In a cohort of 25,957 women, there was an association between perfluorocarbon exposure, decreased estradiol and early menopause in women over age 42.
Abstract
Context:
Perfluorocarbons (PFC) are man-made chemicals used in numerous household products. They have a long half-life in humans and complex animal toxicity, and accumulating evidence points toward associations with multiple human health endpoints.
Objective:
Our objective was to investigate whether PFC are associated with endocrine disruption in women.
Design:
Cross-sectional analyses were made between quintiles of serum PFC, serum estradiol, and menopause onset.
Setting:
The C8 Health Project, with cohort of 69,030 adults and children, was conducted due to PFC contamination of drinking water from six water districts in two states.
Participants:
Participants included 25,957 women aged 18–65 yr.
Main Outcome Measures:
Serum estradiol levels and onset of menopause were assessed. The survey was the result of a class action suit, and survey designers (an independent corporation) had no a priori hypotheses. All hypotheses have been formulated by other investigators after data collection.
Results:
After excluding women who reported hysterectomy and adjusting for age within the group, smoking, alcohol consumption, body mass index, and exercise, the odds of having experienced menopause were significantly higher in the highest quintile relative to the lowest quintile of perfluorooctanoate (PFOA) and perfluorooctane sulfonate (PFOS) in the perimenopausal [PFOS odds = 1.4, confidence interval (CI) = 1.1–1.8; PFOA odds =1.4, CI = 1.1–1.8] and menopausal age groups (PFOS odds = 2.1, CI=1.6–2.8; PFOA odds = 1.7, CI = 1.3–2.3). After appropriate exclusions and adjustment for covariates, there was a significant inverse association between PFOS and estradiol in perimenopausal (β = −3.65; P < 0.0001) and menopausal age groups (β = −0.83; P = 0.007) but not between PFOA and estradiol.
Conclusions:
These data suggest that PFC are associated with endocrine disruption in women and that further research on mechanisms is warranted.
Perfluorooctanoate (PFOA) and perfluorooctane sulfonate (PFOS) are man-made surfactants historically used in a variety of household products, including food containers, clothing, furniture, carpets, paints, fire-fighting foam, and photographic emulsifiers (1). Their broad use has resulted in widespread dissemination in water, air, soil, plant life, animals, and humans, even in remote parts of the world (2). Of even greater concern is that they have a long half-life, and their presence in human blood and internal organs seems to be ubiquitous (2). The National Health and Nutrition Examination Survey (NHANES), a probability sample of U.S. adults, found measurable serum concentrations of PFOS and PFOA in 98% of the participants tested (3). The C8 Health Study cohort used in the present analyses is the largest existing study of a population exposed to perfluorocarbons (PFC) in drinking water. Its mean and median serum PFOA concentrations were substantially higher than those in the NHANES data (4). Regarding the potential importance for human health, one review noted that PFC have become “the highest human exposure to exogenous chemicals, exceeding that of more well-known environmental contaminants such as DDE” (5).
Although research concerning the toxicological effects of PFC is still ongoing, they have been associated with multiple physiological and health outcomes in human and animal studies (2, 6, 7). One of the effects reported in multiple animal species is endocrine disruption, which has been found in rats (7–9), mice (10), and fish (11). It is difficult to extrapolate from animals to humans because the renal clearance of PFOA and PFOS, which is very active in animals, is almost negligible in humans (5), and it is not known how or to what extent this influences interspecies comparisons. Nevertheless, the repeated findings of endocrine disruption in animal studies indicate the importance of this topic and the need for more research in humans. In freshwater minnows, it has been demonstrated that after 14 d of exposure to 3, 10, and 30 mg/liter of PFOA (dosages based on the literature), the expression of estrogen receptor β increased significantly in the livers of both mature males and females but that no difference was observed after 28 d of exposure (11). The ovaries of females in this study underwent degeneration. Additional evidence of estrogenic activity was provided by the development of oocytes in the testes of males. Another study in fish, this time in tilapia, showed that a combination of PFOS or PFOA and 17β-estradiol (a natural receptor ligand) produced antiestrogenic effects in cultured hepatocytes in vitro (12). In female C57BL/6 mice, however, it has been demonstrated that exposure to PFOA at a dosage of 5 mg/kg body weight 5 d a week for 4 wk in prepubertal mice, increased progesterone and enhanced mammary gland responses to exogenous estradiol, increasing growth factors in mammary glands (10). This stimulation was independent of peroxisome proliferator-activated receptor-α, but a study performed on pregnant CD-1 mice found that female pups from dams exposed to PFOA displayed stunted mammary epithelial branching and growth (13). The dosage in this study was also 5 mg/kg. An additional study of PFOA, this time in adult male rats (dosage was either 0 or 25 mg/kg·d for 14 d), showed a decrease in serum and testicular interstitial fluid testosterone levels and increased serum estradiol levels. Additional in vitro experiments with Leydig cells removed from the testes demonstrated that PFOA directly inhibits testosterone release from Leydig cells (6). These studies although not consistent in methodology or results, provide strong evidence of endocrine-disrupting effects of PFC. Investigations of endocrine disruption in humans are unfortunately scant. One cross-sectional study of occupational exposure in male workers (n = 111 one year and n = 80 the next) revealed no significant associations with either estradiol or testosterone after adjustment for confounders (14); however, a 10% increase in mean estradiol levels was observed among those employees who had the highest levels of serum PFOA, although this was confounded by weight. That study did not include women and the number of subjects was small. Larger population-based studies are clearly needed. The goal of the current study was to investigate menopausal status and estradiol concentrations in adult aring age (18–42 yr), perimenopausal age (>42–51 yr), and above the median age of 51 yr for menopause (≥51–65 yr).
Subjects and Methods
Sample selection
The C8 Health Project collected data on 69,030 subjects from six public water districts contaminated by PFOA from the DuPont Washington Works Plant near Parkersburg, WV, between August 2005 and August 2006. Survey methods and demographic data have been reported elsewhere (4). Based on multiple parameters, it has been estimated that 69,030 residents represents a participation rate of about 81% of the residents (15). This is the total sample size. The participants used in the present analyses were limited to 25,957 women who were over age 18. It did not include men or children.
Exposure levels
These data are cross-sectional, and there is no variable for length of exposure. Interpreting exposure levels is complicated by the fact that exposure was not constant because emissions from the DuPont plant increased over time, peaking in the 1990s; this issue has been dealt with in a paper by Seals et al. (16). Their results showed a significant increase in serum PFOA concentrations with cumulative years of residence in exposed water districts. The average increase in PFOA levels for each year of residence was 1.2%, and residential district explained 68% of the variance in PFOA levels after controlling for sex, age, race/ethnicity, body mass index (BMI), smoking, regular exercise, and use of bottled water as the primary source of drinking water. We believe that these data are conservative because they excluded people who also worked in a DuPont plant as well as those who lived in multiple districts and those who had private wells. With respect to former residents, the decay of serum PFOA seems to be nonlinear.
The primary exposure from water in this study was PFOA, and we found PFOA levels that exceeded those in the population-based NHANES by 500% (3, 4). However, the PFOS levels did not differ significantly from those found in NHANES (3, 4) and are, therefore, representative of those in women throughout the United States. The source of PFOS is not primarily water but the multiple products to which we are all exposed in the ambient environment.
Data collection and blood-processing procedures
Temporary modular office units were established within each of the affected water districts, staffed with nurses, phlebotomists, and other Project staff and equipped for venipuncture, blood processing, and short-term record and blood sample storage. A more detailed description of the survey instrument, clinical measures, and data collection is available in the methods paper (4) and online at http://www.hsc.wvu.edu/som/cmed/c8/.
The analytic procedure to quantify PFC (PFOS and PFOA) in serum used a protein precipitation extraction combined with reversed-phase HPLC/tandem mass spectrometry. Spectrometric detection employed a triple-quadrupole mass spectrometer in a selected reaction-monitoring mode, monitoring for the M/2 transitions of the specific PFC and the [13C]PFOA surrogate. A description of quality assurance and data capture methods has been published previously (4). Serum levels of PFOA and PFOS were grouped into quintiles to investigate their associations with menopause and estradiol.
Hormone use and medications
Hormone use was determined by examining all of the prescribed medications reported by each individual. A woman was classified as taking hormones if she was taking birth control pills or hormone replacement therapy, whether orally, transdermally, or via use of vaginal suppositories. Participants who were taking any hormones or selective estrogen receptor modulators and fertility agents such as Clomid or Lupron were excluded from the estradiol analyses.
Menopause and estradiol
Menopause was defined by the participant's answer to the question of whether she had experienced menopause. They were not asked the age of menopause. The objective was to ascertain whether PFC were associated with the odds of having experienced menopause after controlling for other biological factors and behaviors that might influence menopausal onset. Because serum estradiol (picograms per milliliter) is related to ovarian function, it too was assessed to investigate whether it varied with PFC levels. Estradiol and PFC measurements were concurrent; menopausal transitions reported had occurred before the PFC measurements.
Statistical analyses
Because there was an interaction between age and PFC for both PFOS and PFOA, the analyses for menopause were calculated separately for three age groups: those 30–42 yr (childbearing years), those over 42 yr but less than or equal to 51 yr (perimenopausal years), and women over 51 yr and 65 yr or younger (51 being the median age for menopause) (14). The analyses for estradiol were also calculated on three groups except that the younger group was extended to include the childbearing years from 18–42. Although it was not possible to control for stage of cycle in the younger women, given the large size of this population, we believe that the consequences of not adjusting for it are not likely to skew the results. Statistically, the number of subjects (n) is large and dividing by the square root of n should sufficiently adjust for variability.
The odds of having experienced menopause were calculated with SAS logistic regression using quintiles created from the natural log transform of PFC with the lowest quintile as the reference and excluding women who reported hysterectomies. They were adjusted for smoking, age (within group), BMI, alcohol consumption (yes/no), and whether or not the participant had a regular exercise program. For analyses of estradiol, regressions were calculated using the same quintiles and covariates, excluding pregnant women and women with a full hysterectomy as well as women who reported taking hormones and fertility drugs or selective estrogen receptor modulators. Graphs present the quintiles with absolute (not log) PFC values for ease of interpretation.
Results
There were 13,458 women in the 18- to 42-yr age group, 5782 women in the over 42- to 51-yr age group, and 6717 women over 51 and 65 yr or under. Descriptive data for this cohort can be seen in Table 1.
Table 1.
Descriptive data
| Women 18 ≤ 42 yr (n = 13,458) | Women >42 ≤ 51 yr (n = 5782) | Women >51 ≤ 65 yr (n = 6717) | |
|---|---|---|---|
| BMI (kg/m2) | 26.1, 27.9 ± 8.2 | 28.0, 29.4 ± 10.0 | 28.7, 30.0 ± 9.5 |
| Age (yr) | 30.5, 30.3 ± 7.0 | 46.4, 46.5 ± 2.6 | 57.3, 57.4 ± 4.0 |
| PFOA (ng/ml) | 17.6, 44.7 ± 120.1 | 23.4, 72.8 ± 278.0 | 32.5, 94.9 ± 240.1 |
| PFOS (ng/ml) | 15.0, 16.9 ± 9.9 | 16.2, 19.1 ± 12.9 | 21.5, 24.8 ± 16.3 |
| Current smoker [n (%)] | |||
| No | 9,190 (68.4) | 4,013 (69.5) | 5,365 (80.0) |
| Yes | 4,243 (31.6) | 1,764 (30.5) | 1,344 (20.0) |
| Currently drink alcohol [n (%)] | |||
| No | 6,297 (46.8) | 3,024 (52.3) | 4,459 (66.5) |
| Yes | 7,147 (53.2) | 2,757 (47.7) | 2,250 (33.5) |
| Hormones [n (%)] | |||
| No | 12,425 (92.3) | 5,257 (90.9) | 5,832 (86.8) |
| Yes | 1,033 (7.7) | 525 (9.1) | 885 (13.2) |
| Medicationsa [n (%)] | |||
| No | 13,441 (99.9) | 5,753 (99.5) | 6,573 (97.9) |
| Yes | 17 (0.1) | 29 (0.5) | 144 (2.1) |
| Menopause [n (%)] | |||
| No | 12,820 (96.7) | 3,745 (72.3) | 582 (9.2) |
| Yes | 417 (3.2) | 1,434 (27.7) | 5,738 (90.8) |
| Hysterectomy [n (%)] | |||
| No | 12,448 (92.6) | 4,049 (70.1) | 3,742 (55.8) |
| Yes | 996 (7.4) | 1,728 (29.9) | 2,962 (44.2) |
| Exercise [n (%)] | |||
| No | 9,250 (68.7) | 3,934 (68.0) | 4,375 (65.1) |
| Yes | 4,208 (31.3) | 1,848 (32.0) | 2,342 (34.9) |
Unless indicated otherwise, results are presented as median, mean ± sd. Percentages are rounded to the nearest decimal.
Medications influencing estradiol levels.
Menopause
After excluding women who had hysterectomies, and adjusting for smoking, age (within group), BMI, alcohol consumption (yes/no), and whether or not the participant had a regular exercise program, the odds of having experienced menopause in the oldest group of women (>51 to ≤65 yr) exposed to PFOS showed a monotonic increase with all quintiles being significantly higher relative to the lowest. For the perimenopausal age group, there was no monotonic increase by quintile, but the highest three quintiles were significantly higher than the lowest.
The pattern for PFOA was similar but not monotonic in the oldest group of women. All quintiles were significantly higher than the lowest (Table 2). In the perimenopausal age group, the top three quintiles were significantly higher compared with the lowest.
Table 2.
Odds ratios for menopause by quintile of PFOA/PFOS
| Women 18 ≤ 42 yr |
Women >42 ≤ 51 yr |
Women >51 ≤ 65 yr |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Point estimate | Confidence limits | χ2 | Point estimate | Confidence limits | χ2 | Point estimate | Confidence limits | χ2 | |
| PFOAa | 0.009 | 0.839 | 0.041 | ||||||
| 2 vs. 1 | 0.9 | 0.5–1.6 | 1.4 | 1.1–1.8 | 1.5 | 1.1–2.1 | |||
| 3 vs. 1 | 0.9 | 0.5–1.5 | 1.2 | 0.9–1.6 | 1.6 | 1.2–2.2 | |||
| 4 vs. 1 | 0.9 | 0.5–1.7 | 1.4 | 1.1–1.9 | 1.4 | 1.1–1.9 | |||
| 5 vs. 1 | 1.2 | 0.7–2.1 | 1.4 | 1.1–1.8 | 1.7 | 1.3–2.3 | |||
| PFOSa | 0.804 | 0.028 | <0.0001 | ||||||
| 2 vs. 1 | 1.1 | 0.7–1.9 | 1.2 | 0.9–1.5 | 1.5 | 1.1–2.1 | |||
| 3 vs. 1 | 0.8 | 0.4–1.4 | 1.4 | 1.1–1.8 | 1.8 | 1.3–2.5 | |||
| 4 vs. 1 | 1.0 | 0.6–1.8 | 1.4 | 1.1–1.8 | 2.0 | 1.5–2.6 | |||
| 5 vs. 1 | 1.1 | 0.6–2.1 | 1.4 | 1.1–1.8 | 2.1 | 1.6–2.8 | |||
Results are adjusted for age, BMI, alcohol consumption, smoking, and exercise. Women with hysterectomy were excluded from analyses.
Quintiles.
Estradiol concentrations
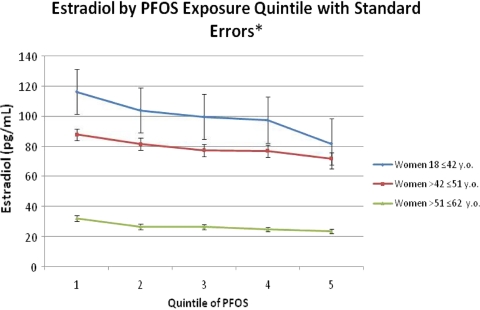
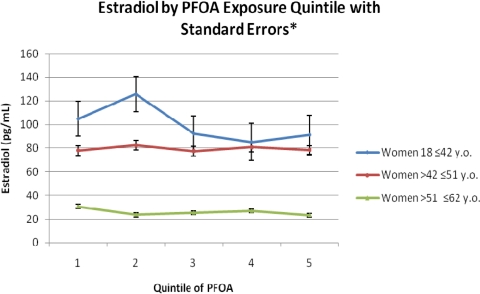
After excluding pregnant women, women on hormones or medications affecting hormones, and women who had experienced hysterectomy and after adjusting for the same covariates as in the menopause analyses, PFOA was not significantly associated with serum estradiol concentrations in any group. However, PFOS was negatively associated with estradiol concentrations in all groups but only significantly in the perimenopausal (β = −3.65; P < 0.0001) and menopausal age groups (β = −0.83; P = 0.007).
Fig. 1.
Mean serum estradiol concentrations by PFOS quintile. Quintiles in ng/mL. Quintile 1 = 0.25 − 11.8, Quintile 2 = 11.9 − 17.0, Quintile 3 = 17.1 − 22.4, Quintile 4 = 22.5 − 30.7, Quintile 5 = 30.8 − 564.3.
Fig. 2.
Mean serum estradiol concentrations by PFOA quintile. Quintiles in ng/mL. Quintile 1 = 0.25 − 11.2, Quintile 2 = 11.3 − 19.8, Quintile 3 = 19.9 − 36.7, Quintile 4 = 36.8 − 84.9, Quintile 5 = 85.0 − 22412.0.
Mean PFC values in women with and without hysterectomy
Mean PFC concentrations in women with and without hysterectomy were compared in women 40–55 yr of age to see whether hysterectomy made a difference. Both PFOS and PFOA were significantly higher (P < 0.0001) in women with a hysterectomy.
Discussion
This study is the largest ever to be done on the endocrine-disrupting effects of PFC in human women. These data show that after controlling for age within the group, women of perimenopausal and menopausal age in this large population are more likely to have experienced menopause if they have high serum concentrations of PFOS and PFOA than their counterparts with lower levels. To help us understand these data, we also examined endogenous estradiol concentrations in relation to PFC in the same age groups. Only PFOS was inversely associated with estradiol levels.
Premature menopause or early menopause is associated with all-cause mortality and cardiovascular mortality (17, 18). A younger age at menopause and a decrease in the years of endogenous estrogen production have been associated with increased cardiovascular risk (19). Women with the highest number of years of endogenous estrogen have also been reported to have a 20% decreased risk of cardiovascular mortality (19). Furthermore, the absolute amount of bone loss has been reported to be greater after early menopause than after normal or late menopause (20).
In addition to cardiovascular risk, the onset of an accelerated decline in ovarian function and menopause is thought to be fixed (14). This means that women who have experienced early menopause also experienced an accelerated decline in fertility before the age of 32 (14). Therefore, an association of a ubiquitous environmental pollutant with decreased estrogen and with earlier menopause is potentially important. What is interesting about these results is that although PFOA levels were much higher in this population than in the rest of the United States because of contamination from manufacturing emissions, PFOS levels were not significantly different. Based on the animal literature, we would have expected PFOA to be inversely associated with estradiol in younger women, but although there was a clear trend, it was not significant. The source of the elevated PFOA levels was primarily contaminated water, but the source of PFOS was primarily sources in the ambient environment. This means that the effects of PFOS in this sample are probably typical for those in the population as a whole.
However, caution is needed in interpreting these findings. Because these analyses are cross-sectional, it is not possible to say whether decreases in estradiol related to PFC during the childbearing years could have caused an increased risk of menopause. The possibility exists that PFC concentrations are higher in postmenopausal women because they are no longer losing blood. Menstrual flow eliminates some of the PFC in blood, and because blood is replaced faster than the toxicant, concentrations measured in menstruating women could be lower than those who aren't menstruating, even with the same exposure level. Thus, the argument could be made that some of the significance associated with an increased risk of menopause associated with increased PFC might be due to reverse causation, i.e. the disappearance of menses causing increases in PFC concentrations rather than an increase in PFC causing early menopause. This argument is given additional support by the fact that women who had experienced hysterectomy in our sample had higher mean levels of PFC than women who had not experienced hysterectomy. However, reverse causation cannot explain the inverse association between PFOS and estradiol nor does it completely account for the monotonic increase in the odds of menopause with PFOS quintile (and to a lesser extent PFOA) in older women because age was controlled for within age groups. Current data do not allow us to completely disentangle the issue of causation, and this should be a goal of future research. However even if the results reported here could be solely explained by reverse causation, they would still be cause for alarm because it would imply that increased PFC exposure is the natural result of menopause or hysterectomy. PFC are known to have multiple toxic effects including increases in lipids other than high-density lipoprotein (21), which increases cardiovascular risk, and immunotoxicity (22, 23). In other words, regardless of mechanism, the results have serious clinical implications for women's health. The additional question that needs to be asked is whether these increased exposure levels are more adverse in an aging body whose systemic defenses may be less robust.
Additional caveats include the fact that information about menopause comes from survey data and was not independently confirmed, nor was it possible to establish the exact age of menopause. However, although the dataset does not contain information about the timing of the blood draw in relation to menstrual cycles, the size of this population should compensate for any possible bias in estradiol concentrations related to timing.
What is unique about this study is its large population size and the availability of multiple laboratory endpoints, including simultaneously obtained PFC and estradiol. Our conclusion is that the mechanism explaining the increased risk of menopause within a group in women with high levels of PFOS and PFOA is still unclear, although the reduction of endogenous estrogen in women exposed to PFOS is provocative. Animal studies have shown that PFOA demonstrates estrogen-like properties, causing degeneration of ovaries in PFOA-exposed females (10). PFOA treatment results in stimulated estrogen synthesis in wild-type and peroxisome proliferator-activated receptor-α-null mice as well as enhanced mammary gland response to exogenous estrogen (10). It has also been shown that hepatocyte exposure to PFC has estrogenic activities and antiestrogenic effects (12).
The C8 dataset does not contain the variables that could help us address some of the issues related to mechanism. We do not know whether PFC in the C8 cohort are toxic to follicles, have estrogen-like properties (e.g. attach to or modify estrogen receptors), or suppress the pituitary release of LH or FSH or whether they modulate the release of GnRH from the hypothalamus. Additional research will be required to understand the associations between PFC and menopause reported here.
Acknowledgments
The C8 Health Project was funded by the Settlement Agreement in the case of Leach vs. E. I. DuPont de Nemours & Co., Civil Action No. 01-C-608 (West Virginia Circuit Court of Wood County, 2004), which resulted from drinking water contamination by the chemical perfluorooctanoic acid (PFOA or C8). The Court authorized the creation of Brookmar, Inc., an independent company created solely to design, publicize, and implement the C8 Health Project, including all enrollment and data collection. Brookmar, Inc., received funding exclusively from the Settlement, administered through a Court-approved financial professional (the Health Project Administrator). Brookmar, Inc., made data from the C8 Health Project available to the C8 Science Panel, also created pursuant to a contractual relationship with Brookmar, Inc.
Disclosure Summary: The design of the project was developed in consultation with, though not subject to, the wishes of the settling parties. All authors declare that their ability to design, conduct, interpret, and publish this research study was unimpeded and fully independent of the Court and settling parties, who had no role in preparing, reviewing, or approving the manuscript.
Footnotes
- BMI
- Body mass index
- NHANES
- National Health and Nutrition Examination Survey
- PFC
- perfluorocarbon
- PFOA
- perfluorooctanoate
- PFOS
- perfluorooctane sulfonate.
References
- 1. Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J. 2007. Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol Sci 99:366–394 [DOI] [PubMed] [Google Scholar]
- 2. Kudo N, Kawashima Y. 2003. Toxicity and toxicokinetics of perfluorooctanoic acid in humans and animals. J Toxicol Sci 28:49–57 [DOI] [PubMed] [Google Scholar]
- 3. Calafat AM, Wong LY, Kuklenyik Z, Reidy JA, Needham LL. 2007. Polyfluoroalkyl chemicals in the U.S. population: data from the National Health and Nutrition Examination Survey (NHANES) 2003–2004 and comparisons with NHANES 1999–2000. Environ Health Perspect 115:1596–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Frisbee SJ, Brooks AP, Jr, Maher A, Flensborg P, Arnold S, Fletcher T, Steenland K, Shankar A, Knox SS, Pollard C, Halverson JA, Vieira VM, Jin C, Leyden KM, Ducatman AM. 2009. The C8 Health Project: design, methods, and participants. Environ Health Perspect 117:1873–1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jensen AA, Leffers H. 2008. Emerging endocrine disrupters: perfluoroalkylated substances. Int J Androl 31:161–169 [DOI] [PubMed] [Google Scholar]
- 6. Biegel LB, Liu RC, Hurtt ME, Cook JC. 1995. Effects of ammonium perfluorooctanoate on Leydig-cell function: in vitro, in vivo, and ex vivo studies. Toxicol Appl Pharmacol 134:18–25 [DOI] [PubMed] [Google Scholar]
- 7. Shi Z, Ding L, Zhang H, Feng Y, Xu M, Dai J. 2009. Chronic exposure to perfluorododecanoic acid disrupts testicular steroidogenesis and the expression of related genes in male rats. Toxicol Lett 188:192–200 [DOI] [PubMed] [Google Scholar]
- 8. Shi Z, Zhang H, Liu Y, Xu M, Dai J. 2007. Alterations in gene expression and testosterone synthesis in the testes of male rats exposed to perfluorododecanoic acid. Toxicol Sci 98:206–215 [DOI] [PubMed] [Google Scholar]
- 9. Shi Z, Zhang H, Ding L, Feng Y, Xu M, Dai J. 2009. The effect of perfluorododecanonic acid on endocrine status, sex hormones and expression of steroidogenic genes in pubertal female rats. Reprod Toxicol 27:352–359 [DOI] [PubMed] [Google Scholar]
- 10. Zhao Y, Tan YS, Haslam SZ, Yang C. 2010. Perfluorooctanoic acid effects on steroid hormone and growth factor levels mediate stimulation of peripubertal mammary gland development in C57BL/6 mice. Toxicol Sci 115:214–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wei Y, Dai J, Liu M, Wang J, Xu M, Zha J, Wang Z. 2007. Estrogen like properties of perfluorooctanoic acid as revealed by expressing hepatic estrogen responsive genes in rare minnows (Gobiocypris rarus). Environ Toxicol Chem 26:2440–2447 [DOI] [PubMed] [Google Scholar]
- 12. Liu C, Du Y, Zhou B. 2007. Evaluation of estrogenic activities and mechanism of action of perfluorinated chemicals determined by vitellogenin induction in primary cultured tilapia hepatocytes. Aquatic Toxicol 85:267–277 [DOI] [PubMed] [Google Scholar]
- 13. White SS, Calafat AM, Kuklenyik Z, Villanueva L, Zehr RD, Helfant L, Strynar MJ, Lindstrom AB, Thibodeaux JR, Wood C, Fenton SE. 2007. Gestational PFOA exposure of mice is associated with altered mammary gland development in dams and female offspring. Toxicol Sci 96:133–144 [DOI] [PubMed] [Google Scholar]
- 14. Nikolaou D, Templeton A. 2004. Early ovarian ageing. European Journal of Obstetrics, Gynecology and Reprod Biol 113:126–133 [DOI] [PubMed] [Google Scholar]
- 15. Olsen GW, Gilliland FD, Burlew MM, Burris JM, Mandel JS, Mandel JH. 1998. An epidemiologic investigation of reproductive hormones in men with occupational exposure to perfluorooctanoic acid. J Occup Environ Med 40:614–622 [DOI] [PubMed] [Google Scholar]
- 16. Seals R, Bartell SM, Steenland K. 2011. Accumulation and clearance of perfluorooctanoic acid (PFOA) in current and former residents of an exposed community. Environ Health Perspect 119:119–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shuster LT, Rhodes DJ, Gostout BS, Grossardt BR, Rocca WA. 2010. Premature menopause or early menopause: long-term health consequences. Maturitas 65:161–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Archer D. 2009. Premature menopause increases cardiovascular risk. Climacteric 12(Suppl 1):26–31 [DOI] [PubMed] [Google Scholar]
- 19. de Kleijn MJ, van der Schouw YT, Verbeek AL, Peeters PH, Banga JD, van der Graaf Y. 2002. Endogenous estrogen exposure and cardiovascular mortality risk in postmenopausal women. Am J Epidemiol 155:339–345 [DOI] [PubMed] [Google Scholar]
- 20. Francucci CM, Romagni P, Camilletti A, Fiscaletti P, Amoroso L, Cenci G, Morbidelli C, Boscaro M. 2008. Effect of natural early menopause on bone mineral density. Maturitas 59:323–328 [DOI] [PubMed] [Google Scholar]
- 21. Steenland K, Tinker S, Frisbee S, Ducatman A, Vaccarino V. 2009. Association of perfluorooctanoic acid and perfluorooctane sulfonate with serum lipids among adults living near a chemical plant. Am J Epidemiol 170:1268–1278 [DOI] [PubMed] [Google Scholar]
- 22. Corsini E, Avogadro A, Galbiati V, dell'Agli M, Marinovich M, Galli CL, Germolec DR. 2011. In vitro evaluation of the immunotoxic potential of perfluorinated compounds (PFCs). Toxicol Appl Pharmacol 250:108–116 [DOI] [PubMed] [Google Scholar]
- 23. DeWitt JC, Shnyra A, Badr MZ, Loveless SE, Hoban D, Frame SR, Cunard R, Anderson SE, Meade BJ, Peden-Adams MM, Luebke RW, Luster MI. 2009. Immunotoxicity of perfluorooctanoic acid and perfluorooctane sulfonate and the role of peroxisome proliferator-activated receptor alpha. Crit Rev Toxicol 39:76–94 [DOI] [PubMed] [Google Scholar]