Post-absorptive non-oxidative free fatty acid (FFA) disposal (FFA flux – fatty acid oxidation) was 50% greater in women than men, but did not correlate with triglyceridemia or insulin sensitivity.
Abstract
Context:
Large increases in systemic free fatty acid (FFA) availability in the absence of a corresponding increase in fatty acid oxidation can create a host of metabolic abnormalities. These adverse responses are thought to be the result of fatty acids being shunted into hepatic very low-density lipoprotein-triglyceride production and/or intracellular lipid storage and signaling pathways because tissues are forced to increase nonoxidative FFA disposal.
Objective:
The objective of the study was to examine whether variations in postabsorptive nonoxidative FFA disposal within the usual range predict insulin resistance and hypertriglyceridemia.
Design:
We measured: systemic FFA turnover using a continuous iv infusion of [9–10, 3H]palmitate; substrate oxidation with indirect calorimetry combined with urinary nitrogen excretion; whole-body and peripheral insulin sensitivity with the labeled iv glucose tolerance test minimal model.
Setting:
the study was conducted at the Mayo Clinic General Clinical Research Center.
Participants:
Participants included healthy, postabsorptive, nonobese adults (21 women and 21 men).
Interventions:
There were no interventions.
Main Outcome Measures:
Nonoxidative FFA disposal (micromoles per minute), defined as the FFA disappearance rate minus fatty acid oxidation.
Results:
Women had 64% greater nonoxidative FFA disposal rate than men but a better lipid profile and similar insulin sensitivity. There was no significant correlation between nonoxidative FFA disposal and whole-body sensitivity, peripheral insulin sensitivity, or fasting serum triglyceride concentrations in men or women.
Conclusions:
Healthy nonobese women have greater rates of nonoxidative FFA disposal than men, but this does not appear to relate to adverse health consequences. Understanding the sex-specific interaction between adipose tissue lipolysis and peripheral FFA removal will help to discover new approaches to treat FFA-induced abnormalities.
In the postabsorptive state, free fatty acids (FFAs) originate primarily from adipose tissue lipolysis in humans. Systemic FFA turnover is most strongly associated with resting energy expenditure (REE) (1), suggesting that the regulation of adipose tissue lipolysis is linked to the needs of lean body tissue for fatty acids as an energy source. However, FFA availability in excess of oxidative needs increases the demand for tissues to dispose of fatty acids through nonoxidative pathways. These nonoxidative pathways for FFA metabolism can lead to increased very low-density lipoprotein (VLDL) triglyceride production (2) and insulin resistance (3, 4) because fatty acids are shunted into signaling pathways (5, 6) rather than being oxidized.
Most studies of fatty acid-induced insulin resistance have used lipid infusions that raise FFA concentrations to levels seen only with prolonged fasting. Whether variations in nonoxidative FFA disposal within the usual overnight postabsorptive range are associated with adverse effects is unknown. Therefore, we measured nonoxidative FFA disposal in healthy, postabsorptive nonobese adults with a wide range of insulin sensitivity and serum triglyceride concentrations. Our goal was to examine whether there are sex differences in nonoxidative FFA disposal and to discover if interindividual differences relate to parameters of lipid metabolism or insulin action.
Subjects and Methods
Subjects
After approval from the Mayo Institutional Review Board, 30 young men and 29 young women gave informed written consent to participate in the study. The following criteria were used to select the volunteers: body mass index (BMI) less than 30 kg/m2; no regular, vigorous physical activity; normal urinalysis; complete blood count; electrolytes; liver and kidney function tests; no medications known to influence lipid metabolism (including oral contraceptives); and no tobacco. All subjects were weight stable at least 2 months before the study. These volunteers were participants as young control subject in studies examining the effects of dehydroepiandrosterone and testosterone on body composition, metabolic function (7), insulin secretion, and action (8) in the elderly.
Experimental design
Body composition
Fat-free mass (FFM) and total and regional body fat mass were measured using dual-energy x-ray absorptiometry (DPX-IQ; Lunar Radiation, Madison, WI), and a single-slice computed tomography of the abdomen at the L2–3 interspace was performed to assess visceral fat.
Intravenous glucose tolerance test (IVGTT)
At approximately 0600 h and after a 12-h overnight fast, an iv 18-gauge cannula was inserted into each arm. One was used for infusion of glucose and insulin, and the other was used for withdrawal of blood. At 0900 h (0 time), glucose (0.3 g/kg total body weight) with 2% [6,6-2H2]glucose was injected iv over 2 min followed by infusion of insulin (0.02 U/kg total body weight, begun at 20 min) given as a square wave over 5 min as previously described (8, 9). Blood was sampled at −120, −30, −20, −10, 0, 2, 4, 6, 8, 10, 15, 20, 22, 25, 26, 28, 31, 35, 45, 60, 75, 90, 120, 180, and 240 min.
Physical performance
Peak aerobic capacity was assessed as maximum volume of oxygen consumed per minute (VO2). The peak VO2 was measured during a graded-intensity treadmill-walking test, with expired gas exchange assessed as previously described (10).
Main study visits
All subjects consumed a weight maintenance diet (55% carbohydrate, 15% protein, and 30% fat) provided by the Mayo Clinic General Clinical Research Center (GCRC) kitchen for 3 d preceding the FFA kinetic studies. The participants were admitted to the GCRC at 1600 h the day before the study and given a standard 10-kcal/kg meal (55% carbohydrate, 15% protein, and 30% fat), which was consumed between 1700 and 1730 h. No additional food was consumed until the next morning.
The following morning at 0600 h, two iv catheters were placed. The first was a forearm vein catheter in one arm and was used for tracer infusion and the second was placed in the contralateral hand vein in a retrograde fashion. The hand was placed in a heated box (55 C) to collect arterialized blood. Both catheters were kept patent with infusions of 0.45% NaCl. The volunteers remained in bed after catheter placement to ensure resting conditions before proceeding with the study. REE was measured at 0700 h using a DeltaTrac metabolic cart (Yorba Linda, CA). After collecting a baseline blood sample, a continuous (∼0.3 μCi/min) infusion of [9–10, 3H]palmitate (NEN Life Science Products, PerkinElmer, Boston, MA) was begun. After 30 min for isotopic equilibration, a series of four arterialized venous blood samples were collected at 10-min intervals for measurement of overnight, postabsorptive FFA flux. Participants remained at the GCRC for the remainder of the day for sample collection related to other aspects of this study. They were provided breakfast, lunch, and supper and underwent a 24-h urine collection for the measurement of nitrogen excretion. The next morning, before dismissal, a repeat measure of overnight, postabsorptive REE was performed. A second assessment of overnight postabsorptive FFA flux and REE was made after a 7- to 10-d interval using the same routine. Thus, each volunteer had two measures of basal FFA flux and four measures of REE. The average of these measures is presented.
To avoid potential confounding influence of negative energy balance on FFA metabolism (11, 12), we reviewed each participant's indirect calorimetry data, as a tool to assure the groups being compared for FFA metabolism were also matched for respiratory exchange ratio (RER). This approach was based on previous observations that, even within individuals fed standardized diets, relatively small variations in daily energy balance contribute substantially to variability in overnight postabsorptive RER and plasma FFA concentrations (11, 12). This is relevant to studies of FFA metabolism because RER is negatively related to plasma FFA concentrations, and in turn, plasma FFA concentrations are highly predictive of FFA flux. To avoid falsely concluding that sex might be the only reason for potential differences in FFA metabolism between men and women, evaluation of participants' energy balance status, as assessed by RER, was undertaken. In the present study, individuals with postabsorptive RER values consistently deviated considerably from 0.80 (approximately that expected based on the food quotient of the prestudy diets) likely incurred a modest energy imbalance despite the metabolic kitchen efforts to ensure otherwise. Based on this factor, we did not include the data from eight women and nine men with average RER values of 0.74–0.79 and 0.78–0.79, respectively. Thus, to obtain groups matched for RER, we evaluated data from 21 women and 21 men. For completeness, we also present the result of the complete groups (29 women and 30 men).
Analytical techniques
Plasma palmitate specific activity and concentration, as well as the concentrations of the other major FFAs, were measured by HPLC (13) using [2H31]palmitate as an internal standard (14). Because FFA concentrations and specific activity are measured independently, FFA flux is not a function of FFA concentration. Plasma glucose concentration was measured with a Beckman glucose analyzer (Beckman Instruments, Fullerton, CA). Plasma insulin, GH, cholesterol, triglyceride, and high-density lipoprotein (HDL)-cholesterol concentrations were measured as previously described (1). Plasma epinephrine was measured using HPLC with electrochemical detection (15).
Calculations
Visceral fat mass was predicted using the computed tomography measures of intraabdominal and sc adipose tissue combined with dual-energy x-ray absorptiometry-measured abdominal fat (16).
Net insulin sensitivity (Si) and peripheral insulin sensitivity (Si*) were assessed with a labeled IVGTT using the minimal model as previously described (8, 9, 17, 18). Net Si measures the ability of insulin to stimulate glucose disposal and inhibit glucose production. The labeled iv glucose minimal model assesses the selective effect of insulin (Si*) on peripheral glucose disposal.
Palmitate flux was calculated using metabolic steady-state formulas, in which the rate of appearance (Ra) (micromoles per minute) equals the rate of disappearance (Rd). The ratio of palmitate to total FFA concentrations was used to convert palmitate Ra and Rd to FFA Ra and Rd.
Fat oxidation was calculated using indirect calorimetry data, taking into consideration protein oxidation as assessed with measurement of urine nitrogen excretion (19). Molar fatty acid oxidation (micromoles per minute) was calculated from the fat oxidation assuming a molecular weight or 860 for a typical triglyceride (palmitoyl-stearoyl-oleoyl-glycerol). Because each triglyceride contains three FFA molecules, this was multiplied by three to obtain molar fatty acid oxidation rates.
Nonoxidative FFA disposal (micromoles per minute) was defined as the FFA Rd minus fatty acid oxidation measured by indirect calorimetry. Using this definition, nonoxidative disposal is the FFA disappearance that exceeds those FFA needed to replace fatty acids that are being oxidized simultaneously. Oxidative and nonoxidative FFA disposal were also expressed as percent of FFA turnover.
Statistical analysis
Values are expressed as means ± sd unless otherwise indicated. Statistical analyses were performed by two-tailed nonpaired Student's t test for between sex comparisons. Univariate correlations were assessed with the Pearson correlation coefficient. Data were checked for normality. Logarithmic transformation was performed to achieve normal distribution of nonnormally distributed data. P < 0.05 was considered statistically significant.
Results
Subject characteristics
When all participants were included in the analysis, men and women had similar age and BMI (by study design), but, as expected, women had more body fat, less FFM, lower VO2 peak per kilogram body weight, and lower REE than men (Table 1). Women had significantly less visceral fat mass and lower plasma epinephrine concentrations, and the expected greater plasma GH and HDL-cholesterol concentrations than men. Women also had significantly greater plasma insulin concentrations, lower Si and Si*, and lower RER than men (Table 1).
Table 1.
Clinical characteristics, body composition, and hormone concentrations of the study participants
| All participants |
P | Excluding low RER |
P | |||
|---|---|---|---|---|---|---|
| Women (n = 29) | Men (n = 30) | Women (n = 21) | Men (n = 21) | |||
| Age (yr) | 22 ± 3 | 24 ± 3 | 23 ± 3 | 24 ± 4 | ||
| BMI (kg/m2) | 24.1 ± 2.8 | 24.9 ± 2.8 | 24.3 ± 2.9 | 25.3 ± 2.6 | ||
| Weight (kg) | 65 ± 9 | 81 ± 9 | <0.0001 | 65 ± 9 | 82 ± 9 | <0.0001 |
| Body fat (%) | 34 ± 6 | 22 ± 7 | <0.0001 | 35 ± 6 | 23 ± 7 | <0.0001 |
| Total fat mass (kg) | 21 ± 7 | 17 ± 7 | 0.01 | 22 ± 7 | 18 ± 7 | 0.04 |
| FFM (kg) | 42 ± 4 | 62 ± 5 | <0.0001 | 42 ± 4 | 62 ± 5 | <0.0001 |
| Visceral fat mass (kg) | 1.5 ± 0.8 | 2.2 ± 1.2 | 0.02 | 1.7 ± 0.9 | 2.1 ± 1.1 | 0.08 |
| Fasting glucose (mmol/liter) | 4.8 ± 0.3 | 4.9 ± 0.3 | 0.09 | 4.75 ± 0.31 | 4.83 ± 0.25 | 0.42 |
| Fasting insulin (pmol/liter) | 29 ± 11 | 23 ± 8 | 0.04 | 27 ± 9 | 25 ± 9 | 0.37 |
| Si IVGTT [×10−4/min−1/(μU/ml)] | 5.0 ± 2.3 | 6.7 ± 3.1 | 0.01 | 5.4 ± 2.4 | 5.8 ± 2.3 | 0.51 |
| Si* IVGTT [×10−4/min−1/(μU/ml)] | 3.6 ± 2.8 | 6.0 ± 3.4 | 0.004 | 4.1 ± 3.1 | 5.2 ± 2.7 | 0.24 |
| VO2 peak (ml/kg · min) | 35 ± 6 | 43 ± 7 | <0.0001 | 34 ± 6 | 43 ± 6 | <0.0001 |
| Epinephrine (pg/ml) | 16 ± 9 | 26 ± 10 | 0.001 | 16 ± 9 | 24 ± 9 | 0.007 |
| GH (μg/liter) | 2.37 ± 1.05 | 0.89 ± 0.57 | <0.0001 | 2.27 ± 1.15 | 0.83 ± 0.46 | <0.0001 |
| Total cholesterol (mmol/liter) | 3.81 ± 0.61 | 3.77 ± 0.79 | 0.80 | 3.81 ± 0.59 | 3.88 ± 0.87 | 0.77 |
| HDL cholesterol (mmol/liter) | 1.40 ± 0.45 | 1.09 ± 0.57 | 0.03 | 1.49 ± 0.49 | 1.05 ± 0.53 | 0.01 |
| Triglycerides (mmol/liter) | 1.12 ± 0.42 | 1.20 ± 0.45 | 0.47 | 1.09 ± 0.46 | 1.26 ± 0.50 | 0.27 |
| REE (kcal/d) | 1443 ± 138 | 1799 ± 197 | <0.0001 | 1422 ± 142 | 1780 ± 178 | <0.0001 |
| RER | 0.81 ± 0.03 | 0.84 ± 0.03 | 0.001 | 0.83 ± 0.02 | 0.84 ± 0.02 | 0.26 |
Comparing women and men matched for RER values (Table 1), women still had greater amounts of body fat, lower FFM, lower VO2 peak, and lower REE than men (Table 1). We observed the expected positive linear relationship between FFM and REE in both women (r = 0.62, P = 0.003) and men (r = 0.45, P = 0.04). Visceral fat mass, insulin concentrations, and insulin sensitivity were not significantly different between men and women. Women had the expected greater plasma GH and HDL-cholesterol concentrations and lower plasma epinephrine concentrations than men (Table 1).
FFA kinetics and fatty acid oxidation
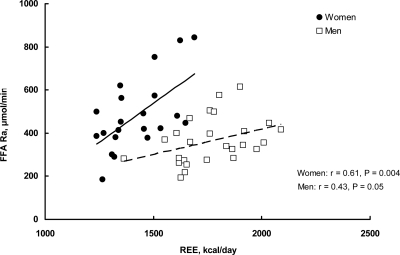
The patterns of FFA kinetics and fatty acid oxidation were similar between the two approaches (all participants vs. matched for RER participants) (Table 2). In the participants matched for RER, plasma palmitate and FFA concentrations were significantly greater in women than in men (Table 2). Average fatty acid oxidation rates (micromoles per minute) were not significantly different between the sexes. Systemic FFA turnover rate was significantly greater in women than in men whether expressed relative to REE (Fig. 1) or as an absolute rate (Table 2). FFA turnover rate was positively associated with REE in a sex-specific manner (Fig. 1), similar to what we have reported previously (1, 20).
Table 2.
Plasma FFA concentration, rate of release, oxidation, and nonoxidative disposal in the study subjects
| All participants |
P | Excluding low RER |
P | |||
|---|---|---|---|---|---|---|
| Women (n = 29) | Men (n = 30) | Women (n = 21) | Men (n = 21) | |||
| Plasma palmitate concentration (μmol/liter) | 97 ± 28 | 68 ± 17 | 0.0001 | 93 ± 27 | 63 ± 15 | 0.0001 |
| Plasma FFA concentration (μmol/liter) | 422 ± 120 | 293 ± 76 | <0.0001 | 406 ± 117 | 273 ± 64 | 0.0001 |
| FFA turnover rate (μmol/min) | 499 ± 156 | 394 ± 135 | 0.007 | 482 ± 169 | 372 ± 109 | 0.02 |
| Fatty acid oxidation rate (μmol/min) | 172 ± 54 | 174 ± 67 | 0.90 | 150 ± 43 | 170 ± 45 | 0.15 |
| Nonoxidative FFA disposal (μmol/min) | 327 ± 139 | 220 ± 102 | 0.001 | 332 ± 151 | 202 ± 95 | 0.002 |
| Nonoxidative FFA disposal (FFA turnover, %) | 64 ± 10 | 55 ± 13 | 0.003 | 67 ± 9 | 53 ± 12 | 0.0001 |
Values are means ± sd.
Fig. 1.
Resting postabsorptive FFA Ra is plotted vs. REE for the women (n = 21) and men (n = 21) participating in the study (r = Pearson correlation coefficient). Multivariate analysis also showed that REE (P = 0.009) and sex (P < 0.0001) were significant and independent predictors of FFA release rates.
Nonoxidative FFA disposal
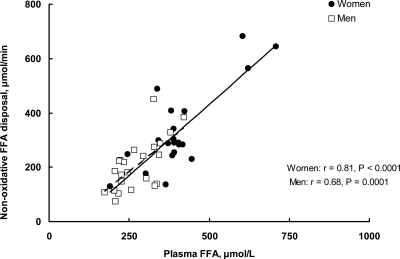
The patterns of nonoxidative FFA disposal were similar between the two approaches (all participants vs. matched for RER participants). In the matched groups, nonoxidative FFA disposal was significantly greater in women than men, whether expressed in absolute terms (micromoles per minute) or as a fraction of FFA turnover rate (Table 2). The rates of nonoxidative FFA disposal ranged from 129–682 μmol/min in women and from 73–451 μmol/min in men. In both sexes, FFA concentration was positively associated with the rate of nonoxidative FFA disposal (Fig. 2).
Fig. 2.
Nonoxidative FFA disposal is plotted vs. plasma FFA concentration for the women (n = 21) and men (n = 21) participating in the study (r = Pearson correlation coefficient).
Nonoxidative FFA disposal in relation to insulin sensitivity and plasma triglycerides
No statistically significant relationships were observed between nonoxidative FFA disposal rates and plasma triglyceride concentrations (women: r = 0.02, P = 0.94; men: r = 0.26, P = 0.15), between nonoxidative FFA disposal rates and Si (women: r = −0.30, P = 0.11; men: r = 0.01, P = 0.60), or between nonoxidative FFA disposal rates and Si* (women: r = 0.19, P = 0.34; men: r = 0.01, P = 0.98), in the complete groups (n = 29 women and n = 30 men).
In the matched women (n = 21) and men (n = 21), Si ranged from 1.5 to 10.6 and 1.2 to 11.3 × 10−4/min−1/(microunits per milliliter), respectively. Si* ranged from 1.18 to 13.70 × 10−4/min−1/(microunits per milliliter) in women and 1.13 to 11.10 × 10−4/min−1/(microunits per milliliter) in men. Fasting serum triglycerides ranged from 0.4 to 2.2 mm in women and 0.7 to 2.7 mm in men. The relationship between nonoxidative FFA disposal rates and plasma triglyceride concentrations were not statistically significant in either women (r = 0.03, P = 0.97) or men (r = 0.42, P = 0.056). Nonoxidative FFA disposal rates also did not correlate with Si in either men (r = 0.17, P = 0.46) or women (r = 0.18, P = 0.44). Likewise, nonoxidative FFA disposal rates did not correlate with Si* in either men (r = 0.13, P = 0.58) or women (r = 0.14, P = 0.55).
Discussion
Basal adipose tissue lipolysis is most strongly associated with resting metabolic rate in lean and obese adults (1, 20), although lipolysis rate is greater in women than men, even in the context of similar overnight postabsorptive RER. This suggested to us that the portion of FFA entering nonoxidative pathways is greater in women than men. Nonoxidative fatty acid trafficking pathways can create a host of metabolic abnormalities because they may lead to increased VLDL triglyceride production (2) and insulin resistance (3, 4) because fatty acids are shunted into intracellular storage and signaling pathways (5, 6) rather than being oxidized. In this study, we report quantitative measures of the differences between nonoxidative FFA disposal in nonobese, premenopausal women and in age- and BMI-matched men. Interestingly, nonoxidative FFA disposal rate was not associated with net (whole body) insulin sensitivity (Si IVGTT), peripheral insulin sensitivity (Si* IVGTT), or plasma triglyceride concentrations in either sex.
We confirmed that systemic FFA turnover rate was correlated with REE in a sex-specific fashion. FFA turnover rate exceeded fatty acid oxidation rate as measured by indirect calorimetry (by a range of 73–682 μmol/min) in every volunteer, consistent with past reports that circulating lipid fuel availability exceeds lipid fuel needs at rest (21, 22). Fatty acid oxidation rates were similar in men and women in this study, yet women had greater plasma FFA concentrations and systemic FFA turnover. Therefore, overnight postabsorptive fatty acid oxidation rates may not be driven solely by plasma FFA availability. On the other hand, it is likely that, if plasma FFA concentration was similar between men and women, men might exhibit greater fatty acid oxidation rates than women. Boden and Jadali (23) reported that physiological increases in plasma FFA concentrations in normal-weight men resulted in a trend toward greater fat oxidation, although the effect was not statistically significant. They also found that if insulin secretion was prevented from increasing by infusing somatostatin together with replacement of insulin and glucagon, these same increases in plasma FFA concentrations resulted in abnormal glucose metabolism (23). On the basis of these results (23), it appears that when FFA concentrations and turnover rates are increased in men to levels seen in women, there are adverse effects on insulin action likely in the context of greater nonoxidative FFA metabolism.
Women had significantly greater FFA concentrations as well as FFA turnover rates relative to energy requirements than men (Fig. 1). We previously studied a more heterogeneous group of adults (lean and obese men and women) and reported greater FFA turnover rates in women in the context of similar FFA concentrations (plasma palmitate averaging 92 μmol/liter in women and 84 μmol/liter in men) (1). In a more recent study (20), we observed both greater FFA turnover rates and plasma FFA concentrations in women than men, which is in line with the present results. The current results support the concept that normal adipose tissue lipolysis is different between sexes, as probably are the mechanisms that allow energy demands to regulate FFA release and removal.
As expected, women had greater GH concentrations than men, although this sex difference is more clearly observed when the 24-h secretion profile of GH is monitored (24). Although GH has lipolytic properties (25), there is little evidence that the greater GH concentrations in women account for their greater lipolysis rates. Interestingly, alterations in GH levels induce significant within-subject differences in lipolysis, however, the major determinant of subject-to-subject variations in the overall lipolytic response appear to be a permissive effect of GH on catecholamine-induced lipolysis rather than a direct lipolytic effect of GH (26). Furthermore, men have been shown to exhibit a higher lipolytic rate in response to a pulsatile GH infusion compared with women (27), indicating that the greater GH concentrations in women do not necessarily translate into enhanced lipolysis (27).
Plasma FFA concentrations correlated with nonoxidative FFA disposal in both sexes (Fig. 2). It is unknown, however, whether the disposal of FFA through nonoxidative pathways is passive, reflecting the balance between lipolysis and oxidation, or whether it represents a dynamic buffering process actively regulating plasma FFA concentrations. The finding of greater nonoxidative FFA disposal in women than men raises the question of which tissue(s) is responsible for this process. Potential sites include reesterification of fatty acids in the liver, adipose tissue, and muscle. It has been estimated that approximately 7% (28) and approximately 6% (29, 30) of systemic FFA enter the VLDL-triglyceride pathway in women and men, respectively. This would not seem to account for the differences in nonoxidative FFA disposal we observed. We recently reported a pathway of direct FFA uptake into adipose tissue that is more active in women than men (31), which could account for a portion of the greater nonoxidative FFA disposal in women. If the greater nonoxidative FFA disposal in women were into skeletal muscle and were not balanced by a greater fatty acid oxidation, we would expect an insulin resistant phenotype (32), which was not seen in women vs. men who were matched for RER.
In the groups matched for RER (n = 21 women and n = 21 men), women had greater nonoxidative FFA disposal rate, yet they had similar Si IVGTT, Si* IVGTT, plasma triglyceride concentrations, and the expected higher HDL-cholesterol concentration than men. In addition, we were unable to detect a significant correlation between nonoxidative FFA disposal rate and plasma triglyceride concentrations or insulin sensitivity despite the fact that our volunteers had a wide range of plasma triglycerides and insulin sensitivity. Given the range of values we observed and the technical and biological reproducibility of our measures, we estimate that we had a power of 0.90 to detect a correlation of r = 0.60 at a P = 0.05 for both triglycerides and Si. The finding that nonoxidative FFA disposal rate was not a predictor of plasma triglyceride concentrations in women and was only a weak predictor in men suggests that plasma triglyceride levels may not be used as a surrogate index of systemic FFA reesterification.
Of interest, among all participants (n = 29 women and n = 30 men), women had lower net (Si) and peripheral (Si*) insulin sensitivity than men. A possible explanation for these results might be because there was a tendency to underfeed women more so than men. To the extent women were in negative energy balance, they might have entered into a temporary insulin-resistant state via the cycle of Randle et al. (33). Indeed, Si was lower (P = 0.07) in the women who were excluded from the analysis [Si 3.9 ± 1.8 × 10−4/min−1 (microunits per milliliter)] compared with those who remained in the analysis based on acceptable RER [Si 5.5 ± 2.3 × 10−4/min−1 (microunits per milliliter)]. In any case, in line with the results from the matched groups (an approach that mainly excluded underfed/low RER individuals), nonoxidative FFA disposal rate did not relate to Si, Si*, or plasma triglyceride concentrations in either sex in the complete groups. This indicates that variations in postabsorptive nonoxidative FFA disposal within the usual range do not predict Si or Si* as well as hypertriglyceridemia in healthy adults.
It has been suggested that the excess mobilization of fatty acids from adipose tissue and the subsequent reesterification to triglycerides may increase the flexibility of fuel regulation (21, 22). In this sense, women may have a more favorable response to changes in energy requirements by changing the portion of released fatty acids that are directed toward reesterification. Understanding the tissues that are involved in nonoxidative FFA uptake in women and the mechanisms that these tissues use to increase FFA removal may provide new insights into the determinants of insulin resistance as it relates to plasma FFA concentrations and kinetics.
In conclusion, we found that the greater rates of lipolysis in nonobese women than men are associated with increased FFA removal through nonoxidative pathways. Over the ranges of nonoxidative FFA disposal we observed, there was no clinically significant association with either insulin sensitivity or fasting serum triglyceride concentrations. It may be that greater FFA excess is needed to create the previously reported abnormalities (3, 4) or that FFA excess combined with sustained hyperinsulinemia (34) is the link between systemic FFA and insulin resistance. Understanding the interaction between adipose tissue lipolysis and peripheral FFA removal will help to discover new approaches to treat FFA-induced abnormalities.
Acknowledgments
We thank the volunteers who participated in this study. We also thank Jean Feehan, Barbara Norby, and the members of the Mayo Clinic Clinical Research Unit nursing, dietary, and support laboratory staff for technical assistance in performing the study.
This work was supported by Grants PO1 AG14283, DK40484, and RR00585 from the U.S. Public Health Service and the Mayo Foundation.
Disclosure Summary: The authors have nothing to declare.
Footnotes
- BMI
- Body mass index
- FFA
- free fatty acid
- FFM
- fat-free mass
- GCRC
- General Clinical Research Center
- HDL
- high-density lipoprotein
- IVGTT
- iv glucose tolerance test
- Ra
- rate of appearance
- Rd
- rate of disappearance
- REE
- resting energy expenditure
- RER
- respiratory exchange ratio
- Si
- net insulin sensitivity
- Si*
- peripheral insulin sensitivity
- VLDL
- very low-density lipoprotein
- VO2
- oxygen consumed per minute.
References
- 1. Nielsen S, Guo Z, Albu JB, Klein S, O'Brien PC, Jensen MD. 2003. Energy expenditure, sex, and endogenous fuel availability in humans. J Clin Invest 111:981–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lewis GF, Uffelman KD, Szeto LW, Weller B, Steiner G. 1995. Interaction between free fatty acids and insulin in the acute control of very low density lipoprotein production in humans. J Clin Invest 95:158–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boden G, Jadali F, White J, Liang Y, Mozzoli M, Chen X, Coleman E, Smith C. 1991. Effects of fat on insulin-stimulated carbohydrate metabolism in normal men. J Clin Invest 88:960–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boden G, Chen X, Ruiz J, White JV, Rossetti L. 1994. Mechanisms of fatty acid-induced inhibition of glucose uptake. J Clin Invest 93:2438–2446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Roden M, Price TB, Perseghin G, Petersen KF, Rothman DL, Cline GW, Shulman GI. 1996. Mechanism of free fatty acid-induced insulin resistance in humans. J Clin Invest 97:2859–2865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dresner A, Laurent D, Marcucci M, Griffin ME, Dufour S, Cline GW, Slezak LA, Andersen DK, Hundal RS, Rothman DL, Petersen KF, Shulman GI. 1999. Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J Clin Invest 103:253–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nair KS, Rizza RA, O'Brien P, Dhatariya K, Short KR, Nehra A, Vittone JL, Klee GG, Basu A, Basu R, Cobelli C, Toffolo G, Dalla Man C, Tindall DJ, Melton LJ, 3rd, Smith GE, Khosla S, Jensen MD. 2006. DHEA in elderly women and DHEA or testosterone in elderly men. N Engl J Med 355:1647–1659 [DOI] [PubMed] [Google Scholar]
- 8. Basu R, Dalla Man C, Campioni M, Basu A, Nair KS, Jensen MD, Khosla S, Klee G, Toffolo G, Cobelli C, Rizza RA. 2007. Two years of treatment with dehydroepiandrosterone does not improve insulin secretion, insulin action, or postprandial glucose turnover in elderly men or women. Diabetes [Erratum (2007) 56:1486] 56:753–766 [DOI] [PubMed] [Google Scholar]
- 9. Basu R, Breda E, Oberg AL, Powell CC, Dalla Man C, Basu A, Vittone JL, Klee GG, Arora P, Jensen MD, Toffolo G, Cobelli C, Rizza RA. 2003. Mechanisms of the age-associated deterioration in glucose tolerance: contribution of alterations in insulin secretion, action and clearance. Diabetes 52:1738–1748 [DOI] [PubMed] [Google Scholar]
- 10. Proctor DN, Beck KC. 1996. Delay time adjustments to minimize errors in breath-by-breath measurement of VO2 during exercise. J Appl Physiol 81:2495–2499 [DOI] [PubMed] [Google Scholar]
- 11. Jensen MD, Bajnárek J, Lee SY, Nielsen S, Koutsari C. 2009. Relationship between postabsorptive respiratory exchange ratio and plasma free fatty acid concentrations. J Lipid Res 50:1863–1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van Aggel-Leijssen DP, van Baak MA, Tenenbaum R, Campfield LA, Saris WH. 1999. Regulation of average 24 h human plasma leptin level: the influence of exercise and physiological change in energy balance. Int J Obes Relat Metab Disord 23:151–158 [DOI] [PubMed] [Google Scholar]
- 13. Miles JM, Ellman MG, McClean KL, Jensen MD. 1987. Validation of a new method for determination of free fatty acid turnover. Am J Physiol 252:E431–E438 [DOI] [PubMed] [Google Scholar]
- 14. Jensen MD, Rogers PJ, Ellman MG, Miles JM. 1988. Choice of infusion-sampling mode for tracer studies of free fatty acid metabolism. Am J Physiol 254:E562–E565 [DOI] [PubMed] [Google Scholar]
- 15. Causon RC, Carruthers ME, Rodnight R. 1981. Assay of plasma catecholamines by liquid chromatography with electrochemical detection. Anal Biochem 116:223–226 [DOI] [PubMed] [Google Scholar]
- 16. Jensen MD, Kanaley JA, Reed JE, Sheedy PF. 1995. Measurement of abdominal and visceral fat with computed tomography and dual-energy x-ray absorptiometry. Am J Clin Nutr 61:274–278 [DOI] [PubMed] [Google Scholar]
- 17. Cobelli C, Vicini P, Toffolo G, Caumo A. 1996. The hot IVGTT minimal models: simultaneous assessment of glucose disposal indices and the hepatic glucose release. In: Lovejoy J, Bergman RN. eds. MinMod 94. Baton Rouge, LA: Louisiana Press; 202–239 [Google Scholar]
- 18. Bergman RN, Ider YZ, Bowden CR, Cobelli C. 1979. Quantitative estimation of insulin sensitivity. Am J Physiol 236:E667–E677 [DOI] [PubMed] [Google Scholar]
- 19. Frayn KN. 1983. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol 55:628–634 [DOI] [PubMed] [Google Scholar]
- 20. Shadid S, Kanaley JA, Sheehan MT, Jensen MD. 2007. Basal and insulin-regulated free fatty acid and glucose metabolism in humans. Am J Physiol 292:E1770–E1774 [DOI] [PubMed] [Google Scholar]
- 21. Klein S, Wolfe RR. 1990. Whole-body lipolysis and triglyceride-fatty acid cycling in cachectic patients with esophageal cancer. J Clin Invest 86:1403–1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wolfe RR, Klein S, Carraro F, Weber JM. 1990. Role of triglyceride-fatty acid cycle in controlling fat metabolism in humans during and after exercise. Am J Physiol 258:E382–E389 [DOI] [PubMed] [Google Scholar]
- 23. Boden G, Jadali F. 1991. Effects of lipid on basal carbohydrate metabolism in normal men. Diabetes 40:686–692 [DOI] [PubMed] [Google Scholar]
- 24. Ho KY, Evans WS, Blizzard RM, Veldhuis JD, Merriam GR, Samojlik E, Furlanetto R, Rogol AD, Kaiser DL, Thorner MO. 1987. Effects of sex and age on the 24-hour profile of growth hormone secretion in man: importance of endogenous estradiol concentrations. J Clin Endocrinol Metab 64:51–58 [DOI] [PubMed] [Google Scholar]
- 25. Raben MS, Hollenberg CH. 1959. Effect of growth hormone on plasma fatty acids. J Clin Invest 38:484–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hansen TK, Gravholt CH, Ørskov H, Rasmussen MH, Christiansen JS, Jørgensen JO. 2002. Dose dependency of the pharmacokinetics and acute lipolytic actions of growth hormone. J Clin Endocrinol Metab 87:4691–4698 [DOI] [PubMed] [Google Scholar]
- 27. Surya S, Horowitz JF, Goldenberg N, Sakharova A, Harber M, Cornford AS, Symons K, Barkan AL. 2009. The pattern of growth hormone delivery to peripheral tissues determines insulin-like growth factor-1 and lipolytic responses in obese subjects. J Clin Endocrinol Metab 94:2828–2834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Eaton RP, Berman M, Steinberg D. 1969. Kinetic studies of plasma free fatty acid and triglyceride metabolism in man. J Clin Invest 48:1560–1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Friedberg SJ, Klein RF, Trout DL, Bogdonoff MD, Estes EH., Jr 1961. The incorporation of plasma free fatty acids into plasma triglycerides in man. J Clin Invest 40:1846–1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Havel RJ, Kane JP, Balasse EO, Segel N, Basso LV. 1970. Splanchnic metabolism of free fatty acids and production of triglycerides of very low density lipoproteins in normotriglyceridemic and hypertriglyceridemic humans. J Clin Invest 49:2017–2035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shadid S, Koutsari C, Jensen MD. 2007. Direct free fatty acid uptake into human adipocytes in vivo: relation to body fat distribution. Diabetes 56:1369–1375 [DOI] [PubMed] [Google Scholar]
- 32. Kelley DE, Mokan M, Simoneau JA, Mandarino LJ. 1993. Interaction between glucose and free fatty acid metabolism in human skeletal muscle. J Clin Invest 92:91–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Randle PJ, Garland PB, Hales CN, Newsholme EA. 1963. The glucose-fatty acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1:785–789 [DOI] [PubMed] [Google Scholar]
- 34. Roust LR, Jensen MD. 1993. Postprandial free fatty acid kinetics are abnormal in upper body obesity. Diabetes 42:1567–1573 [DOI] [PubMed] [Google Scholar]