Deletion of cannabinoid receptors 1 and 2 causes increased sensitivity to immune activation in APC, thereby exacerbating inflammation and cellular immune responses to influenza infection.
Keywords: endocannabinoids, immune regulation, immune homeostasis, host response, pathology, lung
Abstract
We and others have reported that simultaneous targeted deletion of CB1 and CB2 resulted in exacerbation of immune reactivity, suggesting a role of endocannabinoids in down-regulating immune function. In this study, we demonstrate that APC function is enhanced specifically in the absence of CB1 and CB2 signaling, resulting in an exacerbated immune response phenotype. After influenza infection, CB1−/−CB2−/− mice showed more pronounced pulmonary damage, increased inflammatory cell infiltrate, inflammation, and a greater cellular immune responses compared with WT mice, as evidenced by transcriptome analysis, more robust T cell activation, and effector cell cytokine production. After direct activation in vitro, there were no differences in the percentages of cytokine-producing CD4+ T cells between CB1−/−CB2−/− and WT mice. However, untreated CB1−/−CB2−/− mice routinely had fewer naïve T cells compared with WT, suggesting dysregulation of APC immune homeostasis. Moreover, bmDCs and AM isolated from CB1−/−CB2−/− mice exhibited a more mature phenotype, with and without TLR stimulation, and bmDCs elicited T cells more robustly than WT mice. Collectively, these findings implicate a role for CB1 and CB2 on APCs in regulating immune responses and immune homeostasis.
Introduction
The influenza-induced immune response is strictly regulated to ensure effective clearance while minimizing damage to the host [1–3]. Upon influenza infection, resident immune cells, such as AM and DCs, as well as epithelial cells, trigger a proinflammatory response induced by PAMPs to initiate recruitment of neutrophils, macrophages, DCs, and lymphoid cells into the lungs [4].Signaling by PAMP receptors, such as TLRs, and the proinflammatory environment created by innate immune response allows for the maturation of APCs, most importantly DCs, which orchestrate the elicitation of T cell-mediated adaptive immunity [5]. Ultimately, viral clearance depends on the presence of T cells [6] and correlates with the production of cytokines, including IL-17 and IFN-γ, by CD4+ and CD8+ T cells and NK cells. Although IL-17 promotes proinflammatory responses [7–9], IFN-γ exerts potent antiviral activity [4, 10], with both cytokines contributing to the immunopathology associated with influenza infection [11–13].
In light of the potentially deleterious consequences of uncontrolled immune stimulation, maintenance of immune homeostasis is critical in environments continuously exposed to antigens, such as the lung, and involves multiple pathways to minimize damage to the airways [1]. Toward this end, a subclass of arachidonic acid derivatives, termed endocannabinoids, have been implicated as a novel family of immunological mediators [14]. The two identified receptors for endocannabinoids, CB1 and CB2, are ubiquitously expressed by leukocytes, albeit at varying levels [15, 16], and ligation of these receptors by endocannabinoids alters immune responsiveness [17]. Previously, CB1−/−CB2−/− mice were found to exhibit greater sensitivity to induction of type IV hypersensitivity to contact sensitizers [18] and augmented host resistance to influenza challenge [19] through yet-to-be-elucidated mechanisms. Immune responses in both models evoke an overlapping profile of cytokines, which can include IL-17 and IFN-γ [6, 11, 20, 21]. An obvious commonality between contact hypersensitivity reactions and the immune response to influenza is that both are T cell-mediated and thus, require APCs to elicit a T cell response. Despite the hyper-reactivity observed in CB1−/−CB2−/− mice, the cellular entities affected and responsible for the phenotype remained to be understood. In this study, we used a murine-adapted influenza virus as an immunological stimulus to identify the cells responsible for the immune exacerbation in CB1−/−CB2−/− in comparison with WT mice. We present novel insights into the regulation of APC responses by which the endocannabinoid system contributes to the maintenance of immunological balance.
MATERIALS AND METHODS
Mice
Mice were housed at MSU (East Lansing, MI, USA) in pathogen-free animal housing facilities at 21–24°C and 40–60% relative humidity with a 12-h light/dark cycle. Water and lab chow (Nestlé Purina, St. Louis, MO, USA) were provided ad libitum. All protocols and procedures were performed in accordance with guidelines set forth by the MSU Animal Care and Use Committee. CB1−/−CB2−/− mice on a C57Bl/6 genetic background were a kind gift of Dr. Andreas Zimmer (Universität Bonn, Germany) [18] and were maintained at MSU, and C57Bl/6 (WT) mice were ordered from the NCI (Bethesda, MD, USA). Mice were used for experiments between 8 and 12 weeks of age. CB1−/−CB2−/− mice were genotyped to ensure the targeted deletion of both receptors. It is notable that primers from Applied Biosystems (Foster City, CA, USA) were not able to differentiate cnr2 gene expression between WT and CB1−/−CB2−/− mice, and therefore, custom primers were used [22]. OT-1 mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA) and used at 8 weeks of age.
Influenza instillation
On the day of instillation, murine-adapted influenza virus A/PR8/34 (a kind gift of Dr. Allen Harmsen, Montana State University, Bozeman, MT, USA) was diluted in SAL (Hospira, Lake Forest, IL, USA) to 1 PFU/μL. Twenty-five microliters was instilled per nostril, resulting in a total inoculum of 50 PFU/mouse. Mice (n=5 each genotype) received saline or A/PR8/34 under isofluorane anesthesia.
Necropsy and lung tissue collection
Mice were anesthetized by i.p. injection of 250 mg/kg pentobarbital (Fatal-Plus, Vortech, Dearborn, MI, USA) at 1, 3, 5, and/or 7 dpi and killed by exsanguination of the abdominal aorta. Lungs were excised en bloc.
Histological and immunohistochemical staining of lung tissue sections
After en bloc excision, left lung lobes were fixed and inflated under constant pressure with 10% phosphate-buffered formalin. After transverse cuts of the left lung at generation 5 of the axial airway, tissue sections were paraffin-embedded and cut into 4-μM sections. Tissue sections were stained with H&E using a standard procedure or were stained with antibody against CCSP. Glass slides (Fisher, Waltham, MA, USA) were coated with 2% 3-aminopropyltriethoxysilane, and tissue sections were mounted onto slides and dried at 56°C overnight. Xylene was used to deparaffinize the slides, which were hydrated with decreasing concentrations of ethanol to distilled water. Subsequently, the pH was adjusted by soaking slides in TBS, pH 7.4 (Scytek Labs, Logan, UT, USA), for 5 min. Following pH adjustment for CCSP staining, standard ABC for staining was performed at room temperature using a Dako autostainer. All staining steps were performed using TBS with Tween 20 (Scytek Labs) for rinses. In short, nonspecific protein was blocked using normal goat serum (Vector Labs, Burlingame, CA, USA) for 30 min, followed by incubation with an avidin/biotin blocking system for 15 min each (Avidin D, Vector Labs; D-biotin, Sigma-Aldrich, St. Louis, MO, USA). The goat serum-blocked slides were incubated with primary polyclonal rabbit αCCSP antibody diluted 1:1600 (Seven Hills, Cincinnati, OH, USA) in normal antibody diluent (Scytek Labs). Biotinylated goat anti-rabbit IgG (H+L) was diluted to 11.0 μg/ml in NAD and incubated for 40 min, followed by incubation with ready-to-use alkaline phosphatase ABC reagent (Kirkegaard-Perry Labs, Gaithersburg, MD, USA) for 60 min. For the chromatic reaction, Vector Substrate Kit I and counterstain in Gill hematoxylin (Thermo Fisher, Waltham, MA, USA) for 15 s were used. Differentiation, dehydration, clearing, and mounting were performed with synthetic mounting media.
Flow cytometry on lung-isolated cells
Lung tissue was homogenized using a cell dissociation sieve kit (CD-1; Sigma-Aldrich), connective tissue was removed, and cells were immersed in RPMI media. For cytokine production analyses, cells were restimulated in vitro with PMA and Io (both from Sigma-Aldrich) at 40 nM and 0.5 μM, respectively, for 5 h in the presence of Brefeldin A (BioLegend, San Diego, CA, USA) in 2% FBS RPMI media, supplemented with 100 U/mL penicillin and 100 μg/mL streptomycin (all from Gibco Invitrogen, Carlsbad, CA, USA). Cells isolated from lung or restimulated in vitro were washed three times with FACS buffer (1× HBSS, 1% BSA, 0.1% sodium azide, pH adjusted to 7.6), and FcRs were blocked using purified rat anti-mouse CD16/CD32 (BD PharMingen, San Diego, CA, USA). Surface markers were stained with specific antibodies using concentrations of 0.25–0.5 μg/1 × 106 cells. FACS antibodies used were all from BioLegend: CD4 (clone RM4-5), CD8 (53-6.7), CD69 (H1.2F3), and NK1.1 (CD161, PK136). Cells were fixed with Cytofix (Becton Dickinson, Franklin Lakes, NJ, USA) before flow cytometric analysis. Intracellular staining for IFN-γ (XMG1.2) and IL-17 (TC11-18H10.1) was performed using 1× Perm/Wash (Becton Dickinson) for washes and incubation, according to the manufacturer's instructions. Samples were prepared in 96-well round-bottom plates and were analyzed using a BD Biosciences FACSCanto II flow cytometer (San Jose, CA, USA). Obtained data were analyzed individually and concatenated for graphical representation with FlowJo v8.8.6 (Tree Star, Ashland, OR, USA) for Mac.
mRNA extraction and cDNA synthesis
Lung tissues were minced with a dounce tissue grinder set (Sigma-Aldrich) and RNA extracted from right lung tissue using TRIzol reagent (Sigma-Aldrich), according to the manufacturer's instructions. Extracted mRNA was reverse-transcribed into cDNA using 2.5 μg total RNA in a 50-μL reaction volume with a high-capacity cDNA archive kit (Applied Biosystems), according to the manufacturer's instructions.
Mouse immune panel-low-density microarray
Mouse low-density microarray immune panels were obtained from Applied Biosystems and contained primers for 93 immune-related and three housekeeping genes using the TaqMan PCR system. The panels were loaded with 50 ng cDNA/well and centrifuged two times for 1 min at 1100 rpm in a Sorvall Legend T (Thermo Fisher Scientific). 18S gene expression was used to confirm consistent loading (all Ct values between 8 and 9) and was also used to normalize gene expression changes. Data were analyzed with an Applied Biosystems 7900HT real-time PCR machine using the range of two to five cycles for 18S and two to 12 cycles for other genes to set a background level to determine a threshold for the fluorescence emission of the TaqMan probes. Fold expression levels were generated using the ΔΔCt method with respect to WT SAL control samples set to a fold-change level of 1. Genes were normalized with Blom transformations, and statistical analysis was performed with SAS 9.1.3 (SAS Institute, Cary, NC, USA). Gene expression data were log-transformed, mean-centered, and normalized using Cluster version 2.11 and visualized using Treeview version 1.60 [23].
Generation and stimulation of bmDCs in vitro
The isolation and generation of bmDCs were performed as adopted from Lutz et.al. [24] and Brandt et.al. [25]. In short, mice were killed, femurs and tibias were removed aseptically, and bm was flushed with sterile RPMI. Single-cell suspensions were obtained, and connective tissue was removed with a cell strainer (BD Falcon, Becton Dickinson). Cells were cultured at 2.5 × 105 cells/mL in 10% complete RPMI, supplemented with 20 ng/mL GM-CSF (Peprotech, Rocky Hill, NJ, USA). After exchange of 50% of media with fresh media and removal of supernatant cells on Days 3, 6, and 8 after the start of culture, bmDCs were used on Day 9 for treatments. LPS (Salmonella typhimurium, Sigma-Aldrich), 1 μg/mL, and ssRNA (Invivogen, San Diego, CA, USA), 5 μg/mL,were used to stimulate bmDCs for 24 h in 2% FBS RPMI. Subsequently, cells were stained with Live/Dead cell viability dye (Invitrogen), according to the manufacturer's instructions and anti-mouse I-A/I-E (M5/114.15.2), CD11c (N418), CD11b (M1/70), CD80 (16-10A1), CD86 (GL-1), and H2Kb/H2Db (28-8-6), as described above.
bmDC coculture with OT-1 cells
bmDCs, generated as described above, were seeded at 2 × 105 cells/well in a 96-well round-bottom plate and pulsed with SIINFEKL (Anaspec, San Jose, CA, USA) for 2 h in 200 μL RPMI media. Subsequently, bmDCs were washed 3× with RPMI. Spleens from OT-1 mice were aseptically removed and washed in RPMI. OT-1 cells were labeled with CellTrace (Invitrogen) proliferation dye, according to the manufacturer's instructions. OT-1 cells were added into wells with SIINFEKL-pulsed and -unpulsed bmDCs at a final concentration of 5 × 106 cells/mL and cocultured for 4 days. After 4 days in culture, OT-1 cells were restimulated with addition of SIINFEKL at 5 ug/mL for 5 h in the presence of Brefeldin A in 2% FBS RPMI. Staining was done with Live/Dead cell viability dye and antibodies to CD8, IFN-γ, as described above.
Isolation and activation of CD4+ T cells
CD4+ T cells were isolated from splenocyte preparations using a CD4+ T cell isolation kit, according to the manufacturer's instructions (Miltenyi Biotec, Auburn, CA, USA). To immobilize αCD3 (145-2C11, BD Biosciences), flat-bottom 96-well plates were coated with 80 μL αCD3 at 5 μg/mL overnight and washed three times with sterile PBS before addition of cells. After purification, 0.5 × 106 CD4+ T cells were added per well in a total volume of 200 μL RPMI, supplemented with 5% FBS and 1% penicillin/streptomycin (Sigma-Aldrich). To elicit Th0, αCD28 (37.51, BD Biosciences) was added at a final concentration of 1 ug/mL. For Th17-polarizing conditions, additional supplementation with TGF-β (5 ng/mL; R&D Systems, Minneapolis, MN, USA), IL-6 (20 ng/mL; Jena Bioscience, Jena, Germany), and IL-2 (40 U/mL; RocheApplied Science, Indianapolis, IN, USA), as well as αIFN-γ (10 μg/mL; BD PharMingen) and αIL-4 (10 μg/mL; BD PharMingen), was necessary [26]. Four days after elicitation, cell cultures were restimulated with PMA/Io and stained as described above.
Statistical analyses
Statistical analysis was performed with SAS and Graph Pad Prism 4.03 software (GraphPad Software, La Jolla, CA, USA). To determine statistical significance of the effect of treatment (A/PR/8/34 or SAL) with respect to time, one-way ANOVA with comparison with WT 1 dpi SAL was performed. Factorial ANOVA with a Tukey's post-hoc analysis was chosen for the statistical test with a 2 × 2 design, including interactions for comparison between each genotype at each dpi. The independent variables were genotype (WT or CB1−/−CB2−/−) and treatment (A/PR/8/34 or SAL), and the dependent variable was the obtained experimental value. A P value of 0.05 or less was deemed statistically significant. In scenarios where data were nonparametric, such as data represented as percentages, Friedman's test was used as a substitute for ANOVA.
RESULTS
Enhanced leukocyte infiltration and injury in airways of CB1−/−CB2−/− mice after influenza infection
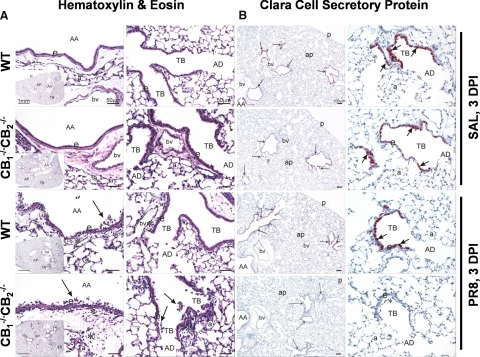
Principal histopathology in the lungs of WT mice instilled with influenza virus was a necrotizing bronchiolitis of large-diameter axial and preterminal bronchioles (Fig. 1A). These airways had conspicuous necrosis and exfoliation of surface epithelium associated with a mixed inflammatory cell infiltration. Inflammatory cell influx, along with edema, was also present in peribronchiolar and perivascular tissues (Fig. 1A). CB1−/−CB2−/− mice instilled with influenza virus developed a similar, but more severe, necrotizing bronchiolitis. No lung lesions were present in WT and CB1−/−CB2−/− SAL control mice (Fig. 1A). Not only were the epithelial and inflammatory responses in large-diameter bronchioles more marked, the virus-induced epithelial necrosis, exfoliation, and accompanying inflammation extended to more distal, small-diameter terminal bronchioles with some extension into adjacent alveolar ducts (Fig. 1A). This difference in severity and extent of viral bronchiolitis between CB1−/−CB2−/− and WT mice was also immunohistochemically demonstrated by a loss of anti-inflammatory CCSP in bronchiolar epithelium lining the terminal bronchioles of virus-treated CB1−/−CB2−/− mice (Fig. 1B). Influenza infection induced airway neutrophil and monocyte infiltration most strongly at 3 dpi, as enumerated in the BALF (Supplemental Fig. 1). The magnitude of the cell infiltrate was enhanced in CB1−/−CB2−/− compared with WT mice 3 dpi, the peak day of inflammatory cell influx into the airways.
Figure 1. More severe virus-induced necrotizing bronchiolitis and loss of CCSP in CB1−/−CB2−/− compared with WT mice, 3 dpi.
Light photomicrographs of axial airways (AA; generation 5) and terminal bronchioles (TB; generation 5) from the left lung lobe of WT and CB1−/−CB2−/− mice intranasally instilled with SAL vehicle alone or PR8 influenza virus in SAL at 3 dpi. The insets are low magnification of tissue sections of the left lung lobe taken perpendicular to the axial airways at generation 5 (see Materials and Methods for microdissection details). All tissues were stained with H&E (A) or immunohistochemically stained for CCSP (red chromagen) located in bronchiolar epithelium (arrows) at 3 dpi (B). Virus-induced necrotizing bronchiolitis is evident in the axial airways of WT and CB1−/−CB2−/− mice with greater necrosis and exfoliation (arrows) of the surface epithelium (e) and interstitial cellular inflammation and edema (asterisks) in the CB1−/−CB2−/− mouse compared with the WT mouse. Similar necrotizing bronchiolitis is evident in the terminal bronchiole of the CB1−/−CB2−/− mouse but not in the virus-treated WT mouse. Compared with SAL-instilled control mice, there was an apparent loss of CCSP staining in the bronchiolar epithelium lining the large-diameter axial airways and smaller-diameter preterminal and terminal bronchioles of the virus-treated CB1−/−CB2−/− mouse. In virus-treated WT mice, there was a loss of CCSP only in large-diameter axial airways, but CCSP was still present in preterminal and terminal bronchioles. All CCSP-stained tissue sections were counterstained with hematoxylin. AD, Alveolar duct; ap, alveolar parenchyma; p, pleura; a, alveolus; bv, blood vessel; asterisk, necrotic cellular debris and inflammatory cells in airway lumen. Light microscopic examinations of histologic tissue sections were conducted using an Olympus BX41 clinical microscope with UPlan Apochromat 10×, 20×, and 40× and Achromat 60× brightfield objectives (www.olympusamerica.com). A 5.0-megapixel Qlympus Q-Color5 camera system was used for all light photomicroscopy. This included a high-resolution FireWire digital charged-coupled device color camera, designed for publication and documentation, with a two-third-inch Bayer color sensor and 2580 × 1944 pixel resolution. The camera was connected to a Dell Precision 380 computer and QCapture Suite software for Windows, allowing for real-time viewing and full computer control of the camera.
Increased mRNA expression levels of proinflammatory-immune mediators in lungs from CB1−/−CB2−/−mice
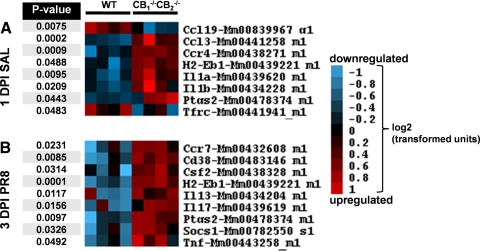
Based on the enhanced immune cell infiltration and increased tissue injury in influenza-infected lungs of CB1−/−CB2−/− mice 3 dpi, gene expression changes were assessed using a PCR-based immune panel with total lung RNA from 1 and 3 dpi (Fig. 2). Differential gene expression was observed in the lungs of uninfected (SAL-instilled) CB1−/−CB2−/− compared with WT mice (Fig. 2A). Specifically, increased expression of transcripts for H2Eb1, cytokines Il1a and IL1b, chemokine Ccl3, chemokine receptor Ccr4, and enzyme Ptgs2 was observed. In addition, decreases in chemokine Ccl19 and Tfrc were evident (Fig. 2A).
Figure 2. Relative transcript levels of genes associated with inflammation are increased in lungs of CB1−/−CB2−/− mice compared with WT mice basally and after influenza infection at 3 dpi.
RNA from lung samples (n=4) was converted to cDNA and analyzed using an Applied Biosystem's mouse immune panel. Genes were included based on 1.5-fold up- or down-regulation, and fold changes in gene expression levels were analyzed using factorial ANOVAs for significance. Genes with significantly different expression between CB1−/−CB2−/− and WT mice were visualized in a heat map with red, indicating an increase, and blue, a decrease, in expression in a log2 relative expression scale, as shown on the right side of the figure. The P value of the factorial ANOVA is shown on the left side of each gene. Arrangement of the heat map is as follows: (A) differentially regulated genes basally, without influenza infection between CB1−/− CB2−/− and WT mice; (B) gene expression differentially expressed in CB1−/−CB2−/− mice after influenza infection.
In influenza-infected mice, most influenza-associated gene expression changes were not evident prior to 3 dpi (data not shown). For the purpose of this study, the focus was placed on genes that were differentially regulated between CB1−/−CB2−/− and WT mice. Exacerbated expression of cytokines Csf2 (GM-CSF), Il13, Il17, and Tnf; negative regulator of cytokines Socs2; chemokine receptor Ccr7; surface marker Cd38; and the previously mentioned H2Eb1 and Ptgs2 was observed (Fig. 2B). Collectively, gene transcript levels indicating increased inflammation and maturation of APCs and the presence of cellular responses involving cytokine secretion in CB1−/−CB2−/− mice defined the overall profile.
T cells from CB1−/−CB2−/− mice undergo accelerated T cell activation
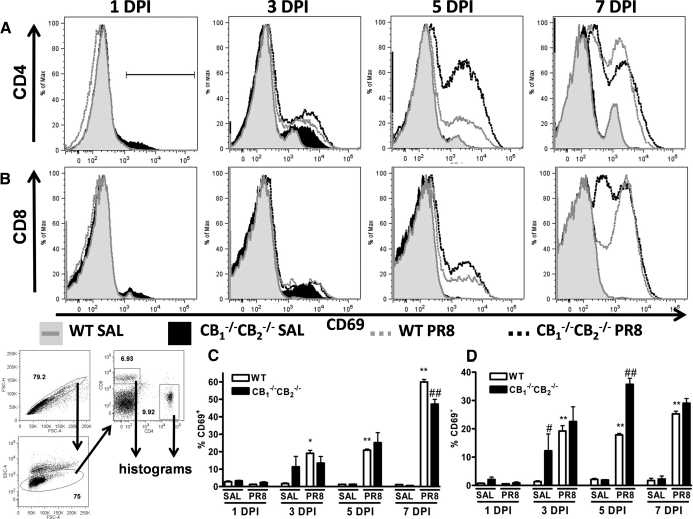
To investigate the effect of gene expression changes on the T cell response in CB1−/−CB2−/− mice after influenza infection, CD69 expression was monitored on 1–7 dpi as a marker of T cell activation in whole lung preparations. In contrast to BALF, in which myeloid cells predominated (shown in Supplemental Fig. 1), whole lung preparations harbored cells of the lymphoid lineage. Induction of surface CD69 was observed 3 dpi in CD4+ and CD8+ T cells, with greater staining intensity observed in CD4+ T cells (Fig. 3). CD69 expression was greater in CD4+ T cells from CB1−/−CB2−/− compared with WT mice as early as 3 dpi (Fig. 3A). The same trend was observed in CD8+ T cells, yet to a lesser degree than in CD4+ T cells (Fig. 3B). Increased CD69 expression was observed in SAL-instilled CB1−/−CB2−/− compared with WT mice. At 7 dpi, WT and CB1−/−CB2−/− mice displayed similar levels of surface CD69 in CD4+ and CD8+ T cells, with CD69 expression starting to wane in cells from CB1−/−CB2−/− mice (Fig. 3A and B).
Figure 3. More rapid T cell activation after influenza infection in CB1−/−CB2−/− compared with WT mice.
At 1, 3, 5, and 7 dpi, cells were isolated from lung tissue (n=5), FcRs were blocked on the surface, and samples were stained for surface markers CD4, CD8, and CD69. FlowJo software was used to analyze samples and create histograms shown. Gating was performed on single cell populations, size, and expression of CD4 and CD8 (bottom left of figure). Within CD4+ (A) and CD8+ (B) populations, histograms depict the fluorescence intensity of CD69 staining. As a result of bimodal expression of CD69, percent positively gated cells, as shown in the first panel (topmost, A), were enumerated (CD4, C; CD8, D). Friedman's test for percentages was performed, and significance was indicated in figures as #P < 0.05 and ##P < 0.01 in comparison with WT and CB1−/−CB2−/−. FSC-H, Forward-scatter-height; FSC-A, forward-scatter-area; SSC-A, side-scatter-area.
Enhanced IL-17 and IFN-γ production in NK and T cells isolated from lungs of CB1−/−CB2−/− mice in response to influenza
The observation of elevated steady-state mRNA levels of IFN-γ and IL-17 and more rapid T cell activation, as assessed by CD69 in CB1−/−CB2−/−, when compared with WT mice, suggested a regulatory function by CBs in antiviral T cell immunity. In light of the increased transcriptional expression of the T cell-produced cytokines, IFN-γ and IL-17 in the lung (Fig. 2) and their pivotal roles in lung inflammation identification of the cellular source(s) of IFN-γ and IL-17 were important in determining the cell types affected by CB1 and CB2 deletion.
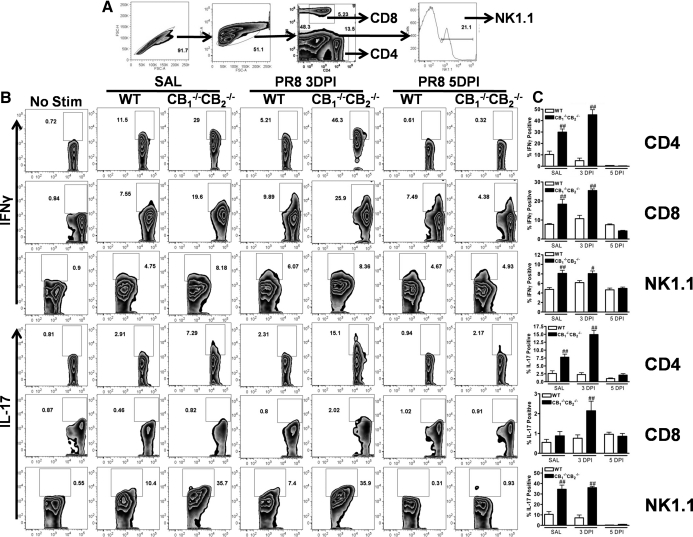
The gating scheme for the analysis of cytokine secretion from whole lung homogenates is shown in Fig. 4A. Minor basal IFN-γ production, mainly from CD4+ and CD8+ cells, was observed in SAL control samples in WT (Fig. 4B). In CB1−/−CB2−/− mice, percentages of IFN-γ-producing cells in the absence of influenza challenge were markedly enhanced compared with WT mice, especially in CD4+ cells (Fig. 4B). The percentage of IFN-γ-producting cells was modestly decreased, 3 and 5 dpi in WT but not CB1−/−CB2−/− CD4+cells, whereas increases in IFN-γ-producing cells were observed in CD8+ and NK1.1+ populations (Fig. 4B). Overall, the percentage of IFN-γ-producing cells was greater in all three (CD4+, CD8+, NK1.1+) delineated effector cell types isolated from CB1−/−CB2−/−mice compared with WT mice.
Figure 4. Greater cytokine production by leukocytes from CB1−/−CB2−/− mice compared with WT mice.
Lungs from mice were mechanically disrupted and passed through a sieve (n=5). After removal of connective tissue, cells were counted and restimulated with PMA/Io for 5 h in the presence of Brefeldin A. (A) Cells were blocked with FcRs and stained with CD4, CD8, and NK1.1, in addition to cytokine staining with IFN-γ and IL-17 (B). Flow cytometry samples were gated as depicted in A. Cytokine secretors were identified as percent of positive cells within surface delineation, and unstimulated samples were used as controls. (C) Immune cell populations were enumerated for percent cytokine expression in FlowJo (type of cytokine indicated on the left) within effector cell populations (surface marker indicated on the right) in FlowJo, and bar graphs were generated using GraphPad Prism. Friedman's test for percentages was performed, and significance is indicated in figures as #P < 0.05 and ##P < 0.01 in comparison with WT mice and CB1−/−CB2−/− mice.
On the other hand, basal IL-17 production was limited predominantly to NK1.1+ cells in WT mice (Fig. 4B). In contrast, CD4+ and NK1.1+ cells from uninfected CB1−/−CB2−/− mice produced significant amounts of IL-17 (Fig. 4B). At 3 dpi, the IL-17-producing cell types did not change, but the percentage of detectable IL-17-producing cells increased, especially in CD4+, and to a lesser extent in CD8+ cells, with greater percentages of IL-17-positive cells detected in CB1−/−CB2−/− mice compared with WT mice (Fig. 4B). By 5 dpi, the percentage of cells staining positive for IL-17 was greatly reduced in NK and CD4+ cells, in WT and CB1−/−CB2−/− mice, and in CD8+ cells of CB1−/−CB2−/− but not WT mice. In summary, IL-17 and IFN-γ hypercytokinemia in effector cells isolated from the lungs was evident in the absence of influenza infection and even more pronounced after infection in CB1−/−CB2−/− compared with WT mice.
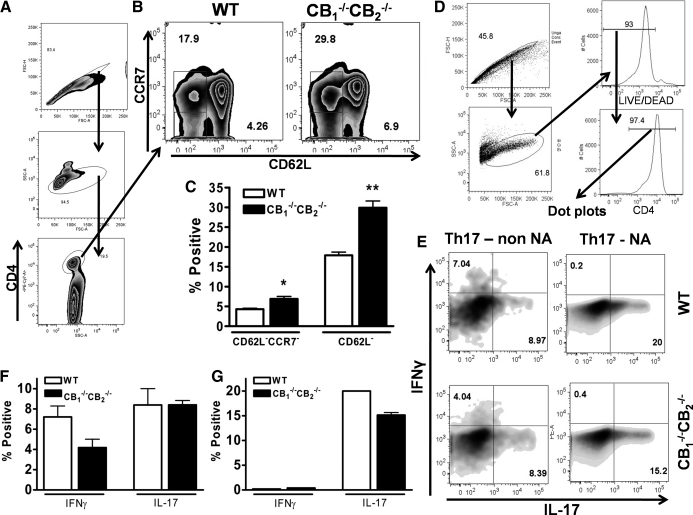
Fewer naïve CD4+ T cells in CB1−/−CB2−/− compared with WT mice
As IFN-γ- and IL-17-secreting CD4+ T cells were found in uninfected, SAL-instilled lungs of CB1−/−CB2−/− mice, we focused on the percent composition of naïve T cells (CD62L+CCR7+). After gating for CD4+ cells from splenocytes (Fig. 5A), CD62L–CCR7– and CD62L–CCR7+ non-naïve CD4+ T cell populations were identified and found in greater percentages in CB1−/−CB2−/− mice (Fig. 5B and C). To assess whether deletion of CB1 and/or CB2 had a direct effect on the ability of T cells to secrete cytokines, naïve CD62L+ CD4+ T cells were isolated from splenocytes and stimulated with immobilized αCD3 and soluble αCD28 under Th17-polarizing conditions to induce IL-17 secretion. Under these conditions, which favor IL-17 production, IL-17 was detected from WT and CB1−/−CB2−/− non-naïve T cells (CD62L–) and naïve T cells (CD62L+; Fig. 5D). No difference in the production of IL-17 was observed in CD4+ T cells from CB1−/−CB2−/− compared with WT (Fig. 5E–G). This suggests that hypercytokinemia experienced in CB1−/−CB2−/−mice in vivo might be a result of interaction with APCs, as T cells intrinsically did not produce a different level of cytokines.
Figure 5. Lower number of naïve CD4+ T cells and no difference in T cell stimulation in vitro in CB1−/−CB2−/−mice compared with WT mice.
Splenocytes (n=3) were obtained from WT and CB1−/−CB2−/− mice and analyzed by flow cytometry with gating shown in A. Non-naïve T cells (CCR7–CD62L– or CD62L–) were gated for in B and analyzed statistically in C. Naïve (NA; CD62L+) and non-naïve (non NA) CD4+ cells were isolated from splenocytes and driven toward a Th17 phenotype in the presence of TGF-β and IL-6, as described in Materials and Methods. Cells were gated as shown in D and then analyzed for cytokine production in E. Statistical analyses of experimental data in Th17-polarizing conditions from E are shown in F and G. The experiment is representative of two repeat in vitro studies. *P = 0.05; **P = 0.01 CB1−/−CB2−/− compared with WT.
Increased APC maturation and ability to induce proliferation and cytokine production in T cells in CB1−/−CB2−/− mice compared with WT mice
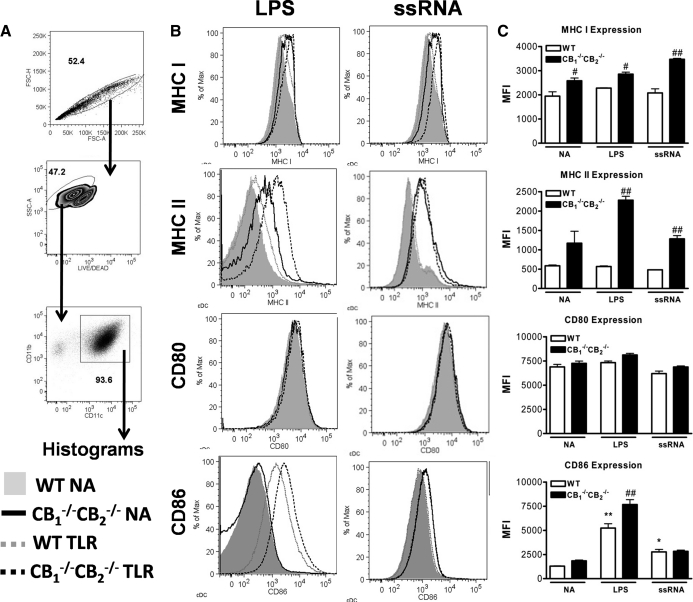
To determine whether APC responses are in fact deregulated in CB1−/− CB2−/− mice, as suggested by a lower percentage of naïve T cells, an in vitro model was used to investigate DCs, which link innate to adaptive immune responses based on their role in T cell elicitation. Immature bmDCs were generated in vitro from femurs and tibias of CB1−/−CB2−/− and WT mice and stimulated with LPS (1 μg/mL) or ssRNA (5 μg/mL) for 24 h to induce a mature phenotype. Flow cytometric staining for CD11b and CD11c was performed to confirm a conventional DC phenotype (CD11b+CD11c+) [27] (Fig. 6A). Staining for MHCI, MHCII, CD80, and CD86 was performed to evaluate the maturation status (Fig. 6B). Increased staining for MHCI, MHCII, and CD86 was found in bmDCs of CB1−/−CB2−/− compared with WT mice without TLR treatment (Fig. 6B). LPS up-regulated surface expression of MHCII and CD86, whereas ssRNA increased CD86 expression in both genotypes. In addition, ssRNA treatment elevated MHCI in bmDCs from CB1−/−CB2−/−mice only. In contrast, CD80 expression did not change with TLR treatment (Fig. 6C). Overall, bmDCs generated in vitro from CB1−/−CB2−/− but not WT mice displayed a preactivated phenotype. Upon TLR activation, maturation markers were induced with greater magnitude in CB1−/−CB2−/−compa red with WT mice.
Figure 6. Increased expression of DC maturation markers in bmDCs from CB1−/−CB2−/− mice.
bmDCs were generated as described in Materials and Methods. In short, bm was isolated from femurs and tibias and cultured in the presence of GM-CSF (20 ng/mL) for 9 days, and then immature bmDCs were washed and stimulated with TLR agonists (n=3). (A) bmDCs gated for singlets, Live/Dead dye, and high CD11b and CD11c expression. (B) Surface expression of maturation markers MHCI, MHCII, CD80, and CD86 was analyzed after stimulation for 24 h with LPS (1 μg/mL) and ssRNA (5 μg/mL). (C) As a result of the Gaussian distribution of the fluorescent staining, mean fluorescence intensity (MFI) was used to compare samples using statistics. The results shown are representative of four identical experiments.
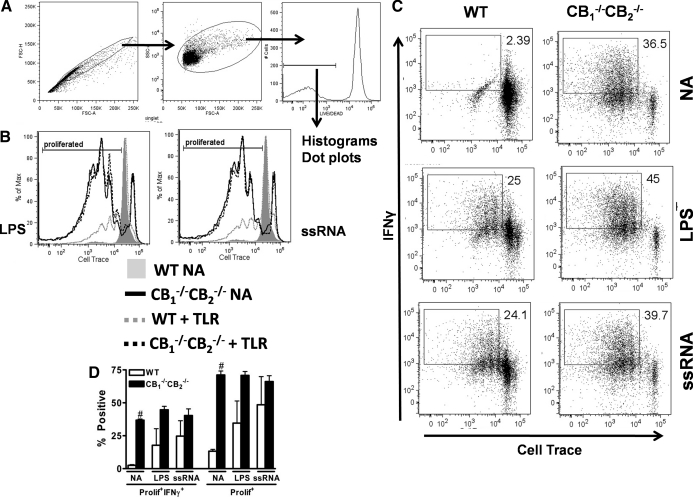
To determine whether the observed phenotypic changes on bmDCs exerted an effect on T cell elicitation and function, bmDCs pulsed with SIINFEKL were cultured with OT-1 splenocytes and evaluated after gating on live cells (Fig. 7A). OT-1 splenocytes were also labeled with a dye for assessment of proliferation by dye dilution and stained for IFN-γ to assess function after restimulation. No proliferation or IFN-γ was detected in OT-1 splenocytes incubated with naïve bmDCs from WT mice (Fig. 7B and C). However, OT-1 splenocytes added to bmDCs from CB1−/−CB2−/− mice were induced to proliferate and secreted IFN-γ in the absence of a maturation stimulus such as LPS or ssRNA (Fig. 7B and C). LPS and ssRNA treatment induced maturation in bmDCs from WT mice sufficiently to detect proliferation and IFN-γ secretion in cocultured OT-1 splenocytes, however still far less than after coculture with bmDCs derived from CB1−/−CB2−/−mice with LPS or ssRNA treatment, as evidenced by the percentage of proliferating cells or percentage of proliferating and IFN-γ+ cells (Fig. 7D). Overall, the contribution of bmDCs to elicit T cell responses is consistent with the overall phenotypic difference observed between WT and CB1−/−CB2−/−mice observed after influenza infection in vivo. AM isolated from BALF of naïve WT and CB1−/−CB2−/− mice were used to extend the findings with bmDCs. As LPS was much more potent, and the amount of cells available in the BALF is limited, we reduced our study design to a single TLR agonist, especially as TLR-initiated signaling by ssRNA and LPS converges at MyD88 [28]. AM, as identified by CD11b–CD11c+ expression [27], were obtained from BALF and stimulated overnight with LPS (Supplemental Fig. 2). As seen previously in the bmDC experiments, AM isolated from CB1−/−CB2−/− mice exhibited a preactivated phenotype compared with macrophages from WT mice, based on the expression of MHCII and CD86 (Supplemental Fig. 2B and C). LPS stimulation again increased maturation markers, especially MHCII (Supplemental Fig. 2B and C), and AM from CB1−/−CB2−/− mice presented a more mature phenotype than WT after TLR stimulation. Overall, the increased maturation of APCs from CB1−/−CB2−/− mice suggests that dysregulation as a result of extinguished CB1 and CB2 signaling predominantly affects the functional regulation of APCs, such as DCs and AM.
Figure 7. bmDCs from CB1−/−CB2−/− elicit OT-1 responses without requirement for maturation stimulus and more potently than WT.
OT-1 cells were elicited as described in Materials and Methods. In short, bmDCs were matured with different TLR agonists for 24 h, pulsed with SIINFEKL peptide for 2 h, washed, incubated with OT-1 cells for 4 days, and then restimulated for 5 h to induce cytokine secretion (n=3). (A) Gating scheme for OT-1 cells; singlet, size, and Live/Dead gating were applied before visualizing data. (B) Proliferation was assessed by Cell Trace dye dilution, and a gate depicting proliferated populations was drawn according to the WT-naive population shown in gray. The legend for the other experimental groups is below the figure, and the TLR agonist used is shown beside the proliferation graph. (C) Proliferation and concurrent IFN-γ secretion were visualized using dot plots. LPS (1 μg/mL) and ssRNA (5 μg/mL) were used to stimulate bmDC maturation as described previously (Fig. 6). Proliferation and IFN-γ secretion gates were drawn according to a WT-naive group without SIINFEKL peptide, which showed no proliferation or IFN-γ secretion. (D) Data from proliferation and cytokine plots are summarized using percentages obtained from shown gates. Statistical significance is indicated as #P ≤ 0.05 comparing WT with CB1−/−CB2−/− using Friedman's test for nonparametric data. The results shown are representative of three identical experiments.
DISCUSSION
CBs and their endogenous ligands have been investigated extensively for their immunomodulatory activity; however, only a few studies have investigated the outcome of concomitant-targeted deletion of CB1 and CB2 on immune competence [18, 19, 29]. Based on a sizable literature demonstrating that plant-derived synthetic and endogenous cannabinoids typically attenuate immune responses [30, 31], it has been long hypothesized that one role of CB1 and CB2 might be to contribute to immune homeostasis by preventing overstimulation of the immune system. Our findings indicate that a principle function of CBs is to modulate APC function, and a lack thereof is critically involved in the described hyper-responsive immune phenotype observed in CB1−/−CB2−/− mice. To assess the role of CB1 and CB2 in the immunopathology induced by influenza, the majority of this investigation was focused on events at 3 dpi, the peak day of the inflammatory response. After influenza infection, immunopathology was evident in lungs of CB1−/−CB2−/− mice to a greater degree than in lungs of WT. The influx of macrophages and neutrophils into the airways was more pronounced with a concomitant increase in necrosis and exfoliation of the surface epithelium in CB1−/−CB2−/− compared with WT mice. The inflammatory mediators of macrophages and neutrophils are likely responsible for damage to the airways [32, 33]. Within the airways, CCSP is thought to perform a protective function, especially involving the reduction of neutrophilia as a result of viral infections, such as respiratory syncytial virus [34]. Thus, the increase in neutrophils in the BALF of CB1−/−CB2−/− mice is consistent with the observed decrease in CCSP and increased airway injury in CB1−/−CB2−/− compared with WT mice.
Several unique gene expression changes were evident in CB1−/−CB2−/− compared with WT mice after infection at 3 dpi. In contrast to basal differences in transcription, several lymphocyte-specific cytokines (IL-13 and IL-17) and indices of lymphocyte activity (CD38, found on cytokine-releasing T cells, especially Th17 [35, 36]) were up-regulated after influenza infection. The presence of cytokine-producing lymphocytes was confirmed by flow cytometry, and greater percentages of IFN-γ- and IL-17-producing CD4+, CD8+, and NK1.1+ were observed in CB1−/−CB2−/− mice compared with WT mice after influenza infection. These findings are in accordance with accelerated activation, as evidenced by CD69 staining on the surface of CD4+ and CD8+ cells. Taken together, exacerbated lymphocyte responses were observed in CB1−/−CB2−/− compared with WT mice.
To investigate whether inherent differences in the function of WT and CB1−/−CB2−/− are present in the lymphocyte compartment, the ability of naïve (CD62L+) and non-naïve (CD62L–) CD4+ T cells to polarize toward a Th17 phenotype after αCD3/αCD28 stimulation was examined. The levels of IL-17 produced from CB1−/−CB2−/−CD4+ T cells were not significantly different from WT. Consistent with in vitro studies of mixed lymphocyte responses and PMA/Io stimulation [29], cytokine production was similar after in vitro stimulation of naïve CD4+ T cells from CB1−/−CB2−/− and WT mice, suggesting no inherent difference in T cell function between the two genotypes.
During T cell isolation, consistently lower numbers of naïve T cells were isolated from CB1−/−CB2−/− mice, and this phenomenon was confirmed by lower percentages of naïve (CD62L+ and CCR7+CD62L+) CD4+ T cells in spleens of CB1−/−CB2−/− compared with WT mice. Accordingly, CCL19 mRNA levels, the chemokine that binds to CCR7, were reduced in CB1−/−CB2−/− mice. In an IκB transgenic mouse model, enhancement of NF-κB signaling reduced the naïve T cell compartments as well, whereas at the same time, greater IL-17 production was observed [37]. In the present study, a similar scenario was observed, in which increased inflammation resulted in the generation of more Th17 cells at the expense of the naïve T cell compartment. Collectively, with no observable difference of in vitro cytokine production from naïve CD4+ T cells, evidence for greater numbers of non-naïve T cells suggested involvement of APCs in eliciting T cell responses rather than inherent differences in the T cell compartment as a result of loss of CB1 and CB2. Thus, the environment encountered and the ability of APCs to elicit stronger lymphocyte responses provided a plausible explanation for the enhanced cytokine production by lymphocytes in vivo and reduction in the naive lymphocyte compartment in vivo observed in CB1−/−CB2−/− mice.
Indeed, profound differences in APC maturation of CB1−/−CB2−/− compared with WT mice were observed. Basal and TLR-inducible maturation of bmDCs and AM was more robust in CB1−/−CB2−/− mice, as assessed by expression of surface markers CD86, MHCI, and MHCII. Consistent with this was the observation of increased mRNA levels of H2Eb1, one of the H2Eb1, one of the MHC II subunits, in the lung. In CB1−/−CB2−/− mice, increased expression of CCL3, IL-1, and COX-2 (Ptgs2) genes was observed, which was often produced by APCs and characteristic of an inflammatory response [38]. Concordantly, but less well understood and characterized, Tfrc, an iron-transporting receptor, has been reported to be down-regulated in macrophages during chronic inflammation, as well as in response to inflammatory stimuli [39, 40]. Taken together, a very consistent profile of increased inflammation and maturation of APCs exists in lungs of CB1−/−CB2−/− compared with WT mice.
The consequence of APC maturation was reflected in the marked differences in the ability of bmDC pulsed with SIINFEKL to stimulate proliferation in OT-1 cells and their ability to become functional effectors, as assessed by IFN-γ secretion. Although this model did not investigate the role of CB1 and CB2 in antigen processing and presentation by DCs, it has been demonstrated previously that cannabinoids, including Δ9-THC, can suppress APC function [41, 42]. Furthermore, it has been reported previously that Δ9-THC-mediated suppression of costimulatory activity by macrophages was dependent on CB2 [43–45]. Most recently, CB1 was also shown to play a role in regulation of costimulation by DCs [46]. Only after TLR stimulation were measurable OT-1 responses elicited by bmDCs from WT, whereas the ability of bmDCs from CB1−/−CB2−/− surpassed that of WT with the same TLR stimulation. These in vitro studies demonstrate that at the cellular level, altered function of APCs, especially DCs, is critical in the observed phenotypic differences between CB1−/−CB2−/− and WT mice.
Although the phenotypic changes as a result of CB1 and CB2 deletion in the immune system described above appear to have improved resistance to virus infection, as evidenced by a reduced lung viral burden after influenza challenge [19], our results indicate that this occurs with a concomitant increase in immunopathology. The drastic loss of CCSP staining and greater inflammation, as evidenced by H&E staining, in addition to cytokine production by NK and T cells and lung mRNA transcript levels for proinflammatory mediators, suggests that although CB1−/−CB2−/− mice have lower viral burden in the lung [19], the lung tissue damage and airway injury were more severe when compared with WT mice. Excessive immune activation can result in lethality from influenza virus infection [47, 48], although in the present study, a sublethal inoculum of influenza virus was administered to avoid lethality to the murine host [49]. Taken together, the loss of a mechanism to negatively regulate immune function, as shown in CB1−/−CB2−/− mice, resulted in increased immunopathology to a pathogenic stimulus, in this case, influenza.
Our results suggest that CB1 and/or CB2, although reducing the magnitude of the influenza-induced immune response, provide protection against excessive airway injury in the lung from exacerbated immunopathology. In humans, SNPs, which can result in a loss of function, have been identified in CB1 and were associated with ulcerative colitis [50]. Similarly a SNP in CB2 was associated with an increased, overall risk of autoimmune disease [51]. In autoimmune diseases, such as Crohn's disease and experimental autoimmune encephalomyelitis, a murine multiple sclerosis model, increased maturation of DCs was observed [52, 53]. Furthermore, it has been demonstrated that DCs can induce and contribute to maintaining autoimmunity [54]. Taken together, with the present studies, CB1 and/or CB2 might be considered host factors that attenuate immune responses, in this case, to influenza, to reduce tissue damage. In humans, who unlike laboratory animals, are routinely exposed to pathogenic bacteria, viruses, fungi, and their PAMPs, DCs confer immunity by acting as accessory cells in the elicitation of effector cell responses (e.g., T cells). However, exacerbated and/or unwanted DC responses can occur through a reduction or absence of signaling through CB1 and/or CB2, for example, as a result of a loss of function SNP. Consequently, in the absence of regulation by the cannabinoid system, DC-elicited effector responses might contribute to the pathologies described above, including autoimmune disease. Overall, these findings provide evidence that the enhanced immune phenotype in CB1−/−CB2−/− mice after influenza infection influences a cascade of events that exists, in part, as a result of altered basal-immune function and functional exacerbation of select immune populations after immune stimulation. As demonstrated with naïve cells in vitro, our results suggest that the lymphocyte compartment is not inherently responsible for exacerbated cytokine production but is dependent on dysregulation of APC function as a result of extinguished CB signaling. Collectively, these results indicate that the CBs play a fundamental role in attenuating inflammation and immune responses orchestrated by APCs.
Supplementary Material
ACKNOWLEDGMENTS
This study was funded in part by NIH DA07908 and DA020402. We thank Dr. Andreas Zimmer at Universität Bonn and Dr. Allen Harmsen at Montana State University for kindly providing us with the CB1–/–CB2–/– mice and the A/PR8/34 virus, respectively. Also, we thank Sonia Dantas (MSU) for assisting with the preparation of BALF samples.
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
- α
- anti
- Δ9-THC
- Δ9-tetrahydrocannabinol
- AM
- alveolar macrophage(s)
- BALF
- BAL fluid
- bmDC
- bone marrow-derived DC
- CB1/2
- cannabinoid receptor 1/2
- CB1–/–CB2–/–
- cannabinoid receptor 1 and 2 knockout mice
- CCSP
- Clara cell secretory protein
- CD62L
- CD62 ligand
- Ct
- comparative threshold
- dpi
- days postinfection
- H2Eb1
- MHCII subunit
- Io
- ionomycin
- MSU
- Michigan State University
- OT-1
- TCR transgenic mice specific for OVA257–264
- Ptgs2
- PG synthase 2
- SAL
- 0.9% NaCl (saline) solution
- SAS
- Statistical Analysis System
- SIINFEKL
- OVA peptide OVA257–264
- SNP
- single nucleotide polymorphism
- Socs1
- suppressor of cytokine signaling 1
- Tfrc
- transferrin receptor
AUTHORSHIP
P.W.F.K. wrote the manuscript and designed and performed experiments. W.C., R.B.C., and B.L.F.K. assisted in performing experiments. J.R.H. contributed the histopathological analyses. B.L.F.K. and N.E.K. designed experiments and edited the manuscript.
DISCLOSURE
The authors report no conflict of interest.
REFERENCES
- 1. Holt P. G., Strickland D. H., Wikstrom M. E., Jahnsen F. L. (2008) Regulation of immunological homeostasis in the respiratory tract. Nat. Rev. Immunol. 8, 142–152 [DOI] [PubMed] [Google Scholar]
- 2. Kobayashi K. S., Flavell R. A. (2004) Shielding the double-edged sword: negative regulation of the innate immune system. J. Leukoc. Biol. 75, 428–433 [DOI] [PubMed] [Google Scholar]
- 3. Wong P., Pamer E. G. (2003) Feedback regulation of pathogen-specific T cell priming. Immunity 18, 499–511 [DOI] [PubMed] [Google Scholar]
- 4. Strieter R. M., Belperio J. A., Keane M. P. (2002) Cytokines in innate host defense in the lung. J. Clin. Invest. 109, 699–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Banchereau J., Steinman R. M. (1998) Dendritic cells and the control of immunity. Nature 392, 245–252 [DOI] [PubMed] [Google Scholar]
- 6. Doherty P. C., Topham D. J., Tripp R. A., Cardin R. D., Brooks J. W., Stevenson P. G. (1997) Effector CD4+ and CD8+ T-cell mechanisms in the control of respiratory virus infections. Immunol. Rev. 159, 105–117 [DOI] [PubMed] [Google Scholar]
- 7. Kolls J. K., Linden A. (2004) Interleukin-17 family members and inflammation. Immunity 21, 467–476 [DOI] [PubMed] [Google Scholar]
- 8. Korn T., Bettelli E., Oukka M., Kuchroo V. K. (2009) IL-17 and Th17 cells. Annu. Rev. Immunol. 27, 485–517 [DOI] [PubMed] [Google Scholar]
- 9. Ye P., Rodriguez F. H., Kanaly S., Stocking K. L., Schurr J., Schwarzenberger P., Oliver P., Huang W., Zhang P., Zhang J., Shellito J. E., Bagby G. J., Nelson S., Charrier K., Peschon J. J., Kolls J. K. (2001) Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J. Exp. Med. 194, 519–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kohlmeier J. E., Woodland D. L. (2009) Immunity to respiratory viruses. Annu. Rev. Immunol. 27, 61–82 [DOI] [PubMed] [Google Scholar]
- 11. Crowe C. R., Chen K., Pociask D. A., Alcorn J. F., Krivich C., Enelow R. I., Ross T. M., Witztum J. L., Kolls J. K. (2009) Critical role of IL-17RA in immunopathology of influenza infection. J. Immunol. 183, 5301–5310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wiley J. A., Cerwenka A., Harkema J. R., Dutton R. W., Harmsen A. G. (2001) Production of interferon-γ by influenza hemagglutinin-specific CD8 effector T cells influences the development of pulmonary immunopathology. Am. J. Pathol. 158, 119–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moskophidis D., Kioussis D. (1998) Contribution of virus-specific CD8+ cytotoxic T cells to virus clearance or pathologic manifestations of influenza virus infection in a T cell receptor transgenic mouse model. J. Exp. Med. 188, 223–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Di Marzo V. (2008) Targeting the endocannabinoid system: to enhance or reduce? Nat. Rev. Drug Discov. 7, 438–455 [DOI] [PubMed] [Google Scholar]
- 15. Bouaboula M., Rinaldi M., Carayon P., Carillon C., Delpech B., Shire D., Le Fur G., Casellas P. (1993) Cannabinoid-receptor expression in human leukocytes. Eur. J. Biochem. 214, 173–180 [DOI] [PubMed] [Google Scholar]
- 16. Galiègue S., Mary S., Marchand J., Dussossoy D., Carriere D., Carayon P., Bouaboula M., Shire D., Le Fur G., Casellas P. (1995) Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur. J. Biochem. 232, 54–61 [DOI] [PubMed] [Google Scholar]
- 17. Pandey R., Mousawy K., Nagarkatti M., Nagarkatti P. (2009) Endocannabinoids and immune regulation. Pharmacol. Res. 60, 85–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Karsak M., Gaffal E., Date R., Wang-Eckhardt L., Rehnelt J., Petrosino S., Starowicz K., Steuder R., Schlicker E., Cravatt B., Mechoulam R., Buettner R., Werner S., Di Marzo V., Tuting T., Zimmer A. (2007) Attenuation of allergic contact dermatitis through the endocannabinoid system. Science 316, 1494–1497 [DOI] [PubMed] [Google Scholar]
- 19. Buchweitz J. P., Karmaus P. W., Williams K. J., Harkema J. R., Kaminski N. E. (2008) Targeted deletion of cannabinoid receptors CB1 and CB2 produced enhanced inflammatory responses to influenza A/PR/8/34 in the absence and presence of Δ9-tetrahydrocannabinol. J. Leukoc. Biol. 83, 785–796 [DOI] [PubMed] [Google Scholar]
- 20. He D., Wu L., Kim H. K., Li H., Elmets C. A., Xu H. (2009) IL-17 and IFN-γ mediate the elicitation of contact hypersensitivity responses by different mechanisms and both are required for optimal responses. J. Immunol. 183, 1463–1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hennet T., Ziltener H. J., Frei K., Peterhans E. (1992) A kinetic study of immune mediators in the lungs of mice infected with influenza A virus. J. Immunol. 149, 932–939 [PubMed] [Google Scholar]
- 22. Kaplan B. L., Lawver J. E., Karmaus P. W., Ngaotepprutaram T., Birmingham N. P., Harkema J. R., Kaminski N. E. (2010) The effects of targeted deletion of cannabinoid receptors CB1 and CB2 on intranasal sensitization and challenge with adjuvant-free ovalbumin. Toxicol. Pathol. 38, 382–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Eisen M. B., Spellman P. T., Brown P. O., Botstein D. (1998) Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. USA 95, 14863–14868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lutz M. B., Kukutsch N., Ogilvie A. L., Rossner S., Koch F., Romani N., Schuler G. (1999) An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods 223, 77–92 [DOI] [PubMed] [Google Scholar]
- 25. Brandt K., Bulfone-Paus S., Foster D. C., Ruckert R. (2003) Interleukin-21 inhibits dendritic cell activation and maturation. Blood 102, 4090–4098 [DOI] [PubMed] [Google Scholar]
- 26. Ivanov I. I., McKenzie B. S., Zhou L., Tadokoro C. E., Lepelley A., Lafaille J. J., Cua D. J., Littman D. R. (2006) The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126, 1121–1133 [DOI] [PubMed] [Google Scholar]
- 27. Gonzalez-Juarrero M., Shim T. S., Kipnis A., Junqueira-Kipnis A. P., Orme I. M. (2003) Dynamics of macrophage cell populations during murine pulmonary tuberculosis. J. Immunol. 171, 3128–3135 [DOI] [PubMed] [Google Scholar]
- 28. Akira S., Uematsu S., Takeuchi O. (2006) Pathogen recognition and innate immunity. Cell 124, 783–801 [DOI] [PubMed] [Google Scholar]
- 29. Springs A. E., Karmaus P. W., Crawford R. B., Kaplan B. L., Kaminski N. E. (2008) Effects of targeted deletion of cannabinoid receptors CB1 and CB2 on immune competence and sensitivity to immune modulation by Δ9-tetrahydrocannabinol. J. Leukoc. Biol. 84, 1574–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Howlett A. C., Barth F., Bonner T. I., Cabral G., Casellas P., Devane W. A., Felder C. C., Herkenham M., Mackie K., Martin B. R., Mechoulam R., Pertwee R. G. (2002) International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol. Rev. 54, 161–202 [DOI] [PubMed] [Google Scholar]
- 31. Cabral G.A., Staab A. (2005) Effects on the immune system. Handb. Exp. Pharmacol., 385–423 [DOI] [PubMed] [Google Scholar]
- 32. Beck-Schimmer B., Schwendener R., Pasch T., Reyes L., Booy C., Schimmer R. C. (2005) Alveolar macrophages regulate neutrophil recruitment in endotoxin-induced lung injury. Respir. Res. 6, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nathan C. (2006) Neutrophils and immunity: challenges and opportunities. Nat. Rev. Immunol. 6, 173–182 [DOI] [PubMed] [Google Scholar]
- 34. Wang S. Z., Rosenberger C. L., Bao Y. X., Stark J. M., Harrod K. S. (2003) Clara cell secretory protein modulates lung inflammatory and immune responses to respiratory syncytial virus infection. J. Immunol. 171, 1051–1060 [DOI] [PubMed] [Google Scholar]
- 35. Sandoval-Montes C., Santos-Argumedo L. (2005) CD38 is expressed selectively during the activation of a subset of mature T cells with reduced proliferation but improved potential to produce cytokines. J. Leukoc. Biol. 77, 513–521 [DOI] [PubMed] [Google Scholar]
- 36. Cox C. A., Shi G., Yin H., Vistica B. P., Wawrousek E. F., Chan C. C., Gery I. (2008) Both Th1 and Th17 are immunopathogenic but differ in other key biological activities. J. Immunol. 180, 7414–7422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chang M., Lee A. J., Fitzpatrick L., Zhang M., Sun S. C. (2009) NF-κ B1 p105 regulates T cell homeostasis and prevents chronic inflammation. J. Immunol. 182, 3131–3138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jenner R. G., Young R. A. (2005) Insights into host responses against pathogens from transcriptional profiling. Nat. Rev. Microbiol. 3, 281–294 [DOI] [PubMed] [Google Scholar]
- 39. Ludwiczek S., Aigner E., Theurl I., Weiss G. (2003) Cytokine-mediated regulation of iron transport in human monocytic cells. Blood 101, 4148–4154 [DOI] [PubMed] [Google Scholar]
- 40. Kim S., Ponka P. (2000) Effects of interferon-γ and lipopolysaccharide on macrophage iron metabolism are mediated by nitric oxide-induced degradation of iron regulatory protein 2. J. Biol. Chem. 275, 6220–6226 [DOI] [PubMed] [Google Scholar]
- 41. McCoy K. L., Gainey D., Cabral G. A. (1995) Δ 9-Tetrahydrocannabinol modulates antigen processing by macrophages. J. Pharmacol. Exp. Ther. 273, 1216–1223 [PubMed] [Google Scholar]
- 42. McCoy K. L., Matveyeva M., Carlisle S. J., Cabral G. A. (1999) Cannabinoid inhibition of the processing of intact lysozyme by macrophages: evidence for CB2 receptor participation. J. Pharmacol. Exp. Ther. 289, 1620–1625 [PubMed] [Google Scholar]
- 43. Buckley N. E., McCoy K. L., Mezey E., Bonner T., Zimmer A., Felder C. C., Glass M. (2000) Immunomodulation by cannabinoids is absent in mice deficient for the cannabinoid CB(2) receptor. Eur. J. Pharmacol. 396, 141–149 [DOI] [PubMed] [Google Scholar]
- 44. Chuchawankul S., Shima M., Buckley N. E., Hartmann C. B., McCoy K. L. (2004) Role of cannabinoid receptors in inhibiting macrophage costimulatory activity. Int. Immunopharmacol. 4, 265–278 [DOI] [PubMed] [Google Scholar]
- 45. Clements D. J., Matveyeva M., McCoy K. L. (1998) Δ9-Tetrahydrocannabinol suppresses macrophage costimulation by decreasing heat-stable antigen expression. Int. J. Immunopharmacol. 20, 415–428 [DOI] [PubMed] [Google Scholar]
- 46. Wacnik P. W., Luhr K. M., Hill R. H., Ljunggren H. G., Kristensson K., Svensson M. (2008) Cannabinoids affect dendritic cell (DC) potassium channel function and modulate DC T cell stimulatory capacity. J. Immunol. 181, 3057–3066 [DOI] [PubMed] [Google Scholar]
- 47. Baskin C. R., Bielefeldt-Ohmann H., Tumpey T. M., Sabourin P. J., Long J. P., Garcia-Sastre A., Tolnay A. E., Albrecht R., Pyles J. A., Olson P. H., Aicher L. D., Rosenzweig E. R., Murali-Krishna K., Clark E. A., Kotur M. S., Fornek J. L., Proll S., Palermo R. E., Sabourin C. L., Katze M. G. (2009) Early and sustained innate immune response defines pathology and death in nonhuman primates infected by highly pathogenic influenza virus. Proc. Natl. Acad. Sci. USA 106, 3455–3460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Brydon E. W., Morris S. J., Sweet C. (2005) Role of apoptosis and cytokines in influenza virus morbidity. FEMS Microbiol. Rev. 29, 837–850 [DOI] [PubMed] [Google Scholar]
- 49. Buchweitz J. P., Harkema J. R., Kaminski N. E. (2007) Time-dependent airway epithelial and inflammatory cell responses induced by influenza virus A/PR/8/34 in C57BL/6 mice. Toxicol. Pathol. 35, 424–435 [DOI] [PubMed] [Google Scholar]
- 50. Storr M., Emmerdinger D., Diegelmann J., Pfennig S., Ochsenkuhn T., Goke B., Lohse P., Brand S. (2010) The cannabinoid 1 receptor (CNR1) 1359 G/A polymorphism modulates susceptibility to ulcerative colitis and the phenotype in Crohn′s disease. PLoS ONE 5, e9453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sipe J. C., Arbour N., Gerber A., Beutler E. (2005) Reduced endocannabinoid immune modulation by a common cannabinoid 2 (CB2) receptor gene polymorphism: possible risk for autoimmune disorders. J. Leukoc. Biol. 78, 231–238 [DOI] [PubMed] [Google Scholar]
- 52. Middel P., Raddatz D., Gunawan B., Haller F., Radzun H. J. (2006) Increased number of mature dendritic cells in Crohn′s disease: evidence for a chemokine mediated retention mechanism. Gut 55, 220–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Serafini B., Columba-Cabezas S., Di Rosa F., Aloisi F. (2000) Intracerebral recruitment and maturation of dendritic cells in the onset and progression of experimental autoimmune encephalomyelitis. Am. J. Pathol. 157, 1991–2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ludewig B., Odermatt B., Landmann S., Hengartner H., Zinkernagel R. M. (1998) Dendritic cells induce autoimmune diabetes and maintain disease via de novo formation of local lymphoid tissue. J. Exp. Med. 188, 1493–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.