. Based on our own evaluations, there was no detectable cross-reactivity of des-acyl ghrelin in the AG assay.
Glucose levels were assayed at bedside in 12 of the 14 subjects using an automated instrument (Yellow Springs model 23A; Yellow Springs Instruments, Yellow Springs, OH) with a CV of less than 2%. Insulin levels were measured using a chemiluminescent enzyme immunoassay (Immulite; Diagnostics Products, Los Angeles, CA) with a limit of sensitivity of 14.4 pmol/liter and intraassay CV of 3.3–5.5%. All samples collected in the same subject were analyzed in the same assay.
Analysis of sleep recordings
Polygraphic sleep recordings were scored at 30-sec intervals in stages wake, I, II, III, IV, and rapid eye movement (REM) according to standard criteria (15). Sleep onset was defined as the time corresponding to the first 30-sec interval scored II, III, IV, or REM provided the subsequent 30-sec interval was not scored wake or stage I. Morning awakening was defined as the time corresponding to the last 30-sec interval scored II, III, IV, or REM.
Analysis of individual profiles of TG, AG, glucose, and insulin
All individual data were interpolated at 10-min intervals to facilitate the quantification of meal responses. Isolated AG values that represented a more than 100% change in comparison with the preceding and following value and that were not concomitant with a similar variation in TG were considered as aberrant and interpolated. Of the total of 938 AG values measured in the present study, only 30 (i.e. 3.2%) were interpolated.
Meal-related peaks of TG and AG were defined as the maximum concentration measured during the 40 min after meal presentation, i.e. before the initiation of postmeal inhibition. Postmeal nadirs of TG and AG were defined as the minimum concentration measured between 40 and 240 min after meal presentation. The postmeal decreases in TG and AG were calculated as the differences between the meal-related peak and the postmeal nadir and expressed as a percentage of the meal-related peak. The rebounds of TG and AG were calculated as the differences between the peak value for the following meal and the preceding nadir and expressed as a percentage of the preceding nadir.
The nocturnal elevations of TG and AG were estimated after smoothing the profiles using a 7-point moving average as the differences between the nadir following the evening meal and the maximum level attained between 2300 and 0500 h.
The premeal levels of plasma glucose and insulin were calculated as the mean of concentrations at −30 min and 0 min relative to meal presentation. The postmeal increments were defined as the difference between maximum levels after the meal and premeal levels. The early and late postmeal responses were estimated, respectively, as the incremental area under the curve (AUC) during the first 1.5 h and during the following 3.5 h.
Statistical analysis
All group values are expressed as mean ± se. Paired comparisons of TG vs. AG summary measures were performed by t test. Correlations were calculated using the Pearson coefficient. ANOVA for repeated measures was used to examine the impact of time of day on meal responses. Factorial ANOVA was used to analyze interactions between ghrelin, insulin and glucose.
Results
Mean levels of TG and AG and relationship AG to TG ratio
The 24-h mean level averaged 1027 ± 108 pg/ml for TG and 80 ± 4 pg/ml for AG. Despite the high homogeneity of the subject group in terms of sex, age, and body mass index, the mean 24-h levels of TG varied widely from one individual to the other, with the highest value being 3- to 4-fold higher than the lowest value. The CV of the mean TG level across individuals was 40%. Individual differences in 24-h mean level were smaller for AG, with a CV of 19%. The ratio of mean AG to mean TG level over the 24-h span averaged 8.7 ± 0.8% (range 5.5–16.2%). The 24-h mean AG and TG levels were correlated (r = 0.74, P < 0.05).
Twenty-four-hour profiles of TG and AG
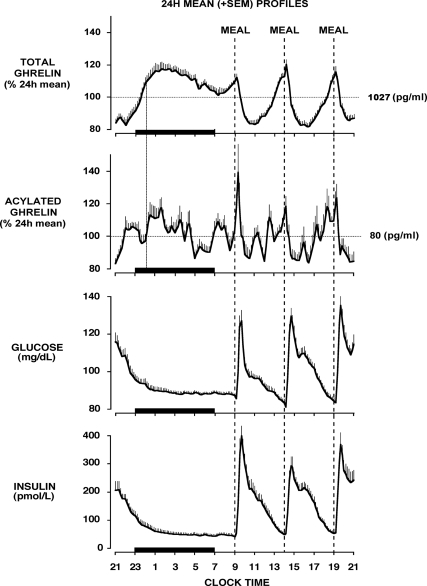
The two upper panels of Fig. 1 show the mean 24-h profiles of TG and AG. Before calculating group means, each individual profile was expressed as a percentage of its 24-h mean to account for the interindividual variability of TG and AG levels. The use of identical and equally spaced carbohydrate-rich meals allowed for the demonstration of consistent and approximately parallel meal-related changes in TG and AG in the morning, midday, and evening.
Fig. 1.
Twenty-four-hour mean (±se) profiles of total ghrelin, acylated ghrelin, glucose, and insulin obtained in healthy young men who were allowed to sleep from 2300 to 0700 h. The meal included identical carbohydrate-rich meals presented at 5-h intervals (0900, 1400, and 1900 h). The dotted line represents the occurrence of a hypothetical fourth identical meal 5 h after the dinner meal, i.e. at midnight. The black bars represent the sleep period.
The concomitant profiles of glucose and insulin levels are shown in the lower panels of Fig. 1. The postprandial decreases in TG and AG levels were coincident with the first phase of glucose and insulin responses and occurred over the first 2 h after meal presentation. Rebounds of TG and AG levels were temporally associated with a slower rate of decline of glucose and insulin concentrations during the second phase of the response.
There is increasing evidence supporting a hyperglycemic effect of AG but not TG (2, 3). We thus examined the impact of individual differences in 24-h mean AG and TG levels on 24-h glucose concentrations. Mean glucose levels were correlated with AG levels (Fig. 2A), but not with TG levels (Fig. 2B). Remarkably, AG levels were a stronger predictor of glucose concentrations than insulin levels (factorial ANOVA; 24-h mean AG: P = 0.016; 24-h mean insulin: P = 0.261). This impact of AG levels on glucose concentrations appeared specific to the daytime period that included the three postprandial phases (0900–2400 h; factorial ANOVA; mean daytime AG: P < 0.04; mean daytime insulin: P = 0.89). Indeed, glucose levels during the overnight fast (2400–0900 h) appeared primarily determined by insulin levels (factorial ANOVA; overnight mean insulin: P < 0.04; overnight mean AG: P = 0.45).
Fig. 2.
A, Association between 24-h glucose levels and 24-h acylated ghrelin levels. B, Association between 24-h glucose levels and 24-h total ghrelin levels. C, Twenty-four-hour mean (±se) profiles of glucose for subjects with high and low 24-h acylated ghrelin levels. The arrows represent identical carbohydrate-rich meals presented at 5-h intervals.
To further explore this impact of AG on glucose levels, we performed a median split of our group of 14 subjects according to the 24-h AG mean. Figure 2C illustrates the mean 24-h glucose profiles for the individuals in the lower range vs. upper range of mean AG values. The impact of AG on glucose concentrations appeared to be primarily exerted during the late postprandial phases. The mean AUC during the late postprandial phases was higher in subjects who had higher vs. lower mean AG levels (12.4 ± 0.1 g/dl · min vs. 13.4 ± 0.3 g/dl · min; P < 0.03), whereas mean AUC values for insulin were comparable (24.1 ± 4.1 nmol/liter · min vs. 25.1 ± 3.2 nmol/liter · min; P = 0.75).
Impact of time of day on postprandial AG and TG responses
Peak levels of TG and AG were similar for each of the three meals (ANOVA for repeated measures; TG: P = 0.17; AG: P = 0.26) and occurred at similar times relative to meal presentation. Postmeal nadir levels of TG and AG were also similar for the three meals and occurred in synchrony 1.5–2 h after meal presentation. As illustrated in Fig. 3A (upper panels), the relative degree of postmeal inhibition of TG and AG was similar in the morning, midday, and evening. This result contrasts with the progressive increase in the glucose response to meals over the course of the day and with a clear impact of time of day on insulin release (Fig. 3A, lower panels).
Fig. 3.
A, Early meal responses of total ghrelin, acylated ghrelin, and incremental AUC of glucose from 0 to 1.5 h postmeal and incremental AUC of insulin from 0 to 1.5 h after the meal (top to bottom), B, Association between the magnitudes of the degree of postmeal inhibition of total ghrelin (upper panel) and acylated ghrelin (lower panel) with the rebounds.
When expressed as percentage of premeal levels and averaged across all meals, the postmeal inhibition was greater for AG (−46.3 ± 2.2%) than for TG (−33.2 ± 2.4%; P = 0.002). For both TG and AG, the magnitude of the rebound was strongly correlated with the degree of postmeal inhibition (Fig. 3B). Similar high correlations between postmeal decrement and rebound were found for both TG and AG when absolute, rather than relative, changes were examined. Thus, when identical high-carbohydrate meals are ingested, both the suppression of TG and AG levels by food intake and the subsequent rebound appeared independent of time of day.
Nocturnal profiles of AG and TG: relationship with postdinner rebound and sleep
On average, sleep onset occurred at 2325 h ± 8 min and morning awakening at 0707 h ± 5 min. Total sleep time averaged 6 h 52 min ± 15 min. The distribution of sleep stages was 3 h 24 min ± 14 min for stages I+II, 1 h 11 min ± 7 min for stages III+IV, and 1 h 18 min ± 8 min for REM sleep. These values are typical of healthy young volunteers. The ratio of AG to TG was significantly higher during the waking period (9.1 ± 0.9%) than during sleep (7.9 ± 0.7%; P < 0.002).
The sleep period was associated with a robust and prolonged increase in TG and a discernable but apparently briefer rise of AG (Fig. 1). The nocturnal TG acrophase occurred, on average, at 2 h 17 ± 20 min (i.e. 7 h 17 ± 20 min after dinner) and represented a rebound of 65.4 ± 8.5% over the postdinner nadir. Overnight levels of AG were more variable, but a nocturnal acrophase could generally be identified (mean timing: 1 h 38 ± 29 min; mean value: 86.8 ± 11.0% over postdinner nadir). After their respective nocturnal acrophases, i.e. approximately 7 h after the last meal, TG and AG levels declined until morning awakening despite the continued fasting condition.
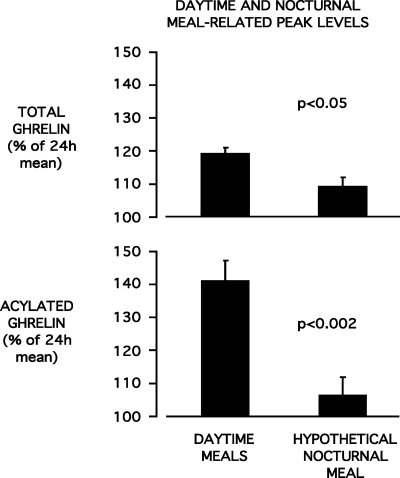
We sought to determine whether the nocturnal acrophases of TG and AG represented an extension of the postdinner rebound commensurate with a steady rise of ghrelin over a longer fasting period. To this effect, we compared TG and AG levels at the time when peak levels would have been observed if a fourth identical meal had been ingested 5 h after the dinner meal, i.e. at midnight, to mean peak levels for daytime meals. For each subject and for both TG and AG, the mean lag between time of daytime meal presentation and peak level was used to estimate what would have been the peak time before ingestion of this hypothetical nocturnal meal. On average, TG levels would have peaked at 00 h 11 ± 1 min and AG levels at 00 h 15 ± 2 min. All but one subject had fallen asleep (mean sleep onset at 2325 h ± 8 min) before midnight. As illustrated in Fig. 4, peak levels of TG and AG levels in the 13 subjects who were sleeping at the time of this hypothetical nocturnal meal were lower than peak TG and AG levels of daytime meals, suggesting an inhibitory effect of sleep on the postdinner rebound of TG and AG. This inhibitory effect of sleep was further evidenced by the clear decline of TG levels, and to a lesser extent AG levels, during the remainder of the sleep period. This decline was abruptly interrupted on polygraphically defined morning awakening (Fig. 1).
Fig. 4.
Mean (±se) levels of total ghrelin (upper panel) and acylated ghrelin (lower panel) at mean peak levels for daytime (0900, 1400, and 1900 h) meals and at hypothetical peak time of a nocturnal (2400 h) meal.
Discussion
The present study used frequent blood sampling under rigorous conditions of energy intake, activity levels, and sleep-wake schedule and observed parallel meal-related variations of AG and TG in a homogenous group of young healthy lean men who ingested three identical carbohydrate-rich meals at 5-h intervals. The study design revealed that postmeal ghrelin suppression and rebound is similar in the morning, midday, and evening and that the nocturnal elevation of ghrelin reflects the postdinner rebound curbed by an inhibitory effect of sleep.
The demonstration of a strong positive association between 24-h mean peripheral concentrations of AG and postprandial glucose levels under normal physiological conditions is a major result of the study. Individuals with higher AG levels had more prolonged glucose responses to carbohydrate-rich meals. Remarkably, the mean AG level was a stronger predictor of overall daytime glucose concentrations than insulin. Because under normal conditions, the overnight period occupies only one third of the 24-h cycle, the mean AG level appears to be an important determinant of the overall physiological glucose concentration, supporting a growing body of evidence for a hyperglycemic effect of AG (16). In contrast, no associations between the 24-h mean TG levels, which represent mostly UAG levels, and glucose and/or insulin concentrations were found, consistent with distinct roles of AG vs. UAG in glucose metabolism. The robust positive relationship between AG levels, but not TG levels, and glucose concentrations under fed conditions could be involved in the remarkable dissociation between AG and UAG that occurs in response to fasting when glucose levels are low (13).
We measured plasma AG and serum TG levels using commercially available RIAs and observed 24-h profiles very similar, both in wave shape and individual variability, to those reported by Liu et al. (13) using two-site sandwich assays for AG and des-acyl ghrelin. A recent comparison of AG and TG concentrations assessed by RIA vs. two-site sandwich assay on more than 800 samples indicated that the RIA provides higher AG and TG values than the sandwich assays but that RIA-based values and sandwich assay-based values are strongly correlated both for AG (r = 0.78) and TG (r = 0.83) (17). The AG and TG values measured in the present study are indeed higher and the ratio of AG to TG levels is smaller than those reported using a two-site sandwich assay, consistent with lower specificity of RIAs. The design of our study involved, however, a within subject comparison of the roles of time of day and sleep on postprandial ghrelin responses to a robust signal, i.e. an 800-kcal meal including 60% of carbohydrates. The signal to noise ratio was high, and it is therefore unlikely that issues of assay specificity confounded our findings.
A robust circadian variation of glucose and insulin responses to mixed meals has been well documented, with a deterioration of glucose tolerance from morning to evening (18). Because insulin release and, to a lesser extent rising glucose levels, have been implicated in the rapid postmeal inhibition of ghrelin, an impact of time of day on ghrelin responses might have been expected. Moreover, the expression of ghrelin and the circadian clock proteins period 1 and period 2 in the oxyntic cells of the stomach is rhythmic (8). Unexpectedly, our data show that the degree of initial suppression of AG and TG levels and their subsequent rebound are independent of time of day. This finding may indicate that ghrelin release and acylation are not sensitive to physiological variations of glucose and insulin levels within the range of circadian variations of meal responses. Alternatively, there may be a circadian variation in ghrelin sensitivity, with greater morning than evening sensitivity, such that postmeal suppression remains essentially constant across the day. The rebound of ghrelin levels after the initial suppression by meal intake is implicated in the increase in hunger and the initiation of the following meal (6, 7). Within this context, our findings would suggest that the orexigenic effect of ghrelin is similar in the morning and evening.
Unlike all previous studies of human ghrelin physiology, our study enforced rigorously controlled sleep-wake and dark-light cycles and used polysomnography to evaluate the effect of sleep-wake transitions on ghrelin levels. Thus, we were able to explore the determinants of the nocturnal ghrelin increase, a robust phenomenon first reported in 2001 (9). Because we used identical meals, we were able to predict the magnitude of the rebounds of AG and TG levels after the dinner meal. During sleep, these rebounds were of lesser magnitude than predicted from meal responses during wake, consistent with a dampening of the rebound. Furthermore, after the nocturnal acrophase, a clear continuous decline of TG and AG levels occurred until morning awakening, which coincided with the resumption of stable concentrations. The AG to TG ratio was lower during sleep than during wake, suggesting that ghrelin-O-acyl-transferase activity may be decreased during sleep, consistent with a reduction of orexigenic signal. Taken together, these findings support an inhibitory effect of sleep on ghrelin, consistent with the association between sleeping and fasting and, conversely, between feeding and arousal. Although opposite findings have been reported in some human studies that concluded that ghrelin is a sleep-promoting factor (19–21), studies in rodents have indeed shown that ghrelin injections promote waking (22, 23). Consistent with an inhibitory effect of sleep on ghrelin, treatment of obstructive sleep apnea with continuous positive airway pressure, a therapy that increases sleep quality, results in lower TG levels (24). Conversely, several studies have linked partial or total sleep deprivation with elevated ghrelin levels (25–27). Studies that manipulate sleep timing and duration under well-controlled dietary conditions are warranted to further elucidate the interactions between sleep regulation and ghrelin release, acylation, and action.
Acknowledgments
We are indebted to Dr. Fabio Broglio (University of Turin, Turin, Italy) for assistance with the analysis and interpretation of a subset of the data. We thank the technical staff of the Endocrine Laboratories of the University of Chicago for assay validation and performance. We also thank the subjects for participating in the study and the nursing and dietary staff of the University of Chicago General Clinical Resource Center for their expert assistance.
This work was supported by U.S. National Institute of Health Grants P01 AG-11412, R01 HL-075025, P60 DK-20595, R01 DK-0716960, and UL1RR024999; U.S. Department of Defense Award W81XWH-07-2-0071; Institut National de la Santé et de la Recherche Médicale Unité 628 (Lyon, France); and Claude Bernard University of Lyon (Lyon, France).
Disclosure Summary: The authors having nothing to declare.
Footnotes
- AG
- Acylated ghrelin
- AUC
- area under the curve
- CV
- coefficient of variation
- REM
- rapid eye movement
- TG
- total ghrelin
- UAG
- unacylated ghrelin.
References
- 1. Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. 1999. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402:656–660 [DOI] [PubMed] [Google Scholar]
- 2. van der Lely AJ. 2009. Ghrelin and new metabolic frontiers. Horm Res 71(Suppl 1):129–133 [DOI] [PubMed] [Google Scholar]
- 3. Gasco V, Beccuti G, Marotta F, Benso A, Granata R, Broglio F, Ghigo E. 2010. Endocrine and metabolic actions of ghrelin. Endocr Dev 17:86–95 [DOI] [PubMed] [Google Scholar]
- 4. Granata R, Baragli A, Settanni F, Scarlatti F, Ghigo E. 2010. Unraveling the role of the ghrelin gene peptides in the endocrine pancreas. J Mol Endocrinol 45:107–118 [DOI] [PubMed] [Google Scholar]
- 5. Lim CT, Kola B, Korbonits M, Grossman AB. 2010. Ghrelin's role as a major regulator of appetite and its other functions in neuroendocrinology. Prog Brain Res 182:189–205 [DOI] [PubMed] [Google Scholar]
- 6. Cummings DE, Frayo RS, Marmonier C, Aubert R, Chapelot D. 2004. Plasma ghrelin levels and hunger scores in humans initiating meals voluntarily without time- and food-related cues. Am J Physiol Endocrinol Metab 287:E297–E304 [DOI] [PubMed] [Google Scholar]
- 7. Callahan HS, Cummings DE, Pepe MS, Breen PA, Matthys CC, Weigle DS. 2004. Postprandial suppression of plasma ghrelin level is proportional to ingested caloric load but does not predict intermeal interval in humans. J Clin Endocrinol Metab 89:1319–1324 [DOI] [PubMed] [Google Scholar]
- 8. LeSauter J, Hoque N, Weintraub M, Pfaff DW, Silver R. 2009. Stomach ghrelin-secreting cells as food-entrainable circadian clocks. Proc Natl Acad Sci USA 106:13582–13587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. 2001. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 50:1714–1719 [DOI] [PubMed] [Google Scholar]
- 10. Dzaja A, Dalal MA, Himmerich H, Uhr M, Pollmächer T, Schuld A. 2004. Sleep enhances nocturnal plasma ghrelin levels in healthy subjects. Am J Physiol Endocrinol Metab 286:E963–E967 [DOI] [PubMed] [Google Scholar]
- 11. Lucidi P, Murdolo G, Di Loreto C, Parlanti N, De Cicco A, Ranchelli A, Fatone C, Taglioni C, Fanelli C, Santeusanio F, De Feo P. 2004. Meal intake similarly reduces circulating concentrations of octanoyl and total ghrelin in humans. J Endocrinol Invest 27:RC12–RC15 [PubMed] [Google Scholar]
- 12. Avram AM, Jaffe CA, Symons KV, Barkan AL. 2005. Endogenous circulating ghrelin does not mediate growth hormone rhythmicity or response to fasting. J Clin Endocrinol Metab 90:2982–2987 [DOI] [PubMed] [Google Scholar]
- 13. Liu J, Prudom CE, Nass R, Pezzoli SS, Oliveri MC, Johnson ML, Veldhuis P, Gordon DA, Howard AD, Witcher DR, Geysen HM, Gaylinn BD, Thorner MO. 2008. Novel ghrelin assays provide evidence for independent regulation of ghrelin acylation and secretion in healthy young men. J Clin Endocrinol Metab 3:1980–1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Foster-Schubert KE, Overduin J, Prudom CE, Liu J, Callahan HS, Gaylinn BD, Thorner MO, Cummings DE. 2008. Acyl and total ghrelin are suppressed strongly by ingested proteins, weakly by lipids, and biphasically by carbohydrates. J Clin Endocrinol Metab 93:1971–1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rechtschaffen A, Kales A. 1968. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. In: Rechtschaffen A, Kales A. eds. Los Angeles: University of California, Los Angeles Brain Information Service/Brain Research Institute [Google Scholar]
- 16. Tong J, Prigeon RL, Davis HW, Bidlingmaier M, Kahn SE, Cummings DE, Tschop MH, D'Alessio D. 2010. Ghrelin suppresses glucose-stimulated insulin secretion and deteriorates glucose tolerance in healthy humans. Diabetes 59:2145–2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Prudom C, Liu J, Patrie J, Gaylinn BD, Foster-Schubert KE, Cummings DE, Thorner MO, Geysen HM. 2010. Comparison of competitive radioimmunoassays and two-site sandwich assays for the measurement and interpretation of plasma ghrelin levels. J Clin Endocrinol Metab 95:2351–2358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Van Cauter E, Polonsky KS, Scheen AJ. 1997. Roles of circadian rhythmicity and sleep in human glucose regulation. Endocr Rev 18:716–738 [DOI] [PubMed] [Google Scholar]
- 19. Kluge M, Gazea M, Schüssler P, Genzel L, Dresler M, Kleyer S, Uhr M, Yassouridis A, Steiger A. 2010. Ghrelin increases slow wave sleep and stage 2 sleep and decreases stage 1 sleep and REM sleep in elderly men but does not affect sleep in elderly women. Psychoneuroendocrinology 35:297–304 [DOI] [PubMed] [Google Scholar]
- 20. Kluge M, Schüssler P, Bleninger P, Kleyer S, Uhr M, Weikel JC, Yassouridis A, Zuber V, Steiger A. 2008. Ghrelin alone or co-administered with GHRH or CRH increases non-REM sleep and decreases REM sleep in young males. Psychoneuroendocrinology 33:497–506 [DOI] [PubMed] [Google Scholar]
- 21. Weikel JC, Wichniak A, Ising M, Brunner H, Friess E, Held K, Mathias S, Schmid DA, Uhr M, Steiger A. 2003. Ghrelin promotes slow-wave sleep in humans. Am J Physiol Endocrinol Metab 284:E407–E415 [DOI] [PubMed] [Google Scholar]
- 22. Szentirmai E, Hajdu I, Obal F, Jr, Krueger JM. 2006. Ghrelin-induced sleep responses in ad libitum fed and food-restricted rats. Brain Res 1088:131–140 [DOI] [PubMed] [Google Scholar]
- 23. Szentirmai E, Kapás L, Krueger JM. 2007. Ghrelin microinjection into forebrain sites induces wakefulness and feeding in rats. Am J Physiol 292:R575–R585 [DOI] [PubMed] [Google Scholar]
- 24. Takahashi K, Chin K, Akamizu T, Morita S, Sumi K, Oga T, Matsumoto H, Niimi A, Tsuboi T, Fukuhara S, Kangawa K, Mishima M. 2008. Acylated ghrelin level in patients with OSA before and after nasal CPAP treatment. Respirology (Carlton, Vic) 13:810–816 [DOI] [PubMed] [Google Scholar]
- 25. Spiegel K, Tasali E, Penev P, Van Cauter E. 2004. Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med 141:846–850 [DOI] [PubMed] [Google Scholar]
- 26. Taheri S, Lin L, Austin D, Young T, Mignot E. 2004. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med 1:e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schmid SM, Hallschmid M, Jauch-Chara K, Born J, Schultes B. 2008. A single night of sleep deprivation increases ghrelin levels and feelings of hunger in normal-weight healthy men. J Sleep Res 17:331–334 [DOI] [PubMed] [Google Scholar]