Abstract
A two-step reconstitution system for the generation of ER cargo exit sites from starting ER-derived low density microsomes (LDMs; 1.17 g/cc) is described. The first step is mediated by the hydrolysis of Mg2+ATP and Mg2+GTP, leading to the formation of a transitional ER (tER) with the soluble cargo albumin, transferrin, and the ER-to-Golgi recycling membrane proteins α2p24 and p58 (ERGIC-53, ER-Golgi intermediate compartment protein) enriched therein. Upon further incubation (step two) with cytosol and mixed nucleotides, interconnecting smooth ER tubules within tER transforms into vesicular tubular clusters (VTCs). The cytosolic domain of α2p24 and cytosolic COPI coatomer affect VTC formation. This is deduced from the effect of antibodies to the COOH-terminal tail of α2p24, but not of antibodies to the COOH-terminal tail of calnexin on this reconstitution, as well as the demonstrated recruitment of COPI coatomer to VTCs, its augmentation by GTPγS, inhibition by Brefeldin A (BFA), or depletion of β-COP from cytosol. Therefore, the p24 family member, α2p24, and its cytosolic coat ligand, COPI coatomer, play a role in the de novo formation of VTCs and the generation of ER cargo exit sites.
Keywords: cell-free assembly, transitional endoplasmic reticulum, endoplasmic reticulum cargo exit sites, α2p24, COPI
Current views on the transport of newly synthesized secretory cargo in eukaryotic cells have been guided by two opposing theories. According to the membrane flow model (Morré and Keenan 1997), membrane migration accompanies the transfer of newly synthesized cargo from the ER to and through the secretory apparatus, towards the plasmalemma. According to the opposing vesicular transport model, small, 60–90-nm coated vesicles ferry newly synthesized secretory cargo between discrete preexisting compartments of the secretory pathway (Rothman and Wieland 1996). The long half-life of resident membrane and intralumenal soluble proteins (e.g., calnexin, KDEL proteins of the ER lumenal matrix, and glycosyl transferases of the ER and Golgi apparatus) compared with the rapidity of cargo transport through the compartments of the secretory apparatus has long supported the second view.
Recently, however, the visualization of large (1 micron diam) structures generated from the ER coincident with cargo transport has given a strong impetus towards establishing the validity of the membrane flow model (Pelham 1997; Presley et al. 1997; Scales et al. 1997). Membrane recycling would account for the discrepancy between the long half-life of resident proteins of the secretory pathway, as compared with the rapidity of cargo transport (Lippincott-Schwartz et al. 1998).
To resolve the controversy, one approach is to identify the proteins that regulate the formation of the early secretory pathway and assign these functions to either the generation of discrete vesicular intermediates or larger membranes undergoing gradual maturation. Identification of such regulatory proteins has relied on three approaches: cell-free intra-Golgi and ER-to-Golgi transport assays to identify and purify molecules regulating protein transport (Rothman 1994; Rothman and Wieland 1996; Schekman and Orci 1996); the selection of Sec mutants of the yeast secretory pathway (Novick et al. 1980); and the sequencing of abundant resident membrane proteins of the secretory pathway (Wada et al. 1991; Bajjalieh and Scheller 1995; Dominguez et al. 1998).
Recently, these three approaches have focused on cytosolic coat proteins, as well as their potential integral membrane protein receptors. Thus, the elucidation of the COPI coatomer coat was deduced from Golgi cell-free transport assays (Rothman and Wieland 1996). COPII coatomer coats were identified as a consequence of yeast genetics and ER-to-Golgi transport assays (Schekman and Orci 1996). Remarkably, the same integral membrane proteins largely localized to the ER-Golgi intermediate compartment (ERGIC)1/cis-Golgi network (p58, the p24 family) have been proposed as receptors for both COPI and COPII coatomers, as deduced from their abundance, location, specificity, and high affinity of binding to these coats in vitro (Kappeler et al. 1997; Dominguez et al. 1998; Bremser et al. 1999).
In the case of the COPI coatomer coat, uncertainty exists as to the direction (direct anterograde versus indirect retrograde) that COPI primarily affects the secretory apparatus (Elazar et al. 1994; Letourneur et al. 1994; Taylor et al. 1994; Lippincott-Schwartz et al. 1998). This likely is a consequence of the assays used to identify COPI functions in vitro and in vivo (Lippincott-Schwartz et al. 1998). However, COPI coatomer and the ARF1-mediated mechanism of coat binding, as well as the regulated mechanism of coat dissociation, have been clearly demonstrated to be essential for trafficking through the early secretory pathway (reviewed in Lippincott-Schwartz et al. 1998). In the present study, a structural role for the Golgi apparatus-derived integral membrane protein α2p24 and its cytosolic COPI ligand is defined in a characterized cell-free assay that reconstitutes the generation of a nascent secretory apparatus from ER-derived low density microsomes (LDMs).
Materials and Methods
Preparation and Characterization of Microsomes
Total microsomes were obtained by differential centrifugation of rat liver homogenates (Paiement and Bergeron 1983). They were resuspended in sucrose to give a final concentration of 1.38 M, placed under a step-gradient of 1.0, 0.86, and 0.25 M sucrose, and centrifuged using a Beckman SW 60 rotor at 300,000 g av for 60 min. A subfraction containing smooth microsomes and low density rough microsomes (1.17 g/cm3) was obtained from the upper half of the 1.38 M sucrose step above the residual pellet after centrifugation. This fraction, characterized as LDMs, was washed once by centrifugation and resuspension in 0.25 M sucrose at 100,000 g av (Lavoie et al. 1996). High density rough microsomes were prepared as previously described (Paiement and Bergeron 1983). These fractions have been shown by electron microscope cytochemistry to contain a uniform distribution of the ER marker glucose-6-phosphatase. Further biochemical characterization for UDP-galactose:ovomucoid galactosyltransferase enzyme activity revealed a 2.2-fold increase over the homogenate for these microsomes, while that of a Golgi apparatus fraction prepared by the method of Dominguez et al. 1998 revealed a 138-fold enrichment in galactosyl transferase activity. Hence, LDMs were at the most 1.6% contaminated by Golgi elements.
Preparation of Rat Liver Cytosol
After centrifugation of the total microsomes at 100,000 g av, 1 mM PMSF, 1 mM DTT, and 0.9 μg/ml leupeptin was added to the supernatant and centrifuged at 200,000 g av for 2 h at 4°C. Ammonium sulfate was added to the resulting supernatant (60% saturation, added from solid). After 1 h of stirring at 4°C, the precipitate was recovered by centrifugation at 10,000 rpm for 30 min at 4°C. Pellets were resuspended in buffer containing 25 mM Tris-HCl, pH 7.4, 50 mM KCl, and 1 mM DTT, desalted in the same buffer by chromatography on a Sephadex G-25 column, and concentrated to 40–50 mg protein/ml using an ultrafiltration stirred cell and Diaflo ultrafilter PM10 (Amicon; W.R. Grace and Co.). The precipitate formed at this stage was removed by centrifugation at 200,000 g av for 60 min. The supernatant obtained was aliquoted and stored at −80°C.
Preparation of COPI-depleted Cytosol
Protein A–Sepharose beads were first incubated in 500 μl KOAc buffer (25 mM Hepes, pH 7.4, 115 mM KOAc, 100 mM NaCl, 2.5 mM MgCl2) containing 15 μg of rabbit anti–mouse IgG for 30 min at 4°C. Beads were washed three times with KOAc buffer and then incubated with or without 10 μl of anti β-COP monoclonal IgG in 500 μl of KOAc buffer for 45 min with agitation at 4°C. Beads were finally washed three times with KOAc buffer devoid of NaCl and incubated with 40 μl of rat liver cytosol (37.5 μg/μl), 160 μl KOAc buffer (without NaCl), and protease inhibitor cocktail (0.25 mM benzamidine, 2.5 μg/ml leupeptin, 1 μg/ml soybean trypsin inhibitor) for 45 min at 4°C. The mixture was centrifuged and the supernatant collected, concentrated with Centricon 10 (Amicon, W.R. Grace and Co.), and frozen in aliquots at −80°C.
Cell-free Incubation Conditions
Unless otherwise indicated, the medium consisted of 0.25 ml of buffer containing 150 μg microsomal protein, 100 mM Tris-HCl, pH 7.4, 5 mM MgCl2, 1 mM GTP, 2 mM ATP, an energy regenerating system (7.3 IU/ml creatine kinase, 2 mM creatine phosphate), 0.1 mM DTT, 0.02 mM PMSF, 1 μg/ml leupeptin, and 50 mM sucrose. Incubations were carried out at 37°C for 180 min. For studies on the effect of cytosol, 750 μg of rat liver cytosolic protein (in the presence or absence of 1 mM GTPγS) was added to the medium described above after 180 min and the incubation was continued for 5–60 min. To assess the effect of Brefeldin A (BFA; 200 μM final concentration), this reagent was added to the medium 10 min before addition of cytosol. For fusion of classical rough ER, high density rough microsomes were incubated at 37°C in the presence of Mg2+GTP (Paiement and Bergeron 1983). For the effects of antibodies, 20 μg (protein) of affinity-purified anti-α2p24 or anticalnexin was added where indicated.
β-COP Binding Reaction
LDMs (300 μg) were incubated for 180 min to form transitional ER (tER). 200 μM BFA was added or methanol (BFA solvent) and incubation continued for 10 min at 37°C. 3 mg of rat liver cytosol in the presence or absence of 1 mM GTPγS were added and reactions were incubated for an additional 15 min at 37°C. The samples were then placed on ice and received 1 ml each of ice-cold washing buffer containing 100 mM Tris-HCl, pH 7.4, 2.5 mM MgCl2, and KOAc (final concentration of 250 mM). The samples were then centrifuged at 16,000 g av for 15 min at 4°C. The supernatant was removed and the pellet subjected to immunoblot analysis using β-COP specific antibodies (Sigma Chemical Co).
SDS-PAGE and Immunoblotting
Proteins were separated by SDS-PAGE using 7–15% polyacrylamide gradients. After electrophoresis, the separated proteins were transferred to nitrocellulose membranes. Electrophoretic blotting procedure and immunodetection were carried out as described in Dominguez et al. 1991.
Antibodies
Rabbit polyclonal antibodies against calnexin (Ou et al. 1993) and α2p24 (Dominguez et al. 1998) have been previously described. Polyclonal antibodies against p58 were a gift from Dr. J. Saraste (Ludwig Institute for Cancer Research, Stockholm, Sweden). Mouse mAbs against β-COP was obtained from Sigma Chemical Co. Antibodies to rat albumin and rat transferrin were obtained from Cappel Laboratories (Organon Teknika Inc.).
Endoglycosidase H and N-Glycosidase F Treatment and Galactosyl Transferase Assay
Deglycosylation experiments were performed according to the recommendation of the supplier (Boehringer Mannheim GmbH). Galactosyl transferase activity using ovomucoid as acceptor was assayed as described by Dominguez et al. 1998.
Thin-Section EM
After incubation, membranes were fixed 12 h using 2.5% glutaraldehyde in cacodylate buffer (100 mM, pH 7.4), recovered onto Millipore membranes (0.45-μm pores) by the random filtration technique of Baudhuin et al. 1967, fixed with reduced osmium (Lavoie et al. 1996), dehydrated, and processed for EM.
Morphometry of ER Membranes and Microsomes
Estimates of the lengths of embedded and sectioned rough and smooth membranes in the membrane networks were obtained by morphometry using the membrane intersection counting procedure (Stäubli et al. 1969) exactly as described previously (Lavoie et al. 1996). Membrane lengths of embedded and sectioned microsomes were obtained by morphometric studies as described previously (Lavoie et al. 1996).
Immunogold Labeling
Immunolocalization of β-COP was modified from that used by Dominguez et al. 1991. LDMs (750 μg protein) were incubated with GTP and ATP for 180 min to reconstitute tER formation and then post-incubated with cytosol in the presence or absence of GTPγS for 10 min for the generation of vesicular tubular clusters (VTCs). After incubation, the membrane fraction was recovered by centrifugation (1500 rpm for 25 min). Sedimented membranes were resuspended in 750 μl containing 100 mM Tris-HCl, pH 7.4, 60 mM sucrose, and 60 μl anti–β-COP. After an incubation of 60 min at room temperature, membranes were fixed using 0.05% glutaraldehyde/cacodylate 0.1 M, pH 7.4, at 4°C for 30 min. After fixation, the membranes were filtered onto Millipore membranes as previously outlined (Paiement and Bergeron 1983). The pellicles were then washed in saline solution (three changes, 10 min each at room temperature, incubations done with mild agitation) and treated again in saline solution containing 3% BSA (three changes, 10 min each), and subsequently incubated with protein A–gold for 1 h at room temperature. The pellicles were washed in saline solution and cacodylate 0.1 M, pH 7.4, fixed overnight in 2.5% glutaraldehyde in 0.1 M cacodylate, pH 7.4, at 4°C and processed for EM as above.
α2p24 immunolocalization was carried out as follows. LDMs (300 μg protein) were incubated with Mg2+GTP and Mg2+ATP for 180 min, and for an additional 10 min in the presence of cytosol at 37°C. Anti-α2p24 antibodies were added and membranes were incubated an additional 30 min at room temperature. Membranes were then fixed using 0.05% glutaraldehyde in 0.1 M cacodylate buffer, pH 7.4, at 4°C and treated for analysis by EM as described above. Unincubated microsomes were incubated with anti-α2p24 for 30 min at 10°C and treated for analysis by EM as described above. For the protein A–gold complexes, 10-nm colloidal gold particles were prepared according to Slot and Geuze 1985 and coated with protein A as described by Ghitescu and Bendayan 1990.
Immunogold Labeling of Cryosections.
After incubation, membranes were fixed using 4% paraformaldehyde and 0.1% glutaraldehyde in 0.1 M cacodylate buffer, pH 7.4, at 4°C. Cryoprotection, freezing, sectioning, immunolabeling, and contrasting were carried out as previously described by Dahan et al. 1994.
Analysis of Gold Label Distribution.
For quantification of COPI labeling after incubation of reconstituted membranes with cytosol gold particles associated with rough membranes, SER and VTCs comprising the ER networks were counted. Rough membrane cisternae were defined as large cisternal profiles limited by ribosome-studded membranes. SER were defined as branching and anastomosing tubules limited by membrane devoid of associated ribosomes. In the presence of cytosol, these tubules are transformed into clusters of closely apposed vesicles and convoluted tubules designated VTCs. The number of gold particles over rough ER membranes, as well as those over the combined SER and VTCs that made up the reconstituted networks, were expressed as average number of gold particles per ER membrane network. Counts were compared for different membrane incubation conditions.
For quantification of α2p24, p58, albumin, transferrin, calnexin, and ribophorin labeling on cryosections, gold particles associated with rough membranes and SER comprising the ER networks were counted. Gold particles were counted over parallel juxtaposed ER cisternae (representing rough ER cisternae) and over the adjacent continuous mass of interconnecting membranes (corresponding to interconnecting smooth ER tubules; see Fig. 2). Surface area measurements of each compartment comprising the reconstituted ER networks were measured as previously described for ER membranes in situ (Paiement et al. 1988). Gold particle densities were calculated as number of particles per compartment of ER network and concentrations expressed in each category of membranes were then expressed as average number of gold particles per surface area for each ER network.
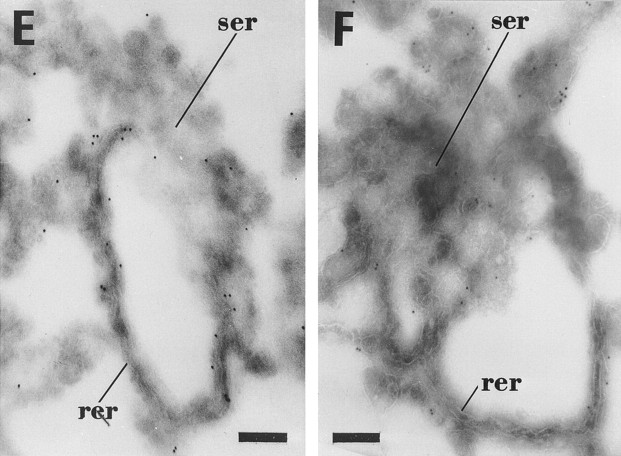
Figure 2.
Concentration of albumin, transferrin, α2p24, p58, ribophorin, and calnexin in tER. LDMs were incubated in the presence of mixed nucleotides to form tER. Antibodies to albumin (A), transferrin (B), α2p24 (C), p58 (D), ribophorin (E), and calnexin (F) were applied to ultrathin cryosections. Gold particle labeling is predominantly distributed over interconnecting ER tubules for all antigens studied, except in the case of ribophorin (E), where gold particle labeling is mainly over rough ER. Inset in C shows α2p24 labeling over a bud in the tER. rer, Parallel rough ER cisternae; ser, interconnecting smooth ER tubules. Bars, 0.2 μm.
Cryoimmune EM of Liver Parenchyma.
Rat liver was prepared for ultracryotomy as described previously (Dahan et al. 1994). For immunolabeling, sections were first floated on drops of PBS (150 mM NaCl, 2.7 mM KCl, 1.5 mM KH2PO4, 6.5 mM Na2HPO4, pH 7.4) containing 0.02 M glycine for 10 min, followed by incubation on primary antibody for 30 min at room temperature. The primary antibodies to α2p24 were diluted 1:5 and those to β-COP were diluted 1:20 in PBS containing 2% BSA/2% casein/0.5% ovalbumin (PBS-BCO). Sections were washed six times for 5 min in PBS followed by blocking in PBS-BCO (5 min) and incubation in appropriate secondary antibodies conjugated to gold particles for 30 min. Sections again were washed six times for 5 min in PBS, six times for 5 min in distilled water, stained for 5 min with uranyl acetate-oxalate solution (pH 7.0), washed twice for 1.5 min in distilled water, and finally transferred to drops of methyl cellulose containing 0.4% aqueous uranyl acetate for 10 min on ice. Grids were picked up with copper loops and excess methyl cellulose was removed with filter paper. Sections were viewed in a Phillips 400 T electron microscope operating at 80 kV. E5A3 mAb to β-COP was kindly provided by the late Dr. Thomas Kreis, (University of Geneva, Geneva).
Results
Cytosol-dependent Formation of ER Cargo Exit Sites
Previously, we have demonstrated that incubation of LDMs with Mg2+GTP and Mg2+ATP leads to the formation of a partially rough, partially smooth transitional region corresponding to the tER (Lavoie et al. 1996). Using this as a basis for a two-step protocol, the generation of ER cargo exit sites was attempted.
In the first step, incubation of LDMs in the presence of Mg2+ATP alone had no effect on the appearance of these microsomes (not shown). However, incubations with Mg2+GTP led to membrane fusion and the formation of large rough ER cisternae (not shown). The majority (75%) of the vesicle profiles closely apposed to the reconstituted cisternae were devoid of associated ribosomes and had an average diameter of 83 ± 29 nm. This vesicle size is similar to the vesicle size of unincubated smooth microsomes (Lavoie et al. 1996) and thus, these vesicles are likely to represent unfused smooth vesicles adhering to the fused rough ER cisternae.
When a mixture of Mg2+GTP and Mg2+ATP was added, membrane differentiation consequent to membrane fusion was observed (Fig. 1 A). As a consequence of mixed nucleotide hydrolysis (Lavoie et al. 1996), fused networks now consisted of an array of rough ER cisternae, continuous with a fenestrated network of anastomosing smooth membranous tubules, i.e., similar morphologically to the rough ER/smooth ER boundary of liver parenchyma (Fawcett 1955; Dallner et al. 1966). Whereas few (1–4) ribosomal particles were observed associated with unincubated vesicle profiles, numerous ribosomal particles (as many as 20 or more) were observed aligned along the cytoplasmic surface of the parallel rough ER cisternae assembled after incubation with Mg2+GTP and Mg2+ATP (not shown). The average total amount of membrane associated with networks generated in the presence of Mg2+GTP and Mg2+ATP (490 ± 89 intersections/network) was significantly higher (P < 0.001) than that for networks generated in the presence of Mg2+GTP alone (320 ± 85 intersections/network). However, the amount of membrane associated with the rough ER cisternae after incubation with Mg2+GTP alone (320 ± 85 intersections/network) was not significantly different (P > 0.05) from that associated with rough ER cisternae after incubation with Mg2+GTP and Mg2+ATP (277 ± 80 intersections/network). Thus, in the presence of Mg2+GTP and Mg2+ATP, nucleotide hydrolysis led to the fusion and incorporation of additional SER to the reconstituted ER networks.
Figure 1.
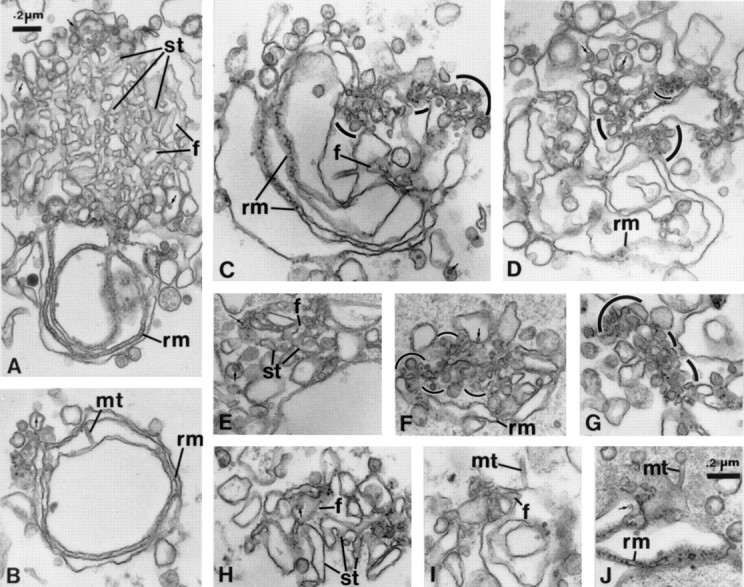
Cytosol-dependent formation of VTCs. Step two in the formation of ER cargo exit sites. (A) tER formed in the presence of 5 mM MgCl2, 2 mM ATP, and 1 mM GTP, showing smooth ER tubules (st) continuous with peripherally located rough membrane cisternae (rm). With subsequent incubation for 10 (C–G), 20 (B and J), or 60 min (H and I) with rat liver cytosol, ER membrane networks showed buds, VTCs (outlined by curved lines), and concomitant reduction of SER. Arrows, lipoprotein particle content; f, fenestrations; mt, minitubules; rm, rough ER membranes; st, smooth ER tubules.
In the second step (Fig. 1), further incubation of such networks with cytosol and the same mixture of nucleotides generated VTCs of identical morphology to ER export sites characterized in situ and in detergent permeabilized cells (Bannykh et al. 1996; Bannykh and Balch 1998). When rat liver cytosol was added to preassembled ER networks (Fig. 1 A) and incubated, the membrane networks became progressively modified. Incubation for 10 min with cytosol was sufficient to provoke loss of the SER and generation of VTCs in many of the membrane networks (Fig. 1, B–J). Depending on the incidence of sectioning through the membrane networks, clusters of closely apposed vesicles and convoluted tubules were found in regions normally containing SER (Fig. 1, C–J). The average size of the vesicles found within the VTCs was 47 ± 19 nm and therefore much smaller than the size of the vesicles associated with networks generated in the presence of Mg2+GTP alone (not shown). Thus, the VTCs represent new structures generated in association with tER during incubation in the presence of Mg2+GTP, Mg2+ATP, and cytosol.
Rough ER cisternae within the membrane networks were least affected by incubation in the presence of cytosol and were often observed as parallel rough cisternae, even after 60 min of incubation (not shown). Minitubules (∼30 nm in diameter) were often observed in association with ER networks, particularly after treatment with cytosol and mixed nucleotides (Fig. 1B, Fig. I, and Fig. J, mt). These represented membranous structures and not microtubules, since unit membranes were seen encompassing the circumference of the minitubules. Hence, LDMs led to the generation of morphological compartments of the early secretory pathway with membrane fusion and transformation (network formation) dependent on both GTP and ATP hydrolysis (Lavoie et al. 1996) and transformation of a specific subdomain (SER) of the network into VTCs dependent on cytosol.
Distribution of Soluble Cargo and Integral Membrane Proteins
To study cargo and membrane protein distribution in reconstituted ER, gold immunolabeling was carried out after the first step generation of tER. The secretory cargo albumin and transferrin revealed a higher density within SER as compared with the rough ER cisternae (Fig. 2A and Fig. B) as verified by quantitation (Table ). A higher concentration in albumin density has also been described in the smooth ER in situ (Dahan, S., M. Dominguez, J. Gushue, P. Melançon, and J.J.M. Bergeron, manuscript submitted for publication).
Table 1.
Amount of Soluble Cargo and Integral Membrane Proteins in Reconstituted tER
| Protein | Networks | Gold particles | Surface area of SER | Labeling over SER | Concentration in SER |
|---|---|---|---|---|---|
| n | n | % | % | ||
| Albumin | 26 | 319 | 60.6 | 73 | 1.6× |
| Transferrin | 15 | 131 | 57.9 | 64.9 | 1.2× |
| α2p24 | 20 | 395 | 66.4 | 76.7 | 2.1× |
| p58 | 12 | 303 | 76.0 | 82.8 | 1.6× |
| Calnexin | 17 | 185 | 61.7 | 74.6 | 1.6× |
| Ribophorin | 19 | 332 | 61.6 | 42.5 | 0.5× |
The distribution of four integral membrane proteins was also assessed, i.e., ribophorin, calnexin, α2p24, and p58. α2p24, a type I integral membrane protein implicated in cargo sorting and membrane biogenesis, is localized in the cis-Golgi network with ∼1/3 found in the ER (Dominguez et al. 1998). The protein p58 is a major constituent of the ERGIC compartment that is also implicated in cargo sorting and membrane biogenesis (Saraste and Kuismanen 1992; Tisdale et al. 1997). Both were found at a higher concentration within the SER portion of the cell-free reconstituted ER networks (Fig. 2C and Fig. D). Occasionally α2p24 was found concentrated over buds (Fig. 2 C, inset). Quantitation revealed a 2.1-fold concentration for α2p24 in the SER compartment, as compared with the surrounding rough membranes (Table ). As for α2p24, p58 immunolabeling was observed in the SER (Fig. 2 D) at a higher concentration to that of the immunolabeled protein in the fused parallel cisternae (Table ). By contrast, ribophorin was more concentrated in the parallel rough membranes juxtaposed to the SER of the networks (Fig. 2 E; Table ). The ribophorin distribution along parallel membranes mimics that of the distribution of the ribosomes (not shown), as would be expected since this rough ER marker has been shown to be in equimolar amounts with ribosomes (Marcantonio et al. 1984). Remarkably, calnexin was found within the SER at a higher concentration than in the surrounding membranes (Fig. 2 F; Table ). In summary, two rough ER markers, ribophorin and ribosomes, were concentrated within reconstituted parallel rough ER cisternae. Two cargo proteins, albumin and transferrin, and three transmembrane proteins, α2p24, p58, and calnexin, were observed in higher concentrations in reconstituted smooth ER tubules. These opposing protein gradients were maintained, despite the fact that the two membrane subcompartments of the ER were continuous. Because the SER containing albumin and transferrin, as well as α2p24 and p58, transforms into VTCs by the addition of cytosol and mixed nucleotides, it was concluded that ER cargo exit site formation was reconstituted.
To address whether protein concentrations were different in the rough and smooth microsomes before incubation, calnexin and α2p24 protein concentrations were studied in the starting membrane preparation. Direct labeling with antibodies to the cytosolic domain of calnexin and α2p24 using preembedding gold immunolabeling was used to determine the concentrations of the proteins in the membranes. Cryosections were ineffective for our studies, since it was not possible to distinguish rough from smooth vesicles in the starting material. The results revealed gold particles over rough and smooth components (Fig. 3A and Fig. B). Labeling density was calculated along the surface of rough and smooth microsomes. For α2p24 labeling, gold particle labeling over smooth microsomes (151 vesicles measured, 4.0 gold particles/μm of membrane) was slightly higher, 1.2 times, compared with that over rough microsomes (136 vesicles measured, 3.3 gold particles/μm of membrane). For calnexin labeling, gold particle labeling over smooth microsomes (175 vesicles measured, 5.5 gold particles/μm of membrane) was also slightly higher, 1.2 times, compared with that over rough microsomes (279 vesicles measured, 4.5 gold particles/μm of membrane). Thus, labeling densities for α2p24 and calnexin were slightly higher in smooth microsomes of the starting membrane preparation. Because of the nature of the preembedding immunogold labeling method, we cannot exclude the possibility that ribosomes associated with the surface of rough microsomes may have partially inhibited labeling by steric hindrance. Thus, the labeling ratios may have been closer to unity for both proteins. In any case, a progressive increase in concentration was found following incubation in the presence of Mg2+GTP and Mg2+ATP in which α2p24 increased to 2.1 times and calnexin increased to 1.6 times (Table ) in density over the SER of the tER, as compared with the surrounding rough ER.
Figure 3.
α2p24 and calnexin distribution in unincubated microsomes. Antibodies to α2p24 and calnexin were applied to unincubated LDMs and gold immunolabeling studied using the preembedding immunolabeling procedure described in Materials and Methods. α2p24 labeling is shown in A and calnexin labeling is shown in B. Gold particle labeling is observed in association with the surface of both rough and smooth microsomes. Arrowheads indicate ribosomes on ER microsomes. Bar, 0.2 μm.
Organization of Cargo Exit Sites by the Cytosolic Domain of α2p24
The effect of antibodies to the cytosolic domain of α2p24 was tested under different conditions of ER assembly and compared with the effects of antibodies to the cytosolic domain of the ER resident membrane protein calnexin. Anticalnexin did not affect Mg2+GTP-dependent membrane fusion or mixed nucleotide-dependent formation of the tER (Fig. 4, a–c, control, d–f, anticalnexin). In contrast, based on the reduced size of the membrane networks, anti-α2p24 inhibited GTP-dependent membrane fusion (Fig. 4 h), as well as mixed nucleotide dependent formation of the tER (Fig. 4i and Fig. j). Quantitation confirmed the effect of anti-α2p24 on the formation of networks with little effect noted by anticalnexin (Fig. 4 k).
Figure 4.
Anti-α2p24, but not anticalnexin antibodies, modulate the structure of the tER. Effect of α2p24 antibody on ER assembly. LDMs incubated with ATP (a, d, and g), GTP (b, e, and h), or ATP/GTP (c, f, i, and j) in the presence of antibodies to the cytosolic domain of calnexin (d–f) or α2p24 (g–j). rer, Rough ER; rm, rough microsomes; ser, smooth ER tubules; sm, smooth microsomes. Quantitation of α2p24 antibody effect by morphometry. (k) Quantitation of rough membranes and SER profiles in networks for conditions described in c, f, i, and j. (l) Conditions are as for i and j, except that antibodies were preincubated with peptides used to produce antibodies against α2p24 or calnexin (CNX). (m) Conditions are as for b, e, and h. (n) Effect of antibodies on the fusion of classical rough ER membranes (high density microsomes prepared and incubated as indicated in Materials and Methods). (o) Binding of anticalnexin antibodies to the cytosolic surface of reconstituted ER membranes. LDMs were incubated in the presence of GTP, ATP, and anticalnexin antibodies. Gold secondary antibody applied using the preembedding immunocytochemical technique reveals calnexin antigenicity on both the surfaces of parallel rough membranes and interconnecting smooth tubules. Bar, 0.2 μm.
As a further control, neutralization experiments were attempted. The effect of anti-α2p24 was neutralized by its antigenic peptide, but not by a peptide corresponding to the cytosolic domain of the ER membrane protein calnexin (Fig. 4 l). Evidence for the efficacy of binding of anticalnexin antibodies to reconstituted ER membranes was observed by immunogold labeling of cryosections (Table ) and reconstituted ER membranes using preembedding immunolabeling (Fig. 3 B and 4 o), and confirmed by immunoblot analysis of unincubated membranes (data not shown). Hence, the cytosolic domain of α2p24, but not of calnexin, modulated step one of the cell-free assembly system that led to the generation of a tER as caused by mixed nucleotide hydrolysis.
Because quantitation also confirmed the effect of the anti-α2p24 antibody on membrane fusion effected by GTP hydrolysis alone (Fig. 4 m), this was compared with GTP-mediated membrane fusion of classical rough ER (high density rough microsomes). Membrane fusion of such rough microsomes required the prior removal of the associated ribosomes (Paiement et al. 1980), and such membrane fusion was unaffected by antibodies to α2p24 (or calnexin; Fig. 4 n). These results further attest to the distinct microdomains that can be distinguished by these membrane fusion assays. Furthermore, when high density microsomes stripped of associated ribosomes were incubated with LDMs, the former were unable to participate in tER formation (data not shown). Therefore, there are two populations of rough ER that can be separated by subcellular fractionation. One is involved in the assembly of tER and is isolated as LDMs, the other is involved in the formation of large rough ER cisternae and is isolated as high density microsomes, and the two exhibit different fusion properties.
Finally, the effect of anti-α2p24 antibodies on cytosol-dependent loss of SER in reconstituted ER networks was tested. Incubation of reconstituted ER networks with anti-α2p24 antibodies before incubation with cytosol led to inhibition of loss of SER within ER networks. In three separate experiments, a total of 106 reconstituted networks were analyzed, and of these networks, 42 ± 4% had recognizable smooth tubules. In contrast, membrane networks incubated with anticalnexin antibodies before treatment with cytosol lost most of their associated smooth tubules. In three separate experiments, a total of 71 reconstituted networks were analyzed, and of these networks, 20 ± 14% had recognizable smooth tubules. Thus, antibodies to the cytosolic tail of α2p24 led to partial inhibition of cytosol-dependent loss of SER in reconstituted ER networks.
Recruitment of COPI Coatomer
The sites of location of α2p24 antigenicity using preembedding gold immunolabeling was studied after induction of VTC formation in the presence of cytosol. Gold immunolabeling revealed α2p24 antigen in vesicular-tubular structures associated with fused rough ER (Fig. 5). The reduced gold immunolabeling is thought to be due to the masking of determinants of the cytosolic domain of α2p24 caused by binding of proteins to the membranes during preincubation in the presence of cytosol. The cytosolic domain of α2p24 binds COPI and COPII coatomer with high affinity and a specificity attributed to the KKXX motif at its COOH-terminal domain for COPI and a diphenylalanine-based motif affecting COPII binding (Dominguez et al. 1998). Because COPI coatomer has been proposed to associate with pre-Golgi apparatus intermediates transporting anterograde cargo early in the secretory pathway (Presley et al. 1997), we elected to pursue the significance of the effect of the α2p24 cytosolic domain on the cell-free system by studying the role of COPI coatomer.
Figure 5.
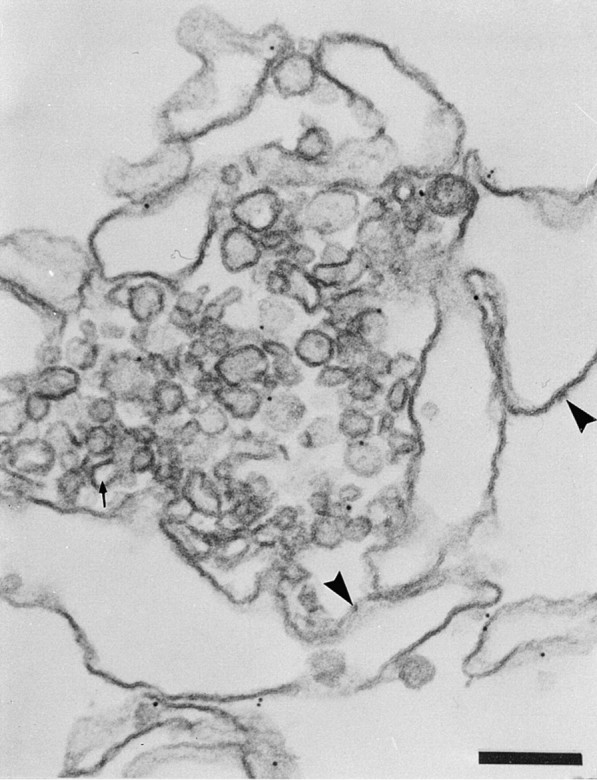
α2p24 distribution in VTCs formed in the presence of cytosol. Formation of VTCs by cytosol plus nucleotides (step two), followed by the addition of primary anti-α2p24 antibody and then gold secondary antibody. α2p24 antigenicity is found within the VTCs. Arrowheads indicate ribosomes on ER membranes. The arrow points to a fenestration of the residual SER. Bar, 0.2 μm.
The predicted binding of COPI to the cell-free system at the ER cargo exit sites (VTCs) was tested following incubations at step two of the reconstitution system with cytosol, Mg2+ATP, and the nonhydrolyzable GTP analogue, GTPγS. Biochemical studies (Fig. 6 A) revealed an augmented association of β-COP to membranes. Visualization of COPI coatomer during these incubations (Fig. 7i) revealed an association of β-COP with the VTCs generated after the tER was incubated with cytosol and Mg2+GTP/ATP (Fig. 7i B) or with Mg2+ATP and Mg2+GTPγS (Fig. 7i, Fig. C and Fig. D). Little labeling with anti–β-COP was found in the absence of cytosol (Fig. 7i A). Quantitation of gold particle distribution confirmed the cytosol and GTPγS-dependent association of β-COP with the VTCs (Fig. 7ii A).
Figure 6.
Content of β-COP in ER membranes and cytosol. (A) Recruitment of β-COP to ER membranes. LDMs were incubated as for Fig. 1, but with BFA or GTPγS as indicated, followed by sedimentation and Western blotting for β-COP. (B) β-COP depletion of cytosol as assessed by β-COP content in control cytosol (lane 1), cytosol incubated with protein A–Sepharose coupled to nonspecific IgG (lane 2), or anti–β-COP (lane 3) followed by Western blotting for β-COP content.
Figure 7.

Coatomer protein-dependent formation of VTCs. (i) Localization of β-COP to VTCs. Immunogold labeling of β-COP with anti–β-COP is shown in association with tER networks post-incubated (step two) in the absence of cytosol (A), the presence of cytosol + ATP/GTP (B), or in the presence of cytosol + ATP + GTPγS (C and D) for 10 min. Arrows, indicate label over smooth membranes; arrowheads, indicate label over clusters of vesicles; f, fenestrations; rm, rough membranes; st, smooth ER tubules. Immunolabeling was done using the preembedding technique described in Materials and Methods. Bar, 0.2 μm. (ii) Modulation of β-COP binding to tER. (A) Effect of GTPγS on the amount of β-COP labeling over VTCs. LDMs were incubated as in i, A–D. Counts are expressed as mean number of gold particles per membrane network over rough ER cisternae (RER) and over vesicular tubular complexes (SER/VTC) formed in the absence of cytosol (−CYT), the presence of cytosol and GTP (+CYT+GTP), or in the presence of cytosol and GTPγS (+CYT+GTPγS). Data is shown from three separate experiments using membranes from separate fractionation experiments. (B) Effect of BFA on the formation of VTCs. LDMs were incubated as for Fig. 1, except for the addition of GTPγS or BFA, as indicated. Except where indicated by *, average values (± SD) are shown from three separate experiments carried out with membranes from different fractionations. The means for VTCs were not significantly different when compared between a–d (P > 0.05, n = 61), but were significantly different when compared between b–d (P < 0.01, n = 61). The means for rough ER membranes were not significantly different (P > 0.05, n = 47) when compared between a and b, b–d, and a–d. The means for smooth ER tubules were not significantly different when compared between a–d, (P > 0.05, n = 47), but were significantly different when compared between a and b (P < 0.001, n = 47) or b and d (0.001 < P < 0.005, n = 47). The means for rough and smooth ER tubules were not significantly different when compared between b and c (P > 0.05, n = 30). RER, rough ER; SER, smooth ER tubules; *, average of two experiments. (C) Effect of β-COP–depleted cytosol on formation of VTCs. Microsomes were incubated with mixed nucleotides (step one) for 180 min to generate tER and then further incubated (step two) for 60 min with or without cytosol, with β-COP–depleted cytosol, or with control-treated cytosol. Amounts of smooth ER tubules associated with the reconstituted membrane networks are compared with the number of VTC structures associated with the same networks. Average of two experiments. A minimum of 32 membrane networks were analyzed for each experimental condition.
Quantitation was also carried out to determine the effects of cytosol on the formation of VTCs. The amount of the SER remaining in the reconstituted membrane networks after treatment with cytosol was used as a measure of the amount of transformation of the tER into VTCs. Percent number of networks with VTCs was also calculated. Thus, a diminution of the amount of SER and a coincident increase in amount of associated VTCs was observed after incubation of reconstituted ER networks in the presence of cytosol plus Mg2+ATP/GTP or Mg2+ATP/ GTPγS (Fig. 7ii B). A prediction of these results is that VTC formation should be sensitive to the fungal metabolite BFA via its action on inhibiting an ARF1-GEF activity (Donaldson et al. 1992; Helms and Rothman 1992). Indeed, BFA significantly inhibited the association of β-COP on tER networks (Fig. 6 A) and significantly diminished the formation of VTCs as evaluated quantitatively (Fig. 7ii B). No effect was observed with BFA alone, when cytosol was omitted (data not shown). None of the incubation conditions affected the amount of rough ER cisternae associated with the networks, as determined by this quantitative assay (Fig. 7ii B). That the cytosol effect was mediated by COPI binding to the tER was tested by partial depletion of COPI coatomer from cytosol with antibody to β-COP (Fig. 6 B). Such cytosol diminished in β-COP content generated fewer VTCs, as compared with cytosol treated with nonimmune IgG (see VTCs compared with loss of SER in Fig. 7ii C).
α2p24 and β-COP Are Localized to ER and Golgi Elements in Rat Liver Hepatocytes
As determined by cryoimmune EM using well characterized antibodies specific to α2p24 (Dominguez et al. 1998), the membrane protein is clearly found in both rough and smooth ER in situ (Fig. 8, A–C). Gold particles decorate the cytoplasmic side of parallel ER cisternae in cryosections immunolabeled with an antibody against the COOH-terminal domain of α2p24 (Fig. 8 A). Tubulo-vesicular smooth ER networks in the Golgi region of hepatocytes, as well as the cis-Golgi intermediate compartment, were also labeled by anti-α2p24 (Fig. 8B and Fig. C). The COPI coatomer subunit β-COP reveals a distribution that overlaps that of α2p24 in liver parenchyma (Fig. 8D and Fig. E). Thus, α2p24 and β-COP are associated with similar structures, including tubular-vesicular elements of the ER often found next to the Golgi apparatus in situ within the rat hepatocyte.
Figure 8.
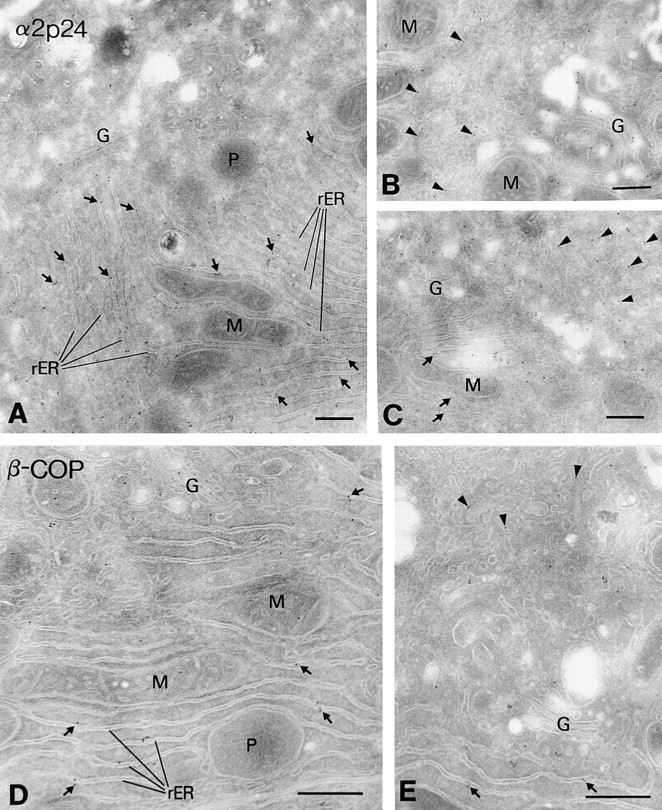
In situ localization of α2p24 and β-COP in rat liver hepatocytes. Rat liver cryosections were immunolabeled with antibodies against the cytosolic tail of α2p24 (A and C), both the cytosolic and lumenal domains of α2p24 (B), or the β-COP subunit of COPI (E5A3 mAb; D and E), followed by goat anti–rabbit IgG 10-nm gold to reveal α2p24 and goat anti–mouse IgG 10-nm gold to reveal β-COP. Immunoreactive α2p24 and β-COP can be detected over the long parallel cisternae of the rough ER (rER, arrows) and tubular-vesicular networks of the smooth ER (arrowheads). These tubular networks can be visualized going in and out of the plane of section (seen clearly in B and E). The Golgi apparatus (G) also reveals gold particle labeling for α2p24 and β-COP, predominantly at the cis face and peripheral distensions. M, mitochondria; P, peroxisomes. Bars, 0.4 μm.
α2p24 in LDMs Is Golgi Apparatus-derived
The cargo molecules albumin and transferrin represent newly synthesized protein cargo of the ER. However, this was unlikely to be the case for α2p24 (and p58) observed in reconstituted ER or for α2p24 observed in hepatocyte ER in situ by immunolabeling. Whether in preparations of LDMs, or even in highly purified stripped rough microsomes (SRM), α2p24 is terminally glycosylated as evident from its lack of sensitivity to endoglycosidase H (endoH), but complete sensitivity to PNGase F (Fig. 9). Equal amounts of protein from each fraction was applied to each lane (100 μg). α2p24 is also highly enriched in Golgi fractions, due to its abundance in the cis-Golgi network that coisolates with hepatic Golgi fractions (Dominguez et al. 1998). Therefore, α2p24 found in the liver ER fraction employed in the in vitro ER reconstitution assay is terminally glycosylated, and thus a molecular derivative of the Golgi apparatus.
Figure 9.
EndoH insensitivity of α2p24 in hepatocyte ER. Low density microsomes (SM), high density stripped rough microsomes (SRM), and Golgi fractions (Gol) were assessed for their α2p24 content by Western blotting and the extent of N-linked glycosylation assessed by endoH or PNGase F sensitivity. 100 μg of protein was applied to each lane.
Discussion
A Cell-free System to Study Assembly of tER and the Formation of ER Exit Sites
A two-step in vitro reconstitution system starting from well-characterized ER-derived LDMs purified from rat liver homogenates (Lavoie et al. 1996) has been used to generate, via nucleotide hydrolysis, membrane structures with the morphological features of the tER. Then, in a second step, cytosol is employed to generate ER cargo exit sites via the formation of VTCs from the preassembled tER. That the in vitro system faithfully reconstituted the early secretory apparatus was established from the following criteria. Morphology: quantitation revealed the nucleotide dependent fusion of LDMs and their transformation into smooth ER and rough ER interfaces of identical morphology to the tER, as seen in liver parenchyma in situ. When the same LDMs as used here were microinjected into the cytoplasm of Xenopus oocytes, a reconstitution of ER identical in structure to that seen in rat liver parenchyma in situ was observed (Paiement et al. 1988). Upon the addition of cytosol and Mg2+ATP/GTP (step two in this study), only the smooth portion of the tER generated VTCs and these correspond to the morphology of pre-Golgi apparatus intermediates based on the morphology of the structures assembled and on the size (47 ± 19 nm) of the associated vesicles (Bannykh and Balch 1998; Morré 1998). Cargo and p58 content: the content of albumin, transferrin, and p58 (ERGIC-53, the marker for the ER-Golgi intermediate compartment; Farquhar and Hauri 1997) was consistent with this compartment as the precursor of the VTCs. In liver parenchyma, two sites of cargo (albumin) concentration have been elucidated. An initial twofold concentration was found at the boundary of the rough ER and smooth ER (Dahan, S., M. Dominguez, J. Gushue, P. Melançon, and J.J.M. Bergeron, manuscript submitted for publication), as observed here. A further fivefold concentration takes place between the cis-Golgi network and stacked flattened cisternae of the Golgi complex (Dahan, S., M. Dominguez, J. Gushue, P. Melançon, and J.J.M. Bergeron, manuscript submitted for publication).
The Role of Membrane Fusion in Assembly of tER
The nucleotide-dependent fusion of LDMs to yield tER is thought to involve both fusion of like (homotypic) and unlike (heterotypic) ER membrane derivatives. Mg2+GTP hydrolysis is required to stimulate fusion of partially rough ER membrane derivatives and Mg2+ATP hydrolysis is required to stimulate fusion of smooth ER membrane derivatives. Hence, these nucleotides contribute to homotypic membrane fusion. At some stage during tER formation, continuity is established between these two ER subcompartments and this would be expected to occur by heterotypic membrane fusion.
However, if partially rough microsomes containing microdomains of smooth ER initially fused in the presence of Mg2+GTP, this would permit subsequent Mg2+ATP-dependent fusion with additional smooth microsomes and obviate a necessity for heterotypic membrane fusion. This possibility has not been ruled out yet. The assays developed here using quantitative morphology and quantitative immunolabeling may now be used to screen for antibodies to proteins that can distinguish between the homotypic and heterotypic membrane fusion events.
Rough ER Membranes within tER Exhibit Unique Fusion Properties
The rough ER subcompartment comprising the tER assembled when LDMs are incubated in the presence of Mg2+GTP and Mg2+ATP is very different from classical rough ER, which is recovered from tissue homogenates as high density rough microsomes. Although high density rough microsomes undergo GTP-dependent fusion, as do low density rough microsomes, the fusion events are different. For example, antibodies to α2p24 inhibit fusion of the partially rough ER comprising tER, but not that of classical rough ER (Fig. 4 n). Fusion of classical rough ER requires prior removal of associated ribosomes (Paiement et al. 1980; Paiement and Bergeron 1983), which transitional rough ER does not (Lavoie et al. 1996). Classical rough ER does not fuse with transitional rough ER when the two types of rough ER are mixed with nucleotides (Lavoie, C., and J. Paiement, unpublished observations). Hence, the fusion machinery associated with the membranes of tER is suggested to be different from that associated with the rest of the ER, and this may be related to the capacity of this compartment to permit formation of ER exit sites. In this scenario, antibodies to α2p24 may affect the generation of tER by influencing the heterooligomerization of p24 family members (Dominguez et al. 1998; Füllekrug et al. 1999; Marzioch et al. 1999). This may be a necessary step for subsequent recruitment of COPI when cytosol is added to generate VTCs.
α2p24 and p58 in tER
The membrane proteins α2p24 and p58 were found in microdomains of the tER. These membrane proteins are found at steady state to be enriched in the cis-Golgi network and ERGIC compartments (Farquhar and Hauri 1997; Dominguez et al. 1998). However, they are also clearly found in the ER and, as for p58 and the p24s cycle through the ER, intermediate compartments, and Golgi complex (reviewed in Farquhar and Hauri 1997; Füllekrug et al. 1999). That α2p24 was terminally glycosylated (endoH resistant) in LDMs, and even in highly purified rough ER membranes, is consistent with a constantly recycling scenario for α2p24.
In the starting preparation of LDMs, α2p24 was found in slightly higher concentration in smooth microsomes. After incubation in step one conditions, a higher concentration of the protein was observed in the SER. The evidence suggests that segregation of α2p24 into SER occurred coincident with tER formation.
The membrane proteins p58 and α2p24 share KKXX motifs at their COOH termini, and in vitro binding assays show that the cytosolic domains of these membrane proteins bind COPI coatomer, and unexpectedly, COPII coatomer as well (Kappeler et al. 1997; Dominguez et al. 1998). These membrane proteins are therefore attractive candidates for coordinating the formation of ER cargo exit sites. As shown here, immunolabeling revealed accumulation of α2p24 in VTCs formed in the presence of cytosol and the in vitro reconstitution assay revealed inhibition of tER formation by antibodies to the cytosolic domain of α2p24. Antibodies to the cytosolic domain of p58 inhibit ER-to-Golgi transport, as well as COPI coatomer binding (Tisdale et al. 1997). In the case of experiments done with anti-p58 (Tisdale et al. 1997) and those reported here with anti-α2p24, possible confounding effects of steric hindrance by antibodies to an abundant protein cannot be excluded. Remarkably, in our studies, antibodies to the cytosolic domain of α2p24 affected the surrounding parallel rough ER cisternae themselves. With Mg2+GTP as sole nucleotide, this membrane fusion step could be studied in isolation. This fusion step was specifically inhibited by antibodies to the cytosolic domain of α2p24, but not calnexin. Because this step was shown to be an early event in the formation of tER (Lavoie et al. 1996) and since formation of ER exit sites occurs from tER (results presented in this paper), we suggest that the influence of α2p24 in affecting the formation of ER cargo exit sites extends to the rough ER portion of the rough/smooth ER boundary of the tER. This boundary is a predicted consequence of incoming retrograde smooth membranes derived from the Golgi apparatus and outgoing anterograde rough membranes transforming into intermediate compartment elements.
Mistargeting of a mutated form of ERGIC-53 to the ER of HeLa cells was shown to impair secretion of a lysosomal enzyme while apparently not affecting gross (light microscope) morphological changes of the early secretory pathway (Vollenweider et al. 1998). The lack of effect on β-COP localization in cells carrying the mutated ERGIC-53 could be due to the compensatory binding of β-COP in the same regions by p24 family members. Consistent with this suggestion is the fact that p24 family members are known to bind COPI coatomer (Kappeler et al. 1997; Dominguez et al. 1998; Bremser et al. 1999) and in our study, both p58 and p24 proteins were observed in the same microdomain of the tER, as shown by immunolocalization of p58 and p24 in cryosections of tER (Fig. 2; Table ).
Role of α2p24 and its COPI Coatomer Ligand in ER Cargo Exit Site Formation
An effect of COPI coatomer on VTC formation was found. This was concluded from the visualization of β-COP in VTCs after the addition of cytosol, the enhancement of VTC formation and β-COP association with VTCs by cytosol with GTPγS, the BFA sensitivity of VTC formation, and the inhibition of VTC formation when β-COP was depleted from cytosol. These coincidental observations are consistent with, but do not prove a direct link between COPI coatomer and the cytosolic domain of α2p24 in the formation of ER cargo exit sites. Indeed, we cannot completely rule out the possibility that binding of antibodies to α2p24, but not calnexin, affects the ability of other abundant membrane proteins in microsomes to access coat proteins. These observations do, however, provide a structural explanation for the observations that ARF1 dominant negative mutants (Dascher and Balch 1994; Peters et al. 1995), BFA (Lippincott-Schwartz et al. 1990), or microinjected antibodies to β-COP (Pepperkok et al. 1993) rapidly inhibit ER-to-Golgi transport of newly synthesized anterograde directed cargo. Furthermore, BFA acts immediately to prevent cargo exit from the ER, suggesting that a locus of COPI binds to ER cargo exit sites (Lippincott-Schwartz et al. 1998). All of these agents may have, as their ultimate target, p24 family members and their effect on structural transformations required for the generation of ER cargo exit sites.
Roles for α2p24 and its COPI coatomer ligand in ER cargo exit site formation have been suggested by results obtained using the novel two-step reconstitution system described. Based on available data, the simplest explanation for the possible involvement of these two proteins is that α2p24 promotes assembly of tER and COPI coatomer is required for subsequent formation of VTCs. The structural modifications implicating α2p24 and COPI involvement are summarized in diagrammatic form (Fig. 10). Because α2p24 is known to bind COPI coatomer, and since anti-α2p24 antibodies were observed to inhibit cytosol-dependent transformation of tER, we cannot exclude a role for α2p24 in the last step of formation of ER exit sites.
Figure 10.
Model for α2p24 and COPI involvement in formation of ER cargo exit sites. The ER-to-Golgi recycling membrane protein α2p24 is suggested to play a role in the assembly of interconnecting smooth tubules (SER) of tER. Coatomer protein COPI promotes formation of VTCs from this ER subcompartment. RER, rough ER.
A Possible Structural Role for the p24 Family of Proteins
A structural role for α2p24 may explain all properties thus far documented for the p24 family of proteins. This would include their enrichment in vesicles derived from ER cargo exit sites and the physical association of p24 family members as heterooligomers in yeast (Belden and Barlowe 1996; Marzioch et al. 1999). The effect of gene disruption of members (one of eight) of the yeast p24 family on the kinetics of a subset of secretory cargo (Schimmöller et al. 1995; Stamnes et al. 1995; Elrod-Erickson and Kaiser 1996), as well as the effect of gene disruption of similar family members on the number of ER-derived vesicles in yeast (Stamnes et al. 1995) may also be attributed to structural consequences of ER cargo exit sites. The ability of at least four (of the eight) p24 family members in yeast to be required for the essential phenotype of sec13 (Elrod-Erickson and Kaiser 1996; Marzioch et al. 1999) are likely a consequence of aberrations in the structural compartment generated at ER cargo exit sites. Because at least four p24 family members are in a biochemical complex in yeast (Marzioch et al. 1999), as well as in mammalian cells (Dominguez et al. 1998; Füllekrug et al. 1999), ER cargo exit site formation may be a function of the balance of p24 family members. The structural role identified here also explains the effect of the addition of antibodies to the cytoplasmic domain of a mammalian p24 family member on the transport of secretory cargo in these cells (Rojo et al. 1997). Alternative models, whereby p24 family members and ERGIC-53 represent specific cargo receptors (Schimmöller et al. 1995; Nichols et al. 1998; Vollenweider et al. 1998), are not completely ruled out by our study, but such models introduce an increased level of complexity and specificity.
COPI and Anterograde Transport
The role of COPI coatomer (and consequently the ARF1 GTPase) as a defining feature required for the generation of membranes of the early secretory apparatus has been argued by Lippincott-Schwartz et al. 1998, as well as by Peter et al. 1993. The hypothesis that COPI affects anterograde transport in the early secretory pathway as supported by the studies shown here also explains why the accumulation of Golgi apparatus resident proteins at the cell surface in yeast strains carrying temperature sensitive alleles of COPI subunits have not been reported (Nickel and Wieland 1998).
The identification of motifs (FF) involved in COPII binding (Dominguez et al. 1998), also shared with p58 (ERGIC-53; Kappeler et al. 1997) in the cytosolic domain of all p24 family members studied thus far, provides a molecular rationale for these ER-Golgi recycling proteins in coordinating cargo transport at ER cargo exit sites formed by the concerted actions of COPII and COPI coatomer. Taken together with the in vivo work of Scales et al. 1997 and Presley et al. 1997, a cooperation between COPI and COPII coatomer at ER cargo exit sites seems clear. The extent of cooperation remains to be determined. Cell-free studies of yeast in which COPI- and COPII-dependent coatomer budding from the isolated nuclear envelope has been reconstituted, secretory cargo was found associated with COPII, but not COPI, decorated buds (Bednarek et al. 1995). In yeast, it remains to be shown whether a COPI mechanism is operational in anterograde ER-to-Golgi transport via an ERGIC compartment. The relative amount of involvement of COPI and COPII in anterograde ER-to-Golgi transport could depend on the relative amount of tER within a particular cell type. Indeed, the basic morphological organization of export complexes has been suggested to vary in different cell types (Bannykh et al. 1996). Clarification, at least with regards to liver, will be obtained when studies are extended using the morphologically based cell-free system reported here to identify further the targets of ATP and GTP hydrolysis and the cooperation between COPII coatomer coating and COPI coats in the early secretory pathway.
Acknowledgments
We thank Dr. Peter McPherson (McGill University) for criticism of the manuscript and Dr. Tommy Nilsson for support and advice throughout these experiments. We thank Dr. Jacopo Saraste for kindly supplying antibodies to p53. We thank Anne Guénette and Ali Fazel for expert assistance, and Jean Léveillé for photographic assistance.
This work was supported by grants from the Medical Research Council of Canada to J. Paiement and J.J.M. Bergeron. C. Lavoie was a recipient of a studentship from the Medical Research Council of Canada.
Footnotes
1.used in this paper: BFA, Brefeldin A; endoH, endoglycosidase H; ERGIC-53, ER-Golgi intermediate compartment protein; SER, interconnecting smooth ER tubules; LDMs, low density microsomes; tER, transitional endoplasmic reticulum; VTCs, vesicular tubular clusters
References
- Bajjalieh S.M., Scheller R.H. The biochemistry of neurotransmitter secretion. J. Biol. Chem. 1995;270:1971–1974. doi: 10.1074/jbc.270.5.1971. [DOI] [PubMed] [Google Scholar]
- Bannykh S.I., Balch W.E. Selective transport of cargo between the ER and Golgi compartments. Histochem. Cell Biol. 1998;109:463–475. doi: 10.1007/s004180050248. [DOI] [PubMed] [Google Scholar]
- Bannykh S.I., Rowe T., Balch W.E. The organization of endoplasmic reticulum export complexes. J. Cell Biol. 1996;135:19–35. doi: 10.1083/jcb.135.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudhuin P., Evrard P., Berthet J. Electron microscopic examination of subcellular fractions. I. The preparation of representative samples from suspensions of particles. J. Cell Biol. 1967;32:181–191. doi: 10.1083/jcb.32.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarek S.Y., Ravazzola M., Hosobushi M., Amherdt M., Perrelet A., Schekman R., Orci L. COPI- and COPII-coated vesicles bud directly from the endoplasmic reticulum in yeast. Cell. 1995;83:1183–1196. doi: 10.1016/0092-8674(95)90144-2. [DOI] [PubMed] [Google Scholar]
- Belden W.J., Barlowe C. Erv25p, a component of COPII-coated vesicles, forms a complex with emp24 that is required for efficient endoplasmic reticulum to Golgi transport. J. Biol. Chem. 1996;271:26939–26946. doi: 10.1074/jbc.271.43.26939. [DOI] [PubMed] [Google Scholar]
- Bremser M., Nickel W., Schweikert M., Ravazzolla M., Amherdt M., Hughes C.A., Söllner T.H., Rothman J.E., Wieland F.T. Coupling of coat assembly and vesicle budding to packaging of putative cargo receptors. Cell. 1999;96:495–506. doi: 10.1016/s0092-8674(00)80654-6. [DOI] [PubMed] [Google Scholar]
- Dahan S., Ahluwalia J.P., Wong L., Posner B.I., Bergeron J.J.M. Concentration of intracellular hepatic apolipoprotein E in Golgi apparatus saccular distensions and endosomes. J. Cell Biol. 1994;127:1859–1869. doi: 10.1083/jcb.127.6.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallner G., Siekevitz P., Palade G.E. Biogenesis of endoplasmic reticulum membranes. I. Structural and chemical differentiation in developing rat hepatocyte. J. Cell Biol. 1966;30:73–96. doi: 10.1083/jcb.30.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dascher C., Balch W.E. Dominant inhibitory mutants of ARFI block endoplasmic reticulum to Golgi transport and trigger disassembly of the Golgi apparatus. J. Biol. Chem. 1994;269:1437–1448. [PubMed] [Google Scholar]
- Dominguez J.M., Lanoix J., Paiement J. Localization of ras antigenicity in rat hepatocyte plasma membrane and rough endoplasmic reticulum fractions. Exp. Cell Res. 1991;192:137–147. doi: 10.1016/0014-4827(91)90168-t. [DOI] [PubMed] [Google Scholar]
- Dominguez M., Dejgaard K., Fullekrug J., Dahan S., Fazel A., Paccaud J-P., Thomas D.Y., Bergeron J.J.M., Nilsson T. gp25L/emp24/p24 protein family members of the cis-Golgi network bind both COP I and II coatomer. J. Cell Biol. 1998;140:751–765. doi: 10.1083/jcb.140.4.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson J.G., Finazzi D., Klausner R.D. Brefeldin A inhibits Golgi membrane-catalysed exchange of guanine nucleotide onto ARF protein. Nature. 1992;360:350–352. doi: 10.1038/360350a0. [DOI] [PubMed] [Google Scholar]
- Elazar Z., Orci L., Ostermann J., Amherdt M., Tanigawa G., Rothman J.E. ADP-ribosylation factor and coatomer couple fusion to vesicle budding. J. Cell Biol. 1994;124:415–424. doi: 10.1083/jcb.124.4.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elrod-Erickson M.J., Kaiser C.A. Genes that control fidelity of endoplasmic reticulum to Golgi transport identified as suppressors of vesicle budding mutations. Mol. Biol. Cell. 1996;7:1043–1058. doi: 10.1091/mbc.7.7.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar M., Hauri H.-P. Protein sorting and vesicular traffic in the Golgi apparatus. In: Berger E., Roth J., editors. The Golgi Apparatus. Birkhauser Verlag; Basel, Switzerland: 1997. pp. 63–128. [Google Scholar]
- Fawcett D.W. Observations on the cytology and electron microscopy of hepatic cells. J. Natl. Cancer Inst. 1955;15:1475–1503. [PubMed] [Google Scholar]
- Füllekrug F., Suganama J.T., Tang T.B.L., Hong W., Storrie B., Nilsson T. Localization and recycling of gp27 (γp24δ3)complex formation with other p24 family members. Mol. Biol. Cell. 1999;In press doi: 10.1091/mbc.10.6.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitescu L., Bendayan M. Immunolabeling efficiency of protein A–gold complexes. J. Histochem. Cytochem. 1990;38:1523–1530. doi: 10.1177/38.11.2212613. [DOI] [PubMed] [Google Scholar]
- Helms J.B., Rothman J.E. Inhibition by brefeldin A of a Golgi membrane enzyme that catalyses exchange of guanine nucleotide bound to ARF. Nature. 1992;360:352–354. doi: 10.1038/360352a0. [DOI] [PubMed] [Google Scholar]
- Kappeler F., Klopfenstein D.R., Foguet M., Paccaud J.-P., Hauri H.-P. The recycling of ERGIC-53 in the early secretory pathwayERGIC-53 carries a cytosolic endoplasmic reticulum-exit determinant interacting with COPII. J. Biol. Chem. 1997;272:31801–31808. doi: 10.1074/jbc.272.50.31801. [DOI] [PubMed] [Google Scholar]
- Lavoie C., Lanoix J., Kan F.W.K., Paiement J. Cell-free assembly of rough and smooth endoplasmic reticulum. J. Cell Sci. 1996;109:1415–1425. doi: 10.1242/jcs.109.6.1415. [DOI] [PubMed] [Google Scholar]
- Letourneur F., Gaynor E.C., Hennecke S., Démollère C., Duden R., Emr S.D., Riezman H., Cosson P. Coatomer is essential for retrieval of dilysine-tagged proteins to the endoplasmic reticulum. Cell. 1994;79:1199–1207. doi: 10.1016/0092-8674(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Donaldson J.G., Schweizer A., Berger E.G., Hauri H.-P., Yuan L.C., Klausner R.D. Microtubule-dependent retrograde transport of proteins into the ER in the presence of brefeldin A suggests an ER recycling pathway. Cell. 1990;60:821–836. doi: 10.1016/0092-8674(90)90096-w. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Cole N.B., Donaldson J.G. Building a secretory apparatusrole of ARF1/COPI in Golgi biogenesis and maintenance. Histochem. Cell Biol. 1998;109:449–462. doi: 10.1007/s004180050247. [DOI] [PubMed] [Google Scholar]
- Marcantonio E., Amar-Costesec E.A., Kreibich G. Segregation of the polypeptide translocation apparatus to regions of the endoplasmic reticulum containing ribophorins and ribosomes. II. Rat liver microsomal subfractions contain equimolar amounts of ribophorins and ribosomes. J. Cell Biol. 1984;99:2254–2259. doi: 10.1083/jcb.99.6.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzioch M., Henthorn D.C., Hermann J.M., Wilson R., Thomas D.Y., Bergeron J.J.M., Solari R.C.E., Rowley A. Erp1p and Erp2p, partners for Emp24p and Erv25p in a yeast p24 complex. Mol. Biol. Cell. 1999;In press doi: 10.1091/mbc.10.6.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morré D.J. Cell-free analysis of Golgi apparatus membrane traffic in rat liver. Histochem. Cell Biol. 1998;109:487–504. doi: 10.1007/s004180050250. [DOI] [PubMed] [Google Scholar]
- Morré D.J., Keenan T.W. Membrane flow revisitedwhat pathways are followed by membrane molecules moving through the Golgi apparatus? BioScience. 1997;47:489–498. [Google Scholar]
- Nichols W.C., Seligsohn U., Zivelin A., Terry V.H., Hertel C.E., Wheatley M.A., Moussalli M.J., Hauri H-P., Ciavarella N., Kaufman R.J. Mutations in the ER-Golgi intermediate compartment protein ERGIC-53 cause combined deficiency of coagulation factors V and VIII. Cell. 1998;93:61–70. doi: 10.1016/s0092-8674(00)81146-0. [DOI] [PubMed] [Google Scholar]
- Nickel W., Wieland F.T. Biosynthetic protein transport through the early secretory pathway. Histochem. Cell Biol. 1998;109:477–486. doi: 10.1007/s004180050249. [DOI] [PubMed] [Google Scholar]
- Novick P., Field C., Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980;21:205–215. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- Ou W.J., Cameron P.H., Thomas D.Y., Bergeron J.J.M. Association of folding intermediates of glycoproteins with calnexin during protein maturation. Nature. 1993;364:771–776. doi: 10.1038/364771a0. [DOI] [PubMed] [Google Scholar]
- Paiement J., Bergeron J.J.M. Localization of GTP-stimulated core glycosylation to fused microsomes. J. Cell Biol. 1983;96:1791–1796. doi: 10.1083/jcb.96.6.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paiement J., Beaufay H., Godelaine D. Coalescence of microsomal vesicles from rat livera phenomenon occurring in parallel with enhancement of the glycosylation activity during incubation of stripped rough microsomes with GTP. J. Cell Biol. 1980;86:29–37. doi: 10.1083/jcb.86.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paiement J., Kan F.W.K., Lanoix J., Blain M. Cytochemical analysis of the reconstitution of endoplasmic reticulum after microinjection of rat liver microsomes into Xenopus oocytes. J. Histochem. Cytochem. 1988;36:1263–1273. doi: 10.1177/36.10.2843593. [DOI] [PubMed] [Google Scholar]
- Pelham H.R.B. Membrane transportgreen light for Golgi traffic. Nature. 1997;389:17–19. doi: 10.1038/37870. [DOI] [PubMed] [Google Scholar]
- Pepperkok R., Scheel J., Horstmann H., Hauri H.-P., Griffiths G., Kreis T.E. β-COP is essential for biosynthetic membrane transport from the endoplasmic reticulum to the Golgi complex in vivo. Cell. 1993;74:71–82. doi: 10.1016/0092-8674(93)90295-2. [DOI] [PubMed] [Google Scholar]
- Peter F., Plutner H., Zhu H., Dreis T.E., Balch W.E. β-COP is essential for transport of protein from the endoplasmic reticulum to the Golgi in vitro. J. Cell Biol. 1993;122:1155–1167. doi: 10.1083/jcb.122.6.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters P.J., Hsu V.W., Ooi C.E., Finazzi D., Teal S.B., Oorschot V., Donaldson J.G., Klausner R.D. Overexpression of wild-type and mutant ARF1 and ARF6distinct perturbations of nonoverlapping membrane compartments. J. Cell Biol. 1995;128:1003–1017. doi: 10.1083/jcb.128.6.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presley J.F., Cole N.B., Schroer T.A., Hirschberg K., Zaal K.J.M., Lippincott-Schwartz J. ER-to-Golgi transport visualized in living cells. Nature. 1997;389:81–85. doi: 10.1038/38001. [DOI] [PubMed] [Google Scholar]
- Rojo M., Pepperkok R., Emery G., Kellner R., Stand E., Parton R.G., Gruenberg J. Involvement of the transmembrane protein p23 in biosynthetic protein transport. J. Cell Biol. 1997;139:1119–1135. doi: 10.1083/jcb.139.5.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J.E. Mechanisms of intracellular protein transport. Nature. 1994;372:55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- Rothman J.E., Wieland F.T. Protein sorting by transport vesicles. Science. 1996;272:227–234. doi: 10.1126/science.272.5259.227. [DOI] [PubMed] [Google Scholar]
- Saraste J., Kuismanen E. Pathways of protein sorting and membrane traffic between the rough endoplasmic reticulum and the Golgi complex. Semin. Cell Biol. 1992;3:343–355. doi: 10.1016/1043-4682(92)90020-V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scales S.J., Pepperkok R., Kreis T.E. Visualization of ER-to-Golgi transport in living cells reveals a sequential mode of action for COPII and COPI. Cell. 1997;90:1137–1148. doi: 10.1016/s0092-8674(00)80379-7. [DOI] [PubMed] [Google Scholar]
- Schekman R., Orci L. Coat proteins and vesicle budding. Science. 1996;271:1526–1533. doi: 10.1126/science.271.5255.1526. [DOI] [PubMed] [Google Scholar]
- Schimmöller F., Singer-Krüger B., Schröder S., Krüger U., Barlowe C., Riezman H. The absence of Emp24p, a component of ER-derived COPII-coated vesicles, causes a defect in transport of selected proteins to the Golgi. EMBO (Eur. Mol. Biol. Organ.) J. 1995;14:1329–1339. doi: 10.1002/j.1460-2075.1995.tb07119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slot J.W., Geuze H.J. A method for preparing gold probes for multiple labeling cytochemistry. Eur. J. Cell Biol. 1985;38:87–93. [PubMed] [Google Scholar]
- Stamnes M.A., Craighead M.W., Hoe M.H., Lampen N., Geromanos S., Tempst P., Rothman J.E. An integral membrane component of coatomer-coated transport vesicles defines a family of proteins involved in budding. Proc. Natl. Acad. Sci. USA. 1995;92:8011–8015. doi: 10.1073/pnas.92.17.8011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stäubli W., Hess R., Weibel E.R. Correlated morphometric and biochemical studies on the liver cell. II. Effects of phenobarbital on rat hepatocytes. J. Cell Biol. 1969;42:92–112. doi: 10.1083/jcb.42.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor T.C., Kanstein M., Weidman P., Melançon P. Cytosolic ARFs are required for vesicle formation but not for cell-free intra-Golgi transportevidence for coated vesicle-independent transport. Mol. Biol. Cell. 1994;5:237–252. doi: 10.1091/mbc.5.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisdale E.J., Plutner H., Matteson J., Balch W.E. p53/58 binds COPI and is required for selective transport through the early secretory pathway. J. Cell Biol. 1997;137:581–593. doi: 10.1083/jcb.137.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollenweider F., Kappeler F., Itin J., Hauri H.-P. Mistargeting of the lectin ERGIC-53 to the endoplasmic reticulum of Hela cells impairs the secretion of a lysosomal enzyme. J. Cell Biol. 1998;142:377–389. doi: 10.1083/jcb.142.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada I., Rindress D., Cameron P.H., Ou W.-J., Doherty J.J., Louvard D., Bell A.W., Dignard D., Thomas D.Y., Bergeron J.J.M. SSRα and associated calnexin are major calcium binding proteins of the endoplasmic reticulum membrane. J. Biol. Chem. 1991;266:19599–19610. [PubMed] [Google Scholar]