Abstract
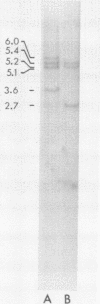
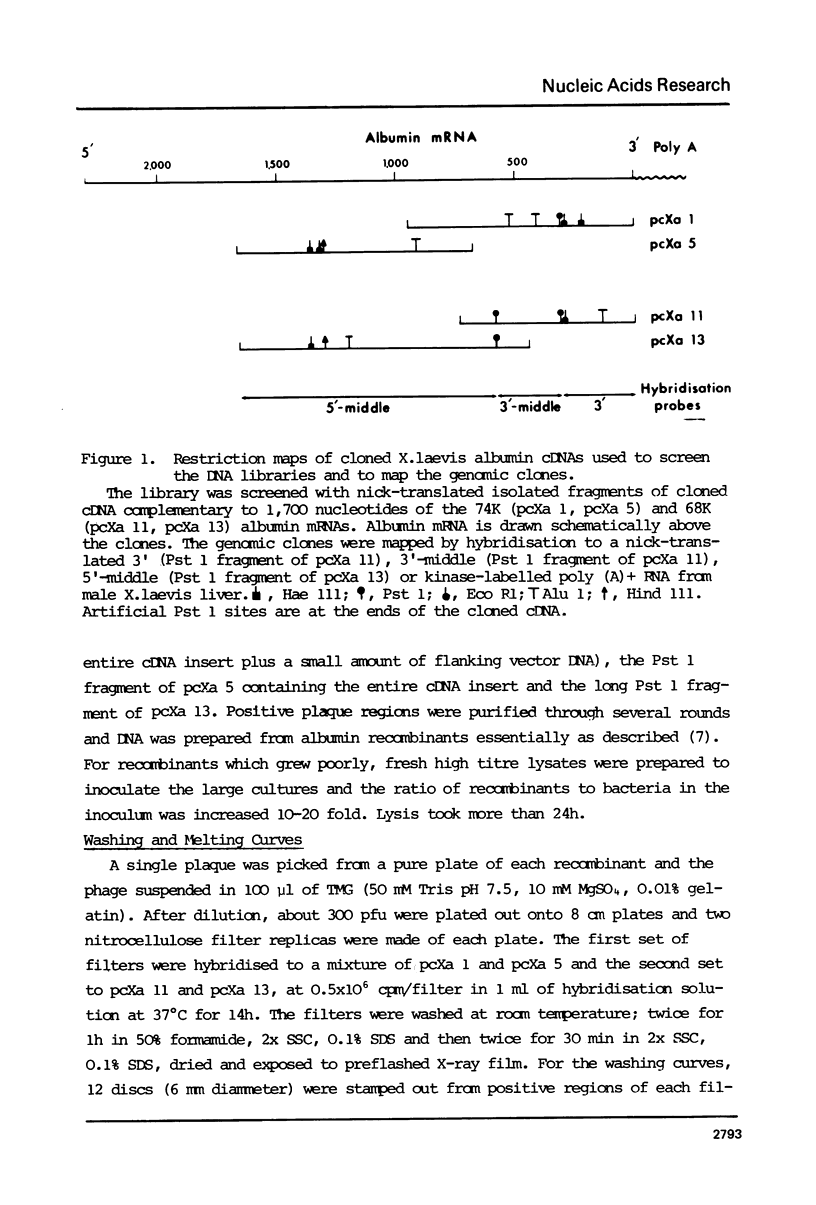
The blood of the frog X.laevis contains 2 albumins of 68,000 and 74,000 daltons which are encoded in the liver by two related mRNAs. When an amplified X.laevis DNA library was screened with cloned albumin cDNA only 68,000 dalton albumin gene sequences were isolated. Hybridisation of the albumin cDNA to Southern-blots of Eco R1 digested X.laevis DNA showed that the sequences present in the recombinants did not account for all the fragments which hybridised on the Southern-blots. This indicated that 74K albumin gene sequences exist but that they are not present in the amplified DNA library. A X.laevis genomic library was therefore constructed and screened for albumin genes without amplification. Both 68K and 74K albumin gene sequences were isolated. Recombinants containing 74K albumin gene sequences grew extremely poorly and this probably explains why the 74K albumin sequences were ot isolated from the amplified library. Characterisation of the cloned DNA indicates that there is one sequence coding for the 68K albumin but two different sequences coding for the 75K albumin.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bisbee C. A., Baker M. A., Wilson A. C., Haji-Azimi I., Fischberg M. Albumin phylogeny for clawed frogs (Xenopus). Science. 1977 Feb 25;195(4280):785–787. doi: 10.1126/science.65013. [DOI] [PubMed] [Google Scholar]
- Bozzoni I., Beccari E., Luo Z. X., Amaldi F. Xenopus laevis ribosomal protein genes: isolation of recombinant cDNA clones and study of the genomic organization. Nucleic Acids Res. 1981 Mar 11;9(5):1069–1086. doi: 10.1093/nar/9.5.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. W., Thomas M., Cameron J., St John T. P., Scherer S., Padgett R. A. Rapid DNA isolations for enzymatic and hybridization analysis. Methods Enzymol. 1980;65(1):404–411. doi: 10.1016/s0076-6879(80)65051-4. [DOI] [PubMed] [Google Scholar]
- Dolan M., Sugarman B. J., Dodgson J. B., Engel J. D. Chromosomal arrangement of the chicken beta-type globin genes. Cell. 1981 Jun;24(3):669–677. doi: 10.1016/0092-8674(81)90093-3. [DOI] [PubMed] [Google Scholar]
- Efstratiadis A., Posakony J. W., Maniatis T., Lawn R. M., O'Connell C., Spritz R. A., DeRiel J. K., Forget B. G., Weissman S. M., Slightom J. L. The structure and evolution of the human beta-globin gene family. Cell. 1980 Oct;21(3):653–668. doi: 10.1016/0092-8674(80)90429-8. [DOI] [PubMed] [Google Scholar]
- Fritsch E. F., Lawn R. M., Maniatis T. Molecular cloning and characterization of the human beta-like globin gene cluster. Cell. 1980 Apr;19(4):959–972. doi: 10.1016/0092-8674(80)90087-2. [DOI] [PubMed] [Google Scholar]
- Herrlich P., Hynes N. E., Ponta H., Rahmsdorf U., Kennedy N., Groner B. The endogenous proviral mouse mammary tumor virus genes of the GR mouse are not identical and only one corresponds to the exogenous virus. Nucleic Acids Res. 1981 Oct 10;9(19):4981–4995. doi: 10.1093/nar/9.19.4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kioussis D., Eiferman F., van de Rijn P., Gorin M. B., Ingram R. S., Tilghman S. M. The evolution of alpha-fetoprotein and albumin. II. The structures of the alpha-fetoprotein and albumin genes in the mouse. J Biol Chem. 1981 Feb 25;256(4):1960–1967. [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Ryffel G. U. Comparison of cytoplasmic and nuclear poly(A)-containing RNA sequences in Xenopus liver cells. Eur J Biochem. 1976 Feb 16;62(2):417–423. doi: 10.1111/j.1432-1033.1976.tb10174.x. [DOI] [PubMed] [Google Scholar]
- Sargent T. D., Wu J. R., Sala-Trepat J. M., Wallace R. B., Reyes A. A., Bonner J. The rat serum albumin gene: analysis of cloned sequences. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3256–3260. doi: 10.1073/pnas.76.7.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Wahli W., Dawid I. B. Isolation of two closely related vitellogenin genes, including their flanking regions, from a Xenopus laevis gene library. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1437–1441. doi: 10.1073/pnas.77.3.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahli W., Dawid I. B., Ryffel G. U., Weber R. Vitellogenesis and the vitellogenin gene family. Science. 1981 Apr 17;212(4492):298–304. doi: 10.1126/science.7209528. [DOI] [PubMed] [Google Scholar]
- Wahli W., Dawid I. B., Wyler T., Jaggi R. B., Weber R., Ryffel G. U. Vitellogenin in Xenopus laevis is encoded in a small family of genes. Cell. 1979 Mar;16(3):535–549. doi: 10.1016/0092-8674(79)90028-x. [DOI] [PubMed] [Google Scholar]
- Westley B., Wyler T., Ryffel G., Weber R. Xenopus laevis serum albumins are encoded in two closely related genes. Nucleic Acids Res. 1981 Aug 11;9(15):3557–3574. doi: 10.1093/nar/9.15.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]