Abstract
Discussion on report that SOCS levels may play an important role in determining macrophage phenotype and function.
Keywords: transcription factors, cytokines, inflammation, PI3K, alternative activation, SOCS
In the early 1990s, Siamon Gordon [1] and colleagues demonstrated that exposure of macrophages to the (TH2) cytokine IL-4 gave rise to a population of cells that was functionally and biochemically distinct from classically activated macrophages. These so-called “alternatively” activated macrophages play important roles in the clearance of helminthic parasites [2]. They also synthesize arginase, an enzyme that inhibits NO production and allows them to produce ornithine, a precursor of hydroxyproline and polyamines. Therefore, these macrophages have been implicated in the wound-healing response [3]. This is in contrast to classically activated macrophages, which do not rapidly induce arginase and therefore, convert arginine into NO, which they use to kill intracellular pathogens.
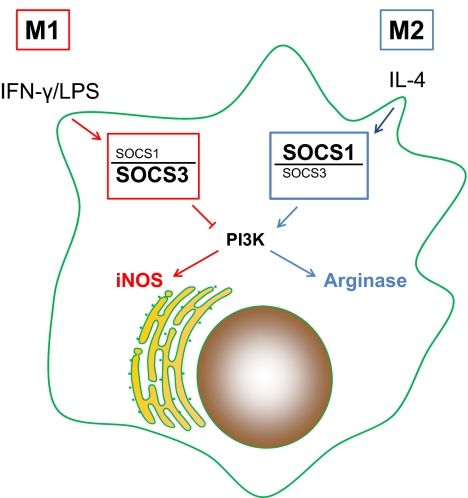
In this issue of the Journal of Leukocyte Biology, Whyte et al. [4] examined SOCS expression in alternatively activated M2 macrophages. The first observation made by these authors was that SOCS1 but not SOCS3 was induced rapidly in macrophages in response to IL-4 treatment in vitro. This observation is consistent with previous observations made by Dickensheets and colleagues [5]. A similar induction occurred in peritoneal macrophages following i.p. infection of mice with the parasitic nematode Brugia malayi. These observations prompted the authors to examine the role of SOCS1 in M2 macrophages. When SOCS1 was knocked down, IL-4 treatment of macrophages no longer resulted in a robust induction of arginase expression. This observation was surprising, as it contrasted with previous work by others [5], demonstrating that SOCS1 knockout mice produced more arginase than their WT counterparts. Instead, the authors observed that SOCS1 knockdown resulted in the induction of iNOS in IL-4-pretreated cells that were stimulated with IFN/LPS. The authors also examined SOCS3 induction in alternatively activated M2 macrophages and showed that SOCS3 levels were not induced in these cells. This led to another key observation: that the ratio of SOCS1:SOCS3 is high in M2 macrophages and lower in classically activated macrophages (Fig. 1). The ratio of SOCS1:SOCS3 may therefore represent another way to identify M2 macrophages. This observation would be particularly useful if it can be extended to human macrophages, as some of the reliable biochemical markers of murine M2 macrophages do not exist in human macrophages.
Figure 1. In M2 macrophages, IL-4 induces an up-regulation of SOCS1 and an induction of arginase activity.
The high ratio of SOCS1:SOCS3 may be exploited as another way to identify M2 macrophages. M1 macrophages fail to induce SOCS1, leading to higher levels of SOCS3 relative to SOCS1 and iNOS induction. How the relative levels of these two SOCS proteins influence NO production in M1 macrophages has not been thoroughly studied, but the involvement of PI3K in determining macrophage phenotype and function has been suggested by this and other work.
The SOCS proteins are a family of proteins that inhibit cytokine signal transduction. The SOCS family proteins contain an Src homology 2 domain and a conserved carboxyterminal SOCS box motif. Macrophages from SOCS1-deficient mice produce increased levels of TNF, IL-12, IFN-γ, and NO in response to TLR ligands [6]. These proteins can inhibit cellular signaling, perhaps by functioning as E3 ubiquitin ligases to target receptors and signaling molecules for proteasomal degradation [7]. Some of the SOCS family members, such as SOCS1 and -3, can also directly inhibit JAK activity to suppress cytokine signaling. SOCS1 binds to IFNRs and modifies signaling through these and other receptors, whereas SOCS3 binds directly to gp130 and thereby, inhibits signaling through the family of gp130-containing receptors [8]. Resting macrophages express low-to-undetectable levels of SOCS1, but expression can be induced rapidly following macrophage stimulation.
In the mouse, reliable biochemical markers for alternatively activated M2 macrophages have been developed. These cells express Relm-α and Ym1, and they up-regulate their expression of the mannose receptor. All three of these markers can be used to identify these macrophages in tissue. Another important observation made by the authors was that these prototypical “markers” of M2 activation were not affected by SOCS1. This suggests that SOCS1 was not involved in the “decision” to become an M2 macrophage but rather, that this molecule was specifically involved in the induction of arginase in M2 macrophages. The molecular biology of arginase 1 regulation in the various macrophage subsets has not been studied exhaustively. A composite STAT6 C/EBPβ element has been identified ∼3 kb upstream of the transcription start site, and this site binds several factors following IL-4 stimulation [9]. This could explain the robust induction of arginase following IL-4 treatment of macrophages, which others have described previously. How knocking down SOCS1 levels in macrophages can lead to a decrease in arginase transcripts (see Fig. 2 in ref. [4]) was not formally determined. However, the authors do demonstrate that IL-4 activates PI3K, and knocking down SOCS1 resulted in a decrease in PI3K activity, resulting in less phosphorylated AKT, the kinase downstream of PI3K. It has been shown previously that PI3K is elevated in alternatively activated macrophages and that blocking PI3K can inhibit the M2 phenotype [10], so the induction of SOCS1 may be a mechanism for regulating PI3K activity in these cells.
Another interesting observation made in this work was the up-regulation of IL-10 in M1 macrophages following SOCS1 knockdown. The mechanism, whereby a decrease in SOCS1 would result in an up-regulation of IL-10, is not evident. The authors refer to the SOCS1 overexpression studies of others, where there is some controversy about whether SOCS1 directly affects NF-κB. One of us demonstrated previously [11] that unlike most of the inflammatory cytokines, whose transcription is induced by the prototypical NF-κB heterodimers (p65/p50), IL-10 transcription is only induced by p50 homodimers. It would therefore be interesting to know whether SOCS1 exerts a specific inhibition on selected Rel family members and not on others.
It is challenging to correlate the findings of this paper to allergic diseases, where SOCS1 appears to be a strong negative regulator of IL-4- or IL-13-dependent pathways. Indeed, mice lacking SOCS1 produce more IL-4 and IL-13 in a variety of allergy models [12], and there is clear evidence that SOCS1 inhibits IL-4 signaling in T cells [6]. This, however, does not appear to be the case in macrophages, as the authors show that IL-4 induces PI3K activity in macrophages, and knocking down SOCS1 actually did the opposite of what was expected and decreased PI3K activity (See Fig. 4 in ref. [4]). How these apparently disparate responses to SOCS1 are balanced in vivo is currently unknown, but they suggest that SOCS can exert unique influences on macrophages that are not observed in T cells.
The expression of arginase in the various macrophage subpopulations has been somewhat controversial. Several groups have used arginase as a marker for alternatively activated macrophages. However, TLR-stimulated macrophages can also express arginase [13], albeit via different signaling pathways and often with different kinetics. Therefore, caution should be exercised when using arginase as a marker for alternative activation of macrophages. As most of the effects observed in this paper focus on arginase, it may be an over-interpretation to deduce that SOCS1 is involved in controlling M1/M2 differentiation. Rather, the more conservative interpretation of this work is that as the authors state, “SOCS1 … can serve as a feedback inhibitor of inflammation.” The lack of alterations in Ym1 and Relm-α following SOCS1 knockdown suggests that the SOCS proteins are not able to “program different states of macrophage activation”, but rather, they specifically affect only a limited number of cellular responses in these cells. This is in contrast to previous observations that SHIP-deficient mice exhibited several characteristics associated with alternatively activated macrophages [10].
Finally, the authors conclude that “SOCS1 regulates the iNOS:arginase balance in both M1 and M2 macrophages.” Another interpretation of these data may be that PI3K is the regulator of arginase induction in M2 macrophages and that SOCS1 and SOCS3 merely serve to regulate PI3K activity. It has been reported previously that SOCS3 can inhibit PI3K, and so, it may simply be that the expression of high SOCS1 and low SOCS3 in M2 macrophages allows for more PI3K activity and more arginase induction in M2 macrophages. A possible test of this hypothesis would have been to use inhibitors that manipulate PI3K without affecting SOCS and vice versa. Regardless of which signaling molecules are downstream, the data in this paper clearly demonstrate that SOCS levels play an important role in determining macrophage phenotype and function.
ACKNOWLEDGMENTS
This work was supported in part by NIH grants AI49383 (D.M.M.) and AI072584 (V.B.).
Footnotes
SEE CORRESPONDING ARTICLE ON PAGE 845
REFERENCES
- 1. Stein M., Keshav S., Harris N., Gordon S. (1992) Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J. Exp. Med. 176, 287–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hesse M., Modolell M., La Flamme A. C., Schito M., Fuentes J. M., Cheever A. W., Pearce E. J., Wynn T. A. (2001) Differential regulation of nitric oxide synthase-2 and arginase-1 by type 1/type 2 cytokines in vivo: granulomatous pathology is shaped by the pattern of L-arginine metabolism. J. Immunol. 167, 6533–6544 [DOI] [PubMed] [Google Scholar]
- 3. Loke P., Gallagher I., Nair M. G., Zang X., Brombacher F., Mohrs M., Allison J. P., Allen J. E. (2007) Alternative activation is an innate response to injury that requires CD4+ T cells to be sustained during chronic infection. J. Immunol. 179, 3926–3936 [DOI] [PubMed] [Google Scholar]
- 4. Whyte C. S., Bishop E. T., Ruckerl D., Gaspar-Pereira S., Barker R. N., Allen J. E., Rees A. J., Wilson H. M. (2011) J. Leukoc. Biol. 90, 845–854 [DOI] [PubMed] [Google Scholar]
- 5. Dickensheets H., Vazquez N., Sheikh F., Gingras S., Murray P. J., Ryan J. J., Donnelly R. P. (2007) Suppressor of cytokine signaling-1 is an IL-4-inducible gene in macrophages and feedback inhibits IL-4 signaling. Genes Immun. 8, 21–27 [DOI] [PubMed] [Google Scholar]
- 6. Yoshimura A., Naka T., Kubo M. (2007) SOCS proteins, cytokine signaling and immune regulation. Nat. Rev. Immunol. 7, 454–465 [DOI] [PubMed] [Google Scholar]
- 7. Piessevaux J., Lavens D., Peelman F., Tavernier J. (2008) The many faces of the SOCS box. Cytokine Growth Factor Rev. 19, 371–381 [DOI] [PubMed] [Google Scholar]
- 8. Lang R., Pauleau A. L., Parganas E., Takahashi Y., Mages J., Ihle J. N., Rutschman R., Murray P. J. (2003) SOCS3 regulates the plasticity of gp130 signaling. Nat. Immunol. 4, 546–550 [DOI] [PubMed] [Google Scholar]
- 9. Pauleau A. L., Rutschman R., Lang R., Pernis A., Watowich S. S., Murray P. J. (2004) Enhancer-mediated control of macrophage-specific arginase I expression. J. Immunol. 172, 7565–7573 [DOI] [PubMed] [Google Scholar]
- 10. Sly L. M., Ho V., Antignano F., Ruschmann J., Hamilton M., Lam V., Rauh M. J., Krystal G. (2007) The role of SHIP in macrophages. Front. Biosci. 12, 2836–2848 [DOI] [PubMed] [Google Scholar]
- 11. Cao S., Zhang X., Edwards J. P., Mosser D. M. (2006) NF-κB1 (p50) homodimers differentially regulate pro- and anti-inflammatory cytokines in macrophages. J. Biol. Chem. 281, 26041–26050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee C., Kolesnik T. B., Caminschi I., Chakravorty A., Carter W., Alexander W. S., Jones J., Anderson G. P., Nicholson S. E. (2009) Suppressor of cytokine signaling 1 (SOCS1) is a physiological regulator of the asthma response. Clin. Exp. Allergy 39, 897–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. El Kasmi K. C., Qualls J. E., Pesce J. T., Smith A. M., Thompson R. W., Henao-Tamayo M., Basaraba R. J., Konig T., Schleicher U., Koo M. S., Kaplan G., Fitzgerald K. A., Tuomanen E. I., Orme I. M., Kanneganti T. D., Bogdan C., Wynn T. A., Murray P. J. (2008) Toll-like receptor-induced arginase 1 in macrophages thwarts effective immunity against intracellular pathogens. Nat. Immunol. 9, 1399–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]