The immunomodulatory activities of LT-IIa-B5 depend upon the adjuvants's capacity to activate and promote migration of DC in mucosal tissues in a strictly TLR2-dependent manner.
Keywords: antigen, migration, CD4+, T cell, CCR7, lymph node, DO11.10 mice
Abstract
A host of human pathogens invades the body at mucosal surfaces. Yet, strong, protective mucosal immune responses directed against those pathogens routinely cannot be induced without the use of adjuvants. Although the strongest mucosal adjuvants are members of the family of HLTs, the inherent toxicities of HLT holotoxins preclude their clinical use. Herein, it is shown that LT-IIa-B5 enhances mucosal immune responses by modulating activities of DCs. i.n. immunization of mice with OVA in the presence of LT-IIa-B5 recruited DCs to the NALT and significantly increased uptake of OVA by those DCs. Furthermore, LT-IIa-B5 increased expression of CCR7 by DCs, which mediated enhanced migration of the cells from the NALT to the draining CLNs. LT-IIa-B5 also enhanced maturation of DCs, as revealed by increased surface expression of CD40, CD80, and CD86. Ag-specific CD4+ T cell proliferation was augmented in the CLNs of mice that had received i.n. LT-IIa-B5. Finally, when used as an i.n. adjuvant, LT-IIa-B5 dramatically increased the levels of OVA-specific salivary IgA and OVA-specific serum IgG. Strikingly, each of the activities induced by LT-IIa-B5 was strictly TLR2-dependent. The data strongly suggest that the immunomodulatory properties of LT-IIa-B5 depend on the productive modulation of mucosal DCs. Notably, this is the first report for any HLT to demonstrate in vivo the elicitation of strong, TLR2-dependent modulatory effects on DCs with respect to adjuvanticity.
Introduction
A majority of human pathogens invaded and/or colonized mucosal surfaces [1]. To protect against these mucosal pathogens, humans evolved highly specialized innate and adaptive mucosal immune systems. The importance of these systems to human life is reflected in the observation that ∼80% of all immune cells in a healthy adult human are found in the various inductive and effector sites of the gut, respiratory, nasal, and other mucosae [2]. Yet, endogenous cellular and molecular mechanisms in these mucosae commonly down-regulate mucosal immune responses to foreign Ag [3], likely as a means of avoiding elicitation of immune responses to the plethora of harmless Ags that are routinely encountered by the mucosae. As a result of the effects of these mucosal immunosuppressive mechanisms, it is often difficult to elicit protective immune responses against invading pathogens by the use of mucosal vaccines. In fact, a large number of experimental mucosal vaccines have failed to evoke strong, protective, Ag-specific mucosal immune responses [4]. The immunosuppressive activities of the mucosae, however, can usually be circumvented by the use of mucosal adjuvants.
A common route for mucosal immunization is the i.n. cavity [5], which in the mouse, takes advantage of inductive sites located in the nasal sinus, where collections of epithelial cells and immune cells are situated within a specific mucosal inductive site, referred to as the NALT. Immune cells located within the murine NALT modulate the initial direction of the mucosal immune response to nasally encountered foreign Ags [5, 6]. The NALT is neither encapsulated nor supplied by afferent lymphatics [7]. Rather, the NALT has a specialized dome epithelium containing membranous cells (M-type cells), which transport Ag from the luminal surface of the epithelium to the APCs, which are located directly underneath the dome [7]. Various types of immune cells reside in the NALT, including T cells, B cells, and APCs [8].
A variety of studies indicates that the major APCs residing in the NALT are DCs [9]. Sentinel DCs located in the NALT and in proximally located nasal mucosal sites are critical for acquiring and presenting foreign Ags to T cells as a prelude to initiating an Ag-specific adaptive immune response [8]. Once having encountered, acquired, and processed Ags, DCs in the NALT migrate to the CLN, the initial effector site for the NALT, where the DCs engage T cells and promote Ag-specific T cell proliferation [6, 10–12]. Thus, the process of Ag uptake by resident DCs or by DCs that are recruited to the NALT is a critical step in a cascade of immunological events that promote Ag-specific adaptive immune responses in the mucosa [13].
To date, the most potent mucosal adjuvants belong to the family of bacterial HLTs, which is comprised of two distinctive subfamilies [14–17]. The type I subfamily consists of LT-I produced by Escherichia coli and choleratoxin (CT) produced by Vibrio cholerae. The type II subfamily is composed of LT-IIa, LT-IIb, and LT-IIc. All members of the HLT family are oligomeric proteins, comprised of a single A polypeptide (∼28 kDa), which encodes a strong ADP-ribosylase activity, and five B polypeptides (∼12 kDa), which are arranged into a pentamer. The B pentamer is noncovalently bound to the A subunit to form the ∼90-kDa oligomeric protein [14, 15]. Each of the members of the HLT superfamily exhibits strong immunomodulatory activities. For example, in contrast to mice i.n.-administered AgI/II [18], mice i.n.-coimmunized with AgI/II and LT-IIa, the prototypical type II HLT, produced high levels of Ag-specific IgA in saliva and in vaginal fluids and enhanced levels of Ag-specific IgG in serum [19, 20]. Based on the patterns of cytokine expression and IgG subclass distributions, the immunomodulatory properties of LT-IIa are clearly distinctive from those elicited by CT, LT-I, or the other type II HLTs [4, 14–16].
Recently, it has been shown that the nontoxic LT-IIa-B5 and the closely related LT-IIb-B5 interact productively with TLR2, a molecule of the innate immune system that is expressed on the surface of mouse and human monocytes [21, 22]. In vitro treatment of monocytes with the B pentamers activates NF-κB in those cells in a TLR2-dependent manner. In silico mapping and mutational evaluation revealed a small domain located on LT-IIa-B5 and LT-IIb-B5, which is essential for the physical interaction of the pentamers with TLR2 [22]. The importance of the capacity of LT-IIa-B5 (or of LT-IIb-B5) to functionally interact with TLR2, however, has not been evaluated in vivo.
With the use of a mouse i.n. immunization model, it is demonstrated herein that LT-IIa-B5, when i.n.-coadministered with Ag, dramatically altered a variety of cellular immune responses by DCs in the NALT and the CLN. Furthermore, these in vivo data support a model in which the adjuvant properties of LT-IIa-B5 are dependent on the capacity of the nontoxic pentamer to modulate the cellular activities of DCs in a manner that is strictly dependent on cellular expression of TLR2.
Of note is that this study is the first report for which the in vivo immunomodulatory properties of any HLT have been shown to be dependent on TLR2.
MATERIALS AND METHODS
Mice
BALB/c mice were purchased from Harlan Laboratories (Somerville, NJ, USA). C57Bl/6 mice, B6.129-Tlr2tm1Kir/J knockout mice (TLR2−/−) [23], B6.129P(2)-CCR7tm1Rfor/J(CCR7−/−) mice, and DO11.10 mice [24], expressing the αβ-TCR specific for the OVA epitope (323–339), recognized in the context of the MHC II molecule I-Ad, were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). To generate BALB/c(TLR2−/−) mice, the C57Bl/6 mouse B6.129-Tlr2tm1Kir/J mouse was backcrossed for seven generations with BALB/c mice using the Max Bax speed congenic method (Charles River Laboratories, Wilmington, MA, USA). Seventh-generation backcrossed mice were 99.52% BALB/c in genotype. Females (6–8 weeks of age) were used for all experiments. All animal experiments were approved by the Institutional Animal Care and Use Committee at the University at Buffalo (Buffalo, NY, USA).
Antibodies and other reagents
Allophycocyanin-conjugated anti-mouse CD11c (clone HL3), PE-conjugated anti-mouse CD80 (clone 16-10A1), PE-conjugated anti- mouse CD86 (clone GL1), PE-conjugated anti-mouse CCR6 (clone 29-2L17), and PE-conjugated anti-hamster IgG, κ isotype control (clone B81-3), were obtained from BD Biosciences (Franklin Lakes, NJ, USA). PerCP-conjugated anti-mouse CD11c (clone N418), PerCP-conjugated anti-mouse CD4 (clone RM4-5), allophycocyanin-conjugated anti-mouse CD4 (clone RM4-5), PE-conjugated anti-mouse CD40 (clone 3/23), and PE-conjugated rat IgG2a, κ isotype control (clone RTK2758) were purchased from BioLegend (San Diego, CA, USA). Allophycocyanin-conjugated anti-mouse DO11.10 TCR (clone KJ1-26) was purchased from eBioscience (San Diego, CA, USA). The Vyrant CFDA succinimidyl ester cell tracer kit (CFSE) was obtained from Molecular Probes (Eugene, OR, USA).
Cloning and purification of LT-IIa-B5
Methods were reported previously for cloning and purifying the His-tagged LT-IIa-B5 [19]. Briefly, a plasmid designated pHN1.1 encoding LT-IIa-B5 was introduced into Escherichia coli DH5αF'Kan (Life Technologies, Gaithersburg, MD, USA). Expression of LT-IIa-B5 by the transformant was induced by addition of 1 mM isopropyl-β-d-thiogalactoside to log-phase cultures. LT-IIa-B5 was extracted from the cells using isotonic shock and purified by nickel affinity chromatography (Qiagen, Valencia, CA, USA) [25, 26]. If contaminating proteins were evident in the eluate, the pentamer was purified further by gel filtration using a Sephacryl S-100 gel filtration column (GE Healthcare, Piscataway, NJ, USA). SDS-PAGE and immunoblotting using polyclonal antibodies against LT-IIa holotoxin [26] were used to confirm homogeneity of the purified LT-IIa-B5. Purified protein was analyzed for endotoxin by use of an end-point quantitative Limulus amoebocyte lysate assay (Charles River Endosafe, Charleston, SC, USA). The level of endotoxin in purified preparation of LT-IIa-B5 was <0.003 ng/μg protein, a level considerably below the level required to evoke an immune response in cells. LT-IIa-B5 purified in this manner fails to activate TLR4-expressing cells, further confirming lack of biologically active endotoxin [21].
In vivo Ag uptake assay
Ag uptake by DC in NALT was measured using an established method with minor modifications [27]. To stain nasal cells, 20 μl of a 500-μM CFSE solution was i.n.-administered to the external nares of female C57Bl/6 mice and B6.129-Tlr2tm1Kir/J knockout mice. After 2 h, PBS or 200 μg Alexa 647-OVA (Invitrogen-Molecular Probes, Eugene, OR, USA), dissolved in PBS, with or without 10 μg LT-IIa-B5, was i.n.-administered at 15 μl vol, divided between each external naris. After 18 h, NALTs were removed and the tissue digested for 1 h in a PBS solution containing 1 mg/ml collagenase D and 0.5 mg/ml DNase I. Total cells from NALT were resuspended in FACS buffer (PBS containing 2% BSA) by meshing the tissue through a 40-μm nylon cell strainer (BD Falcon, Franklin Lakes, NJ, USA). After staining for PerCP-conjugated anti-mouse CD11c, cells were washed and resuspended in FACS buffer. Fluorescence of the CD11c-positive and CFSE-positive DC was measured using a FACSCaliber four-color flow cytometer (Becton Dickinson, Bedford, MA, USA). Fluorescence values were reported as MFI.
Migration assay
Migration of DC from NALT and nasal mucosae into the CLN was analyzed by use of an established method, with minor modifications [27]. To stain nasal cells, 20 μl of a 500-μM solution of CFSE was i.n.-administered to the external nares. After 2 h, female C57Bl/6 mice were immunized by the i.n. route with PBS or with 100 μg chicken OVA (Sigma-Aldrich, St. Louis, MO, USA) in the presence or absence of 10 μg LT-IIa-B5 in a 15-μl vol, divided equally between both nares. After 2 days, CLN were removed and digested for 1 h in a PBS solution containing 1 mg/ml collagenase D and 0.5 mg/ml DNase I. Total cells from CLN were resuspended in FACS buffer (PBS containing 2% BSA) by meshing the tissue through a 40-μm nylon cell strainer (BD Falcon). After staining for allophycocyanin-conjugated anti-mouse CD11c, cells were washed and resuspended in FACS buffer. The number of the CD11c- and CFSE-positive DCs in the cell mixture was measured using a FACSCaliber four-color flow cytometer (Becton Dickinson) with Flow-Count fluorospheres (Beckman Coulter, Fullerton, CA, USA) used as a standard.
To examine whether LT-IIa- and LT-IIa-B5-mediated migration of DCs into CLNs requires CCR7 [28–30], the number of the CD11c- and CFSE-positive DCs in CLNs from B6.129P(2)-CCR7tm1Rfor/J(CCR7−/−) mice was determined, as above.
Costimulatory molecule expression
Ten micrograms of LT-IIa-B5 or PBS in a final volume of 15 μl was i.n.-administered to the external nares of mice at a point 2 h after i.n. administration of 20 μl of a 500-μM solution of CFSE. After 2 days, CLNs were removed and digested for 1 h in PBS containing 1 mg/ml collagenase D and 0.5 mg/ml DNase I. Cells were obtained by suspending the tissues in FACS buffer (PBS containing 2% BSA) and meshing through a 40-μm nylon cell strainer (BD Falcon). After staining for allophycocyanin-conjugated anti-mouse CD11c, PE-conjugated anti-mouse CD80 (clone 16-10A1), PE-conjugated anti-mouse CD40 (clone 3/23), PE-conjugated anti-mouse CD86 (clone GL1), PE-conjugated anti-hampster IgG isotype control (clone B81-3), and PE-conjugated anti-rat IgG2a, κ isotype control (clone RTK2758), cells were washed and resuspended in FACS buffer. Expression of CD86 and CD80 on the CD11c+CFSE+ cells was quantified using a FACSCaliber four-color flow cytometer (Becton Dickinson).
In vivo Ag-specific CD4+ T cell proliferation assay
CFSE-stained, naïve CD4+ T cells (2×106), isolated from the spleen of DO11.10 mice using a CD4+ T cell negative-selection isolation kit (Miltenyi Biotec, Auburn, CA, USA), were i.p.-transferred into female BALB/c mice or BALB/c(TLR2−/−) mice. After 1 day, mice were administered PBS or with 100 μg OVA (Sigma-Aldrich) in the presence or absence of 10 μg LT-IIa-B5 in a 15-μl final volume that was divided between both external nares. After 3 days, CLNs were removed and total cells isolated, as detailed above. After staining with PerCP-conjugated anti-mouse CD4 and allophycocyanin-conjugated anti-mouse DO11.10 TCR, cells were washed and resuspended in FACS buffer. The levels of proliferation of the DO11.10 CD4+ T cells in the mixtures were determined by measuring the dilution of CFSE fluorescence using a FACScaliber four-color flow cytometer (Becton Dickinson).
Immunization assay
i.n. immunizations were performed using a well-established mouse mucosal immunization model [19]. Immunizations were administered in a standardized volume (15 μl), which was applied slowly to both external nares of nonanesthetized mice. Female BALB/c mice (6–8 weeks of age) were immunized with a solution containing 100 μg OVA (Sigma-Aldrich), with or without 10 μg LT-IIa-B5. Groups were boosted with the same combination of OVA and LT-IIa-B5 at Days 10 and 20. Samples of serum and saliva were collected from mice at 1 week before the initial immunization (preimmune) and at Day 35 after the primary immunization, which has been shown to be the peak of the humoral immune response [16, 20]. Prior to use in assays, saliva and serum samples were stored at –80°C. The levels of Ag-specific IgA and IgG antibodies in saliva and sera were determined by ELISA [20].
Statistical analysis
ANOVA and the Tukey multiple-comparison test were used for multiple comparisons. Unpaired t tests were performed to analyze differences between two groups. Statistical analyses were performed using the InStat software package (GraphPad Software, San Diego, CA, USA). Differences were considered significant at P ≤ 0.05.
RESULTS
Enhanced uptake of Ag by DC
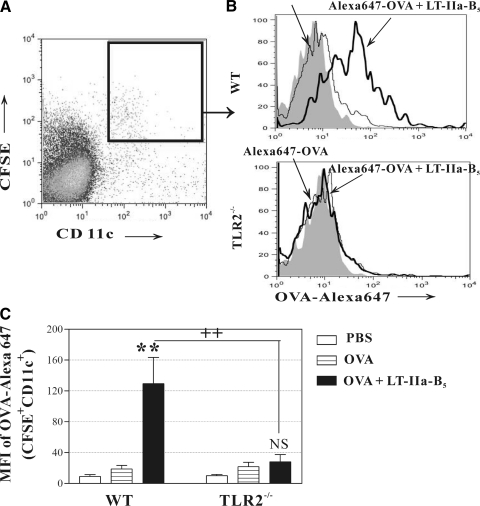
Uptake of Ag by DCs and other APCs is the initial step in a cascade of cellular events that initiates Ag-specific adaptive immune responses [31]. To determine whether i.n.-administered LT-IIa-B5 had the capacity to influence the efficiency of Ag uptake by DCs located in the NALT, mice were i.n.-administered CFSE, a nonspecific fluorescent staining agent that covalently couples to intracellular molecules, to enable simultaneous tracking of cells. After 2 h to allow CFSE to stain all cells in the nasal cavity, CFSE-painted mice were i.n.-immunized with Alexa 647-conjugated OVA in the presence or absence of LT-IIa-B5. After 18 h, cells were isolated from the NALT, and the fluorescent intensity of the CFSE+CD11c+OVA+ cells in each group of mice was analyzed (Fig. 1A). In comparison with DCs from mice immunized only with Alexa 647-OVA, DCs from mice immunized with Alexa 647-OVA in combination with LT-IIa-B5 exhibited a significant enhancement (6.84-fold) in uptake of the Ag (Fig. 1B and C; OVA vs. OVA+LT-IIa-B5). It should be noted that uptake of OVA by macrophages, the other CD11c-expressing APC, was not enhanced by LT-IIa-B5 (unpublished results).
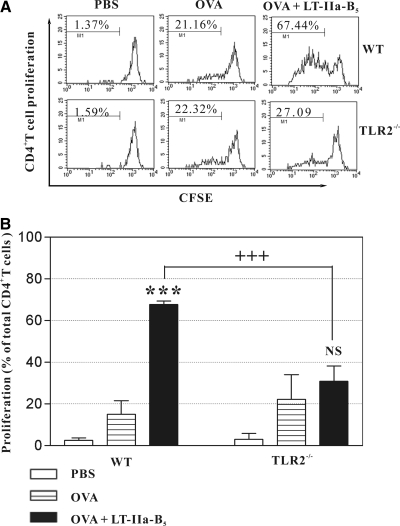
Figure 1. Uptake of OVA by DCs.
Conventional C57Bl/6 or C57Bl6(TLR2−/−) mice were i.n.-administered Alexa 647-OVA (OVA; control) or Alexa 647-OVA in combination with LT-IIa-B5 (OVA+LT-IIa-B5) at a point 2 h after nasal administration of CFSE. Eighteen hours after immunization, NALT was removed. Total cells from NALT were stained with PerCP-anti-mouse CD11c. The amount of Alexa 647-OVA associated with CFSE+CD11c+ DCs from the NALT was measured using flow cytometry. (A) CFSE+CD11c+ cells for subsequent analysis were gated, as shown. (B) Uptake of Alexa 647-OVA by CFSE+CD11c+ DCs from the NALT. Data from one of three independent experiments, each of which exhibited consistent results, are shown. The solid histogram indicates the fluorescence of cells acquired from PBS-administered mice as an untreated control. (C) Graphical comparison of uptake of Alexa 647-OVA by CFSE+CD11c+ DCs from the NALT. Error bars denote 1 sd from the mean for groups of three mice. Data are shown from one of two independent experiments, each of which yielded consistent results. WT, TLR2-proficient C57Bl/6 mice; TLR2−/−, TLR2-deficient mice; **statistical difference from the control (OVA; P<0.01); ++statistical difference (OVA+LT-IIa-B5-treated, WT vs. TLR2-deficient) control (P<0.01); NS, OVA versus OVA + LT-IIa-B5, TLR2−/− mice.
Prior experiments demonstrated that TLR agonists have the capacity to enhance cellular uptake by bone marrow-derived DCs of fluorescently labeled dextran [32]. Studies from our laboratory demonstrated that general activation of human THP-1 monocytic cells by LT-IIb-B5 required cellular expression of TLR2 [21, 33]. As a domain identical to the TLR2-binding domain of LT-IIb-B5 is located in LT-IIa-B5 (unpublished results), Ag uptake studies were repeated in a strain of mice deficient in expression of TLR2 to determine whether TLR2 was required for augmented uptake of Ag by DC in mice i.n.-administered LT-IIa-B5 [34]. In the absence of cellular expression of TLR2, LT-IIa-B5 was unable to stimulate DCs from the NALT to increase uptake of Alexa 647-OVA (Fig. 1C).
These experiments clearly demonstrated that the LT-IIa-B5 had the capacity to enhance uptake of Ag by DC in the NALT and that the augmented uptake was dependent on cellular expression of TLR2 in the immunized mice.
Enhanced numbers of DC in the NALT and CLN
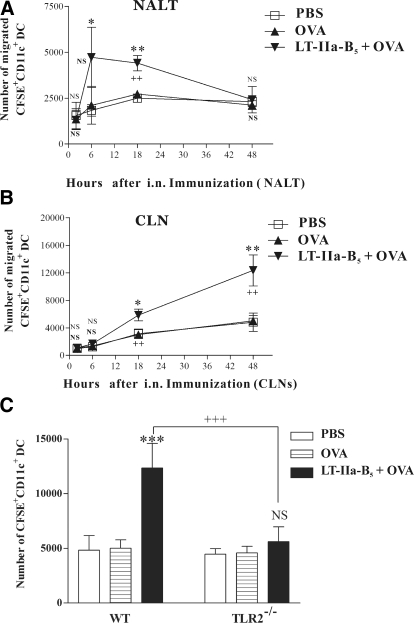
To initiate Ag-specific immune responses, DCs located in Ag-inductive sites migrate into DLNs to interact with naïve CD4+ and CD8+ T cells [35, 36]. To determine whether LT-IIa-B5, when used as a mucosal adjuvant, alters the populations of DCs in the NALT and DLNs, the nasal tissues of BALB/c mice were painted with CFSE, and changes in the number of CFSE+ DCs at various time-points were determined in lymphoid tissues after i.n. administration of the mice with OVA in the presence or absence of LT-IIa-B5.
At 2 h postimmunization, similar numbers of CD11c+ cells were detected in the NALT of mice that had been immunized with OVA or with OVA + LT-IIa-B5 (Fig. 2A). By 6 h postimmunization, distinctive differences in the number of CD11c+ cells in the NALT of the immunized mice were observed. In comparison with the NALT of mice receiving PBS or OVA, the NALT of mice immunized with OVA + LT-IIa-B5 had a significant increase in the numbers of CD11c+ cells entering into the site (Fig. 2A). By 18 h, the differences of the number of DCs in the NALT of mice receiving OVA or OVA + LT-IIa-B5 began to diminish. By 48 h, the numbers of CD11c+ cells in the NALT of the two groups of immunized mice were similar (Fig. 2A).
Figure 2. Time-course of DC migration.
At a time-point 2 h after i.n. administration of CSFE, C57Bl/6 mice were i.n.-immunized with OVA (control) or with OVA in combination with LT-IIa-B5 (OVA+LT-IIa-B5). CLN and NALT were removed at time-points 2, 6, 18, and 48 h after immunization. The number of CFSE+CD11c+ DCs in NALTs and CLNs was enumerated using flow cytometry by using Flow-Count fluorospheres as an external control particle. (A) Number of CFSE+CD11c+ DCs in NALTs. Error bars denote 1 sd from the mean for groups of three mice. (B) Number of CFSE+CD11c+ DCs in CLNs. Error bars denote 1 sd from the mean for groups of three mice. Data are shown from one of two independent experiments, each of which yielded consistent results. *Statistical difference from the untreated control (PBS) at P < 0.05; **statistical difference from the untreated control (PBS) at P < 0.01; ++statistical difference from the un-treated control (OVA) at P < 0.01; NS, boldface; PBS versus OVA + LT-IIa-B5; OVA versus OVA + LT-IIa-B5. (C) Enhanced migration of DCs induced by LT-IIa-B5 requires TLR2. Conventional C57Bl/6 mice or C57Bl/6(TLR2−/−) mice were immunized with OVA (control) or with OVA in combination with LT-IIa-B5 (OVA+LT-IIa-B5) at a time-point 2 h after i.n. administration of CSFE. Cells obtained from the CLN at a point 2 days after immunization were stained with allophycocyanin-anti-mouse CD11c, and the numbers of CFSE+CD11c+ DCs from the CLNs were determined using flow cytometry and Flow-Count fluorospheres as an external control. Error bars denote 1 sd from the mean for groups of three mice. Data are shown from one of two independent experiments, each of which yielded consistent results. ***Statistical difference from control (OVA vs. OVA+LT-IIa-B5; P<0.001); +++statistical difference from the matched control (WT vs. TLR2-deficient); NS, OVA versus OVA + LT-IIa-B5, TLR2−/− mice.
Similar experiments were performed to determine if i.n. administration of LT-IIa-B5 altered the numbers of nasally derived DCs in the CLN. Similar numbers of nasally derived DCs were observed in the CLN of mice receiving OVA or the mock immunization (PBS) at 6, 18, and 24 h after receiving LT-IIa-B5 (Fig. 2B). By 18 h, however, the numbers of nasally derived DCs were elevated substantially in the CLN of mice that had been i.n.-immunized with OVA in combination with LT-IIa-B5, a difference that continued to increase for at least 48 h (Fig. 2B).
These results were consistent with a model in which i.n. administration of LT-IIb-B5 enhanced recruitment into the NALT of local DCs from other nasal tissues and enhanced recruitment of those NALT-associated DCs into the CLNs.
To ascertain the importance, if any, of TLR2 in the enhanced numbers of DCs in the NALT and CLN of mice immunized with OVA in the presence of LT-IIa-B5, similar experiments to enumerate DCs in those tissues were performed. WT and TLR2-deficient mice, which had been i.n.-painted with CFSE, were immunized subsequently with OVA or with OVA + LT-IIa-B5, and the numbers of nasally derived DCs in the CLNs were determined at a time-point 48 h after immunization. As observed earlier, mice immunized with OVA + LT-IIa-B5 had increased numbers (2.46-fold) of DCs in the CLNs in comparison with the numbers of DCs in the CLNs of mice immunized solely with OVA (Fig. 2C). In contrast, no differences in the numbers of DCs in the CLNs were observed in TLR2-deficient mice that had been immunized with OVA or with OVA + LT-IIa-B5 (Fig. 2C). These data demonstrated that LT-IIa-B5 had the capacity to influence migration of DCs from the NALT to the CLN but only when the mouse expressed TLR2.
Enhanced expression of CCR7
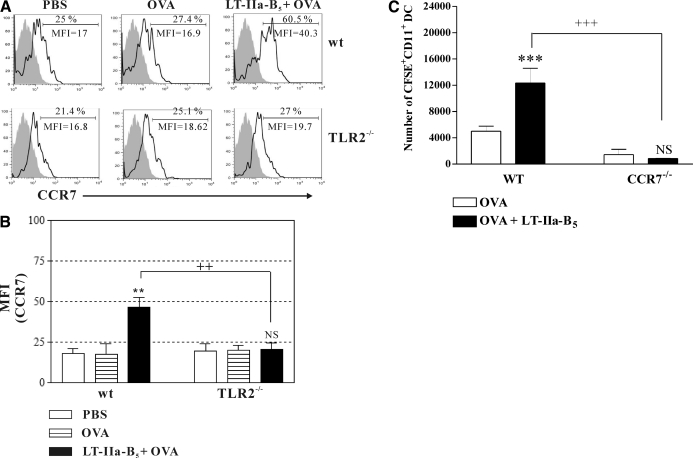
The enhanced numbers of nasally derived DCs observed in the CLN of mice receiving LT-IIa-B5 could have been a result of induction of a more efficient migratory activity by those cells. Migration of DCs into secondary LNs is modulated by the CCR7 [37, 38], a surface molecule that is up-regulated upon maturation of DCs [39, 40]. To determine if enhanced migration of nasal DCs into the CLN could be explained by an increased expression of CCR7 by those cells, mice, in which nasal tissues had been painted with CFSE, were i.n.-immunized with OVA in the presence or absence of LT-IIa-B5. After 2 days, CLNs were removed from the immunized mice, and the levels of expression of CCR7 were measured in CFSE+CD11c+ cells obtained from that lymphoid tissue. In comparison with mice immunized only with OVA, expression of CCR7 by CLN DCs, which had migrated from the NALTs, was increased dramatically in mice receiving i.n. administration of OVA + LT-IIa-B5 (MFIs: 16.9 vs. 40.3; Fig. 3A).
Figure 3. LT-IIa-B5 enhances expression of CCR7 in a TLR2-dependent manner.
Conventional C57Bl/6 mice or C57Bl/6(TLR2−/−) mice were immunized with OVA (OVA; control) or OVA in combination with LT-IIa-B5 (OVA+LT-IIa-B5) at a time-point 2 h after i.n. administration of CFSE. CLNs were removed at 2 days, and the dispersed cells stained with allophycocyanin-anti-mouse CD11c and PE-anti-mouse CCR7. Levels of CCR7 on CFSE+CD11c+ DCs derived from CLNs were measured by flow cytometry. (A) Histographic analysis of expression of CCR7 on CFSE+CD11c+ DCs from the CLNs. Data are presented from one of three independent experiments, each of which yielded consistent results. The MFI values for CCR7 are denoted within each histogram. The isotype control is indicated by the solid histogram. (B) Graphical comparison of the expression of CCR7 on CFSE+CD11c+ DCs from the CLN. Error bars denote 1 sd from the mean for groups of three mice. Data are shown from one of two independent experiments, each of which yielded consistent results. WT, TLR2-proficient C57Bl/6 mice; TLR2−/−, TLR2-deficient mice; **statistical difference from the untreated control (P<0.01); ++statistical difference from the matched (WT vs. TLR2-deficient) control; NS, OVA versus OVA + LT-IIa-B5, TLR2−/− mice. (C) Enhanced migration of DCs into CLNs induced by LT-IIa-B5 requires CCR7. C57Bl/6 mice or C57Bl/6(CCR7−/−) mice were immunized with OVA (control) or with OVA in combination with LT-IIa-B5 at a time-point 2 h after i.n. administration of CFSE. CLNs were removed at 2 days after i.n. immunization, and total cells were stained with allophycocyanin-anti-mouse CD11c. The numbers of CFSE+CD11c+ DCs from CLNs were determined using flow cytometry and Flow-Count fluorospheres as an external control. Error bars denote 1 sd from the mean for groups of three mice. WT, CCR7-proficient C57Bl/6 mice; CCR7−/−, CCR7-deficient mice; ***statistical difference from the control (OVA; P<0.001); +++statistical difference from the matched control (WT vs. CCR7-deficient); NS, OVA versus OVA + LT-IIa-B5, CCR7−/− mice.
Thus, the capacity of LT-IIa-B5 to increase the numbers of DCs in the CLN was correlated directly with the capacity of the pentamer to augment expression of CCR7 on those cells, which likely altered the efficiency of DC migration from the inductive site (NALT) to the effector site (CLN).
The capacity of LT-IIa-B5 to enhance expression of CCR7 by NALT-derived DCs in the CLNs also required expression of TLR2. In comparison with TLR2-proficient mice, no enhanced expression of CCR7 was evident in NALT-derived DCs obtained from TLR2−/− mice that had been mucosally immunized with LT-IIa-B5 + OVA (Fig. 3B).
To confirm the importance of CCR7 in LT-IIa-B5-enhanced migration, similar mucosal immunization experiments were performed using CCR7−/− mice. The absence of CCR7 in the mice abrogated the capacity of LT-IIa-B5 to enhance migration of DCs from the NALT to the CLN (Fig. 3C).
Collectively, these data indicated that NALT-derived DCs stimulated by LT-IIa-B5 did not use alternative chemokine/chemokine receptor systems in any significant manner to enhance migration into the CLN.
Increased expression of costimulatory molecules
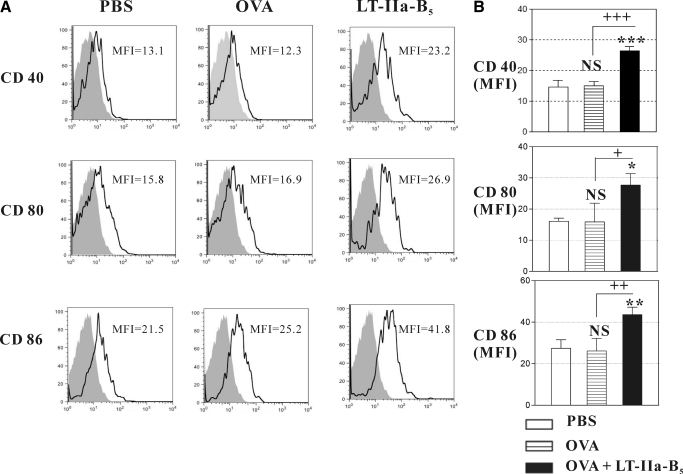
For full activation of naïve T cells, DCs express a number of costimulatory molecules, including CD40, CD80, and CD86 [41]. To determine whether LT-IIa-B5 had the capacity to alter in vivo expression of costimulatory molecules by DCs, mice were i.n.-administered OVA in the presence or absence of LT-IIa-B5, and the expression status of CD40, CD80, and CD86 was determined on nasally derived DCs isolated from the CLN (Fig. 4A). In comparison with mice administered only OVA, mice receiving LT-IIa-B5 + OVA exhibited elevated expression of CD40 (1.75-fold), CD80 (1.82-fold), and CD86 (1.67-fold; Fig. 4B).
Figure 4. Expression of costimulatory molecules.
C57Bl/6 mice were immunized with OVA (control) or with a combination of OVA and LT-IIa-B5 at a time-point 2 h after i.n. administration of CFSE. CLNs were removed at Day 2, at which time, total cells were stained with allophycocyanin-anti-mouse CD11c, PE-anti-mouse CD40, PE-anti-mouse CD80, or PE-anti-mouse CD86. Fluorescence levels of PE in CFSE+CD11c+ DCs from the CLN were measured by flow cytometry. (A) Histographic analysis of expression of CD40, CD80, and CD86 on CFSE+CD11c+ DCs. Data are from one of three independent experiments, each of which yielded consistent results. The MFI values for CD40, CD80, and CD86 are denoted within each histogram. The isotype control is denoted by the solid histogram. (B) Graphical comparison of the expression of CD40, CD80, and CD86 of CFSE+CD11c+ DCs. Error bars denote 1 sd from the mean for three mice/group. Data are shown from one of two independent experiments, each of which yielded consistent results. *, **, and ***Statistical difference from control (OVA; P<0.05, P<0.01, and P<0.001, respectively); +, ++, and +++statistical difference from untreated (PBS; P<0.05, P<0.01, and P<0.001, respectively); NS, not significant in comparison to mice receiving only OVA.
The elevated levels of these three costimulatory molecules on DCs, induced by LT-IIa-B5, would be expected to improve the capacity of APCs to interact with naïve T cells in effector sites such as the CLN.
Augmented Ag-specific proliferation of CD4+ T cells
CD4+ T cells orchestrate a broad range of cellular and humoral adaptive immune responses. Thus, Ag-specific proliferation of naïve CD4+ T cells induced by interactions with APCs is a critical event in the processes required to initiate adaptive immune responses [42]. From the results of the prior experiments, it was clear that LT-IIa-B5 enhanced uptake of Ags by DCs, increased migration of DCs from the NALT to the CLN, and elevated expression of the costimulatory molecule on nasally derived DCs in the CLN. To determine whether those effects of LT-IIa-B5 on DC phenotypes translated into increased activation of Ag-specific CD4+ T cells in the CLN, a syngeneic, adoptive transfer mouse model was used. CFSE-labeled DO11.10 CD4+ T cells were adoptively transferred into naïve BALB/c mice, which were subsequently i.n.-immunized with OVA or with OVA + LT-IIa-B5. Three days after i.n. administration, CLNs were removed, and the relative degrees of proliferation of the CFSE-stained DO11.10 CD4+ T cells residing in that lymphoid tissue were analyzed.
In comparison with DO11.10 CD4+ T cells from the CLN of mice that had been i.n.-immunized only with OVA, Ag-specific clonal expansion of DO11.10 CD4+ T cells was elevated in the CLN of mice that had been i.n.-immunized with OVA + LT-IIa-B5 (67.44% vs. 21.16%; Fig. 5). When similar experiments were performed using TLR2-deficient mice as recipients for adoptive transfer, no significant increase in clonal expansion of DO11.10 CD4+ T cells was observed in response to immunization with OVA + LT-IIa-B5 (27.09% vs. 22.32%; Fig. 5).
Figure 5. i.n. administration of LT-IIa-B5 induces enhanced Ag-specific proliferation of CD4+ T cells in the CLN.
Syngeneic CFSE-labeled DO11.10 CD4+ T cells (2×106) were adoptively transferred into WT BALB/c or BALB/c(TLR2−/−) mice. Subsequently, mice were i.n.-immunized with OVA (control) or with a combination of OVA and LT-IIa-B5. Three mice were used for each group. CLNs were removed at Day 3 after immunization, and total cells were stained with allophycocyanin-anti-mouse DO11.10 and PerCP-anti-mouse CD4. CFSE fluorescence of the DO11.10+CD4+ T cells was measured as a determinant of proliferation. (A) Histograms of the proliferative responses of CFSE-labeled DO11.10 CD4+ T cells in the CLN. In each histogram, the percentage of dividing cells in each population is denoted above the bracket. Data are shown from one of three independent experiments, each of which yielded consistent results. (B) Graphical comparison of the percentage of dividing CFSE-labeled DO11.10 CD4+ T cells in the CLN. Error bars denote 1 sd from the mean for groups of three mice. Data from one of two independent experiments, each of which yielded consistent results, are shown. WT, TLR2-proficient BALB/c mice; TLR2−/−, TLR2-deficient mice; ***statistical difference from the control (OVA; P<0.001); +++statistical difference (OVA+LT-IIa-B5-treated, WT vs. TLR2-deficient; P<0.001); NS, OVA versus OVA + LT-IIa-B5, TLR2-deficient mice.
These data demonstrated that the immunostimulatory effects of LT-IIa-B5 on nasally derived DCs cooperatively augmented Ag-specific CD4+ T cell clonal expansion in the CLN of i.n.-immunized mice.
Enhanced Ag-specific antibody responses
It was clear that LT-IIa-B5 exerted potent immunomodulatory effects on the major phenotypes of DCs, which are considered to be essential for stimulating Ag-specific adaptive immune responses. To determine whether the effects of LT-IIa-B5 on DCs could be correlated with the capacity of the pentamer to augment Ag-specific immune responses in vivo, a well-established mouse mucosal immunization model was used [15, 19]. Female BALB/c mice were i.n.-immunized with OVA or with OVA + LT-IIa-B5 and boosted with the same combination of immunogens at Days 10 and 20. At Day 35, the point of peak immune response [16, 19, 20], saliva and serum were analyzed for OVA-specific IgA and OVA-specific IgG, respectively (Fig. 6). To compensate for dilution factors inherent in determining the amounts of Ag-specific IgA, the levels of Ag-specific IgA in saliva were reported as a percentage of the amount of Ag-specific IgA to the amount of total IgA in the samples.
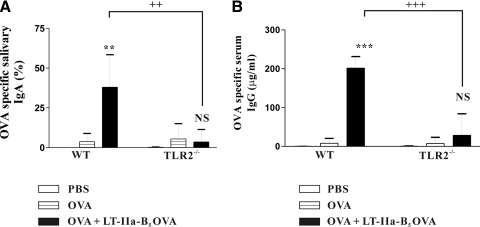
Figure 6. Mucosal adjuvant activity of LT-IIa-B5.
BALB/c mice or BALB/c(TLR2−/−) mice were i.n.-immunized at Day 0 and boosted at Days 10 and 20 with OVA (control) or with OVA in combination with LT-IIa-B5 (OVA+LT-IIa-B5). (A) Salivary IgA responses. Amounts of IgA are reported as the ratio of anti-OVA IgA to total IgA. (B) Serum IgG responses. Error bars denote 1 sd from the mean for groups of five mice. Similar results to those shown in the figure were obtained in an independent experiment. WT, TLR2-proficient BALB/c mice; TLR2−/−, TLR2-deficient mice; **, ***statistical differences from the OVA (control) at P < 0.01 and P < 0.001, respectively; ++, +++statistical difference from the matched control (WT vs. TLR2-deficient) at P < 0.01 and P < 0.001, respectively; NS, OVA versus OVA + LT-IIa-B5, TLR2−/− mice.
As expected for OVA, a weak Ag in mice, only low levels of OVA-specific IgA (3.73% of total IgA) were detected in the saliva of mice immunized solely with OVA. This pattern of minimal responsiveness to OVA was also observed systemically, in which only low levels of serum OVA-specific IgG (7.88 μg/ml) were detected. In contrast, mice that were mucosally immunized with OVA + LT-IIa-B5 exhibited significantly elevated levels of Ag-specific IgA (38.01% of total IgA) in the salivary mucosal compartment and elevated levels of Ag-specific IgG (201.52 μg/ml) in the serum.
Those significant enhancements of OVA-specific salivary IgA and serum IgG induced by LT-IIa-B5 disappeared when TLR2−/− mice were i.n.-immunized with OVA or with OVA + LT-IIa-B5 (Fig. 6). Only low levels of OVA-specific salivary IgA (3.53% of total IgA) and serum IgG (28.70 μg/ml) were detected in TLR2−/− mice, which had been immunized with OVA + LT-IIa-B5. Those levels were not significantly different from the levels of IgA and IgG, which were induced in the TLR2−/− mice or in conventional mice after i.n. immunization solely with OVA.
These immunization experiments clearly demonstrated that LT-IIa-B5 is a potent immunomodulatory agent that has the capacity, when used as an i.n. adjuvant, to elevate Ag-specific mucosal and systemic immune responses.
DISCUSSION
One of the most important sentinel APCs in the mucosae for detecting and responding to pathogens is the DC. When mucosal DCs encounter a pathogen-derived Ag, the cell internalizes the Ag, processes the Ag, and presents the Ag on the surface of the cell in the context of MHC I or MHC II. During this process, DCs undergo maturation, during which, the cells up-regulate expression of costimulatory surface proteins (e.g., CD40, CD80, CD86), which are required for DC:T cell immunosynapse formation. Up-regulated expression of chemokines, such as CCR7, on the surface of the DC induces migration of the mature APCs to proximal draining lymphoid tissues [31, 35, 36], where the DCs interact with naïve T cells [41]. In those lymphoid sites, DCs help to modulate polarization of T cells to Th1 or Th2 phenotypes by the interactions of costimulatory molecules and by secretion of specific cytokines [43]. Boosting the efficacy of DCs at any one (or more) of these stages would be expected to augment cellular and/or humoral immune responses against the acquired Ag and thereby, enhance protection against the pathogen. In this study, we investigated the in vivo properties of LT-IIa-B5 that modulate the developmental and regulatory events of DCs and as a result, promote strong Ag-specific immune responses.
Clearly, the results of experiments reported herein demonstrate that LT-IIa-B5 has strong immunomodulatory effects on multiple stages of DC activation, including Ag acquisition, maturation, and intercellular signaling. DCs in the NALT of mice, administered LT-IIa-B5 by the i.n. route, displayed an enhanced capacity to acquire Ag. Furthermore, LT-IIa-B5 also enhanced the capacity of DCs to migrate from the NALT to the CLN. With the use of a syngeneic DO11.10 CD4+ T cell-adoptive transfer mouse model, LT-IIa-B5 enhanced Ag-specific clonal expansion of CD4+ T cells. Interestingly, in experiments not described herein, LT-IIa holotoxin was found to exert equivalent enhancement on DC migration and Ag-specific CD4+ T cell proliferation but had no detectible effects on Ag uptake (data not shown). Notably, LT-IIa-B5 and LT-IIa holotoxin bind to identical sets of gangliosides, which are situated on the surface of various cells. Binding of LT-IIa holotoxin and LT-IIb-B5 to one or more of those ganglioside receptors has been shown to be essential for modulating the immune properties of both adjuvants [15, 16, 19, 33, 44, 45]. Thus, we surmise that the effects on migration of NALT DCs and on the capacity of NALT DCs to enhance Ag-specific T cell proliferation are induced, in part, by signaling events associated with binding of LT-IIa-B5 (or LT-IIa holotoxin) to ganglioside receptors located on those DCs. In contrast, we believe the effects of LT-IIa-B5 on Ag uptake require binding to the ganglioside receptor and to additional engagement with cellular TLR2 [33].
LT-IIa-B5 and LT-IIb-B5 have been shown to interact physically with TLR2, one of the family of pattern recognition receptors (PRR) expressed on lymphoid and other cell types, using a short hydrophobic domain located within a loop situated distally to the ganglioside-binding surface of the pentamer [22]. Physical engagement of LT-IIa-B5 and LT-IIb-B5 with TLR2 on immunocompetent cells cultured in vitro initiates a series of signal transduction events that promote several important immunological responses in various cells of the immune system. Treatment of murine or human monocytes in vitro with LT-IIa-B5 induced TLR2-dependent activation of NF-κB [21]. Furthermore, human THP-1 monocytic cells treated with LT-IIb-B5 [21] or with LT-IIa-B5 (unpublished results) elaborated IL-1β, IL-6, IL-8, and TNF-α in a manner that required cellular expression of TLR2. Genetic disruption of the TLR2-binding domain in LT-IIb-B5 by introduction of a charged amino acid abrogated the physical and functional interactions of that pentamer with human monocytes [22]. Whether this in vitro requirement of LT-IIa-B5 (or LT-IIb-B5) for TLR2 in immunomodulation was mirrored in vivo, however, had not been examined. Experiments described herein clearly demonstrated that interaction of LT-IIa-B5 with TLR2 was required for the capacity of the pentamer to enhance the activities of DCs in the mouse mucosal immunization model. Interestingly, coadministration of LT-IIb holotoxin inhibited the capacity of LT-IIb-B5 to stimulate monocytes [26]. Use of a mutant LT-IIb holotoxin, which had been detoxified by genetic alteration of the catalytic site for ADP-ribosylation activity in experiments with monocytes, indicated that the capacity of the holotoxin to inhibit the TLR2 activities of LT-IIb-B5 in vitro was likely a result of the ability of the holotoxin to increase the levels of cAMP in the treated cells. That model will be tested directly by performing similar in vitro and in vivo experiments that will use a genetically detoxified mutant LT-IIb holotoxin having critical amino acid substitutions in the enzymatic site of the A polypeptide [e.g., LT-IIb(S59K/E110K)].
A critical event in promoting Ag-specific immune responses is the capacity of DCs to migrate from immune induction sites, in which Ag is encountered, to immune effector sites, in which mature DCs interact with T cells. Migration is controlled by the production of chemokines and chemokine receptors. Immature DCs respond to many CC and CXC chemokines [e.g., MIP-1α (CCL3), MIP-1β (CCL4), MIP-5, MCP-3 (CCL7), MCP-4, RANTES, thymic-expressed chemokine, and stromal cell-derived factor-1] and in particular, to MIP-3α/liver and activation-regulated chemokine, which interacts with CCR6, one of the CCRs [46–48]. Upon maturation, however, responsiveness to most of these chemokines is lost by DCs that acquire responsiveness to MIP-3β/EBl-1 ligand chemokine (ECL; CCL19) and chemokine with 6 cysteines/secondary lymphoid-tissue chemokine (6Ckine; CCL21) by up-regulation of CCR7. CCL19 and CCL21 are produced in LNs, the appendix, and in tonsil [46, 48–50]. Thus, CCR7, which interacts with CCL19 and CCL21, is a major regulator of chemotaxis and migratory speed, two factors that direct mature DCs to secondary lymphoid sites [27, 48, 50]. i.n. administration of LT-IIa-B5 enhanced recruitment of DCs from the NALT to the CLN, an effect that was correlated directly with increased expression of CCR7 by DCs in vivo. When CCR7−/− mice were used in the mucosal immunization model, no differences in DC migration were observed, regardless of the presence or absence of LT-IIa-B5 in the immunizing mixture. These results provided compelling evidence that in mice receiving i.n. LT-IIa-B5, no chemoattractant other than CCR7 is significantly involved in the enhanced migration of DCs from the NALT to the CLN, stimulated by the pentamer.
To produce a standard Ag-specific immune response, a highly sophisticated interplay of immune cells located at various sites in the body is required. To enhance those Ag-specific immune responses, however, the cellular and molecular immune responses of one or more types of those immune cell types must be facilitated. As LT-IIa-B5 enhanced Ag uptake, maturation, and migration of DC, and Ag-specific CD4+ T cell proliferation, it was hypothesized that LT-IIa-B5 also had the capacity to augment production of Ag-specific Igs. With the use of a well-established mouse mucosal immunization model, we demonstrated that i.n. coadministration of a weak Ag with LT-IIa-B5 stimulated significantly higher levels of Ag-specific IgA in saliva in comparison with the levels detected in mice receiving only the Ag. The immunopotentiating effects of LT-IIa-B5, however, were not limited to increasing Ig levels in the fluids of the proximal mucosa (nasal and oral cavity). LT-IIa-B5 also induced enhanced Ag-specific IgG immune responses in the systemic compartment (serum) of the i.n.-immunized mice. At this point, it is not clear whether the enhanced systemic immune response was a result of migration of DCs from the NALT and CLN into nonmucosal-inductive sites for interaction with naïve T cells, migration of T cells, which had interacted with DCs from the NALT, into distal effector sites, or a combination of both processes. Adoptive transfer experiments are ongoing to determine whether CFSE-painted NALT DCs are found in distal DLNs and whether those DCs have the capacity to enhance Ag-specific CD4+ T cell proliferation within those distal LNs.
The in vivo capacities of LT-IIa-B5 to functionally interact with TLR2, enhance acquisition of Ag by DCs, augment expression of important costimulatory surface molecules, promote Ag-specific CD4+ T cell proliferation, and promote Ag-specific mucosal and systemic immune responses are a distinctive immunomodulatory characteristics that may be clinically exploitable. Furthermore, it is unlikely that the nontoxic pentamer, unlike the toxic holotoxin [15], when used as an i.n. adjuvant, will traffic to the brain or inflame neurological tissues. The pentamer's nontoxic nature, in combination with its strong immunomodulatory characteristics, makes it an intriguing new candidate as a safe and effective mucosal adjuvant. Furthermore, it will be interesting to use LT-IIa-B5 and the respective mutants of that pentamer, in subsequent experiments to probe in more depth the immunomodulatory responses of DCs. Hitherto, the adjuvant effects of HLTs have been considered to depend solely on their ganglioside-binding and/or catalytic-toxic activities.
This study is the first report to experimentally demonstrate in vivo that the adjuvant properties of a HLT depend on TLR signaling.
ACKNOWLEDGMENTS
This research was supported by NIH Research grants DE013833 (T.D.C.) and DE017138 (G.H.). The authors are grateful for the efforts of Ms. Lorrie Mandell for coordinating the backcrossing production of the BALB/c(TLR2–/–) mice, Dr. M. O. Kilinc for his advice and instruction of the adoptive transfer experiments, and Dr. Natalie King-Lyons for proofreading the manuscript. Dr. Hesham F. Nawar provided exceptionally helpful discussions and suggestions.
Footnotes
- AgI/II
- surface antigen from Streptococcus mutans
- Alexa 647-OVA
- Alexa 647-labeled chicken OVA
- CLN
- cervical LN
- CT
- cholera toxin
- DLN
- draining LN
- HLT
- heat-labile enterotoxin
- i.n.
- intranasal
- LT-I
- Escherichia coli type I heat-labile enterotoxin
- LT-IIa
- type IIa enterotoxin of Escherichia coli
- LT-IIa-B5/LT-IIb-B5
- pentameric B subunit of LT-IIa/LT-IIb
- MFI
- mean fluorescence intensity
- NALT
- nasal-associated lymphoid tissue
AUTHORSHIP
C.H.L. designed and performed the experiments; P.M-W., G.H., and T.D.C. aided C.H.L. in designing the experiments; C.H.L. and T.D.C. wrote the manuscript; and P.M-W. and G.H. edited the manuscript.
REFERENCES
- 1. Holmgren J., Czerkinsky C. (2005) Mucosal immunity and vaccines. Nat. Med. 11, S45–S53 [DOI] [PubMed] [Google Scholar]
- 2. Mestecky J., Ogra P. L., McGhee J. R., Lambrecht B. N., Strober W. (2005) Mucosal Immunology, 3rd ed., Academic Press, San Diego, CA, USA [Google Scholar]
- 3. Matzinger P. (1994) Tolerance, danger, and the extended family. Annu. Rev. Immunol. 12, 991–1045 [DOI] [PubMed] [Google Scholar]
- 4. Connell T. D., Metzger D., Sfintescu C., Evans R. T. (1998) Immunostimulatory activity of LT-IIa, a type II heat-labile enterotoxin of Escherichia coli. Immunol. Lett. 62, 117–120 [DOI] [PubMed] [Google Scholar]
- 5. Wu H. Y., Russell M. W. (1997) Nasal lymphoid tissue, intranasal immunization, and compartmentalization of the common mucosal immune system. Immunol. Res. 16, 187–201 [DOI] [PubMed] [Google Scholar]
- 6. Wiley J. A., Tighe M. P., Harmsen A. G. (2005) Upper respiratory tract resistance to influenza infection is not prevented by the absence of either nasal-associated lymphoid tissue or cervical lymph nodes. J. Immunol. 175, 3186–3196 [DOI] [PubMed] [Google Scholar]
- 7. Park H. S., Francis K. P., Yu J., Cleary P. P. (2003) Membranous cells in nasal-associated lymphoid tissue: a portal of entry for the respiratory mucosal pathogen group A streptococcus. J. Immunol. 171, 2532–2537 [DOI] [PubMed] [Google Scholar]
- 8. Kiyono H., Fukuyama S. (2004) NALT- versus Peyer′s-patch-mediated mucosal immunity. Nat. Rev. Immunol. 4, 699–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Iwasaki A. (2007) Mucosal dendritic cells. Annu. Rev. Immunol. 25, 381–418 [DOI] [PubMed] [Google Scholar]
- 10. Liang B., Hyland L., Hou S. (2001) Nasal-associated lymphoid tissue is a site of long-term virus-specific antibody production following respiratory virus infection of mice. J. Virol. 75, 5416–5420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wu H. Y., Nikolova E. B., Beagley K. W., Eldridge J. H., Russell M. W. (1997) Development of antibody-secreting cells and antigen-specific T cells in cervical lymph nodes after intranasal immunization. Infect. Immun. 65, 227–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tilney N. L. (1971) Patterns of lymphatic drainage in the adult laboratory rat. J. Anat. 109, 369–383 [PMC free article] [PubMed] [Google Scholar]
- 13. Drakes M. L., Czinn S. J., Blanchard T. G. (2006) Regulation of murine dendritic cell immune responses by Helicobacter felis antigen. Infect. Immun. 74, 4624–4633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hajishengallis G., Arce S., Gockel C. M., Connell T. D., Russell M. W. (2005) Immunomodulation with enterotoxins for the generation of secretory immunity or tolerance: applications for oral infections. J. Dent. Res. 84, 1104–1116 [DOI] [PubMed] [Google Scholar]
- 15. Connell T. D. (2007) Cholera toxin, LT-I, LT-IIa and LT-IIb: the critical role of ganglioside binding in immunomodulation by type I and type II heat-labile enterotoxins. Expert Rev. Vaccines 6, 821–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nawar H. F., Arce S., Russell M. W., Connell T. D. (2005) Mucosal adjuvant properties of mutant LT-IIa and LT-IIb enterotoxins that exhibit altered ganglioside-binding activities. Infect. Immun. 73, 1330–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nawar H. F., King-Lyons N. D., Hu J. C., Pasek R. C., Connell T. D. (2010) LT-IIc, a new member of the type II heat-labile enterotoxin family encoded by an Escherichia coli strain obtained from a nonmammalian host. Infect. Immun. 78, 4705–4713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Russell M. W., Bergmeier L. A., Zanders E. D., Lehner T. (1980) Protein antigens of Streptococcus mutans: purification and properties of a double antigen and its protease-resistant component. Infect. Immun. 28, 486–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nawar H. F., Arce S., Russell M. W., Connell T. D. (2007) Mutants of type II heat-labile enterotoxin LT-IIa with altered ganglioside-binding activities and diminished toxicity are potent mucosal adjuvants. Infect. Immun. 75, 621–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Martin M., Metzger D. J., Michalek S. M., Connell T. D., Russell M. W. (2000) Comparative analysis of the mucosal adjuvanticity of the type II heat-labile enterotoxins LT-IIa and LT-IIb. Infect. Immun. 68, 281–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hajishengallis G., Tapping R. I., Martin M. H., Nawar H., Lyle E. A., Russell M. W., Connell T. D. (2005) Toll-like receptor 2 mediates cellular activation by the B subunits of type II heat-labile enterotoxins. Infect. Immun. 73, 1343–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liang S., Hosur K. B., Lu S., Nawar H. F., Weber B. R., Tapping R. I., Connell T. D., Hajishengallis G. (2009) Mapping of a microbial protein domain involved in binding and activation of the TLR2/TLR1 heterodimer. J. Immunol. 182, 2978–2985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wooten R. M., Ma Y., Yoder R. A., Brown J. P., Weis J. H., Zachary J. F., Kirschning C. J., Weis J. J. (2002) Toll-like receptor 2 is required for innate, but not acquired, host defense to Borrelia burgdorferi. J. Immunol. 168, 348–355 [DOI] [PubMed] [Google Scholar]
- 24. Murphy K. M., Heimberger A. B., Loh D. Y. (1990) Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo. Science 250, 1720–1723 [DOI] [PubMed] [Google Scholar]
- 25. Hajishengallis G., Nawar H., Tapping R. I., Russell M. W., Connell T. D. (2004) The type II heat-labile enterotoxins LT-IIa and LT-IIb and their respective B pentamers differentially induce and regulate cytokine production in human monocytic cells. Infect. Immun. 72, 6351–6358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liang S., Wang M., Triantafilou K., Triantafilou M., Nawar H. F., Russell M. W., Connell T. D., Hajishengallis G. (2007) The A subunit of type IIb enterotoxin (LT-IIb) suppresses the proinflammatory potential of the B subunit and its ability to recruit and interact with TLR2. J. Immunol. 178, 4811–4819 [DOI] [PubMed] [Google Scholar]
- 27. Song J. H., Kim J. I., Kwon H. J., Shim D. H., Parajuli N., Cuburu N., Czerkinsky C., Kweon M. N. (2009) CCR7-CCL19/CCL21-regulated dendritic cells are responsible for effectiveness of sublingual vaccination. J. Immunol. 182, 6851–6860 [DOI] [PubMed] [Google Scholar]
- 28. Villablanca E. J., Mora J. R. (2008) A two-step model for Langerhans cell migration to skin-draining LN. Eur. J. Immunol. 38, 2975–2980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bromley S. K., Thomas S. Y., Luster A. D. (2005) Chemokine receptor CCR7 guides T cell exit from peripheral tissues and entry into afferent lymphatics. Nat. Immunol. 6, 895–901 [DOI] [PubMed] [Google Scholar]
- 30. Fainaru O., Shseyov D., Hantisteanu S., Groner Y. (2005) Accelerated chemokine receptor 7-mediated dendritic cell migration in Runx3 knockout mice and the spontaneous development of asthma-like disease. Proc. Natl. Acad. Sci. USA 102, 10598–10603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Niess J. H., Reinecker H. C. (2006) Dendritic cells in the recognition of intestinal microbiota. Cell. Microbiol. 8, 558–564 [DOI] [PubMed] [Google Scholar]
- 32. West M. A., Wallin R. P., Matthews S. P., Svensson H. G., Zaru R., Ljunggren H. G., Prescott A. R., Watts C. (2004) Enhanced dendritic cell antigen capture via Toll-like receptor-induced actin remodeling. Science 305, 1153–1157 [DOI] [PubMed] [Google Scholar]
- 33. Liang S., Wang M., Tapping R. I., Stepensky V., Nawar H. F., Triantafilou M., Triantafilou K., Connell T. D., Hajishengallis G. (2007) Ganglioside GD1a is an essential coreceptor for Toll-like receptor 2 signaling in response to the B subunit of type IIb enterotoxin. J. Biol. Chem. 282, 7532–7542 [DOI] [PubMed] [Google Scholar]
- 34. Wooten R. M., Ma Y., Yoder R. A., Brown J. P., Weis J. H., Zachary J. F., Kirschning C. J., Weis J. J. (2002) Toll-like receptor 2 plays a pivotal role in host defense and inflammatory response to Borrelia burgdorferi. Vector Borne Zoonotic Dis. 2, 275–278 [DOI] [PubMed] [Google Scholar]
- 35. Sekine S., Kataoka K., Fukuyama Y., Adachi Y., Davydova J., Yamamoto M., Kobayashi R., Fujihashi K., Suzuki H., Curiel D. T., Shizukuishi S., McGhee J. R., Fujihashi K. (2008) A novel adenovirus expressing Flt3 ligand enhances mucosal immunity by inducing mature nasopharyngeal-associated lymphoreticular tissue dendritic cell migration. J. Immunol. 180, 8126–8134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Enioutina E. Y., Bareyan D., Daynes R. A. (2008) TLR ligands that stimulate the metabolism of vitamin D3 in activated murine dendritic cells can function as effective mucosal adjuvants to subcutaneously administered vaccines. Vaccine 26, 601–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schumann K., Lammermann T., Bruckner M., Legler D. F., Polleux J., Spatz J. P., Schuler G., Forster R., Lutz M. B., Sorokin L., Sixt M. (2010) Immobilized chemokine fields and soluble chemokine gradients cooperatively shape migration patterns of dendritic cells. Immunity 32, 703–713 [DOI] [PubMed] [Google Scholar]
- 38. Yen J. H., Kong W., Ganea D. (2010) IFN-β inhibits dendritic cell migration through STAT-1-mediated transcriptional suppression of CCR7 and matrix metalloproteinase 9. J. Immunol. 184, 3478–3486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lapteva N., Seethammagari M. R., Hanks B. A., Jiang J., Levitt J. M., Slawin K. M., Spencer D. M. (2007) Enhanced activation of human dendritic cells by inducible CD40 and Toll-like receptor-4 ligation. Cancer Res. 67, 10528–10537 [DOI] [PubMed] [Google Scholar]
- 40. Cyster J. G. (1999) Chemokines and cell migration in secondary lymphoid organs. Science 286, 2098–2102 [DOI] [PubMed] [Google Scholar]
- 41. Leitner J., Grabmeier-Pfistershammer K., Steinberger P. (2010) Receptors and ligands implicated in human T cell costimulatory processes. Immunol. Lett. 128, 89–97 [DOI] [PubMed] [Google Scholar]
- 42. Huang X., Reynolds A. D., Mosley R. L., Gendelman H. E. (2009) CD 4+ T cells in the pathobiology of neurodegenerative disorders. J. Neuroimmunol. 211, 3–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kapsenberg M. L. (2003) Dendritic-cell control of pathogen-driven T-cell polarization. Nat. Rev. Immunol. 3, 984–993 [DOI] [PubMed] [Google Scholar]
- 44. Berenson C. S., Nawar H. F., Yohe H. C., Castle S. A., Ashline D. J., Reinhold V. N., Hajishengallis G., Connell T. D. (2010) Mammalian cell ganglioside-binding specificities of E. coli enterotoxins LT-IIb and variant LT-IIb(T13I). Glycobiology 20, 41–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nawar H. F., Greene C. J., Lee C. H., Mandell L. M., Hajishengallis G., Connell T. D. (2011) LT-IIc, a new member of the type II heat-labile enterotoxin family, exhibits potent immunomodulatory properties that are different from those induced by LT-IIa or LT-IIb. Vaccine 29, 721–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Caux C., Ait-Yahia S., Chemin K., de Bouteiller O., Dieu-Nosjean M. C., Homey B., Massacrier C., Vanbervliet B., Zlotnik A., Vicari A. (2000) Dendritic cell biology and regulation of dendritic cell trafficking by chemokines. Springer Semin. Immunopathol. 22, 345–369 [DOI] [PubMed] [Google Scholar]
- 47. Le Borgne M., Dubois B., Kaiserlian D. (2007) Dendritic cells of mucosa and skin: ″recruited for vaccination″. Med. Sci. (Paris) 23, 819–825 [DOI] [PubMed] [Google Scholar]
- 48. Dieu-Nosjean M. C., Vicari A., Lebecque S., Caux C. (1999) Regulation of dendritic cell trafficking: a process that involves the participation of selective chemokines. J. Leukoc. Biol. 66, 252–262 [DOI] [PubMed] [Google Scholar]
- 49. Xin H. M., Peng Y. Z., Yuan Z. Q., Guo H. (2009) In vitro maturation and migration of immature dendritic cells after chemokine receptor 7 transfection. Can. J. Microbiol. 55, 859–866 [DOI] [PubMed] [Google Scholar]
- 50. Caux C., Vanbervliet B., Massacrier C., Ait-Yahia S., Vaure C., Chemin K., Dieu-Nosjean M. C., Vicari A. (2002) Regulation of dendritic cell recruitment by chemokines. Transplantation 73, S7–S11 [DOI] [PubMed] [Google Scholar]