Loss of CaMKK2 in myeloid progenitor cells results in enhanced and accelerated granulopoiesis suggesting an inhibitory role for CaMKK2 in early granulocyte development.
Keywords: myelopoiesis, myeloid progenitors, calcium-calmodulin-dependent kinases
Abstract
Granulocytes serve a critical function in host organisms by recognizing and destroying invading microbes, as well as propagating and maintaining inflammation at sites of infection. However, the molecular pathways underpinning the development of granulocytes are poorly understood. Here, we identify a role for CaMKK2 in the restriction of granulocytic fate commitment and differentiation of myeloid progenitor cells. Following BMT, engraftment by Camkk2−/− donor cells resulted in the increased production of mature granulocytes in the BM and peripheral blood. Similarly, Camkk2−/− mice possessed elevated numbers of CMP cells and exhibited an accelerated granulopoietic phenotype in the BM. Camkk2−/− myeloid progenitors expressed increased levels of C/EBPα and PU.1 and preferentially differentiated into Gr1+Mac1+ granulocytes and CFU-G in vitro. During normal granulopoiesis in vivo or G-CSF-induced differentiation of 32D myeloblast cells in vitro, CaMKK2 mRNA and protein were decreased as a function of time and were undetectable in mature granulocytes. Expression of ectopic CaMKK2 in Camkk2−/− CMPs was sufficient to rescue aberrant granulocyte differentiation and when overexpressed in 32D cells, was also sufficient to impede granulocyte differentiation in a kinase activity-dependent manner. Collectively, our results reveal a novel role for CaMKK2 as an inhibitor of granulocytic fate commitment and differentiation in early myeloid progenitors.
Introduction
Hematopoiesis is the lifelong process in which HSCs self-renew or differentiate into all mature blood cell Lin through the production of intermediate progenitor blood cells [1, 2]. This process is tightly regulated in the BM by several factors, including microenvironment, paracrine interactions, cytokines, transcriptional programs, and signal transduction pathways. Although the signaling cascades that regulate hematopoietic cell maintenance and Lin specification are largely unclear, there have been implications for Ca2+ and its intracellular receptor, CaM, to be important mediators of HSC-progenitor cell processes such as growth and migration [3–5], suggesting that Ca2+/CaM-dependent pathways may play significant and diverse roles in hematopoiesis.
Indeed, we previously identified a CaMK, CaMKIV, as a critical, cell-intrinsic signaling component that regulates HSC survival and maintenance [6]. Camk4−/− mice possessed a significantly decreased number of KLS cells, which include HSCs and MPPs, and Camk4−/− HSCs failed to reconstitute hematopoiesis when transplanted into lethally irradiated mice. These HSC defects were a result of deregulated proliferation and increased apoptosis as a result of deficiencies in pCREB, CREB-binding protein, and Bcl-2. Furthermore, our previous studies demonstrated that CaMKIV signaling through CREB regulates the lifespan and function of several mature hematopoietic cell types, including DCs, T cells, and thymocytes, indicating a broader role for this CaMK in hematopoiesis [7–9].
CaMKIV belongs to a family of Ca2+/CaM-dependent, multifunctional serine/threonine kinases, which also include CaMKI and CaMKII [10, 11]. However, only CaMKI and CaMKIV require phosphorylation on a single activation-loop threonine to achieve maximal activation. This phosphorylation event occurs via an upstream CaMKK, CaMKK1 and/or CaMKK2, which is also a Ca2+/CaM-binding protein [12]. This method of regulation has been termed a ″CaM kinase cascade″ in cells, analogous to the MAPK cascade [13]. Indeed, we showed recently that CaMKK2-CaMKIV signaling to CREB is required for normal cerebellar granule cell development [14]. However, the importance of CaMKK2-mediated signaling in hematopoiesis has not been evaluated.
To elucidate the physiological relevance of CaMKK2 and the CaMK cascade in early HSC-progenitor cells, we investigated the effects of CaMKK2 deletion on the mouse hematopoietic system. Similar to CaMKIV, mice null for CaMKK2 possessed reduced numbers of total KLS and BM cells. However, depletion of CaMKK2 did not elicit abnormal cell proliferation or impaired survival or function of HSCs. Interestingly, unlike Camk4−/− mice, Camkk2−/− mice displayed marked defects in early myeloid progenitor (CMP and GMP) populations in the BM. Engraftment of Camkk2−/− HSCs in BMT led to increased repopulation of myeloid Lin and particularly in the granulocyte compartment. Moreover, Camkk2−/− myeloid progenitors differentiated into greater numbers of CFU-G and Gr1+Mac1+ granulocytes in vitro. Restoration of CaMKK2 in Camkk2−/− CMP cells was sufficient to rescue the enhanced granulopoietic phenotype of Camkk2−/− progenitors. Similarly, overexpression of WT but not kinase-deficient CaMKK2 in a 32D myeloid progenitor cell line resulted in a pronounced inhibition of granulocyte differentiation. Thus, our findings demonstrate a novel, cell-intrinsic role for CaMKK2 in myeloid progenitors as a negative regulator of granulopoiesis and provide key insights for potential therapies of granulopoietic and/or myelogenous disorders.
MATERIALS AND METHODS
Mice
WT and Camkk2–/– mice were generated as described previously [15]. B6.SJL-Ptprca Pep3b/BoyJ (CD45.1+) mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). The animals were housed under a 12-h light, 12-h dark cycle and provided food and water ad libitum. All animal care and experimental procedures were performed in compliance with NIH (Bethesda, MD, USA) and Duke University (Durham, NC, USA) Institutional Animal Care and Use Committee guidelines. Unless otherwise noted, experiments were performed using 8- to 12-week-old, gender-matched mice.
Flow cytometry
Murine BM hematopoietic cell subsets were analyzed and/or isolated by FACS, based on the expression pattern of cell-surface markers, as described previously [16–19]. Briefly, nucleated BM cell suspensions were prepared from hindlimb bones in HBSS (Mediatech, Manassas, VA, USA), plus 5% FBS (Gemini Bio-Products, West Sacramento, CA, USA). Prior to staining and sorting stem progenitor cells by FACS, c-Kit+ (CD117) cells were enriched using an autoMACS cell sorter to separate cells bound to anti-CD117 microbeads (both from Miltenyi Biotec, Auburn, CA, USA). All fluorescent antibodies herein came from eBioscience (San Diego, CA, USA), unless otherwise noted. For KLS cell isolation and/or analysis by FACS, c-Kit+ BM cells were stained with c-Kit-APC, Sca-1-PE-Cy5, and Lin cocktail in PE (Lin: CD3, CD4, CD5, CD8, B220, Mac1, Gr1, and Ter119). HSCs and MPPs were stained with c-Kit-APC, Sca-1-Pacific Blue (BioLegend, San Diego, CA, USA), CD48-FITC, and CD150-PE-Cy7. CLPs were stained with c-Kit-FITC, Sca-1-PE-Cy5, B220-PE-Cy7, and IL-7Rα-biotin, followed by streptavidin APC-Cy7. Myeloid progenitors were stained with c-Kit-APC, Sca-1-PE-Cy5, CD16/32-eFluor450, and CD34-FITC. Primary granulocytes and differentiating 32D cells were stained with Gr1-FITC and Mac1-PE or -Pacific Blue. Stem and progenitor cell populations were immunophenotypically defined as follows: HSC, CD150+c-kit+Sca-1+CD48–Lin–; MPP, CD150–c-kit+Sca-1+CD48–Lin–; KLS, c-Kit+Sca-1+Lin–/lo; CLP, Lin−IL-7R+B220−Sca-1loc-Kitlo; CMP, Lin–Sca-1–c-Kit+CD34+CD16/32lo; GMP, Lin–Sca-1–c-Kit+CD34+CD16/32+; and MEP, Lin–Sca-1–c-Kit+CD34–CD16/32–. Analyses were performed using a FACScan or FACSCanto II cytometer (Becton Dickinson, San Jose, CA, USA) and cell sorting performed with a FACSVantage cell sorter (Becton Dickinson). Data analyses were performed using CellQuest Software (Becton Dickinson) and FlowJo Software (Tree Star, Ashland, OR, USA).
In vivo BrdU incorporation
Mice were administered a single i.p. injection of BrdU (Sigma-Aldrich, St. Louis, MO, USA) at 1 mg/6 g mouse weight [20]. Three hours later, the mice were killed, and KLS cells were isolated and fixed in 70% ethanol at –20°C. Analysis of in vivo BrdU incorporation was performed using a FITC BrdU flow kit (Becton Dickinson), according to the manufacturer′s instructions, followed by FACS.
Annexin V apoptosis assay
Freshly isolated KLS cells were stained with anti-Annexin V-FITC (Becton Dickinson) and PI (Sigma-Aldrich) for 15 min, according to the manufacturer′s instructions (Becton Dickinson). Stained cells were analyzed by flow cytometry within 30 min.
BMT
CD45.1+ mice were lethally irradiated with two doses of 5 Gy whole-body irradiation (10 Gy total) from a 137Cs source administered ∼24 h apart. On the following day, CD34–KLS donor cells were isolated from three WT or Camkk2–/– mice (CD45.2+) and then pooled, respectively. Subsequently, 1000 CD45.2+ donor cells along with 300,000 CD45.1+ Sca-1-depleted BM cells obtained from nonirradiated recipient mice [21] were injected retro-orbitally into five to 10 irradiated recipients/cohort. To measure repopulation, peripheral blood was obtained from recipient tail veins every 3 weeks, and nucleated blood cells were stained and analyzed by FACS. After 15 weeks, recipient BM cells were harvested and stained for FACS analysis of CD45.2+ Lin populations.
Cytological analysis of BM
To examine the morphology of developing granulocytes, nucleated BM cells were obtained from individual WT and Camkk2–/– mice, and early-stage granulocytes were sorted based on B220–CD11c–Gr1+Mac1–/lo cell surface expression. Isolated granulocytes were cytospun, air-dried, fixed in methanol, and subjected to Giemsa staining (Sigma-Aldrich) for analysis of nuclear morphology.
In vitro CMP differentiation assays
To assess differentiation potential, sorted WT and Camkk2–/– CMPs were cultured, as described previously [22], on murine OP9 BM stromal cells, plated the day before at a density of 20,000 cells/well in a 24-well plate. OP9 cells were propagated in α-MEM (Invitrogen, Carlsbad, CA, USA) containing 20% FBS and 1% penicillin-streptomycin (Mediatech). For ectopic-expression experiments, cells were transduced with lentiviruses containing control (empty vector) or CaMKK2, as described previously [14], and after 3 days, were selected by GFP marker and Lin– expression using FACS. Five hundred CMPs were co-cultured with OP9 cells for 8–12 days in RPMI-1640 medium (Invitrogen) containing 10% FBS, 1% penicillin-streptomycin, and cytokines which support cell growth and myeloid differentiation including rmSCF (50 ng/mL), rm fetal liver tyrosine kinase 3 ligand (30 ng/mL), rmIL-3 (10 ng/mL), rmIL-7 (10 ng/mL), and rmGM-CSF (10 ng/mL; R&D Systems, Minneapolis, MN, USA). All cells were harvested from each well, enumerated using a Z1 Coulter Counter (Beckman Coulter, Brea, CA, USA), stained with Gr1-FITC, Mac1-Pacific Blue, and CD45-PE (to exclude OP9 CD45– cells), and then analyzed by FACS.
In vitro CFU assays
To measure proliferation and differentiation potential, 1500 sorted GMP cells were plated in triplicate into gridded, 35-mm cell-culture dishes (Nalgene Nunc, Rochester, NY, USA) and grown in methylcellulose media (Complete Methocult Media M3434, StemCell Technologies, Vancouver, Canada) containing SCF, IL-3, IL-6, and erythropoietin. Seven to 10 days later, colonies were classified and enumerated based on morphology.
Granulocyte isolation from BM
BM cells were collected from WT and Camkk2−/− mice as described above. Granulocytes were isolated by performing two sequential density-gradient centrifugations. First, whole BM cells were stratified on Lympholyte (Cedarlane, Burlington, NC, USA). The high-density fraction containing granulocytes and erythrocytes was recovered and fractionated further by using a 50% Percoll (GE Healthcare, Piscataway, NJ, USA) gradient. Granulocytes were obtained from the less-dense fraction, and purity was confirmed shortly thereafter by FACS analysis.
32D cell differentiation assays
32D cells were obtained from American Type Culture Collection (Manassas, VA, USA) and propagated in RPMI 1640 containing 10% FBS, 1% penicillin-streptomycin, and 5 ng/mL rmIL-3. For knockdown studies, cells were lentivirally transduced with CaMKK2-shRNA or empty vector, pLKO.1 (OpenBiosystems, Huntsville, AL, USA), and then selected with 2 μg/mL puromycin (Sigma-Aldrich) for 5–7 days. For overexpression experiments, cells were transduced with lentiviruses containing CaMKK2 (WT) or CaMKK2D311A (inactive), which were constructed as described previously [14] and after 3 days, were selected by GFP marker expression using FACS. To assess granulocytic differentiation potential, cells were washed with HBSS to remove IL-3, and 200,000 cells were plated on Day 0 in RPMI 1640 containing 10% FBS, 1% penicillin-streptomycin, and 40 ng/mL rmG-CSF. For experiments using KN-93 (EMD Chemicals, Gibbstown, NJ, USA), cells cultured in normal growth media or G-CSF-containing media were treated on Day 0 with 2.5 μM KN-93 (or as otherwise specified), and during differentiation assays, the drug-containing media were refreshed every 2 days. After 6 days of differentiation in culture, cells were harvested, cytospun, and then Wright′s-stained (EMD Chemicals) for analysis of nuclear morphology to determine the extent of granulocytic differentiation. For experiments using ionomycin (EMD Chemicals), cells in normal growth media were treated with 2 μM ionomycin for 5 min and then prepared and harvested for immunoblot analysis.
Immunoblot analyses
Equal amounts of protein sample/lane (unless otherwise noted) were denatured and resolved by SDS-PAGE. Proteins were transferred to Immobilon-FL membrane (Millipore, Billerica, MA, USA), and quantitative Western blotting was performed using the Odyssey Infrared Western blotting detection system (LI-COR Biosciences, Lincoln, NE, USA). Primary antibodies used were anti-CaMKK (Becton Dickinson), anti-β-actin (Sigma-Aldrich), anti-pAMPKα (Thr172; Cell Signaling, Danvers, MA, USA), anti-AMPKα (Cell Signaling), anti-CaMKI (generated by Christina Kahl, laboratory of A. R. Means, Department of Pharmacology and Cancer Biology, Duke University Medical Center), anti-CaMKIV (Becton Dickinson), anti-pCaMKII (Thr286; Thermo Scientific, Rockford, IL, USA), anti-CaMKII (D11A10; Cell Signaling), and antieukaryotic translation initiation factor 4E (Becton Dickinson). Secondary antibodies used were anti-mouse IgG Alexa Fluor 680 (Invitrogen) and anti-rabbit IgG IRDye800 conjugated (Rockland Immunochemicals, Gilbertsville, PA, USA).
Real-time qRT-PCR
Total RNA was isolated from myeloid progenitor and 32D cells using the RNAqueous-Micro kit (Ambion, Austin, TX, USA) and RNeasy Mini kit (Qiagen, Valencia, CA, USA), respectively. cDNA synthesis was subsequently performed using SuperScript III RT (Invitrogen), and real-time PCR was carried out using iQ SYBR Green Supermix (Bio-Rad, Hercules, CA, USA), according to the manufacturer′s instructions. All RT-PCR primer sequences are available upon request.
Statistical analysis
Bar graphs represent mean ± sem, with the number of experiments indicated in the figure legends. Student′s t test was used for all statistical analyses, and level of significance was set at P < 0.05.
Online Supplemental material
Camkk2−/− mice maintained a similar percentage of KLS cells in the BM compared with WT mice but displayed decreased total numbers of nucleated BM cells (Supplemental Fig. 1). After 15 weeks post-transplantation, spleens from mice transplanted with Camkk2−/− HSCs contained increased numbers of donor myeloid cells, including granulocytes, as well as B cells (Supplemental Fig. 2). In the BM of Camkk2−/− mice, CMP cells exhibited increased proliferation compared with WT. Although Camkk2−/− mice had increased percentages of mature myeloid cells in BM, there was no significant difference in the number of granulocytes in the peripheral blood (Supplemental Fig. 3). In 32D cells, CaMKI but not CaMKIV was detected, whereas KN-93 failed to reverse CaMKK2-mediated inhibition of 32D differentiation. Furthermore, treatment of 32D cells with ionomycin did not alter the levels of pAMPK (Supplemental Fig. 4).
RESULTS
CaMKK2 is not required for homeostatic KLS cell proliferation or survival
We confirmed that CaMKK2 mRNA is expressed in KLS cells, a population enriched for HSCs (Fig. 1A), and next evaluated whether the hematopoietic compartment was altered in the absence of CaMKK2 by analyzing the KLS and BM cellularity of WT and Camkk2–/– mice. Although our results showed a similar frequency of KLS cells in WT and Camkk2–/– BM (Fig. 1B and Supplemental Fig. 1A), the total number of KLS and BM cells was reduced significantly in Camkk2−/− mice (Fig. 1C and Supplemental Fig. 1B), similar to what was observed in Camk4–/– mice [6]. This decrease in Camkk2–/– BM cellularity did not result from a runted body size, as these mice were comparable in weight with WT on a standard chow diet as described previously [15]. As the relative decrease in total BM cellularity was proportional to the reduction in KLS cells, the frequency of KLS cells was not significantly different in WT versus Camkk2–/– mice. However, given that the total number of KLS cells was similarly reduced in Camkk2–/– and Camk4–/– mice, we tested whether CaMKK2-deficient KLS cells exhibited defective proliferation and/or survival under homeostatic conditions such as Camk4–/– KLS cells. BrdU incorporation experiments revealed no significant differences in the percentage of proliferating KLS cells between WT and Camkk2–/– mice (Fig. 1D and E). To test whether CaMKK2 was necessary for KLS cell survival, freshly isolated WT and Camkk2–/– KLS cells were analyzed for Annexin V and PI reactivity to evaluate the percentage of apoptotic cells. We observed no significant differences in the percentage of early apoptotic (Annexin V+PI–) cells in WT versus Camkk2–/– mice (Fig. 1F and G). As KLS cell proliferation and survival did not appear altered, the decreased number of KLS cells in the Camkk2−/− BM may reflect the overall decrease in total BM cellularity and/or be suggestive of altered self-renewal or repopulation ability. Collectively, these data indicate that CaMKK2 is not necessary for regulating the proliferation or survival of KLS cells.
Figure 1. Loss of CaMKK2 does not affect cell proliferation or survival of KLS cells.
(A) RT-PCR analysis of Camkk2 mRNA expression in WT and Camkk2−/− KLS cells. WT hypothalamus (hypoth.) cDNA was included as a positive control and H2O as a negative control. (B) Representative FACS analysis of KLS cells in c-Kit+-enriched BM from WT and Camkk2−/− mice. FL1, -2, -3, and -5, Fluorescence 1, 2, 3, and 5, respectively. (C) Total numbers of KLS cells in WT and Camkk2–/– mice (n≥8; *P<0.05). (D) To assess proliferation, KLS cells in WT and Camkk2–/– mice were pulse-labeled with BrdU in vivo, then isolated, stained with anti-BrdU-FITC and PI, and analyzed by FACS. (E) Quantification of Panel D showing percentages of BrdU-positive KLS cells (n≥9). (F) To evaluate apoptosis, WT and Camkk2–/– KLS cells were freshly isolated and stained with anti-Annexin V-FITC and PI and then analyzed by FACS. FL1/2-H, Fluorescence 1/2-height. (G) Quantification of Panel F showing percentages of (Annexin V+PI–) KLS cells undergoing apoptosis (n≥9). (A–G) Data are representative of at least three independent experiments.
CaMKK2 deficiency does not impair HSC repopulating ability but causes enhanced myelopoiesis in vivo during BMT
Although the loss of CaMKK2 did not affect KLS cell proliferation or viability, the reduced number of Camkk2–/– KLS cells suggested the possibility that CaMKK2 null HSCs were functionally compromised relative to WT. Moreover, the long-term self-renewal potential and multi-Lin repopulating properties of Camkk2–/– HSCs remained unknown. Therefore, to determine whether loss of CaMKK2 affects the in vivo repopulation potential of HSCs, we performed a noncompetitive transplantation assay in the same manner that allowed us to identify marked defects in the blood reconstitution ability of Camk4−/− HSCs [6]. To this end, we transplanted an equal number of CD34–KLS cells (enriched for long-term HSCs [17]) from CD45.2+ WT and Camkk2–/– mice along with Sca-1-depleted BM cells from nonirradiated WT CD45.1+ mice into lethally irradiated CD45.1+ recipients. Donor:host blood cell chimerism (CD45.2+ vs. CD45.1+) and hematopoietic Lin in the peripheral blood were analyzed by FACS once every 3 weeks for 15 weeks. Based on the mean percentage of CD45.2+ cells in the blood, overall donor engraftment was significantly higher in Camkk2–/–-reconstituted mice compared with WT (Fig. 2A and B), suggesting that repopulation and/or self-renewal abilities of Camkk2–/– HSCs were not impaired but perhaps enhanced.
Figure 2. Loss of CaMKK2 alters hematopoietic engraftment and results in the expansion of myeloid and granulocyte Lin during in vivo BMT.
Donor WT and Camkk2–/– (CD45.2+) HSCs were transplanted into irradiated WT (CD45.1+) recipient mice and monitored for ≥15 weeks post-BMT. (A) Donor (CD45.2+) chimerism in recipients was measured by FACS analysis of peripheral blood cells stained with anti-CD45.2-APC and anti-CD45.1-FITC. KK2 KO, CaMKK2 knockout. (B) Representative FACS plots of WT and Camkk2–/– donor engraftment in Panel A. (C) Numbers of CD45.2+ B cells (B220+), T cells (CD3+), and myeloid cells (Mac1+) in recipients' peripheral blood 6 weeks (left) and 15 weeks (right) after transplantation. (D–F) Analysis of recipient BM populations after 15 weeks post-transplantation. (D) Total number of whole BM cells and CD45.2+ BM cells. (E) Total number of CD45.2+ stem and progenitor cells of different subsets. (F) Total number of CD45.2+ granulocytes. (A–F) Data are representative of at least three independent experiments (n≥15; *P<0.03).
However, further analysis showed that increased engraftment by Camkk2–/– donor cells in irradiated recipients was Lin-specific and largely a result of increases in the total number of myeloid (Mac1+) rather than lymphoid cells in the peripheral blood, although by Week 15, recipient mice also exhibited a significant increase in circulating Camkk2–/– B cells (B220+; Fig. 2C). Although assessing the short-term (6 weeks post-BMT), rapid repopulation potential of donor cells [23] (Fig. 2C, left), we observed engraftment by Camkk2–/– HSCs, which was three-fold higher in the myeloid Lin when compared with WT HSC transplantation. In addition, a transient decrease in Camkk2–/– engraftment of T cells (CD3+) was observed at this time-point but later became comparable with WT CD3+ numbers. By 15 weeks after BMT, the long-term reconstitution ability, as well as the self-renewal potential of HSCs, could be evaluated [17], and these results showed a persistent increase in the number of CD45.2+ Camkk2–/– myeloid cells compared with CD45.2+ WT (Fig. 2C, right). We additionally observed a significant enhancement in the long-term B cell repopulation potential of Camkk2–/– donor cells. Furthermore, analysis of recipient spleens revealed differences consistent with those found in the peripheral blood (Supplemental Fig. 2A). Interestingly, we also found that host T cells (CD3+) were increased significantly in the spleens of recipients with Camkk2–/– but not WT donor cells. This suggested that an accumulation of T lymphocytes occurred, possibly resulting from the increased number of supporting myeloid cells (including macrophages and DCs) from Camkk2–/– engraftment.
To understand whether the increases in Camkk2–/– mature myeloid and B cells in the recipients were derived from an elevation of stem or progenitor cells, we examined the composition of the donor-reconstituted recipient BM after 15 weeks post-BMT. Although the total BM cellularity in WT and Camkk2–/– recipients was not different, we found an approximate two-fold increase in total Camkk2–/– donor cells compared with WT (Fig. 2D), which correlates with the higher engraftment by Camkk2–/– cells observed in the peripheral blood. In our analysis of donor stem/progenitor populations, we did not find statistically significant differences in the number of CD45.2+ Camkk2–/– HSC, MPP, or KLS cells in the recipient BM, compared with engraftment by WT (Fig. 2E), which suggested that the increase in total Camkk2–/– engraftment was not a result of any proliferation or survival advantages of Camkk2–/– HSCs. Furthermore, there were no significant differences in the number of CD45.2+ CLP or MEP cells in WT- and Camkk2–/–-engrafted recipients. Interestingly, our data did indicate a significant two-fold increase in the number of CD45.2+ Camkk2–/– CMPs and conversely, a four-fold reduction of GMPs (differentiated progeny of CMPs) in the recipient BM compared with WT. Additionally, we observed a significant two-fold increase in Gr1+Mac1+ donor granulocytes in Camkk2–/– recipient BM relative to WT (Fig. 2F), presumably contributing to the increased number of myeloid cells in the Camkk2–/–-repopulated peripheral blood. Altogether, these data suggest that CaMKK2 plays a modulatory role in myelopoiesis, particularly in the suppression of granulocyte development. Thus, based on the intriguing differences in myeloid progenitor subsets and mature myeloid cells without a change in lymphoid progenitor cell number compared with WT, we began to evaluate a potential role for CaMKK2 in myelopoiesis and granulocyte development.
Loss of CaMKK2 promotes cell autonomous granulocyte commitment and differentiation from myeloid progenitors
As the transplantation of Camkk2−/− HSCs resulted in increased engraftment in the CMP and granulocyte Lin in the recipient BM and peripheral blood, we surmised that the absence of CaMKK2 results in the accelerated differentiation of CMPs to mature granulocytes. Consistent with our findings in Camkk2−/−-reconstituted, recipient BM, Camkk2−/− mice possessed significantly increased frequency and number of CMPs, as well as decreases in GMPs compared with WT (Fig. 3A–C). A possible explanation of this anomaly is that in the absence of CaMKK2, GMPs undergo more rapid differentiation into mature granulocytes, which would trigger an expansion of the parental CMPs to compensate for the decrease in GMPs. To test this, we first measured the percentage of proliferating CMPs in the BM of WT and Camkk2−/− mice and found a significantly higher percentage of BrdU-positive CMPs in Camkk2−/− mice, suggesting that an increased expansion of Camkk2−/− CMPs does occur (Supplemental Fig. 3A).
Figure 3. Camkk2–/– mice display defects in early myeloid progenitors and enhanced granulopoiesis in the BM.
(A) Percentages of HSC and progenitor populations in BM of WT and Camkk2–/– mice (n>8; *P<0.01). (B) Representative FACS analysis of Lin–Sca-1–c-Kit+-gated myeloid progenitor populations (CMP, GMP, and MEP) in BM of WT and Camkk2–/– mice. (C) Total number of stem and progenitor cells of different subsets in the BM (n>8; *P<0.01). (D) Cytological images demonstrating the morphology of immature, developing granulocytes (Gr1loMac1+),which were sorted from the BM of WT and Camkk2–/– mice and then subjected to Giemsa staining. Arrowheads indicate mature granulocytes, which exhibit band or segmented nuclear morphology. Images were acquired with an Axio Observer Z1 microscope (Carl Zeiss) at original magnification, ×630. (E) Quantification of Panel D, showing ratios of mature granulocytes over immature granulocytes (i.e., promyelocytes/myelocytes/metamyelocytes; n=4; ≥300 cells scored/individual; *P<0.001). (F) Representative FACS analysis of mature myeloid, B, and erythroid Lin cells in BM. (G) Total number of mature granulocytes (Gr1+Mac1+), monocyte Lin myeloid cells (Gr1lo/–Mac1+), B cells (B220+), and erythroid cells (Ter119+) in the BM of WT and Camkk2–/– mice, 3–4 weeks old (n=5; *P<0.05).
To confirm whether loss of CaMKK2 resulted in accelerated granulocyte differentiation, we performed Giemsa staining of the immature, developing Gr1loMac1lo/+ granulocytes [24, 25] isolated from the BM of WT and Camkk2−/− mice. As indicated in Fig. 3D and E, the Gr1loMac1lo/+ population of early committed granulocytes from Camkk2−/− mice consisted of a four-fold higher number of cells displaying condensed band or segmented nuclear morphology, a hallmark of terminally differentiated, mature granulocytes [25]. Moreover, Camkk2−/− mice had a significantly higher frequency and number of mature granulocytes (Gr1+Mac1+) in the BM than WT mice (Fig. 3F and G and Supplemental Fig. 3B), as seen before in the BM and peripheral blood of Camkk2−/−-transplanted recipients (Fig. 2C and F). These developmental defects in the BM of Camkk2−/− mice appeared to be specific to the myeloid and granulocyte populations, as there were no significant differences in B lymphocyte and erythroid (Ter119+) Lin (Fig. 3F and G and Supplemental Fig. 3B). We also measured circulating granulocytes in the peripheral blood to assess if the Camkk2−/− mice exhibited neutrophilia but found no significant differences (Supplemental Fig. 3C and D). Nonetheless, our data corroborate that the in vivo deficiency of CaMKK2 leads to enhanced granulocytic differentiation in the BM.
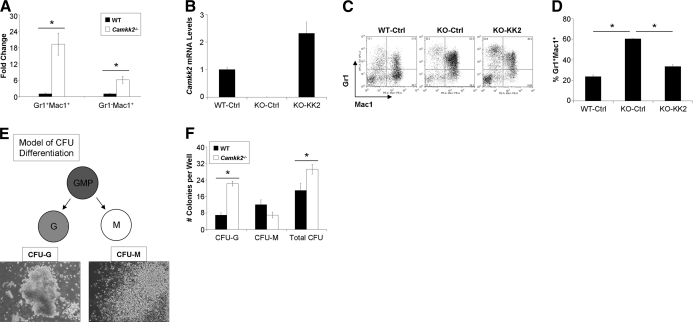
The accelerated granulocyte differentiation observed in Camkk2−/− mice suggested that the intrinsic deficiency of CaMKK2 in myeloid progenitors increases their commitment and differentiation toward the granulocyte Lin. To test this hypothesis directly, WT and Camkk2−/− CMP cells were isolated and co-cultured with OP9 cells in media supplemented with myeloid growth factors to induce differentiation. The cells were then harvested and stained for FACS analysis of Gr1+Mac1+ granulocytes versus Gr1–Mac1+ monocytic populations. Similar to our earlier results of enhanced myeloid repopulation in Camkk2−/−-reconstituted recipients, these results indicated a significant increase in granulocyte and monocyte populations (Fig. 4A). Yet, although we observed a 19-fold increase in Camkk2−/− versus WT granulocytes, the increase in monocytic cells was smaller (i.e., six-fold) in Camkk2−/− versus WT, suggesting a preferential Lin commitment of Camkk2−/− myeloid progenitors to differentiate into granulocytes rather than monocytes. To ensure that the granulocytic differentiation phenotype was specifically a result of the absence of CaMKK2, we re-expressed CaMKK2 in Camkk2−/− CMPs and assessed their function in vitro. After confirming that CaMKK2 was restored (∼2.5-fold relative to WT control) in Camkk2−/− CMPs (Fig. 4B), we tested their differentiation potential and confirmed that the add-back of CaMKK2 to Camkk2−/− CMPs was able to reverse enhanced granulopoiesis significantly (Fig. 4C and D).
Figure 4. CaMKK2 deficiency in CMPs and GMPs results in increased granulocytic fate commitment and differentiation.
(A) Fold change relative to WT of Gr1+Mac1+ and Gr1–Mac1+ cells that arose from Camkk2–/– CMPs cultured and differentiated for 8–12 days in vitro (n=3; *P<0.001). (B) qRT-PCR analysis of Camkk2 mRNA in WT control (WT-Ctrl), CaMKK2 knockout control, and CaMKK2 knockout-CaMKK2 (added back) CMP cells. (C) Representative FACS analysis of Gr1/Mac1 cells differentiated in vitro from WT control, CaMKK2 knockout control, and CaMKK2 knockout-CaMKK2 (added back) CMP cells. (D) Quantified average percentage of Gr1+Mac1+ cells from Panel C.(n=3; *P<0.05). (E) Schematic model of in vitro CFU differentiation. Representative images of CFU-G and CFU-M were acquired with a CK40 microscope (Olympus) at original magnification, ×100. (F) Total numbers of Lin-specific and total CFUs that developed from WT and Camkk2–/– GMPs after 7 days of culture in Complete Methocult Media M3434 (n=4; *P<0.05).
In addition to evaluating the effects of the absence of CaMKK2 in CMPs, we examined the differentiation potential of WT and Camkk2−/− GMPs via in vitro methylcellulose-based CFU assays. In the presence of growth factors, GMPs differentiate into CFU-G and CFU-M of the granulocyte and monocyte/macrophage Lin, respectively (Fig. 4E). We found that Camkk2−/− GMP cells gave rise to a significant, three-fold higher number of CFU-G and 1.5-fold higher number of total colonies than WT (Fig. 4F). There was also a decrease, albeit not significant, in the number of CFU-M that formed from Camkk2−/− GMPs compared with WT. Collectively, our findings thus far indicate that the absence of CaMKK2 in myeloid progenitors leads to increased granulocytic commitment and differentiation in a cell autonomous manner.
CaMKK2 is expressed in myeloid progenitors but is absent in terminally differentiated granulocytes
The enhanced granulopoiesis phenotype, resulting from the cell-intrinsic loss of CaMKK2 in myeloid progenitors, supports the idea that CaMKK2 functions as an inhibitor of granulocyte differentiation in myeloid progenitors. To test this idea during granulopoiesis, we first evaluated CaMKK2 gene expression in progenitor cells by qRT-PCR and found that Camkk2 mRNA was expressed in WT CMP, GMP, and MEP cells but was vastly decreased (>30-fold) in mature granulocytes, suggesting that CaMKK2 expression becomes down-regulated when myeloid progenitors differentiate into granulocytes (Fig. 5A). Next, we evaluated whether C/EBPα and PU.1, two master regulators of early myeloid Lin commitment that are essential for granulocyte development [26–29], were altered between WT and Camkk2−/− CMP cells. Compared with WT, Camkk2−/− CMPs showed a significant, two-fold increase in C/EBPα and PU.1 mRNA, whereas the levels of Pax5 and E2A, which are involved in early lymphoid Lin differentiation and included in the experiment as negative controls [30, 31], were not different (Fig. 5B). These data confirm our earlier contention that the absence of CaMKK2 primes myeloid progenitors for granulocyte fate commitment and accelerated granulopoiesis.
Figure 5. CaMKK2 is expressed in myeloid progenitors but is undetectable in mature granulocytes from the BM.
(A) qRT-PCR analysis of Camkk2 mRNA levels in myeloid progenitor subsets and mature granulocytes from the BM, normalized by β2-microglobulin (n≥3; *P<0.01). (B) CMPs were isolated and analyzed by qRT-PCR for the expression of genes involved in hematopoietic cell differentiation and fate commitment, normalized by β2-microglobulin (n=6 mice from two independent experiments; *P<0.05). (C) Representative FACS analysis of granulocytes purified by density-gradient centrifugation from WT, Camkk2–/–, and Camkk1–/– BM. (D) Representative immunoblot of two experiments to evaluate expression of CaMKK2 in Gr1+Mac1+ terminally differentiated granulocytes purified from the BM of WT, Camkk2–/–, and Camkk1–/– mice (the latter generated in our lab and shown here as a control for CaMKK band discrimination). Lysates of 32D myeloblast cells ± control-shRNA (32D-ctrl) or CaMKK2-shRNA (32D-sh-KK2) served as controls for CaMKK2 immunoblotting, and β-actin served as a loading control.
We next questioned the presence of CaMKK2 in mature Gr1+Mac1+ BM granulocytes from WT, Camkk2−/−, and Camkk1−/− mice (where both knockout genotypes were used as a control). After cell isolation, we determined the purity of the granulocyte preparation from BM to be >90% Gr1+Mac1+ by FACS (Fig. 5C). Granulocytes were then immunoblotted for CaMKK2 using a pan-CaMKK antibody, and as a control for CaMKK2 immunoblotting, we included lysates from 32D myeloid progenitor cells that expressed control- or CaMKK2-shRNA. These results revealed CaMKK2 to be undetectable at the protein level in terminally differentiated granulocytes (Fig. 5D). Taken together, these data reinforce the hypothesis that loss of CaMKK2 in myeloid progenitor cells promotes differentiation, as evidenced by increased levels of C/EBPα and PU.1, and reveal that CaMKK2 is decreased during granulocyte development in vivo.
Granulocyte differentiation of 32D myeloblast cells results in decreased CaMKK2
To better understand the role of CaMKK2 in myeloid progenitors at the transition point of early granulocyte differentiation, we used the well-established murine 32D myeloid progenitor cell line as a secondary model to study the role of CaMKK2 in granulocyte development [32, 33]. When 32D cells were treated with G-CSF, they terminally differentiated into granulocytes, as indicated by the continuous increase in percentage of Gr1+Mac1+ cells over at least 9 days following G-CSF treatment (Fig. 6A and B). To evaluate whether CaMKK2 decreased during granulocyte differentiation of 32D cells, as was suggested by the preceding in vivo data, we examined CaMKK2 mRNA and protein in G-CSF-treated 32D cells as a function of time. Our results revealed that Camkk2 mRNA is decreased significantly (∼2.3-fold) after 6 days of G-CSF treatment (Fig. 6C). However, CaMKK2 protein was markedly down-regulated (∼65%) within 3 days and became undetectable after 6 days of G-CSF treatment (Fig. 6D and E). These data are consistent with our earlier observations that CaMKK2 cannot be detected in mature BM granulocytes (Fig. 5D) and suggest that G-CSF stimulates the post-transcriptional down-regulation of CaMKK2 during granulocyte differentiation.
Figure 6. CaMKK2 is markedly down-regulated during early granulocyte differentiation of 32D myeloblast cells.
(A) Percentages of 32D cells that differentiated into Gr1+Mac1+ cells over a time-course of 9 days after G-CSF treatment (n=3; plated in triplicate). (B) Representative FACS plots demonstrating 32D cell differentiation in Panel A. (C) qRT-PCR analysis of Camkk2 mRNA levels during 32D cell differentiation over 9 days, normalized by β2-microglobulin (n=2; plated in triplicate; *P<0.01). (D) CaMKK2 protein levels assessed by immunoblot analysis as a function of time during G-CSF-induced 32D cell differentiation [32D-uninfected (U)]. (E) Quantification of Panel D showing CaMKK2 protein levels relative to Day 0, normalized by β-actin (n=3; *P<0.02).
CaMKK2 represses granulocytic differentiation independently of AMPK, CaMKI, or CaMKIV
Since a reduction in CaMKK2 occurs as 32D cells undergo granulocytic differentiation, we investigated whether forced expression of CaMKK2 in these cells would inhibit granulocyte differentiation. To this end, 32D cells were transduced with lentiviruses encoding control GFP, WT CaMKK2, or catalytically inactive CaMKK2 (D311A; ref. [12]). Uninfected and lentivirus-infected 32D cells were stimulated with G-CSF for 6 days, and cell samples in each category from Day 0 and Day 6 of treatment were Wright's stained and then analyzed to quantify immature and mature granulocytes based on nuclear morphology. Immature granulocytes such as myeloblasts, promyelocytes, and myelocytes contain rounded nuclei, whereas mature granulocytes such as metamyelocytes and neutrophils display band or segmented-shaped nuclei [34]. Although there were no significant differences in the percentage of mature granulocytes among 32D-uninfected, -control, or -D311A after 6 days of G-CSF treatment, we observed a significant, three-fold decrease in the percentage of mature granulocytes among 32D-CaMKK2 cells (Fig. 7A and B), indicating repression of granulopoiesis by CaMKK2 in a kinase-dependent manner. To determine whether this CaMKK2-mediated effect occurred through its downstream targets, CaMKI or CaMKIV, we first evaluated if these substrates are expressed in 32D cells by immunoblot (Supplemental Fig. 4A). These results indicate that CaMKI, which has been reported to be ubiquitously expressed [10, 35], is present in 32D cells. However, CaMKIV was not detectable in these cells, which is consistent with previous findings that it is more tissue-restricted in its expression than is CaMKI [36, 37]. We next investigated whether inhibiting the function of CaMKI would be sufficient to relieve CaMKK2-mediated inhibition of granulocyte differentiation and performed G-CSF-dependent granulocyte differentiation of 32D-CaMKK2 cells in the presence of KN-93, a pharmacological inhibitor of CaMKs I, II, and IV [11, 12]. KN-93 did not abrogate CaMKK2-mediated repression of granulocyte differentiation, suggesting that neither CaMKI (nor IV or II) was functioning downstream of CaMKK2 (Supplemental Fig. 4B and Fig. 7B). As a positive control for CaM kinase inhibition of 32D cells, we confirmed that 2.5 μM KN-93 (which was the maximal dose that these cells could tolerate without cytotoxicity) inhibited the Ca2+-induced autophosphorylation of CaMKII, a CaMK not phosphorylated by CaMKK2 but inhibited by KN-93 with a similar inhibition constant to CaMKI and CaMKIV (Supplemental Fig. 4C and D) [36]. Altogether, these data indicate that CaMKK2 represses granulocyte differentiation of 32D cells in a CaMK-independent manner, although this function requires CaMKK2 activity as the kinase-inactive CaMKK2D311A mutant failed to impede granulopoiesis.
Figure 7. CaMKK2 overexpression in 32D cells is sufficient to repress granulocyte differentiation in a kinase-dependent manner but is independent of CaMKI, CaMKIV, and AMPK.
(A) Uninfected or 32D cells transduced with lentiviral empty vector-GFP control (Ctrl), CaMKK2 (KK2), or kinase-inactive CaMKK2D311A (D311A) were induced to differentiate with G-CSF and then Wright′s-stained. Arrowheads indicate cells with mature nuclear morphology. Images were taken at original magnification, ×630. (B) Quantification of Panel A and Supplemental Fig. 4B showing percentages of mature cells on Day 6 (n=3; ≥150 cells scored/sample; *P<0.01). (C) Undifferentiated cell lines from Panel A were analyzed by immunoblot for levels of pAMPKα, total AMPKα, CaMKK2, and β-actin. (D and E) Quantification of Panel C showing fold change of (D) pAMPK levels normalized by total AMPKα and (E) CaMKK2 levels normalized by β-actin in transduced cells relative to 32D-uninfected (n=3; *P<0.05). (F) Immunoblot analysis of pAMPKα and CaMKK2 in CaMKK2-knockdown (sh-KK2) 32D cells compared with 32D-uninfected and -control cells. (G) Quantification of Panel F showing fold change of pAMPK and CaMKK2 levels relative to 32D-uninfected, normalized as in Panels D and E (n=3; *P<0.001).
As KN-93 treatment of 32D-CaMKK2 cells did not rescue granulocyte differentiation and CaMKIV was undetectable in 32D cells (Supplemental Fig. 4A), these data suggest that CaMKI and CaMKIV are unlikely to be relevant downstream substrates of CaMKK2 during granulopoiesis. We next investigated whether AMPK, the third well-established substrate of CaMKK2 [38–40], was involved in blocking granulocyte development downstream of CaMKK2. To this end, we first evaluated p-AMPK in 32D-CaMKK2, 32D-D311A, and control cells and found no significant differences in pAMPK in any of the cell lines (Fig. 7C and D). In addition, we evaluated the amount of CaMKK2 in these cells and found it to be overexpressed 12- to 18-fold in 32D-CaMKK2 and -D311A lines relative to the control level (Fig. 7E). Next, we knocked down CaMKK2 by shRNA interference in 32D cells and also found pAMPK unchanged (Fig. 7F and G). To further examine whether p-AMPK is regulated by Ca2+-signaling in these cells, we treated 32D cells with ionomycin to increase intracellular calcium. This induced increase of intracellular Ca2+ in 32D cells did not change the levels of pAMPK (Supplemental Fig. 4E and F). Taken together, these results suggest that the pAMPK is unlikely to depend on CaMKK2 in 32D myeloid progenitor cells. Thus, although we cannot identify the substrate responsible, our collective data support the notion that CaMKK2 plays a cell-intrinsic role in the restriction of granulocyte differentiation in myeloid progenitor cells of homeostatic BM.
DISCUSSION
Granulocytes serve a critical function in the innate immune system by maintaining a frontline defense against bacterial and fungal pathogens. However, precise control of granulocyte number is important to provide an appropriate balance between effective immunity and immunosuppression or inflammation [41, 42]. In this study, we identify a role for CaMKK2 in the repression of granulocytic fate commitment and differentiation during early granulopoiesis. Furthermore, we also show that unlike CaMKIV, CaMKK2 is not an essential regulator of HSC homeostasis. These results, taken together with the myeloid defects shown here in Camkk2–/– mice, which are not present in Camk4–/– mice [6], suggest that the CaMKK2-CaMKIV signaling cascade is not functionally relevant in the biology of early hematopoietic cells. Rather, our findings demonstrate that CaMKK2 functions as a critical regulator of myeloid cell fate.
The absence of CaMKK2 in mice results in increased CMPs, decreased GMPs, and increased granulocytes in the BM under basal conditions. Similarly, when Camkk2–/– HSCs are transplanted into lethally irradiated recipient mice, an expansion of granulocytes occurs in the BM and peripheral blood. These results demonstrate that in basal and stressed conditions (i.e., BMT), the loss of CaMKK2 leads to an aberrant increase in granulopoiesis in vivo. Based on our results, we suggest that the accelerated differentiation of Camkk2–/– GMPs into granulocytes results in the reduction of the GMP population and concomitant increase in CMPs in the null mice, possibly as a feed-forward mechanism to compensate for a paracrine-detected deficiency of GMPs. Indeed, cytological examination of immature granulocytes (Gr1loMac1lo/+) from Camkk2–/– mice revealed robust, accelerated granulocyte differentiation compared with WT. Moreover, Camkk2–/– GMPs formed significantly more CFU-G in vitro. Altogether, these data support a cell autonomous role for CaMKK2 in restricting granulocytic commitment and differentiation during early granulopoiesis.
Consistent with these findings, our results also show that C/EBPα and PU.1 levels are significantly increased in Camkk2–/– CMPs. Studies have shown that under homeostatic conditions, C/EBPα is required for the transition from CMP to GMP [43], as its genetic ablation in neonatal mice resulted in the complete absence of neutrophils and eosinophils whereas other hematopoietic Lin, including monocytes were not affected [27], and during hematopoiesis, it is expressed predominantly in the granulocyte and monocyte Lin [44, 45]. Additionally, expression of low levels of PU.1 in PU.1 (−/−) myeloprogenitor cells induces granulopoiesis, whereas high levels can induce monopoiesis [46, 47]. Moreover, it has been well-documented that PU.1 physically interacts with and represses GATA-1, a transcription factor that promotes erythroid differentiation, thereby inhibiting the erythroid differentiation program during Lin commitment decisions in myeloerythroid cells [48–50]. In light of these findings, our data suggest that the depletion of CaMKK2 leads to altered fate commitment in progenitor cells as early as CMPs to favor differentiation of CMPs into granulocyte/monocyte precursor cells instead of megakaryocyte/erythrocyte Lin cells.
Our studies reveal that CaMKK2 is not expressed in terminally differentiated granulocytes, and CaMKK2 expression is progressively down-regulated during granulocytic differentiation. Conversely, when CaMKK2 is overexpressed in Camkk2−/− CMP cells or undifferentiated 32D myeloprogenitor cells, it is sufficient to block granulocyte differentiation, and, at least in 32D cells does so in a manner that requires its kinase activity. Together, these data support a role for CaMKK2 as a selective inhibitor of early granulopoiesis.
However, our results suggest that this effect may not be mediated by any of the three known targets of CaMKK2, i.e., CaMKIV, CaMKI, or AMPK. In the analysis of Camkk2−/− mice, we identified myeloid progenitor and granulopoiesis phenotypes that are not exhibited by Camk4−/− mice [6]. Moreover, CaMKIV was not detected in 32D cells, suggesting that it may not be expressed in myeloid progenitor cells. Although a specific isoform of CaMKI, CKLiK, has been implicated previously in late neutrophil maturation and function [51–53], it does not seem a likely target of CaMKK2 in early granulopoiesis, as our CaMK-inhibition studies using KN-93 showed that KN-93 treatment of CaMKK2-overexpressing cells did not abrogate the CaMKK2-mediated inhibition of granulocyte differentiation. These results suggest that neither CaMKI nor CaMKIV is the potential effector substrate of CaMKK2 in the signaling pathway that represses granulocyte differentiation.
Although AMPK can also be phosphorylated by CaMKK2, reduction of CaMKK2 expression with shRNA and overexpression of CaMKK2 did not alter the phosphorylation status of AMPK. We believe that this could be for a number of reasons. In other studies, we have found that there is relatively little CaMKK2 expressed in a number of non-neuronal cell types compared with the amount of AMPK present, and CaMKK2 binds to and interacts with only a small pool of the AMPK in the cell. Furthermore, several other kinases, in addition to CaMKK2, function as AMPK kinases, including the liver kinase B1, TGF-β-activated kinase 1, and Ataxia telangiectasia mutated [54]. Thus, given these factors and the appreciation that many pathways involve and cross-regulate AMPK, it is not surprising that alteration of CaMKK2 expression in myeloid progenitor fails to affect the level of pAMPK. Collectively, these results suggest that CaMKK2 may regulate early granulopoiesis through yet-to-be-identified targets. We also speculate that following the down-regulation of CaMKK2 in developing granulocytes, CaMKK1-CKLiK signaling may become important in modulating late-stage neutrophil development and effector functions, given that we detected CaMKK1 expression in mature BM granulocytes (Fig. 5D), a finding that confirms a previous study [51]. Taken together, these observations suggest an important role for the CaMKK cascade members to regulate appropriate granulocyte production and function throughout granulopoiesis.
In humans, granulocyte production can be impaired by immunosuppressive chemotherapy or severe congenital disorders [55]. The lack of neutrophils, or neutropenia, can cause extreme susceptibility to life-threatening infections. Hence, G-CSF, which stimulates granulocyte production, has become a primary therapeutic agent to treat neutropenia. However, in some cases of severe congenital neutropenia, individuals possess a mutant G-CSFR allele leading to neutrophil maturation arrest at the promyelocyte/myelocyte stage of development, a correlated onset of high-risk myelodysplastic syndrome and AML, and reduced responsiveness to G-CSF therapy [55, 56]. Our results may be relevant to such conditions, as a potentially useful treatment option could be the combined therapy of G-CSF with a specific CaMKK2 inhibitor to serve as a more effective, postreceptor means of stimulating neutrophil production in these patients. Moreover, the specific inhibition of CaMKK2 would not interfere with the biological function of fully mature neutrophils, as one would predict to occur with the use of KN-93. The combination treatment might also decrease the risks of purported G-CSF-induced leukemia [55], as smaller doses of G-CSF could be used, and cell differentiation would be accelerated.
Finally, the combination of G-CSF and a CaMKK2 inhibitor might also be useful for the treatment of AML, in which the blocked differentiation of myeloid precursors is a hallmark of the disease. Interestingly, recent studies suggest CaMKK2 to be a potentially relevant target in prostate cancer cells, where CaMKK2 is expressed in an androgen-dependent manner and is required for invasiveness [57]. Thus, further research is warranted to ascertain the role of CaMKK2 in hematologic malignancies, as its deregulated activity in myeloprogenitor cells may conceivably promote a maturation defect in this population, leading to increased risk of additional genetic mutations and leukemic transformation.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by NIH R01 Grant DK-074701 to A.R.M. We thank Dr. Jeffrey Rathmell and Dr. Uma Sankar for reviewing this manuscript and providing helpful feedback. We are grateful to Dr. Akiko Watanabe for advice and assistance with isolation of HSC and progenitor subsets and Dr. Motonari Kondo for advice, as well as providing the OP9 cell line. We thank Dr. Tannishtha Reya and members of her laboratory for helpful discussions and particularly, Dr. William Lento for assistance with BMT studies. We also thank Dr. Michael Cook, Dr. Beth Harvat, and Lynn Martinek for assistance with flow cytometry. We appreciate members of the Means laboratory for reagents, advice, and discussions and in particular, thank Thomas Ribar and Brian Teng for assistance with BMT studies and Pamela Noeldner and Dr. Kristin Anderson for generation of the Camkk1–/– mice.
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
- 32D
- 32D Clone 3
- AMPK
- AMP-activated protein kinase
- APC
- allophycocyanin
- BM
- bone marrow
- BMT
- bone marrow transplantation
- CFU-M/G
- monocyte/macrophage/granulocyte CFU
- CKLiK
- CaMKI-like kinase
- CLP
- common lymphoid progenitor
- CMP
- common myeloid progenitor
- GMP
- granulocyte-monocyte progenitor
- HSC
- hematopoietic stem cell
- KLS
- c-Kit+Sca1+Lin–/lo
- Lin
- lineage
- MEP
- megakaryocyte-erythroid progenitor
- MPP
- multipotent progenitor
- p
- phospho
- qRT-PCR
- quantitative RT-PCR
- rm
- recombinant mouse
- Sca-1
- stem cell antigen 1
- SCF
- stem cell factor
- shRNA
- small hairpin RNA
AUTHORSHIP
E.C.T. designed and performed research, analyzed and interpreted data, and wrote the paper. L.R. performed research, interpreted data, and edited the manuscript. A.R.M. participated in the analysis and interpretation of all data and edited the manuscript.
REFERENCES
- 1. Metcalf D. (2007) Concise review: hematopoietic stem cells and tissue stem cells: current concepts and unanswered questions. Stem Cells 25, 2390–2395 [DOI] [PubMed] [Google Scholar]
- 2. Till J. E., McCulloch E. A. (1961) A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat. Res. 14, 213–222 [PubMed] [Google Scholar]
- 3. Adams G. B., Chabner K. T., Alley I. R., Olson D. P., Szczepiorkowski Z. M., Poznansky M. C., Kos C. H., Pollak M. R., Brown E. M., Scadden D. T. (2006) Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature 439, 599–603 [DOI] [PubMed] [Google Scholar]
- 4. Katayama N., Nishikawa M., Komada F., Minami N., Shirakawa S. (1990) A role for calmodulin in the growth of human hematopoietic progenitor cells. Blood 75, 1446–1454 [PubMed] [Google Scholar]
- 5. Ueda S., Mizuki M., Ikeda H., Tsujimura T., Matsumura I., Nakano K., Daino H., Honda Z., Sonoyama J., Shibayama H. (2002) Critical roles of c-Kit tyrosine residues 567 and 719 in stem cell factor-induced chemotaxis: contribution of src family kinase and PI3-kinase on calcium mobilization and cell migration. Blood 99, 3342–3349 [DOI] [PubMed] [Google Scholar]
- 6. Kitsos C. M., Sankar U., Illario M., Colomer-Font J., Duncan A., Ribar T., Reya T., Means A. (2005) Calmodulin-dependent protein kinase IV regulates hematopoietic stem cell maintenance. J. Biol. Chem. 280, 33101–33108 [DOI] [PubMed] [Google Scholar]
- 7. Anderson K. A., Means A. R. (2002) Defective signaling in a subpopulation of CD4+ T cells in the absence of Ca2+/calmodulin-dependent protein kinase IV. Mol. Cell. Biol. 22, 23–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Anderson K. A., Ribar T. J., Illario M., Means A. R. (1997) Defective survival and activation of thymocytes in transgenic mice expressing a catalytically inactive form of Ca2+/calmodulin-dependent protein kinase IV. Mol. Endocrinol. 11, 725–737 [DOI] [PubMed] [Google Scholar]
- 9. Illario M., Giardino-Torchia M. L., Sankar U., Ribar T. J., Galgani M., Vitiello L., Masci A. M., Bertani F. R., Ciaglia E., Astone D. (2008) Calmodulin-dependent kinase IV links Toll-like receptor 4 signaling with survival pathway of activated dendritic cells. Blood 111, 723–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chow F. A., Means A. R. (2007) The calcium/calmodulin-dependent protein kinase cascades. In Calcium: A Matter of Life or Death (Krebs J., Michalak M., eds.), Vol. 41, Elsevier, Amsterdam, The Netherlands, 345–364 [Google Scholar]
- 11. Colomer-Font J., Means A. R. (2007) Physiological roles of the Ca2+/CaM-dependent protein kinase cascade in health and disease. In Calcium Signaling and Disease (Carafoli E., Brini M., eds.), Vol. 45, Springer Science, Dordrecht, The Netherlands, 169–214 [DOI] [PubMed] [Google Scholar]
- 12. Anderson K. A., Means R. L., Huang Q. H., Kemp B. E., Goldstein E. G., Selbert M. A., Edelman A. M., Fremeau R. T., Means A. R. (1998) Components of a calmodulin-dependent protein kinase cascade. J. Biol. Chem. 273, 31880–31889 [DOI] [PubMed] [Google Scholar]
- 13. Means A. R. (2008) Commentary: the year in basic science: calmodulin kinase cascades. Mol. Endocrinol. 22, 2759–2765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kokubo M., Nishio M., Ribar T. J., Anderson K. A., West A. E., Means A. R. (2009) BDNF-mediated cerebellar granule cell development is impaired in mice null for CaMKK2 or CaMKIV. J. Neurosci. 29, 8901–8913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Anderson K. A., Ribar T. J., Lin F., Noeldner P. K., Green M. F., Muehlbauer M. J., Witters L. A., Kemp B. E., Means A. R. (2008) Hypothalamic CaMKK2 contributes to the regulation of energy balance. Cell Metab. 7, 377–388 [DOI] [PubMed] [Google Scholar]
- 16. Christensen J. L., Weissman I. L. (2001) Flk-2 is a marker in hematopoietic stem cell differentiation: a simple method to isolate long-term stem cells. Proc. Natl. Acad. Sci. USA 98, 14541–14546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Osawa M., Hanada K., Hamada H., Nakauchi H. (1996) Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science 273, 242–245 [DOI] [PubMed] [Google Scholar]
- 18. Akashi K., Traver D., Miyamoto T., Weissman I. L. (2000) A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature 404, 193–197 [DOI] [PubMed] [Google Scholar]
- 19. Kondo M., Weissman I. L., Akashi K. (1997) Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell 91, 661–672 [DOI] [PubMed] [Google Scholar]
- 20. Jankovic V., Ciarrocchi A., Boccuni P., DeBlasio T., Benezra R., Nimer S. D. (2007) Id1 restrains myeloid commitment, maintaining the self-renewal capacity of hematopoietic stem cells. Proc. Natl. Acad. Sci. USA 104, 1260–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Duncan A. W., Rattis F. M., DiMascio L. N., Congdon K. L., Pazianos G., Zhao C., Yoon K., Cook J. M., Willert K., Gaiano N., Reya T. (2005) Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance. Nat. Immunol. 6, 314–322 [DOI] [PubMed] [Google Scholar]
- 22. Zhou L., Li L., Yan Q., Petryniak B., Man Y., Su C., Shim J., Chervin S., Lowe J. (2008) Notch-dependent control of myelopoiesis is regulated by fucosylation. Blood 112, 308–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang L., Bryder D., Adolfsson J., Nygren J., Månsson R., Sigvardsson M., Jacobsen S. E. W. (2005) Identification of Lin-Sca1+ kit+ CD34+ Flt3– short-term hematopoietic stem cells capable of rapidly reconstituting and rescuing myeloablated transplant recipients. Blood 105, 2717–2723 [DOI] [PubMed] [Google Scholar]
- 24. Kastner P., Lawrence H., Waltzinger C., Ghyselinck N., Chambon P., Chan S. (2001) Positive and negative regulation of granulopoiesis by endogenous RARα. Blood 97, 1314–1320 [DOI] [PubMed] [Google Scholar]
- 25. Hestdal K., Ruscetti F., Ihle J., Jacobsen S., Dubois C., Kopp W., Longo D., Keller J. (1991) Characterization and regulation of RB6–8C5 antigen expression on murine bone marrow cells. J. Immunol. 147, 22–28 [PubMed] [Google Scholar]
- 26. Wang X., Scott E., Sawyers C. L., Friedman A. D. (1999) C/EBPα bypasses granulocyte colony-stimulating factor signals to rapidly induce PU. 1 gene expression, stimulate granulocytic differentiation, and limit proliferation in 32D cl3 myeloblasts. Blood 94, 560–571 [PubMed] [Google Scholar]
- 27. Zhang D. E., Zhang P., Wang N., Hetherington C. J., Darlington G. J., Tenen D. G. (1997) Absence of granulocyte colony-stimulating factor signaling and neutrophil development in CCAAT enhancer binding protein-deficient mice. Proc. Natl. Acad. Sci. USA 94, 569–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. DeKoter R. P., Walsh J. C., Singh H. (1998) PU. 1 regulates both cytokine-dependent proliferation and differentiation of granulocyte/macrophage progenitors. EMBO J. 17, 4456–4468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Scott E. W., Simon M., Anastasi J., Singh H. (1994) Requirement of transcription factor PU.1 in the development of multiple hematopoietic lineages. Science 265, 1573–1577 [DOI] [PubMed] [Google Scholar]
- 30. Urbánek P., Wang Z. Q., Fetka I., Wagner E. F., Busslinger M. (1994) Complete block of early B cell differentiation and altered patterning of the posterior midbrain in mice lacking Pax5/BSAP. Cell 79, 901–912 [DOI] [PubMed] [Google Scholar]
- 31. O′Riordan M., Grosschedl R. (1999) Coordinate regulation of B cell differentiation by the transcription factors EBF and E2A. Immunity 11, 21–31 [DOI] [PubMed] [Google Scholar]
- 32. Nakajima H., Ihle J. N. (2001) Granulocyte colony-stimulating factor regulates myeloid differentiation through CCAAT/enhancer-binding protein ε. Blood 98, 897–905 [DOI] [PubMed] [Google Scholar]
- 33. Valtieri M., Tweardy D., Caracciolo D., Johnson K., Mavilio F., Altmann S., Santoli D., Rovera G. (1987) Cytokine-dependent granulocytic differentiation. Regulation of proliferative and differentiative responses in a murine progenitor cell line. J. Immunol. 138, 3829–3835 [PubMed] [Google Scholar]
- 34. Bainton D. F., Ullyot J. L., Farquhar M. G. (1971) The development of neutrophilic polymorphonuclear leukocytes in human bone marrow. J. Exp. Med. 134, 907–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hook S. S., Means A. R. (2001) Ca(2+)/CaM-dependent kinases: from activation to function. Annu. Rev. Pharmacol. Toxicol. 41, 471–505 [DOI] [PubMed] [Google Scholar]
- 36. Means A. R. (2000) Regulatory cascades involving calmodulin-dependent protein kinases. Mol. Endocrinol. 14, 4–13 [DOI] [PubMed] [Google Scholar]
- 37. Means A. R., Ribar T. J., Kane C. D., Hook S. S., Anderson K. A. (1997) Regulation and properties of the rat Ca2+/calmodulin-dependent protein kinase IV gene and its protein products. Recent Prog. Horm. Res. 52, 389–406 [PubMed] [Google Scholar]
- 38. Hurley R. L., Anderson K. A., Franzone J. M., Kemp B. E., Means A. R., Witters L. A. (2005) The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J. Biol. Chem. 280, 29060–29066 [DOI] [PubMed] [Google Scholar]
- 39. Witters L. A., Kemp B. E., Means A. R. (2006) Chutes and ladders: the search for protein kinases that act on AMPK. Trends Biochem. Sci. 31, 13–16 [DOI] [PubMed] [Google Scholar]
- 40. Hawley S. A., Pan D. A., Mustard K. J., Ross L., Bain J., Edelman A. M., Frenguelli B. G., Hardie D. G. (2005) Calmodulin-dependent protein kinase kinase-β is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2, 9–19 [DOI] [PubMed] [Google Scholar]
- 41. Boxer L., Dale D. (2002) Neutropenia: causes and consequences. Semin. Hematol. 39, 75–81 [DOI] [PubMed] [Google Scholar]
- 42. Nakamura H., Ueki Y., Sakito S., Matsumoto K., Yano M., Miyake S., Tominaga T., Tominaga M., Eguchi K. (2000) High serum and synovial fluid granulocyte colony stimulating factor (G-CSF) concentrations in patients with rheumatoid arthritis. Clin. Exp. Rheumatol. 18, 713–718 [PubMed] [Google Scholar]
- 43. Zhang P., Iwasaki-Arai J., Iwasaki H., Fenyus M. L., Dayaram T., Owens B. M., Shigematsu H., Levantini E., Huettner C. S., Lekstrom-Himes J. A. (2004) Enhancement of hematopoietic stem cell repopulating capacity and self-renewal in the absence of the transcription factor C/EBPα. Immunity 21, 853–863 [DOI] [PubMed] [Google Scholar]
- 44. Radomska H. S., Huettner C. S., Zhang P., Cheng T., Scadden D. T., Tenen D. G. (1998) CCAAT/enhancer binding protein α is a regulatory switch sufficient for induction of granulocytic development from bipotential myeloid progenitors. Mol. Cell. Biol. 18, 4301–4314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Scott L. M., Civin C. I., Rorth P., Friedman A. D. (1992) A novel temporal expression pattern of three C/EBP family members in differentiating myelomonocytic cells. Blood 80, 1725–1735 [PubMed] [Google Scholar]
- 46. Dahl R., Walsh J. C., Lancki D., Laslo P., Iyer S. R., Singh H., Simon M. C. (2003) Regulation of macrophage and neutrophil cell fates by the PU.1:C/EBPα ratio and granulocyte colony-stimulating factor. Nat. Immunol. 4, 1029–1036 [DOI] [PubMed] [Google Scholar]
- 47. Laslo P., Spooner C. J., Warmflash A., Lancki D. W., Lee H. J., Sciammas R., Gantner B. N., Dinner A. R., Singh H. (2006) Multilineage transcriptional priming and determination of alternate hematopoietic cell fates. Cell 126, 755–766 [DOI] [PubMed] [Google Scholar]
- 48. Rhodes J., Hagen A., Hsu K., Deng M., Liu T. X., Look A. T., Kanki J. P. (2005) Interplay of PU. 1 and Gata1 determines myelo-erythroid progenitor cell fate in zebrafish. Dev. Cell 8, 97–108 [DOI] [PubMed] [Google Scholar]
- 49. Stopka T., Amanatullah D. F., Papetti M., Skoultchi A. I. (2005) PU. 1 inhibits the erythroid program by binding to GATA-1 on DNA and creating a repressive chromatin structure. EMBO J. 24, 3712–3723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhang P., Zhang X., Iwama A., Yu C., Smith K. A., Mueller B. U., Narravula S., Torbett B. E., Orkin S. H., Tenen D. G. (2000) PU. 1 inhibits GATA-1 function and erythroid differentiation by blocking GATA-1 DNA binding. Blood 96, 2641–2648 [PubMed] [Google Scholar]
- 51. Gaines P., Lamoureux J., Marisetty A., Chi J., Berliner N. (2008) A cascade of Ca2+/calmodulin-dependent protein kinases regulates the differentiation and functional activation of murine neutrophils. Exp. Hematol. 36, 832–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Verploegen S., Lammers J. W. J., Koenderman L., Coffer P. J. (2000) Identification and characterization of CKLiK, a novel granulocyte Ca++/calmodulin-dependent kinase. Blood 96, 3215–3223 [PubMed] [Google Scholar]
- 53. Verploegen S., Ulfman L., van Deutekom H. W. M., van Aalst C., Honing H., Lammers J. W. J., Koenderman L., Coffer P. J. (2005) Characterization of the role of CaMKI-like kinase (CKLiK) in human granulocyte function. Blood 106, 1076–1083 [DOI] [PubMed] [Google Scholar]
- 54. Jensen T. E., Wojtaszewski J., Richter E. (2009) AMP activated protein kinase in contraction regulation of skeletal muscle metabolism: necessary and/or sufficient? Acta Physiol. (Oxf.) 196, 155–174 [DOI] [PubMed] [Google Scholar]
- 55. Panopoulos A. D., Watowich S. S. (2008) Granulocyte colony-stimulating factor: molecular mechanisms of action during steady state and ″emergency″ hematopoiesis. Cytokine 42, 277–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Germeshausen M., Ballmaier M., Welte K. (2007) Incidence of CSF3R mutations in severe congenital neutropenia and relevance for leukemogenesis: results of a long-term survey. Blood 109, 93–99 [DOI] [PubMed] [Google Scholar]
- 57. Frigo D. E., Howe M. K., Wittmann B. M., Brunner A. M., Cushman I., Wang Q., Brown M., Means A. R., McDonnell D. P. (2011) CaM kinase kinase-mediated activation of the growth regulatory kinase AMPK is required for androgen-dependent migration of prostate cancer cells. Cancer Res. 71, 528–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.