The activated coagulation factor XI may play a role in regulating the directional migration of PMNs.
Keywords: inflammation, innate immunity, contact system
Abstract
PMN leukocytes are the most abundant leukocytes in the circulation and play an important role in host defense. PMN leukocyte recruitment and inflammatory responses at sites of infection are critical components in innate immunity. Although inflammation and coagulation are known to have bidirectional relationships, little is known about the interaction between PMN leukocytes and coagulation factors. Coagulation FXI participates in the intrinsic coagulation pathway upon its activation, contributing to hemostasis and thrombosis. We have shown previously that FXI-deficient mice have an increased survival and less leukocyte accumulation into the peritoneum in severe polymicrobial peritonitis. This result suggests a role for FXI in leukocyte trafficking and/or function. In this study, we characterized the functional consequences of FXIa binding to PMN leukocytes. FXIa reduced PMN leukocyte chemotaxis triggered by the chemokine, IL-8, or the bacterial-derived peptide, fMLP, perhaps as a result of the loss of directed migration. In summary, our data suggest that FXIa modulates the inflammatory response of PMN leukocytes by altering migration. These studies highlight the interplay between inflammation and coagulation and suggest that FXIa may play a role in innate immunity.
Introduction
PMN leukocytes play a central role in innate immunity and are responsible for mediating the inflammatory response to bacterial infection and tissue injury [1]. PMN leukocyte recruitment and transmigration across the endothelial cell lining of the vasculature are orchestrated by the secretion of chemoattractants and cytokines from infected, inflamed, or injured tissues. The antimicrobial activity of PMN leukocytes is characterized by phagocytosis, degranulation of cytolytic enzymes, and production of superoxide anion. The antimicrobial function of PMN leukocytes is critical to host defense against bacterial infections; however, dysregulation of PMN leukocyte influx can result in severe collateral tissue damage [2].
Enzymes of the blood coagulation cascade are known to be activated at sites of inflammation [3], where they are thought to play a role in regulating the trafficking and function of PMN leukocytes. For instance, mice deficient in coagulation FXI have higher survival rates after induction of severe polymicrobial peritonitis and reduced leukocyte accumulation at the site of infection [4]. However, it is not known whether coagulation factors, such as FXI, play a direct role in the regulation of PMN leukocyte defense mechanisms.
FXI is a 160-kDa homodimer that plays a key role in the early phase of the intrinsic blood coagulation cascade [5]. In the intrinsic phase, FXI is activated to FXIa by FXIIa, thrombin, or autocatalytic activation. FXIa functions as a serine protease to cleave and activate FIX, leading to sustained thrombin generation. FXI circulates in the plasma as a complex with HK, a feature shared in common with PK, the monomeric homologue of FXI. PMN leukocytes have been reported to bind FXI directly [6]; however, the functional consequences of the FXI–PMN leukocyte interaction have not been described. In this study, our data suggest that FXIa modulates the inflammatory response of PMN leukocytes by altering migration. These studies highlight the interplay between inflammation and coagulation and suggest that FXIa may play a role in innate immunity.
MATERIALS AND METHODS
Reagents
Plasma-derived FXIa and FXII were purchased from Hematologic Technologies (Essex Junction, VT, USA). HK and PK were purchased from Enzyme Research Laboratories (South Bend, IN, USA). Polymorphprep was from Axis-Shield PoC AS (Olso, Norway). Fluo-4 AM was purchased from Molecular Probes (Eugene, OR, USA). All other reagents were from Sigma-Aldrich (St. Louis, MO, USA) or previously named sources [7].
Preparation of human PMN leukocytes
Human venous blood was collected from healthy volunteers into citrate-phosphate-dextrose (1:7 vol/vol). Blood was layered over an equal volume of Polymorphprep and centrifuged at 500 g for 45 min at 18°C. The lower layer containing PMN leukocytes was collected and washed with HBSS by centrifugation at 400 g for 10 min. To remove contaminating RBCs, the pellet was resuspended in sterile H2O for 30 s, followed by the immediate addition of 10× PIPES buffer (250 mΜ PIPES, 1.1 mΜ NaCl, 50 mΜ KCl, pH 7.4). After centrifugation at 400 g for 10 min, the pellet was resuspended in PMN leukocyte buffer (HBSS containing 2 mΜ CaCl2, 2 mΜ MgCl2, and 1% w/v BSA).
Static adhesion assays
Nontissue, culture-treated 24-well plates were incubated with proteins for 1 h at room temperature. Surfaces were then blocked with BSA (2% w/v) for 1 h and washed with PBS. Purified human PMN leukocytes (1×106/ml) were incubated on protein-coated surfaces at 37°C for 30 min. After washing with PBS and fixation with 4% paraformaldehyde, adherent cells were imaged using 10× phase microscopy and counted using ImageJ software.
Intracellular Ca2+ measurement
Human PMN leukocytes (2×106 /ml) were loaded with fluo-4 AM (final 2 μΜ) and plated on a fibronectin surface for 30 min at 37°C. After washing with Ca2+-free HBSS, cells were incubated for another 30 min at 37°C in PMN leukocyte buffer. Intracellular Ca2+ spikes in adherent PMN leukocytes were monitored for 15 min at 40× using fluorescent microscopy. Vehicle (PMN leukocyte buffer) or FXIa (final10 μg/ml) was added at 5 min, followed by the addition of fMLP (final 10 nΜ) at 10 min.
Transwell assays
Upper and bottom chambers of 24-well Transwell plates (Corning, Corning, NY, USA; 3.0 μm pore) were coated with fibronectin (5 μg/ml) for 1 h, washed with PBS, and dried overnight. After incubation with indicated reagents, purified PMN leukocytes were placed onto the upper chamber in the presence of fMLP (10 nΜ) or IL-8 (100 ng/ml) in the lower chamber and incubated at 37°C for 90 min. After incubation, EDTA (final 50 mΜ) was added to the bottom chamber at 4°C for 10 min. The supernatants in the bottom chambers were collected and pelleted by centrifugation at 500 g for 10 min. Migratory cells were resuspended and counted using a hemocytometer.
Insall migration chamber assays
Acid-washed, 22-mm square coverslips (#1.5, 0.16–0.18 mm) were coated with fibronectin (20 μg/ml) for 1 h and blocked with heat-inactivated BSA (1% w/v) for 1 h. Purified human PMN leukocytes were incubated at 37°C for 30 min on the coverslips. Inner and outer wells of an Insall chamber [8] were filled with vehicle (PMN leukocyte buffer), and the coverslips with adherent cells were inverted onto the chamber. After the vehicle in outer wells was aspirated, fMLP-containing medium was added. Images were obtained every 10 s for 30 min at 40× using DIC microscopy. In selected experiments, cells were pretreated with indicated reagents for 30 min at room temperature prior to the experiment. Images were analyzed using ImageJ plugin MTrackJ and quantified using the CircStat toolbox [9] for MATLAB (MathWorks, Natick, MA, USA).
Analysis of data
Data are shown as means ± sem. Statistical analysis was performed using paired Student′s t test. Probability values of P < 0.05 were selected to be statistically significant.
RESULTS AND DISCUSSION
Characterization of FXIa–PMN leukocyte interactions
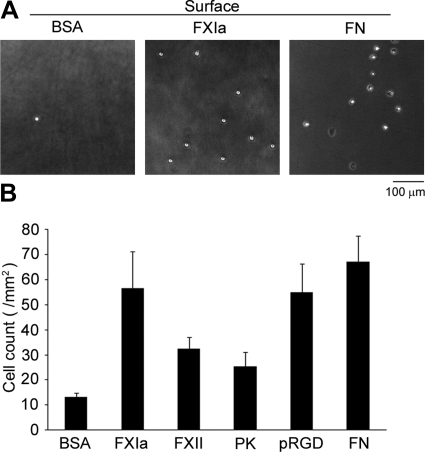
We first performed static adhesion assays using purified human PMN leukocytes to evaluate the ability of PMN leukocytes to bind FXIa. We found that immobilized FXIa supported binding of 59.7 PMN leukocytes/mm2 (Fig. 1). A similar degree of PMN leukocyte binding was observed on fibronectin or synthetic polypeptide analogs containing the RGD sequence found in the functional domain of fibronectin. In contrast, only a limited degree of PMN leukocyte adhesion was observed to immobilized PK, which has structural similarity to FXI [5] or FXII (Fig. 1B).
Figure 1. Human PMN leukocyte binding to FXIa.
Purified human PMN leukoctyes (1×106/ml) were placed on protein-coated surfaces for 30 min at 37°C and imaged using 10× phase microscopy for quantification. (A) Representative images of phase-bright PMN leukoctyes on BSA (2% w/v), FXIa (50 μg/ml), or fibronectin (FN; 50 μg/ml)-coated surfaces. (B) Adherent PMN leukoctyes on immobilized BSA, FXIa, FXII (50 μg/ml), PK (50 μg/ml), polyRGD (pRGD; 20 μg/ml), or fibronectin (50 μg/ml) were calculated and are reported as mean cell count/mm2 ± sem. Results were obtained from at least three independent experiments.
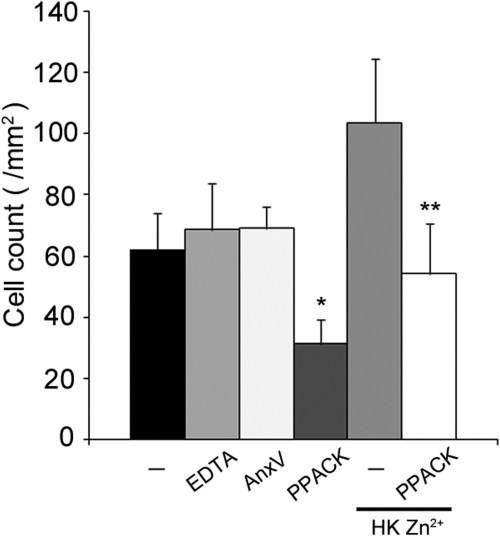
We next investigated the molecular mechanisms of FXIa binding to PMN leukocytes. As shown in Fig. 2, the serine protease inhibitor PPACK reduced PMN leukocyte binding to FXIa by >50%, suggesting that the protease activity of FXIa or alternatively, its catalytic domain plays a role in FXIa–PMN leukocyte binding. Equivalent results were observed whether PPACK were present in solution (31.3 PMN leukocytes/mm2) or FXIa were pretreated with PPACK (21.4 PMN leukocytes/per mm2). In contrast, chelation of extracellular divalent cations with EDTA or saturation of surface-expressed phosphatidylserine with Annexin V did not affect PMN leukocyte binding to FXIa (Fig. 2).
Figure 2. Characterization of PMN leukoctye–FXIa interaction.
Purified human PMN leukoctyes (1×106/ml) were incubated over surfaces of FXIa (50 μg/ml) for 30 min at 37°C in the presence of vehicle (PMN leukoctye buffer; –), EDTA (5 mΜ), AnnexinV (AnxV; 10 μg/ml), or serine protease inhibitor PPACK (40 μΜ). In selected experiments, cells were treated with Zn2+ (25 μΜ) and HK (42 nΜ). Results are reported as mean cell count/mm2 ± sem of three independent experiments. P < 0.05 compared with adhesion of vehicle-treated cells in the absence (*) or presence (**) of Zn2+/HK.
Zymogen FXI circulates in complex with HK. In the presence of the divalent cation Zn2+, HK has been shown to regulate FXIa binding to blood platelets [10–12] and endothelial cells [11]. Along these lines, our data show that the presence of HK/Zn2+ enhanced PMN leukocyte binding of FXIa (Fig. 2). This interaction remained sensitive to the presence of PPACK.
Attenuation of fMLP-induced Ca2+mobilization by FXIa
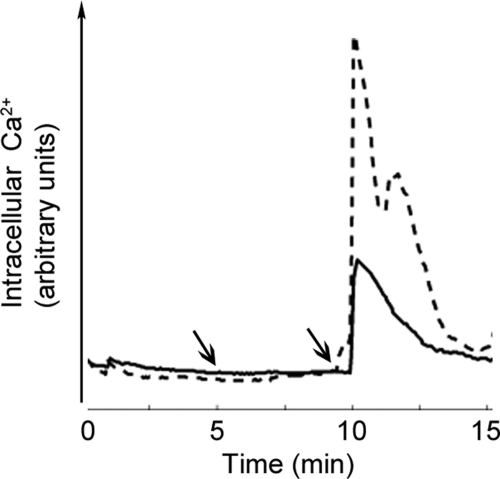
It is known that PMN leukoctye activation is associated with an increase in cytosolic Ca2+, resulting from the mobilization of intracellular Ca2+ stores. In agreement with previous reports, our data show that the exposure of PMN leukocytes to the bacterial peptide, fMLP, resulted in a rapid spike in intracellular Ca2+ (Fig. 3), followed by a rapid decay in intracellular Ca2+ (exponential time constant=3.29±0.22 min). Experiments were designed to determine whether FXIa regulates fMLP-induced Ca2+ mobilization. Our data showed that FXIa substantially inhibited PMN leukoctye intracellular Ca2+ mobilization in response to fMLP. FXIa alone did not induce intracellular Ca2+ mobilization (Fig. 3, first arrow). Taken together, our data suggest that FXIa sequesters the release of intracellular Ca2+ induced by fMLP.
Figure 3. FXIa attenuates fMLP-induced Ca2+mobilization.
Human PMN leukoctyes were loaded with fluo-4 AM (2 μΜ) and plated on a fibronectin surface. Intracellular Ca2+ spikes were monitored for 15 min. Vehicle (PMN leukoctye buffer; dashed line) or FXIa (10 μg/ml; solid line) was added at 5 min (first arrow), followed by the addition of fMLP (10 nΜ) at 10 min (second arrow). Data are presented as mean intensity of five PMN leukoctyes in a field of view from each treatment.
Inhibition of directional chemotaxis by FXIa
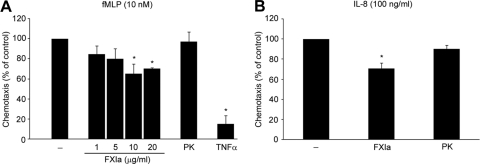
It is well established that PMN leukoctyes migrate to the sites of inflammation in the response to chemoattractants such as fMLP and IL-8. We assessed the effect of FXIa on PMN leukoctye chemotaxis toward fMLP and IL-8 using a Transwell assay. Our data show that FXIa reduced PMN leukoctye migration to fMLP or IL-8 by ∼30% (Fig. 4A and B, respectively). In agreement with previous studies, we show that TNF-α abrogated the chemotactic activity of fMLP (Fig. 4A). In contrast, PK failed to inhibit fMLP-driven chemotaxis (Fig. 4). FXIa itself did not drive PMN leukoctye migration (data not shown).
Figure 4. Inhibitory effects on fMLP-driven PMN leukocyte chemotaxis.
(A) Human PMN leukoctyes, pretreated with vehicle (PMN leukoctye buffer; –), FXIa (1, 5, 10, 20 μg/ml), PK (20 μg/ml), or TNF-α (10 ng/ml), were placed on fibronectin (5 μg/ml)-coated transwell inserts and allowed to migrate toward the fMLP (10 nΜ)-containing lower chamber for 90 min at 37°C. (B) PMN leukoctyes pretreated with vehicle, FXIa (10 μg/ml), or PK (10 μg/ml) were allowed to migrate toward the IL-8 (100 ng/ml)-containing lower chamber for 90 min at 37°C. The results are presented as mean percentage ± sem of the migratory population for each treatment relative to chemoattractant-driven chemotaxis. *P < 0.05 compared with the migration of vehicle-treated cells to the indicated chemoattractants.
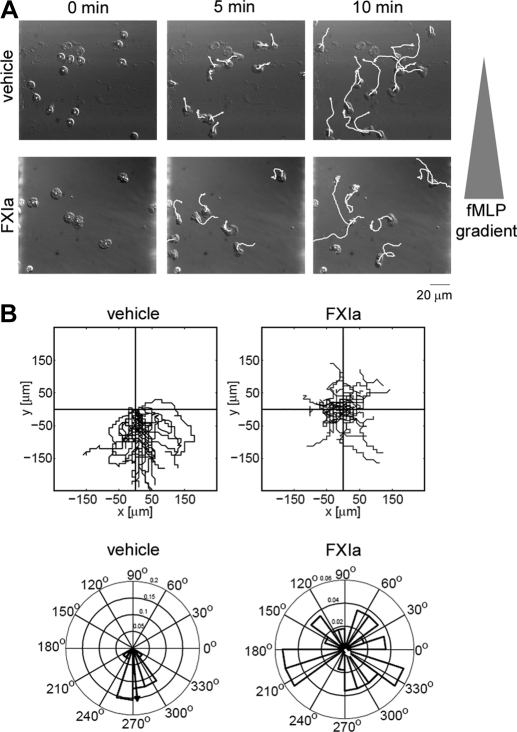
Next, we directly visualized and tracked migrating PMN leukoctyes in real time using an Insall chamber [8]. The linear gradient of the chemoattractant was established by adding fMLP to the outer well, and the time-lapse images were taken at the central area of the wide (1.0 mm) bridge for 30 min. We observed directional migration of vehicle-treated PMN leukoctyes toward the chemoattractant gradient of fMLP (Fig. 5). FXIa significantly diminished PMN leukoctye migration toward the fMLP gradient, resulting in random movement (Fig. 5). In contrast, PK treatment did not affect the guided chemotaxis, whereas only random movement was observed in the absence of fMLP (data not shown). The inhibitory effect of FXIa treatment was confirmed further by Rayleigh's test, vehicle (P=3.43, 3.43×10−15) versus FXIa (P=0.89), indicating that vehicle-treated PMN leukocytes demonstrated a highly significant unimodal deviation (directed migration) from a uniform angular distribution (random migration). The mean resultant vector (arrows in Fig. 5B) for vehicle-treated PMN leukocytes had a magnitude of 0.916 and pointed in the direction of the fMLP gradient (275°) with narrow 95% confidence bounds (±9.5°), whereas the FXIa mean resultant vector was smaller in magnitude (0.062) and pointed away from the gradient (310°) with larger 95% confidence bounds (±86°).
Figure 5. FXIa inhibits directed migration.
Adherent human PMN leukoctyes on fibronectin-coated coverslips were pretreated with vehicle (PMN leukoctye buffer) or FXIa (10 μg/ml) and placed on an Insall chamber. Chemotaxis was induced by adding fMLP (10 nΜ) in the outer well of the chamber. Time-lapse images were taken every 10 s for 30 min. (A) Time-lapse images at the first 0, 5, and 10 min with cell paths (white lines). (B) The PMN leukoctye migration was analyzed using the ImageJ plugin MTrackJ and quantified using the CircStat toolbox for MATLAB. Spider plots indicate the paths of individual cells (upper panels), and rose plots indicate the angular histograms associated with the trajectories (lower panels). The mean resultant vectors are shown in rose plots (arrows).
Concluding remarks
PMN leukoctyes are key mediators of inflammation, and their migration is strictly regulated for host defense and the prevention of tissue damage. In this study, we showed that the coagulation FXIa binds to human PMN leukoctyes and modulates chemotaxis.
Crosstalk between coagulation and inflammation is increasingly thought to be mediated by the contact system, which consists of FXII, FXI, PK, and HK [13]. The activation of FXII leads to the enzymatic generation of FXIa from FXI, as well as the generation of kallikrein from PK. These enzymes in turn are responsible for generation of FIXa and generation of the potent inflammatory and vasodilator peptide BK from HK. Local accumulation of BK triggers the classical signs of inflammation, including vasodilation, increased permeability, pain, and reduced tissue function. FIXa can activate the common pathway of blood coagulation, leading to the generation of the enzyme, thrombin, which can then lead to platelet activation, fibrin formation, and vaso-occlusive thrombi.
In patients with a clinical suspicion of disseminated intravascular coagulation, markers of contact system activation are elevated [14, 15]. In a baboon model of lethal bacteremia, an inhibitory mAb against FXII extended animal survival time [16]. Although the limited treatment failed to prevent disseminated intravascular coagulation, the data suggested that reducing FXII activity lessened the systemic inflammatory response to sepsis. FXII has been shown to induce monocyte IL-1 production and secretion [17], neutrophil aggregation and degranulation [18], and complement activation [19]. Moreover, HK has been shown to drive the secretion of proinflammatory cytokines such as TNF-α, IL-6, and IL-8 from human mononuclear cells [20]. Our study provides the first evidence that FXIa modulates PMN leukocyte intravascular coagulation inflammatory responses. It remains to be determined which receptors and signaling pathway(s) are involved in this interaction. Future studies will be aimed to identify the molecular mechanisms that contribute to the action of FXIa on PMN leukocytes.
Sepsis is characterized by the failure to maintain the necessary balance between excessive and inadequate PMN leukocyte migration to tissues. We have shown previously that FXI-deficient mice have increased survival relative to WT mice in a model of severe polymicrobial peritonitis, and FXI-deficient mice exhibit a reduction in coagulopathy and leukocyte infiltration at the site of infection [4]. Another study using mice deficient in Plg and FXI found an increased leukocyte infiltration in lungs of Plg−/− FXI−/− mice in contrast to Plg−/− FXI+/+ mice [21]. Our in vitro experiments in the present study demonstrate the inhibitory effect of FXIa on human PMN leukocyte chemotaxis to the exogenous chemotactic factors, fMLP and IL-8. These findings imply that FXIa may arrest migrating PMN leukocytes to those sites where coagulation is initiated. Although inflammation promotes the activation of coagulation, perhaps accumulated FXIa on the clot surface promotes PMN leukocyte retention by reducing their ability to leave the area. FXIa may also promote additional coagulation by limiting PMN leukocyte migration and concentrating the PMN leukocyte-associated tissue factor [22, 23], which is a key driver of pathological coagulation [24]. These data implicate a potential mechanism of disease development via the coupling of coagulation and inflammation in sepsis.
ACKNOWLEDGMENTS
This work was supported by NIH (R01HL101972, R43AI088937, and 1U54CA143906-01), the American Heart Association (09GRNT2150003), and Cancer Research UK. We thank Dr. Michelle Berny-Lang and Sawan Hurst for insightful discussions.
Footnotes
- BK
- bradykinin
- FXI/FXII/FIX
- factor XI/XII/IX
- FXIa/XIIa/IXa
- activated factor XI/XII/IX
- HK
- high MW kininogen
- PK
- prekallikrein
- Plg
- plasminogen
- PPACK
- D-Phe-Pro-Arg chloromethyl ketone
- RGD
- arginine-glycine-aspartic acid
AUTHORSHIP
A.I. and O.J.T.M. conceived of and designed the experiments. A.I. performed the experiments. A.I. and K.G.P. analyzed the data. R.H.I. and D.G. contributed reagents/materials/analysis tools. A.I., N.G.V., K.G.P., E.I.T., A.G., and O.J.T.M. wrote the paper. All authors read and approved the final version of the manuscript.
DISCLOSURES
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- 1. Borregaard N. (2010) Neutrophils, from marrow to microbes. Immunity 33, 657–670 [DOI] [PubMed] [Google Scholar]
- 2. Reddy R. C., Standiford T. J. (2010) Effects of sepsis on neutrophil chemotaxis. Curr. Opin. Hematol. 17, 18–24 [DOI] [PubMed] [Google Scholar]
- 3. Levi M., van der Poll T. (2005) Two-way interactions between inflammation and coagulation. Trends Cardiovasc. Med. 15, 254–259 [DOI] [PubMed] [Google Scholar]
- 4. Tucker E. I., Gailani D., Hurst S., Cheng Q., Hanson S. R., Gruber A. (2008) Survival advantage of coagulation factor XI-deficient mice during peritoneal sepsis. J. Infect. Dis. 198, 271–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Emsley J., McEwan P. A., Gailani D. (2010) Structure and function of factor XI. Blood 115, 2569–2577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Henderson L. M., Figueroa C. D., Muller-Esterl W., Bhoola K. D. (1994) Assembly of contact-phase factors on the surface of the human neutrophil membrane. Blood 84, 474–482 [PubMed] [Google Scholar]
- 7. Itakura A., Aslan J. E., Sinha S., White-Adams T. C., Patel I. A., Meza-Romero R., Vandenbark A. A., Burrows G. G., Offner H., McCarty O. J. (2010) Characterization of human platelet binding of recombinant T cell receptor ligand. J. Neuroinflammation 7, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Muinonen-Martin A. J., Veltman D. M., Kalna G., Insall R. H. (2010) An improved chamber for direct visualization of chemotaxis. PLoS ONE 5, e15309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Berens P. (2009) CircStat: a MATLAB toolbox for circular statistics. J. Stat. Softw. 31, 1–21, http://www.jstatsoft.org/v31/i10/paper [Google Scholar]
- 10. Greengard J. S., Heeb M. J., Ersdal E., Walsh P. N., Griffin J. H. (1986) Binding of coagulation factor XI to washed human platelets. Biochemistry 25, 3884–3890 [DOI] [PubMed] [Google Scholar]
- 11. Baird T. R., Walsh P. N. (2002) The interaction of factor XIa with activated platelets but not endothelial cells promotes the activation of factor IX in the consolidation phase of blood coagulation. J. Biol. Chem. 277, 38462–38467 [DOI] [PubMed] [Google Scholar]
- 12. White-Adams T. C., Berny M. A., Tucker E. I., Gertz J. M., Gailani D., Urbanus R. T., de Groot P. G., Gruber A., McCarty O. J. (2009) Identification of coagulation factor XI as a ligand for platelet apolipoprotein E receptor 2 (ApoER2). Arterioscler. Thromb. Vasc. Biol. 29, 1602–1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Colman R. W., Schmaier A. H. (1997) Contact system: a vascular biology modulator with anticoagulant, profibrinolytic, antiadhesive, and proinflammatory attributes. Blood 90, 3819–3843 [PubMed] [Google Scholar]
- 14. Kaufman N., Page J. D., Pixley R. A., Schein R., Schmaier A. H., Colman R. W. (1991) α 2-Macroglobulin-kallikrein complexes detect contact system activation in hereditary angioedema and human sepsis. Blood 77, 2660–2667 [PubMed] [Google Scholar]
- 15. Wuillemin W. A., Fijnvandraat K., Derkx B. H., Peters M., Vreede W., ten Cate H., Hack C. E. (1995) Activation of the intrinsic pathway of coagulation in children with meningococcal septic shock. Thromb. Haemost. 74, 1436–1441 [PubMed] [Google Scholar]
- 16. Pixley R. A., De La Cadena R., Page J. D., Kaufman N., Wyshock E. G., Chang A., Taylor F. B., Jr., Colman R. W. (1993) The contact system contributes to hypotension but not disseminated intravascular coagulation in lethal bacteremia. In vivo use of a monoclonal anti-factor XII antibody to block contact activation in baboons. J. Clin. Invest. 91, 61–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Toossi Z., Sedor J. R., Mettler M. A., Everson B., Young T., Ratnoff O. D. (1992) Induction of expression of monocyte interleukin 1 by Hageman factor (factor XII). Proc. Natl. Acad. Sci. USA 89, 11969–11972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wachtfogel Y. T., Pixley R. A., Kucich U., Abrams W., Weinbaum G., Schapira M., Colman R. W. (1986) Purified plasma factor XIIa aggregates human neutrophils and causes degranulation. Blood 67, 1731–1737 [PubMed] [Google Scholar]
- 19. Ghebrehiwet B., Silverberg M., Kaplan A. P. (1981) Activation of the classical pathway of complement by Hageman factor fragment. J. Exp. Med. 153, 665–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Khan M. M., Bradford H. N., Isordia-Salas I., Liu Y., Wu Y., Espinola R. G., Ghebrehiwet B., Colman R. W. (2006) High-molecular-weight kininogen fragments stimulate the secretion of cytokines and chemokines through uPAR, Mac-1, and gC1qR in monocytes. Arterioscler. Thromb. Vasc. Biol. 26, 2260–2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cheng Q., Zhao Y., Lawson W. E., Polosukhin V. V., Johnson J. E., Blackwell T. S., Gailani D. (2005) The effects of intrinsic pathway protease deficiencies on plasminogen-deficient mice. Blood 106, 3055–3057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Giesen P. L., Rauch U., Bohrmann B., Kling D., Roque M., Fallon J. T., Badimon J. J., Himber J., Riederer M. A., Nemerson Y. (1999) Blood-borne tissue factor: another view of thrombosis. Proc. Natl. Acad. Sci. USA 96, 2311–2315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Maugeri N., Brambilla M., Camera M., Carbone A., Tremoli E., Donati M. B., de Gaetano G., Cerletti C. (2006) Human polymorphonuclear leukocytes produce and express functional tissue factor upon stimulation. J. Thromb. Haemost. 4, 1323–1330 [DOI] [PubMed] [Google Scholar]
- 24. Todoroki H., Nakamura S., Higure A., Okamoto K., Takeda S., Nagata N., Itoh H., Ohsato K. (2000) Neutrophils express tissue factor in a monkey model of sepsis. Surgery 127, 209–216 [DOI] [PubMed] [Google Scholar]