Data show that the insights that improve obesity prevention and treatment will almost certainly benefit the incidence and care of type 2 diabetes.
Abstract
Objective:
This report examines what is known about the relationship between obesity and type 2 diabetes and how future research in these areas might be directed to benefit prevention, interventions, and overall patient care.
Research Design and Methods:
An international working group of 32 experts in the pathophysiology, genetics, clinical trials, and clinical care of obesity and/or type 2 diabetes participated in a conference held on 6–7 January 2011 and cosponsored by The Endocrine Society, the American Diabetes Association, and the European Association for the Study of Diabetes. A writing group comprising eight participants subsequently prepared this summary and recommendations. Participants reviewed and discussed published literature and their own unpublished data.
Results:
The writing group unanimously supported the summary and recommendations as representing the working group's majority or unanimous opinions.
Conclusions:
The major questions linking obesity to type 2 diabetes that need to be addressed by combined basic, clinical, and population-based scientific approaches include the following: 1) Why do not all patients with obesity develop type 2 diabetes? 2) Through what mechanisms do obesity and insulin resistance contribute to β-cell decompensation, and if/when obesity prevention ensues, how much reduction in type 2 diabetes incidence will follow? 3) How does the duration of type 2 diabetes relate to the benefits of weight reduction by lifestyle, weight-loss drugs, and/or bariatric surgery on β-cell function and glycemia? 4) What is necessary for regulatory approval of medications and possibly surgical approaches for preventing type 2 diabetes in patients with obesity? Improved understanding of how obesity relates to type 2 diabetes may help advance effective and cost-effective interventions for both conditions, including more tailored therapy. To expedite this process, we recommend further investigation into the pathogenesis of these coexistent conditions and innovative approaches to their pharmacological and surgical management.
Most patients with type 2 diabetes are obese, and the global epidemic of obesity largely explains the dramatic increase in the incidence and prevalence of type 2 diabetes over the past 20 years. Currently, over a third (34%) of U.S. adults are obese (defined as BMI >30 kg/m2), and over 11% of people aged ≥20 years have diabetes (1), a prevalence projected to increase to 21% by 2050 (2). However, the precise mechanisms linking the two conditions remain unclear, as does our understanding of interindividual differences. Improved understanding will help advance identification and development of effective treatment options.
Excess weight is an established risk factor for type 2 diabetes, yet most obese individuals do not develop type 2 diabetes. Recent studies have identified “links” between obesity and type 2 diabetes involving proinflammatory cytokines (tumor necrosis factor and interleukin-6), insulin resistance, deranged fatty acid metabolism, and cellular processes such as mitochondrial dysfunction and endoplasmic reticulum stress. These interactions are complex, with the relative importance of each unclearly defined. Further genetic studies may elucidate additional common pathophysiological pathways for obesity and diabetes and identify promising new treatment targets. As physicians frequently prescribe glucose-lowering medications associated with weight gain, trade-offs between glycemic control and body weight with current therapeutic options need more consideration. This issue is particularly pressing given accumulating evidence that even modest weight reduction—whether through lifestyle/behavioral interventions, obesity medications, or bariatric surgery—can improve glycemic control and reduce diabetes risk.
These intriguing, but still largely unexplored, connections between obesity and type 2 diabetes suggested the timely need to convene a group of scientific experts in the fields to more closely examine underlying pathophysiology and treatment options for patients with type 2 diabetes addressing issues of excess weight and glycemic control simultaneously. Participants in the January 2011 conference (see Appendix) were tasked with examining what is known about the relationship between obesity and type 2 diabetes and the heterogeneity of these conditions, what needs to be learned, and how to direct future research in these areas to advance effective interventions and improve patient care. What follows summarizes the major issues addressed and the outcomes of the discussion.
Mechanisms of obesity-associated insulin resistance
The influence of obesity on type 2 diabetes risk is determined not only by the degree of obesity but also by where fat accumulates. Increased upper body fat including visceral adiposity, as reflected in increased abdominal girth or waist-to-hip ratio, is associated with the metabolic syndrome, type 2 diabetes, and cardiovascular disease (3), although underlying mechanisms remain uncertain. Whether subcutaneous fat lacks the pathological effects of visceral fat or is simply a more neutral storage location, for example, requires further study. Beyond differences in body fat distribution, emerging evidence suggests that different subtypes of adipose tissue may be functionally distinct and affect glucose homeostasis differentially. Adult humans have limited and variable numbers of brown fat cells (4), which play a role in thermogenesis and potentially influence energy expenditure and obesity susceptibility (5). Improved understanding of the function of different fat cell types and depots and their roles in metabolic homeostasis is a priority for investigation into the pathogenesis and complications of obesity. Likewise, adipose tissue is composed of heterogeneous cell types. Immune cells within adipose tissue also likely contribute to systemic metabolic processes. As the study of adipose biology progresses, it will be important to consider whether additional subtypes of adipocytes or other cell types can be identified to refine our understanding of obesity complications and generate novel approaches to prevention.
At least three distinct mechanisms have been proposed to link obesity to insulin resistance and predispose to type 2 diabetes: 1) increased production of adipokines/cytokines, including tumor necrosis factor-α, resistin, and retinol-binding protein 4, that contribute to insulin resistance as well as reduced levels of adiponectin (6); 2) ectopic fat deposition, particularly in the liver and perhaps also in skeletal muscle, and the dysmetabolic sequelae (7); and 3) mitochondrial dysfunction, evident by decreased mitochondrial mass and/or function (8). Mitochondrial dysfunction could be one of many important underlying defects linking obesity to diabetes, both by decreasing insulin sensitivity and by compromising β-cell function.
Mechanisms of progressive β-cell dysfunction in obese individuals
The link between obesity and hyperinsulinemia, first identified ∼50 years ago (9), reflects compensation by insulin-secreting β-cells to systemic insulin resistance. Although mechanisms underlying this coupling (e.g., mild hyperglycemia and raised levels of circulating free fatty acids) remain elusive, obese normoglycemic individuals have both increased β-cell mass and function (9–12). Obesity-induced glucose intolerance reflects failure to mount one or more of these compensatory responses (13).
Factors predisposing to β-cell decompensation could also be primarily genetic or epigenetic. A clear, mechanistic basis for this decompensation has remained elusive. Genetic studies have helped identify the role of some key molecules in β-cell biology that may be important in this regard. For example, recent rodent studies have demonstrated diabetogenic effects of reduced pancreatic expression of the Pdx1 gene (14, 15). While these animal studies have demonstrated that PDX1 deficiency relates mechanistically to diabetes through β-cell apoptosis, and PDX1 deficiency is linked to MODY4 (16), it is not clear yet that PDX1 deficiency has a role in common forms of type 2 diabetes in humans. This example illustrates how a growing understanding of genetics and cellular function of the β-cell can identify potential mediators predisposing obese individuals to type 2 diabetes and further may provide insights for the development of new therapeutic agents.
Genetic factors linking obesity and diabetes
Genome-wide association scans (GWAS) and candidate gene approaches now have identified ∼40 genes associated with type 2 diabetes (17, 18) and a similar number, albeit largely different, with obesity. Most type 2 diabetes genes appear to be related to β-cell dysfunction, with many fewer involved in pathways related to insulin resistance independent of obesity (19, 20). Not surprisingly, many obesity gene variants appear to be involved in pathways affecting energy homeostasis. Although numerous diabetes- and obesity-associated genes have been identified, the known genes are estimated to predict only 15% of type 2 diabetes and 5% of obesity risk (21). Although additional genes with important roles will undoubtedly be discovered, this low predictive power may reflect the importance of environmental factors, less frequent genetic variants with stronger effects, or gene-environment, gene-gene, and epigenetic interactions that are not readily identified through methods based on population genetics. Methods for detecting gene-gene interactions exist, but the population size needed to detect them is substantially greater than is required for detection of single genes of relatively small effect. Alternatively, pathway analyses or a systems biology approach combining information from DNA variations with transcript, protein, and metabolite profiles may better capture the genetic influences on metabolism than studying single genes. One should also keep in mind that the missing heritability could be an illusion of inferring additive genetic effects from epidemiological data (22).
Does a shared pathogenesis underlie both obesity and type 2 diabetes?
Although the link between obesity and type 2 diabetes is widely held to involve two discrete lesions—obesity-induced insulin resistance and β-cell failure—both disorders may share an underlying defect. This “unified field theory” raises questions about whether defects favoring progressive weight gain and metabolic impairment also contribute to β-cell decompensation.
One potential link could be sustained cell exposure to nutrient concentrations exceeding energy requirements. Deleterious cellular effects of nutrient excess can include impaired inflammatory signaling, endoplasmic reticulum stress, excess production of reactive oxygen species, mitochondrial dysfunction, accumulation of triglycerides and/or fatty acyl intermediates, and activation of serine-threonine kinases (23). These responses are not mutually exclusive, and induction of one may trigger another, leading to a cascade of damage. Obesity-associated cellular injury can in turn recruit and activate macrophages and other immune cells that exacerbate tissue inflammation (23, 24). Collectively, these responses contribute to the pathogenesis of insulin resistance in the liver, skeletal muscle, and adipose tissue, and some (e.g., acquired mitochondrial dysfunction and inflammation) may occur in β-cells as well via mechanisms discussed above. In susceptible individuals, therefore, obesity-induced metabolic impairment can favor insulin resistance on the one hand and progressive β-cell dysfunction on the other. Reduced insulin secretion can in turn worsen the nutrient excess problem by raising circulating concentrations of glucose, free fatty acids, and other nutrients. In this way, a vicious cycle arises whereby obesity-induced nutrient excess triggers inflammatory responses that cause insulin resistance, placing a greater demand on the β-cell, and as β-cell function declines the cellular toll taken by nutrient excess increases. Since not all obese individuals develop hyperglycemia, however, an underlying abnormality of the β-cell must coexist with nutrient excess to promote type 2 diabetes (13).
Brain neurocircuits governing energy homeostasis also affect insulin sensitivity in the liver and perhaps other peripheral tissues (25), and inflammation similar to that induced by obesity in peripheral insulin-sensitive tissues also occurs in these areas of the brain (26). If obesity is associated with impairment of neurocircuits regulating both energy balance and insulin action, obesity-induced insulin resistance may arise not only as a direct consequence of excessive adipose mass but via neuronal mechanisms as well. Whether disturbed neurocircuits also contribute to deteriorating β-cell dysfunction as obesity and its sequelae progress is an active area of investigation (27).
Managing body weight by behavioral change and medications
The dramatic increase in incidence and prevalence of obesity over the past 50 years, associated in part with major worldwide changes in caloric intake and dietary composition, has focused attention on lifestyle intervention to reverse or ameliorate caloric imbalance. In general, programs including individual or group counseling to modify behavior result in 5–10% weight loss and are effective for 6–12 months, after which weight regain is the rule. Some longer-term lifestyle intervention studies with sustained interventions demonstrate more durable weight loss (28, 29), with extent of weight loss in the first 3–6 months generally predicting longer-term success. Successful lifestyle intervention programs typically involve self-monitoring of weight, dietary intake, and activity; behavioral modification; frequent contact; and caloric balance through diet, with or without exercise. For example, short-term intervention studies suggest that dietary changes, which emphasize less fat and refined carbohydrates, make it easier to reduce total caloric intake in obese adults and overweight children (30, 31).
Medications have been used to assist in weight loss for almost 80 years, but adverse effects frequently restrict utility. Medications have been developed based on physiological insights, more recently targeting central nervous system control of appetite and metabolism, or opportunistically when weight loss was noted as a side effect of approved medications. Table 1 lists medications that have been available and others under development. In general, weight loss achieved with these medications ranges from 2 to 8% greater than placebo, with some suggestion that combination therapy may either increase weight loss or ameliorate side effects and increase tolerability. However, most drug trials last only 6–12 months, and thus there are few long-term data that weight loss can be sustained. Moreover, high drop-out rates, which approach 50%, are characteristic of many weight-loss trials and result in survivor effects in efficacy analyses, thereby potentially amplifying drug benefits and limiting generalizability. Furthermore, concern regarding adverse effects, including cardiovascular disease risk and central effects (e.g., depression) in drugs crossing the blood-brain barrier, continue to limit approval and application.
Table 1.
Weight-loss medications: past, current, and future
| Medication | Availability | Serious adverse effects |
|---|---|---|
| Withdrawn | ||
| Fenfluramine | 1973–1997 | Cardiac valvular insufficiency and pulmonary hypertension |
| Dexfenfluramine | 1996–1997 | Cardiac valvular insufficiency and pulmonary hypertension |
| Phenylpropanolamine* | 1960–2000 | Hemorrhagic stroke |
| Rimonabant | 2006–2009 | Depression and suicidal ideation |
| Sibutramine* | 1997–2010 | Nonfatal myocardial infarction and nonfatal stroke (in subjects with preexisting cardiovascular conditions) |
| Current | ||
| Phentermine# | 1959–present | Palpitations and elevated blood pressure |
| Orlistat | 1999–present | Liver injury |
| Phase 3 trials and current applications to FDA/EMA | ||
| Lorcaserin | Potential valvular heart disease and psychiatric and cognitive disorders | |
| Bupropion/naltrexone | Seizures, palpitations, and transient blood pressure elevations | |
| Topiramate/phentermine | Depression, suicidal ideation, cardiovascular events, memory loss, and birth defects | |
| GLP-1 analogs | Pancreatitis |
EMA, European Medicines Agency; FDA, U.S. Food and Drug Administration; GLP-1, glucagon-like peptide 1.
Phenylpropanolamine is still available in some European countries and sibutramine in some South American countries.
Phentermine is one of a class of sympathomimetic drugs that also includes benzphetamine, diethylpropion, and phendimetrazine.
Managing body weight by bariatric surgery
Health benefits of bariatric surgery, determined largely from nonrandomized studies, are being increasingly recognized. These benefits include substantial and sustained weight loss (32), resolution of comorbidities such as diabetes, hypertension, and dyslipidemia (33, 34), and reduced myocardial infarction, cancers, and associated mortality (35). For extreme obesity, surgery is now the preferred and currently only effective treatment modality. Acute morbidity and mortality of surgical approaches have been dramatically reduced, enabling widespread use of these procedures. Furthermore, over the long term, bariatric surgery might reduce aggregate health care expenditures (36). There is also a growing movement toward using surgery to control diabetes, independent of severe excess weight, but there are currently few scientifically valid data to support this clinical path.
Bariatric surgery falls into two general categories: purely restrictive procedures such as the laparoscopic adjustable gastric band devices, which appear to improve diabetes via weight loss, and procedures bypassing the proximal gut, such as the Roux-en-Y gastric bypass (RYGB) or newer gastric sleeve procedures. The latter approaches (“metabolic” surgery) appear to produce unique effects on enteroendocrine hormones and neuronal signaling pathways and produce more weight loss and diabetes remission than banding alone (34, 37). Metabolic surgeries are associated with increases in anorexigenic and decreases in orexigenic hormones, changes largely absent in band or restrictive procedures, and may explain the differential outcomes (38). Although mechanisms leading to weight loss and diabetes remission are only beginning to be understood, the above endocrine, peptide, and neural effects may mediate these benefits because of structural changes including isolation of the gastric cardia; exclusion of the distal stomach, duodenum, and proximal jejunum; exposure of the distal intestine to undigested nutrients; and partial vagotomy. Longer duration of diabetes and insulin use, both typically associated with decreased β-cell function and possibly surrogates for reduced β-cell mass, are associated with reduced postsurgical remission rates, thus suggesting that residual β-cell function may be a critical factor for metabolic benefits (39).
Known differences in mechanism and efficacy, along with risks and patient priorities (e.g., weight loss vs. metabolic/diabetes goals) already inform the choice of surgical procedure. However, many questions remain, including the following: How much weight loss is required for health benefits? What is the effect of different interventional methods on long-term outcomes? What mechanisms underlie the heterogeneous responses? Further, regarding diabetes, Is the optimal timing for treatment the same or different from obesity? Are β-cells preserved or do they even grow? Why do not we see the same efficacy and durability of response for other obesity-related pathologies (e.g., hypertension) as for glycemic control? Ongoing randomized clinical trials (40) promise to answer many questions regarding patient selection, optimal procedure, when to intervene, and where initial and chronic care should be delivered.
Barriers to effective management
A vast array of barriers—ranging from deficits in basic research to socioeconomic and individual psychological factors beyond the scope of the conference—undermines current efforts to manage obesity, particularly in individuals with type 2 diabetes. Lessons learned from efforts such as those applied to tobacco cessation may be quite relevant (41).
Lifestyle programs (especially long-term) are often plagued by inadequate reimbursement. Further, there is a lack of evidence-based individualized goals and strategies combining lifestyle and medications, or appreciation of sequential (stepped) therapy. As mechanisms leading to obesity and its maintenance are not fully understood, questions remain about which interventions, be they lifestyle or pharmacological, might be most effective during various stages of weight gain, loss, and regain. In addition, medications under development may carry indeterminate risk. Likewise, surgery is an imperfect remedy due in part to perceived risks and high cost. With laparoscopic banding now approved for BMI >30 kg/m2 with a comorbidity such as diabetes or hypertension, 27 million Americans would be eligible for surgery. However, the large-scale feasibility of such an approach is uncertain and compounded by issues related to reimbursement. Thus, the search must continue for how to implement optimal lifestyle interventions and to find effective drugs and/or minimally invasive devices.
These barriers are further complicated in the context of type 2 diabetes. Obese patients with hyperglycemia are often poorly characterized not only in terms of their history of obesity but also in the duration of their glucose intolerance. Further, interventions are typically started late in the disease, with minimal preventive efforts. In addition, as initial weight loss is the main determinant of longer-term weight loss, the typical initial goal of ∼5–10% weight loss may be inadequate to produce glycemic control (42). Furthermore, although controlling body weight (either by reduction or by prevention of further rise) improves glycemic control by ameliorating both insulin resistance and β-cell dysfunction, the impact of pharmacologically induced improved glycemic control on body weight varies by individual drug. Glucose-lowering medications can be broadly categorized into those associated with weight gain and those essentially weight neutral or promoting weight loss (Table 2). Whether weight gain offsets any benefit of reduced glycemia on cardiovascular risk needs to be determined. Further, weight changes do not necessarily predict changes in glycemic control (43), and while specific therapies may work in certain diabetes subtypes, the response to glucose-lowering medications varies considerably. This latter topic was the focus of a similar workshop in 2009 on individualizing therapies in type 2 diabetes (44).
Table 2.
Weight effects of glucose-lowering medications
| Medication class | Weight effects |
|---|---|
| GLP-1 analogs | ↓ |
| Pramlintide | ↓ |
| Metformin | ± or ↓ |
| α-Glucosidase inhibitors | ± |
| DPP-4 inhibitors | ± |
| Insulin | ← |
| Sulfonylureas | ← |
| Glinides | ← |
| Thiazolidinediones | ← |
DPP-4, dipeptidyl peptidase-4; GLP-1, glucagon-like peptide 1.
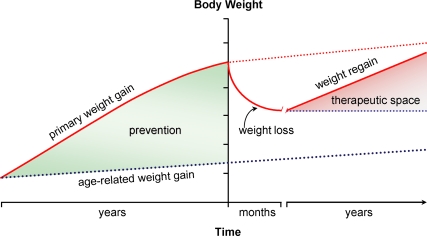
Equally challenging is the problem of weight regain, which usually follows any degree of weight loss, however achieved (Fig. 1). Well studied and viewed as a normal response in lean individuals, this phenomenon is equally robust among the obese. It involves complex, highly integrated physiological responses that are similar to those invoked in weight-reduced, nonobese individuals. The biologic basis appears to be the tendency to defend attained weight, whether normal or excessive, which seems to be wired in multiple central nervous system defenses against weight loss. Current models of energy homeostasis predict genetic or acquired defects in key neurocircuits that undermine the normal response to adiposity-related humoral signals. Much of the basic science in this area has been performed in animal models of obesity (genetic or overfeeding); extrapolation to the pathophysiology of human obesity remains uncertain.
Fig. 1.
Schematic representation of the natural history of obesity. Primary (excess) weight gain occurs usually over years against the typical background of mild age-related increase in weight in the general population. Intentional weight loss frequently is at least partially successful, but in the vast majority of cases, is followed by weight regain. Weight loss and its maintenance is the therapeutic goal; prevention of primary weight gain is a societal endeavor.
The panoply of potential mechanisms defending body weight helps explain why the field is moving toward targeting multiple pathways by harnessing additive effects of current drugs, which individually produce ∼5% weight loss (45). A number of compounds, old and new, alone or in combination, are being developed. It is hoped that they may safely achieve the magnitude of change in body weight, as well as other beneficial effects such as glucose control, that has been obtained with some of the surgical approaches.
Recommendations
Elucidate the pathogenesis linking obesity and type 2 diabetes
A better understanding of mechanisms linking obesity, insulin resistance, and type 2 diabetes may ultimately facilitate more individualized treatment. One future research priority is to clarifty how identified gene variants affect glucose, fatty acid, and energy metabolism at both cellular and whole-body levels. Rather than searching for a single factor or theory explaining the predisposition to β-cell decompensation in obese individuals, a multifactorial, synergistic explanation seems more compatible with current knowledge. Multiple mechanisms may link β-cell dysfunction to systemic insulin resistance, including differing cellular responses to nutrient excess and impaired brain neurocircuits governing energy homeostasis. One way to approach this complex pathophysiology is to examine glucose-tolerant obese patients and study the association with and progression to β-cell decompensation.
Expand research on heterogeneity
So far, genetic studies have been limited by a lack of accurate assessments of phenotype. Additional large-scale population-based analyses addressing more complex disease determinants of obesity and diabetes (beyond single genetic polymorphisms) might improve understanding of the relative impact of genetic and environmental factors linking them. Other priorities include clarifying the genetic basis for differences in fat distribution across ethnic groups (46); identifying factors that control homing of adipose tissue to the different—visceral versus subcutaneous—fat depots (47) and adipose tissue angiogenesis (48); and understanding the time course and extent of transdifferentiation of brown and white adipocytes in humans (5).
Human β-cells, including those from patients with type 2 diabetes, need to be made more widely available for investigational use. An additional approach would be the creation of patient-specific stem cell–derived β-cells. Moreover, longitudinal studies of β-cell dysfunction in humans should address differences in the amount of weight loss required to durably improve β-cell function. Finally, research to elucidate the intrauterine environment's impact on β-cell development and function may provide further strategic approaches to protecting progressive β-cell dysfunction.
Develop innovative approaches to pharmacological and surgical management
Innovative approaches to managing obesity may lower certain barriers undermining treatment of both obesity and type 2 diabetes. For example, modulating the incretin axis may benefit both energy balance and glycemia. Novel pharmacological development may depend on information gained from more efficient use of genomic, proteomic, and metabolomic approaches and from information learned from studying weight-loss mechanisms in bariatric surgery. In addition, co-opting less traditional organs such as the brain and gut into the core pathophysiology of type 2 diabetes may reveal new biomarkers and/or targets for therapeutic intervention. Finally, safe and effective centrally acting drugs that decrease appetite or increase satiety are urgently needed. However, as regulatory agencies increase the need for safety testing, fewer new and innovative approaches for weight loss are being developed because of the prolonged time and immense expense involved.
Emphasize primary prevention of obesity and type 2 diabetes
Current clinical approaches to obesity continue to focus on secondary and tertiary intervention. Physicians often introduce secondary interventions when patients surpass some dichotomous BMI threshold or when patients self-identify, for cosmetic or health reasons. They introduce tertiary intervention when obesity-related complications responsive to weight loss, such as diabetes, hypertension, or sleep apnea, develop. Because weight problems develop over the entire life span, however, emphasizing obesity prevention is urgent and must include cooperation of public health institutions, the school systems, and the private (e.g., food industry) sector. The likelihood of sustained benefits of weight reduction on β-cell function and glycemia in patients with early-onset versus more prolonged durations of type 2 diabetes needs to be determined.
Although intensive lifestyle modifications and medications have been conclusively demonstrated to slow the development of type 2 diabetes in those with impaired glucose metabolism (28, 49), regulatory authorities have still not approved medications for preventing type 2 diabetes, nor have they provided a regulatory framework to do so. Guidance on what would be required to approve medications for treating high-risk individuals would foster more scientific investment in this area and subsequent availability of additional preventive options.
Adopt a chronic disease model linking obesity to diabetes care
Current understanding of both pathophysiology and management suggests the need to adopt a chronic disease model of care linking obesity and diabetes care management systems. Besides including stepped-care approaches similar to those used for other chronic diseases, this model involves basing interventional (pharmacological and surgical) approaches on severity, duration, and individual risk/benefit. The common perception that the obesity problem is insurmountable leads to some degree of clinical inertia. What is needed is similar to what occurred with tobacco—a comprehensive social, economic, and workplace approach to prevention and intervention. In addition, community-setting approaches supplemented by physician involvement can work when combining treatment modalities (50). Furthermore, multidisciplinary teams including nutritionists, exercise physiologists, and behavioral/mental health professionals can achieve both initial and sustained weight management and glucose control (28, 29). This approach to attaining and maintaining weight reduction is critically important both in alleviating the intensive defense of body weight by multiple biological systems and in reducing risk of β-cell decompensation and, over the long term, diabetes complications.
Summary and Conclusions
Improved understanding of obesity's heterogeneity, including interindividual differences in pathogenesis, propensity to regain lost weight, development of obesity-related complications including diabetes, and response to therapy, is critical to advance the development of effective and cost-effective interventions. The insights that improve obesity prevention and treatment will almost certainly benefit the incidence and care of type 2 diabetes. The converse may not be true since current treatments of diabetes can have differential effects on weight. Even so, we have reached a point when we can begin to consider innovative and potentially more effective approaches to managing both obesity and type 2 diabetes. Increased understanding of the pathogenesis of obesity and type 2 diabetes, for example, should not only help differentiate responders from nonresponders but also make tailored therapy a reality. Equally beneficial will be incorporating these ideas into a chronic disease model of care linking obesity management to diabetes care systems, including multidisciplinary approaches to patient care designed to prevent weight regain that is almost universal when therapy is stopped.
Presently, some of the major questions linking obesity to type 2 diabetes that need to be urgently addressed include the following:
Why do not all patients with obesity develop type 2 diabetes?
Through what mechanisms do obesity and insulin resistance contribute to β-cell decompensation, and if/when obesity prevention ensues, how much reduction in type 2 diabetes incidence will follow?
How does the duration of type 2 diabetes relate to the benefits of weight reduction by lifestyle, weight-loss drugs, and/or bariatric surgery on β-cell function and glycemia?
What is necessary for regulatory approval of medications and possibly surgical approaches for preventing type 2 diabetes in patients with obesity?
Acknowledgments
This article is based on a conference jointly sponsored by The Endocrine Society, the American Diabetes Association, and the European Association for the Study of Diabetes, with the financial support of an unrestricted educational grant from Novo Nordisk.
The authors are grateful for the contributions of the speakers and participants in the January 2011 conference, who are listed, together with affiliations, in the Appendix. In addition, the authors acknowledge the editorial assistance of Dr. Terra Ziporyn, medical editor, in writing the manuscript.
Disclosure Summary: R.H.E. received grant/research support from Sanofi Research Grant (fellowship educational grant), diaDexus, and GlaxoSmithKline; compensation for working as a consultant for Amylin, GTC Nutrition, Genfit, Eli Lilly, Pfizer, Johnson & Johnson, and Esperion; financial or material support from Cardiometabolic Health Congress and Metabolic Syndrome Institute; and honoraria from Vindico, CME Incite, and Voxmedia. S.E.K. received consulting fees from Eli Lilly, GlaxoSmithKline, Intarcia Therapeutics, and Novo Nordisk for acting as an advisory board member; consulting fees and honoraria from Boehringer Ingelheim and Merck for acting as an advisory board member and speaker; and grant support from Daiichi Sankyo. E.F. received consulting fees from Merck, Boehringer Ingelheim, Bristol-Myers Squibb/AstraZeneca, sanofi-aventis, Novartis, GlaxoSmithKline, and Daiichi Sankyo and grant support from Merck and Eli Lilly. A.B.G. acted as Site Principal Investigator for a clinical trial funded by Eli Lilly that is now complete with results published. M.W.S. received consulting fees from Merck, Pfizer, and Orexigen. R.J.S. received consulting fees from GI Dynamics and royalties from Baxter and Fresenius Kabi. S.R.S. received consulting fees from Amylin, Bristol-Myers Squibb, Eli Lilly, and Novartis and consulting fees as an advisory board member for Arena. No other potential conflicts of interest relevant to this article were reported.
Appendix
Participants in the meeting “The Pathogenesis and Treatment of Obesity and Type 2 Diabetes: What Can Be Unified and What Needs to Be Individualized” held on 6–7 January 2011 were the following:
Meeting Co-chairs:
Robert H. Eckel, MD, University of Colorado Anschutz Medical Campus
Steven E. Kahn, MB, ChB, VA Puget Sound Health Care System and University of Washington
Session Co-chairs:
Ele Ferrannini, MD, University of Pisa, Italy
Allison B. Goldfine, MD, Joslin Diabetes Center and Harvard Medical School
David M. Nathan, MD, Harvard Medical School and Massachusetts General Hospital
Michael W. Schwartz, MD, University of Washington
Robert J. Smith, MD, Brown University
Steven R. Smith, MD, Florida Hospital–Burnham Clinical Research Institute
Speakers:
Caroline M. Apovian, MD, Boston University School of Medicine and Boston Medical Center
Jamy D. Ard, MD, University of Alabama
Arne V. Astrup, MD, DMSc, University of Copenhagen, Denmark
David A. D'Alessio, MD, University of Cincinnati School of Medicine and Cincinnati VA Medical Center
Judith E. Fradkin, MD, National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institutes of Health (NIH)
Leif Groop, MD, PhD, Lund University, Sweden
Steven B. Heymsfield, MD, Pennington Biomedical Research Center, Louisiana State University System
Lee M. Kaplan, MD, PhD, Massachusetts General Hospital and Harvard Medical School
Kenneth S. Polonsky, MD, University of Chicago
Robert E. Ratner, MD, Georgetown University Medical School and MedStar Health Research Institute
Nancy A. Rigotti, MD, Massachusetts General Hospital and Harvard Medical School
Bruce M. Spiegelman, PhD, Harvard Medical School and Dana-Farber Cancer Institute
Panelists:
Rexford S. Ahima, MD, PhD, University of Pennsylvania
George A. Bray, MD, Pennington Biomedical Research Center, Louisiana State University System
John B. Buse, MD, PhD, University of North Carolina
John B. Dixon, MBBS, PhD, Monash University and Baker IDI Heart & Diabetes Institute, Australia
Robert W. Jeffery, PhD, University of Minnesota
Barbara B. Kahn, MD, Beth Israel Deaconess Medical Center and Harvard Medical School
Samuel Klein, MD, Washington University in St. Louis
John L. Leahy, MD, University of Vermont College of Medicine and Vermont Regional Diabetes Center
Rudolph L. Leibel, MD, Columbia University Medical Center and Naomi Berrie Diabetes Center and New York Obesity Research Center
Francesco Rubino, MD, Weill Cornell Medical College and New York-Presbyterian Hospital
Philip R. Schauer, MD, Cleveland Clinic
Nicholas J. Wareham, MD, PhD, University of Cambridge
References
- 1. Centers for Disease Control and Prevention National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta, GA, U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2011 [Google Scholar]
- 2. Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr 2010;8:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Björntorp P. Metabolic implications of body fat distribution. Diabetes Care 1991;14:1132–1143 [DOI] [PubMed] [Google Scholar]
- 4. Cypess AM, Lehman S, Williams G, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 2009;360:1509–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Frontini A, Cinti S. Distribution and development of brown adipocytes in the murine and human adipose organ. Cell Metab 2010;11:253–256 [DOI] [PubMed] [Google Scholar]
- 6. Deng Y, Scherer PE. Adipokines as novel biomarkers and regulators of the metabolic syndrome. Ann N Y Acad Sci 2010;1212:E1–E19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Larson-Meyer DE, Newcomer BR, Ravussin E, et al. Intrahepatic and intramyocellular lipids are determinants of insulin resistance in prepubertal children. Diabetologia 2011;54:869–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bournat JC, Brown CW. Mitochondrial dysfunction in obesity. Curr Opin Endocrinol Diabetes Obes 2010;17:446–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bagdade JD, Bierman EL, Porte D., Jr The significance of basal insulin levels in the evaluation of the insulin response to glucose in diabetic and nondiabetic subjects. J Clin Invest 1967;46:1549–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Polonsky KS, Given BD, Hirsch L, et al. Quantitative study of insulin secretion and clearance in normal and obese subjects. J Clin Invest 1988;81:435–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Klöppel G, Löhr M, Habich K, Oberholzer M, Heitz PU. Islet pathology and the pathogenesis of type 1 and type 2 diabetes mellitus revisited. Surv Synth Pathol Res 1985;4:110–125 [DOI] [PubMed] [Google Scholar]
- 12. Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. β-Cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes 2003;52:102–110 [DOI] [PubMed] [Google Scholar]
- 13. Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006;444:840–846 [DOI] [PubMed] [Google Scholar]
- 14. Johnson JD, Ahmed NT, Luciani DS, et al. Increased islet apoptosis in Pdx1+/- mice. J Clin Invest 2003;111:1147–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Leibowitz G, Ferber S, Apelqvist A, et al. IPF1/PDX1 deficiency and β-cell dysfunction in Psammomys obesus, an animal with type 2 diabetes. Diabetes 2001;50:1799–1806 [DOI] [PubMed] [Google Scholar]
- 16. Stoffers DA, Ferrer J, Clarke WL, Habener JF. Early-onset type-II diabetes mellitus (MODY4) linked to IPF1. Nat Genet 1997;17:138–139 [DOI] [PubMed] [Google Scholar]
- 17. Rampersaud E, Damcott CM, Fu M, et al. Identification of novel candidate genes for type 2 diabetes from a genome-wide association scan in the Old Order Amish: evidence for replication from diabetes-related quantitative traits and from independent populations. Diabetes 2007;56:3053–3062 [DOI] [PubMed] [Google Scholar]
- 18. Hayes MG, Pluzhnikov A, Miyake K, et al. Identification of type 2 diabetes genes in Mexican Americans through genome-wide association studies. Diabetes 2007;56:3033–3044 [DOI] [PubMed] [Google Scholar]
- 19. Scherag A, Dina C, Hinney A, et al. Two new loci for body-weight regulation identified in a joint analysis of genome-wide association studies for early-onset extreme obesity in French and German study groups. PLoS Genet 2010;6:e1000916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lindgren CM, Heid IM, Randall JC, et al. ; Wellcome Trust Case Control Consortium; Procardis Consortia; Giant Consortium Genome-wide association scan meta-analysis identifies three loci influencing adiposity and fat distribution. PLoS Genet 2009;5:e1000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bogardus C. Missing heritability and GWAS utility. Obesity (Silver Spring) 2009;17:209–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lander ES. Initial impact of the sequencing of the human genome. Nature 2011;470:187–197 [DOI] [PubMed] [Google Scholar]
- 23. Hotamisligil GS, Erbay E. Nutrient sensing and inflammation in metabolic diseases. Nat Rev Immunol 2008;8:923–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003;112:1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Obici S, Zhang BB, Karkanias G, Rossetti L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med 2002;8:1376–1382 [DOI] [PubMed] [Google Scholar]
- 26. Thaler JP, Schwartz MW. Minireview: Inflammation and obesity pathogenesis: the hypothalamus heats up. Endocrinology 2010;151:4109–4115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Calegari VC, Torsoni AS, Vanzela EC, et al. Inflammation of the hypothalamus leads to defective pancreatic islet function. J Biol Chem. 21 January 2011. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28. Knowler WC, Barrett-Connor E, Fowler SE, et al. ; Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Look AHEAD Research Group Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four-year results of the Look AHEAD trial. Arch Intern Med 2010;170:1566–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Larsen TM, Dalskov SM, van Baak M, et al. ; Diet, Obesity, and Genes (Diogenes) Project Diets with high or low protein content and glycemic index for weight-loss maintenance. N Engl J Med 2010;363:2102–2113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Papadaki A, Linardakis M, Larsen TM, et al. ; DiOGenes Study Group The effect of protein and glycemic index on children's body composition: the DiOGenes randomized study. Pediatrics 2010;126:e1143–e1152 [DOI] [PubMed] [Google Scholar]
- 32. Sjöström L, Lindroos AK, Peltonen M, et al. ; Swedish Obese Subjects Study Scientific Group Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004;351:2683–2693 [DOI] [PubMed] [Google Scholar]
- 33. Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med 2007;357:753–761 [DOI] [PubMed] [Google Scholar]
- 34. Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med 2009;122:248–256.e5 [DOI] [PubMed] [Google Scholar]
- 35. Sjöström L, Narbro K, Sjöström CD, et al. ; Swedish Obese Subjects Study Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 2007;357:741–752 [DOI] [PubMed] [Google Scholar]
- 36. Makary MA, Clarke JM, Shore AD, et al. Medication utilization and annual health care costs in patients with type 2 diabetes mellitus before and after bariatric surgery. Arch Surg 2010;145:726–731 [DOI] [PubMed] [Google Scholar]
- 37. Vetter ML, Cardillo S, Rickels MR, Iqbal N. Narrative review: effect of bariatric surgery on type 2 diabetes mellitus. Ann Intern Med 2009;150:94–103 [DOI] [PubMed] [Google Scholar]
- 38. Korner J, Inabnet W, Febres G, et al. Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y gastric bypass. Int J Obes (Lond) 2009;33:786–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schauer PR, Burguera B, Ikramuddin S, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg 2003;238:467–484; discussion 84–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lautz D, Halperin F, Goebel-Fabbri A, Goldfine AB. The great debate: medicine or surgery: what is best for the patient with type 2 diabetes? Diabetes Care 2011;34:763–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rigotti NA. Clinical practice. Treatment of tobacco use and dependence. N Engl J Med 2002;346:506–512 [DOI] [PubMed] [Google Scholar]
- 42. Nathan DM, Buse JB, Davidson MB, et al. ; American Diabetes Association; European Association for the Study of Diabetes Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009;32:193–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kahn SE, Haffner SM, Heise MA, et al. ; ADOPT Study Group Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006;355:2427–2443 [DOI] [PubMed] [Google Scholar]
- 44. Smith RJ, Nathan DM, Arslanian SA, Groop L, Rizza RA, Rotter JI. Individualizing therapies in type 2 diabetes mellitus based on patient characteristics: what we know and what we need to know. J Clin Endocrinol Metab 2010;95:1566–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Eckel RH. Clinical practice. Nonsurgical management of obesity in adults. N Engl J Med 2008;358:1941–1950 [DOI] [PubMed] [Google Scholar]
- 46. Shai I, Jiang R, Manson JE, et al. Ethnicity, obesity, and risk of type 2 diabetes in women: a 20-year follow-up study. Diabetes Care 2006;29:1585–1590 [DOI] [PubMed] [Google Scholar]
- 47. Gálvez BG, San Martín N, Rodríguez C. TNF-α is required for the attraction of mesenchymal precursors to white adipose tissue in ob/ob mice. PLoS ONE 2009;4:e4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cao Y. Angiogenesis modulates adipogenesis and obesity. J Clin Invest 2007;117:2362–2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. DeFronzo RA, Tripathy D, Schwenke DC, et al. ; ACT NOW Study Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med 2011;364:1104–1115 [DOI] [PubMed] [Google Scholar]
- 50. Ryan DH, Johnson WD, Myers VH, et al. Nonsurgical weight loss for extreme obesity in primary care settings: results of the Louisiana Obese Subjects Study. Arch Intern Med 2010;170:146–154 [DOI] [PubMed] [Google Scholar]