Abstract
Expression of the canine 180-kD ribosome receptor (p180) in yeast cells resulted in a marked proliferation of intracellular membranes. The type of membranes observed varied with the expression of specific portions of p180. Rough membranes predominated when the ribosome binding domain of p180 was present, whereas expression constructs lacking this region resulted in smooth membranes. Northern analysis indicated that expression of the NH2-terminal 767 amino acids (ΔCT), which include the ribosome binding domain, upregulated the transcription and translation of genes involved in exocytosis. The membranes that were proliferated were functional as these cells overcame a temperature-sensitive translocation defect. Most significantly, cells that overexpressed ΔCT and proliferated rough endoplasmic reticulum exhibited severalfold higher levels of secretion of an ectopically expressed secretory protein. We conclude that p180 expression triggers a cascade of events leading to an increase in secretory potential akin to the terminal differentiation of mammalian secretory cells and tissues.
Keywords: secretion, endoplasmic reticulum, membrane biogenesis, yeast, ribosome receptor
The entry of proteins into the secretory pathway via the rough endoplasmic reticulum (ER) is essential for intracellular transport of proteins to the extracellular milieu as well as to a number of compartments within the cell (for review see Brodsky 1998). Depending on their functional differentiation, different cell types possess intracellular membranes that comprise this pathway to a lesser or greater extent. Thus, in tissues with high secretory activity, such as pancreas or liver, or the plasma cells of the immune system, the secretory apparatus is developed to a level at which its membranous elements dominate the morphological landscape. A question central to both cell and developmental biology is: What is the molecular basis of the biogenesis of the secretory pathway in such tissues? This is clearly a complex problem that would benefit greatly from a simple, genetically manipulable experimental system. An interesting option has presented itself that relies on the ability of a ribosome binding protein, p180, to induce the proliferation of membranes in yeast (Wanker et al. 1995).
Pioneering studies by Palade, Rutter, and others documented a massive proliferation of membranes during the ontogeny of hepatocytes and cells of the pancreas (Dallner et al. 1966; Wessels and Evans 1968; Pictet et al. 1972). Through a combination of morphological and biochemical studies they showed that around the time of birth, extensive membrane proliferation is accompanied by an increase in marker enzymes. Most important is the fact that these changes coincide with the onset of the animal's ability to secrete tissue specific proteins. In B lymphocytes, similar changes in morphology and cellular physiology occur during antigen-induced maturation into plasma cells (Shohat et al. 1973). As with pancreas and liver, these changes result in a dramatic increase in secretory capacity.
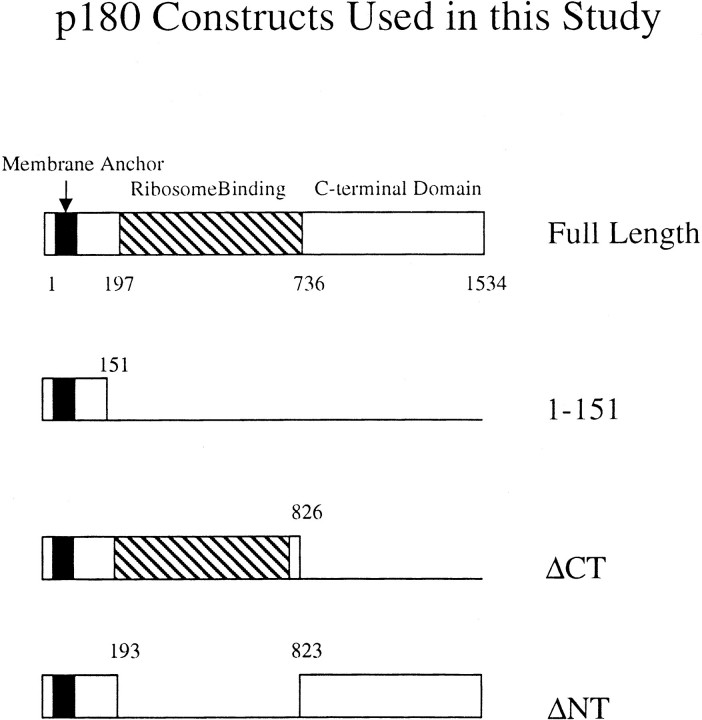
The most obvious morphological change seen in liver, pancreas, and B cells is the proliferation of rough ER. Studies by Sabatini and others have shown that in such tissues the polysomes associated with the ER are predominantly synthesizing secretory products (for review see Hortsch and Meyer 1986). These polysomes are anchored to the ER both by nascent polypeptide chains and by interactions with ER membrane proteins (Rosbash and Penman 1971; Adelman et al. 1973; Borgese et al. 1974). One such ER membrane protein, having a molecular mass of 180 kD, was originally isolated in a functional assay from pancreatic microsomes, and has been shown to induce ribosome binding both in vivo and in vitro (Savitz and Meyer 1990, Savitz and Meyer 1993; Wanker et al. 1995). It displays tissue-specific expression, with highest levels observed in typical secretory tissues, e.g., pancreas, liver, and placenta (Langley et al. 1998). This receptor, referred to here as p180, consists of three domains (see Fig. 1). At the NH2 terminus is its membrane anchor. It is followed by a highly repetitive basic domain that is responsible for its ribosome binding ability (Wanker et al. 1995). In the canine as well as the human form of p180, the number of uninterrupted consensus decapeptide motifs in the ribosome binding domain is 54 (Wanker et al. 1995; Langley et al. 1998). The function of its acidic COOH-terminal domain is currently unknown, although based on the presence of typical heptad repeats, it is believed to form an α-helical double-stranded coiled-coil rod (Leung et al. 1996; Langley et al. 1998). The p180 protein has a highly conserved primary structure, as the overall level of amino acid identities between the canine and human forms is 93%, rising to 99% for the NH2-terminal 86 amino acids.
Figure 1.
Constructs used in this study. A description of cloning strategies can be found in Materials and Methods. The striped area, encoding a domain with 54 tandem decapeptides, has been shown previously to be essential for ribosome binding (Wanker et al. 1995). Numbers shown correspond to amino acids in the primary structure. In the text, the full-length construct is referred to as p180FL. When p1-151 is expressed, a protein is produced that comprises the NH2-terminal 151 amino acids of p180. The Membrane Anchor designation refers to amino acids 6–33, which are uncharged and mainly hydrophobic, and whose deletion leads to the production of a soluble form of p180.
A remarkable feature of p180 was first seen in studies in which the gene was expressed in yeast. When constructs encoding p180 were expressed under the control of a regulatable promoter, a rapid and significant proliferation of membranes occurred (Wanker et al. 1995). Constructs whose primary sequence included the repetitive second domain induced the proliferation of rough membranes, while those lacking this region induced smooth membrane proliferation. The expression of membrane proteins in yeast was shown previously to result in the production of membranes of varying morphologies, but never rough ER (Ohkuma et al. 1995; Koning et al. 1996). Obvious questions arising from these studies include: Are the observed membranes functional rough ER? Does the cell show an increased secretory capacity? Is what we have observed in yeast comparable to the induction of the secretory pathway in developing mammalian cells?
In this study we have ectopically expressed constructs encoding different forms of p180 in yeast. The transfected cells take on morphologies reminiscent of highly developed mammalian secretory cells. Our molecular and biochemical studies indicate that an upregulation of the secretory pathway has taken place with a striking increase in secretory capacity. These results support using yeast as a genetically manipulable model for the study of the differentiation of (mammalian) secretory cells.
Materials and Methods
Construction of Expression Plasmids
Regulated expression of the constructs used in this study was achieved through the use of the vector pYEX-BX that utilizes the copper-inducible promoter from the CUP1 gene (Amrad Biotech). For the construction of the p180FL, ΔCT, and ΔNT we cloned BamHI-SalI fragments of the vectors pRRFL-EW1, pBSRRΔCT, and pBSRRΔNT (Wanker et al. 1995), respectively, into pYEX-BX. For the construction of pYEX-BX-AA1-151 we amplified a fragment from pRRFL-EW1 using the primers 5′-AAGGATCCATGGATATTTACGACACTCAGACC-3′ and 5′-AAGTCGACTTACTCCTTGGGAGCAGTTTCTAA-3′.
The fragment was cloned into pYEX-BX using BamHI and SalI sites. Plasmids were transfected into Escherichia coli XL1-Blue by the method of Cohen et al. 1972. The Saccharomyces cerevisiae strain SEY 6210 (MATαleu2-3,112 ura3-52 his3-Δ200 trp1-Δ901 lys2-801 suc2-Δ9) (Wilsbach and Payne 1993) was transfected using a lithium acetate based method (Ito et al. 1983). The copper-dependent expression of all constructs was assayed by Northern and Western blot analyses. HMG1 and HMG2 clones were obtained from Robin Wright (University of Washington, Seattle, WA) and subcloned into the BamHI and EcoRI sites of pYEX-BX for copper-regulated expression.
Cells were grown on SD or Sgal medium for experiments using galactose induction. SD medium, with or without 50 mM copper sulfate, was used for experiments in which proteins were expressed under control of the CUP1 promoter. SEY 6210 was the strain used for all experiments. After 5 h of growth, transformed cells were in logarithmic phase and were used for further study.
Electron Microscopy
Yeast cells were spheroplasted with oxalyticase in SD sorbitol buffer, fixed in 2% glutaraldehyde, and postfixed with 1% OsO4 in sodium cacodylate solution. Samples were dehydrated in ethanol and embedded in Spurr (Ted Pella, Inc.). Sections ∼60 nm thick were made with an MT6000-XL ultamicrotome (RMC, Inc.) and stained with uranyl acetate and lead citrate. Sections were examined with a JEM-1200EX electron microscope (JEOL).
RNA Isolation and Northern Blot Analysis
RNA isolation was performed by the method of Hollingworth et al. (1990). Total RNA (10 μg) was separated on a 1.2% formamide containing agarose gel (Maniatis et al. 1982) and transferred to Magna Membranes (Micron Separations). Probes were generated either from restriction fragments of cloned DNA, or through PCR using the primer pairs below using yeast genomic DNA as a template: 28S RNA, 5′-TGACCTCAAATCAGGTAGGA-3′ and 5′-TGTACTTGTTCGCTATCGGT-3′; PYK1, 5′-AAAGATTGACCTCATTAAACG-3′ and 5′-GAGACTTGCAAAGTGTTGGA-3′; KAR2, 5′-AGTCTCAAGGGAAAAAGCGT-3′ and 5′-AGCTTCCAGCAGCAAAAATT-3′; SEC61, 5′-ATGTCCTCCAACCGTGTTCTA-3′ and 5′-AAAATCCTGGAACGAGGTTC-3′; OST1, 5′-GGTATATATCATTCAAACGTG-3′ and 5′-CAAGACGCAAACTACAAAA-3′; and GDA1, 5′-TGGCGCCCATCTTTAGAAAT-3′ and 5′-CAAGCTGATTGAATTTTAC-3′.
Northern blots were quantified using a PhosphorImager and Imagequant software (Molecular Dynamics). All lanes were corrected for loading inconsistencies by normalization to expression of PYK1. Values expressed are relative to vector-only controls.
Immunofluorescence Microscopy
The antiserum to RRp has been described previously (Wanker et al. 1995). The antiserum against Sec61p was kindly provided by R. Schekman (University of California, Berkeley, Berkeley, CA) and the anti-Gda1p antisera by G. Payne (University of California, Los Angeles, Los Angeles, CA). Immunofluorescent staining of yeast cells was performed according to Adams and Pringle 1984 and Pringle et al. 1991. FITC-conjugated goat anti–rabbit antibodies were purchased from Sigma Chemical Co. The images were generated using a Zeiss fluorescence laser microscope.
Complementation of sec63
The temperature-sensitive strain ptl1/sec63 (Toyn et al. 1988) was transformed with the expression plasmids and grown at the permissive temperature on selective media. Following the induction of the expression for 4 h, 10-fold serial dilutions were plated and incubated at 24°C or 37°C for 6 or 3 d, respectively.
Rescue from BPTI Toxicity
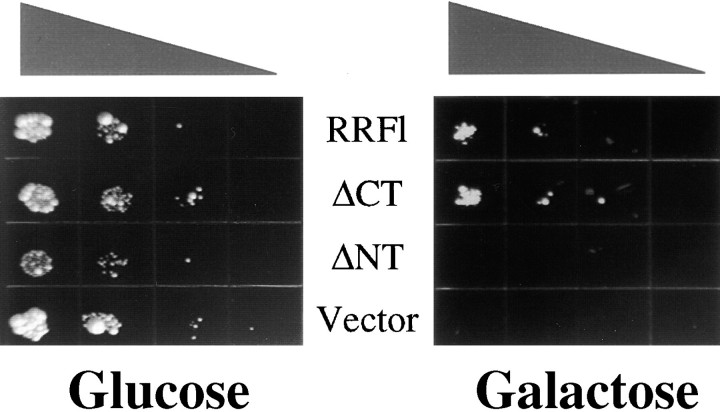
SEY 6210 were cotransformed with a galactose-inducible high copy plasmid expressing secreted bovine pancreatic trypsin inhibitor (BPTI)1 (Parekh et al. 1995) and pYEX-BX, pYEX-BX-p180FL, -ΔNT, or -ΔCT. Following induction of copper-dependent expression of the p180 constructs, 10-fold serial dilutions were plated on glucose or galactose containing media and incubated for 3 or 5 d at 30°C. For quantification of the secreted BPTI in the supernatant, aliquots were tested as described by Parekh et al. 1995.
Results
p180 Expression Results in Extensive Rough Membrane Proliferation in Yeast
Preliminary observations indicated that the expression of various domains of p180 led to membrane proliferation in yeast (Wanker et al. 1995). To refine this system for the study of membrane biogenesis, we subcloned p180-encoding constructs under control of the regulatable CUP1 promoter (Fig. 1). High levels of expression were achieved by growing cells in copper-containing medium. Expression of the full-length ribosome receptor led to the generation of cells whose morphology (Fig. 3) was quite different from vector-only controls (Fig. 2). The cytoplasm was filled with rough (ribosome-studded) membranes that appeared in many places to have direct connections to the nuclear envelope. There was no restriction of the membranes to certain parts of the cell; rough membranes were seen in all areas between the nucleus and the periphery of the cell.
Figure 3.
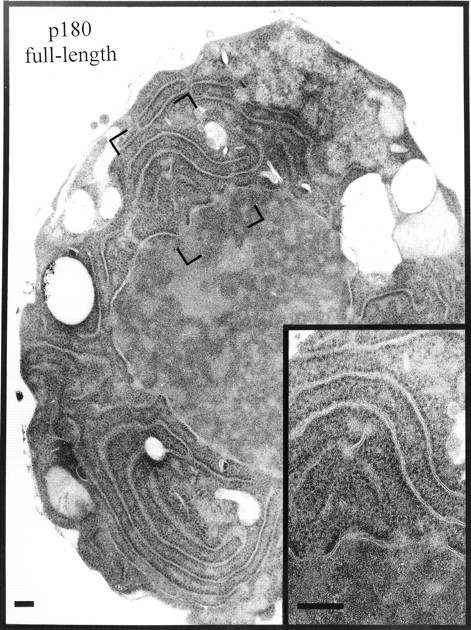
Expression of full-length p180 induces rough membrane proliferation. Cells were transformed with pYEX-BX containing full-length p180 cDNA. Transformants were selected and grown in Cu2+-containing medium for 5 h before preparation for electron microscopy. Visible are extensive arrays of ribosome-studded membranes. Bars, 200 nm.
Figure 2.
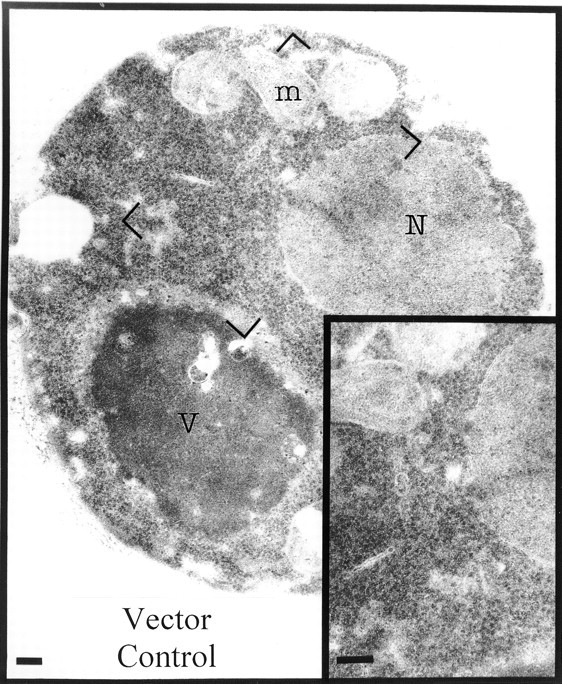
Vector-only control yeast cells have few intracellular membranes. Cells were transformed with pYEX-BX. Transformants were selected and grown in Cu2+-containing medium for 5 h before preparation for electron microscopy. Visible are mitochondria, the nucleus, the vacuole, and substantial quantities of free ribosomes in the cytosol. Bars, 200 nm.
This result was quite different from the types of membranes observed in cells where proliferation had been induced by the expression of the HMG1 gene (Wright et al. 1988). In that case, tightly packed layers of membranes were observed in the perinuclear region, and were accordingly named karmellae. When only the first 151 amino acids of p180 were expressed, corresponding to a stretch of amino acids from the NH2 terminus to the repeat region (including the membrane-spanning domain (see Fig. 1), we obtained cells with proliferated membranes identical in appearance to karmellae (Fig. 4). Many, but not all, proteins with membrane spanning domains can induce karmellae (Vergères et al. 1993; Ohkuma et al. 1995; Parrish et al. 1995). The expression of soluble forms of p180, i.e., ones lacking the NH2-terminal membrane anchor (see Fig. 1), did not induce membrane proliferation (data not shown). In close agreement with previously published data (Wanker et al. 1995), the frequency with which proliferated membranes could be seen in thin sections—in all of the studies described here—was between 40 and 60%.
Figure 4.
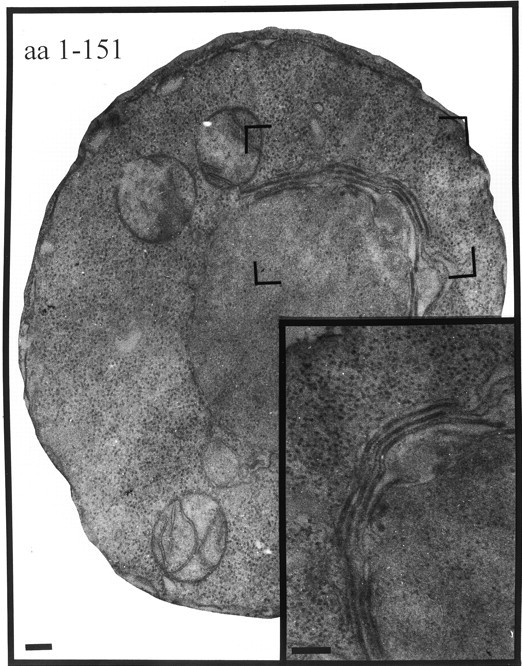
Expression of amino acids 1–151 of p180 induces membrane proliferation having a karmellar morphology. Cells were transfected with pYEX-BX-AA1-151. Transformants were selected and grown in Cu2+-containing medium for 5 h before preparation for electron microscopy. Visible are perinuclear membranes similar in appearance to karmellae. Bars, 200 nm.
Expression of Different Parts of p180 Results in the Proliferation of Intracellular Membranes Having Different Morphologies
We constructed two versions of p180 harboring deletions in major domains (Fig. 1). The first lacks the ribosome binding domain (the 54 tandem repeats of the decapeptide motif) and is referred to as ΔNT. The second lacks the COOH-terminal coiled-coil forming domain and is referred to as ΔCT (see Materials and Methods and Wanker et al., 1995 for details). Expression of ΔNT resulted in the proliferation of smooth membranes, with consistent 80–100 nm spacing, from the perinuclear region to the cell periphery (Fig. 5). The smooth appearance would be expected, as the ΔNT construct lacks the ribosome binding domain. In fact, ribosomes are selectively excluded from areas of the cell where the smooth membranes are located, and are instead restricted to more peripheral areas of the cell. This is quite different from the random distribution of ribosomes throughout the cytosol of wild-type yeast (Fig. 2). It is interesting to note, however, that the presence of the COOH-terminal domain on the ΔNT construct results in a definitely nonkarmellar type of membrane proliferation. In this case, the spacing between membranes is far greater than in that of karmellae, and could be a feature of the ability of ΔNT to interact through its putative coiled-coil domains with cytosolic components.
Figure 5.
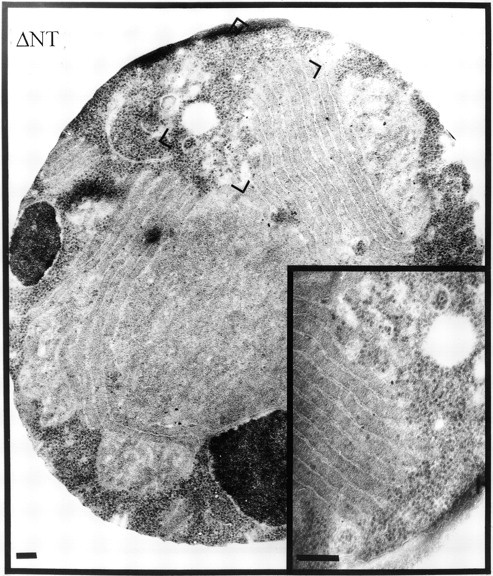
Expression of p180 without its ribosome binding domain produces extensive smooth membrane proliferation different from karmellae. Cells were transfected with pYEX-BX containing the ΔNT version of p180. Transformants were selected and grown in Cu2+-containing medium for 5 h before preparation for electron microscopy. Visible are parallel arrays of smooth membranes. Note the exclusion of cytoplasmic ribosomes from the areas where membranes are evident, and their high density in membrane-free zones. Bars, 200 nm.
A different, yet equally striking morphology was observed in the case of the ΔCT construct. In this case, membrane spacing collapses to that closely resembling karmellae, yet there is no restriction to the perinuclear area, and several areas of proliferated membranes are observed at the cell periphery (Fig. 6). In contrast to ΔNT, the membranes have attached ribosomes, as indicated by the dense staining in the intermembrane space, and a lower density of free cytosolic ribosomes compared with ΔNT.
Figure 6.
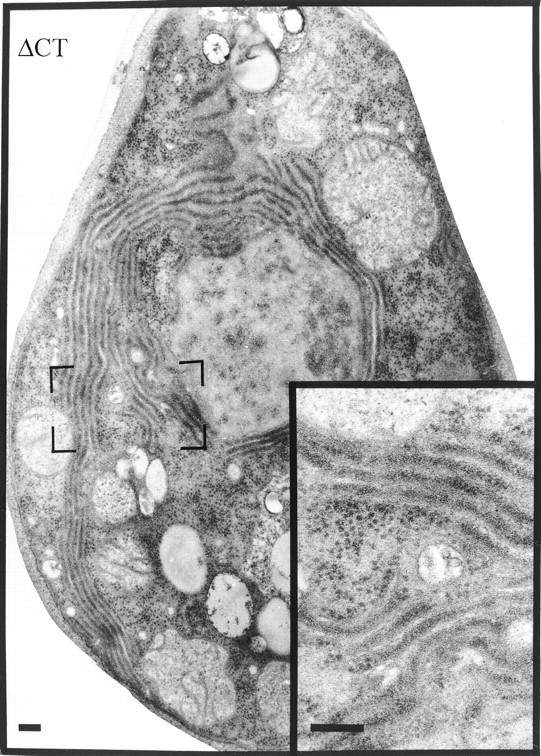
Expression of the NH2-terminal half of p180 induces extensive, closely spaced rough membranes. Cells were transfected with pYEX-BX containing the ΔCT version of p180. Transformants were selected and grown in Cu2+-containing medium for 5 h before preparation for electron microscopy. Visible are closely opposed rough membranes with a lower density of ribosomes in membrane-free zones (compared with cells shown in Fig. 4). Bars, 200 nm.
From these data, obtained through the expression of the various domains of p180, we can make the following provisional conclusions about the morphology of the induced membranes: The presence of the NH2-terminal 151 amino acids enables membrane proliferation in general, with the closely spaced perinuclear appearance of karmellae. The presence of the repeat region enables ribosome binding irrespective of membrane distribution or spacing, and the presence of the COOH-terminal domain enables a large intermembrane separation.
p180 Expression Results in the Coordinate Expression of Rough ER–specific Genes
These results beg the question as to the functionality of the proliferated membranes. Are they merely lipid bilayers produced to soak up an excess of ectopically expressed membrane protein, or do they represent bona fide rough and smooth ER? The first answer to this question comes from an examination of levels of expression of genes encoding resident proteins of these membrane systems. Northern blotting (Table ) was carried out on RNA derived from p180FL, ΔNT, ΔCT expressing strains, vector-only controls, and cells that overexpressed HMG1 and HMG2 genes. Overexpression of HMG1 induces karmellae, whereas HMG2 expression induces short karmellae, parallel membrane strips near the cell periphery, or membranous whorls in the cytoplasm (Koning et al. 1996). Transcripts examined included ones encoding pyruvate kinase (PYK1), a cytosolic marker, and KAR2, SEC61, SEC72, and OST1 that encode rough ER–specific proteins. KAR2 encodes the luminal HSP-70 required for translocation (Vogel et al. 1990), whereas the products of SEC61 and SEC72 are membrane proteins that participate in protein translocation into the rough ER (Rothblatt et al. 1994). OST1 is part of the oligosaccharyl transferase complex, which mediates N-linked oligosaccharide addition to glycoproteins (Silberstein et al. 1995). Should any of the proliferated membranes represent real rough ER, one would predict that these markers would be induced.
Table 1.
Quantification of Northern Analysis of Gene Expression Induced by p180 Constructs
| Expression of: | Northern Analysis Probed with: | |||||||
|---|---|---|---|---|---|---|---|---|
| Gene: | PYK1 | KAR2 | SEC61 | SEC72 | OST1 | SAC1 | GDA1 | SEC1 |
| Location: | Cytosol | RER/L | RER/M | RER/M | RER/M | RER/G | Golgi | SV |
| Vector-only | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| HMG1 | 1.0 | 3.5 | 1.3 | 0.9 | 0.5 | 1.7 | 0.8 | 1.4 |
| HMG2 | 1.0 | 3.7 | 1.3 | 0.5 | 0.3 | 1.1 | 0.6 | 0.8 |
| p180 Full-L | 1.0 | 3.2 | 10.3 | 3.5 | 5.3 | 3.9 | 2.4 | 1.7 |
| p180 ΔNT | 1.0 | 2.2 | 2.9 | 2.7 | 4.1 | 2.7 | 1.8 | 1.2 |
| p180 ΔCT | 1.0 | 3.8 | 14.9 | 3.6 | 6.9 | 9.1 | 5.0 | 6.1 |
Overexpression of all of the constructs resulted in an upregulation of KAR2, ranging from a low of 2.2-fold in the case of ΔNT to a high of 3.8-fold for ΔCT (Table ). In contrast, transcripts encoding rough ER membrane proteins were upregulated to the greatest extent in strains in which the ectopically expressed versions of p180 contained the ribosome binding domain (p180FL and ΔCT). SEC61 was the most highly expressed of all, where close to 15-fold higher levels were achieved in cells expressing ΔCT. In the same strain, SEC72 and OST1 expression increased 3.6- and 6.9-fold, respectively. In contrast, the HMG1 HMG2 overexpressing strains showed significant increases only in KAR2 expression. Cells expressing the p180 construct lacking the ribosome binding domain (ΔNT) showed only a (comparatively) moderate upregulation of ER markers, despite high levels of membrane proliferation (see Fig. 5).
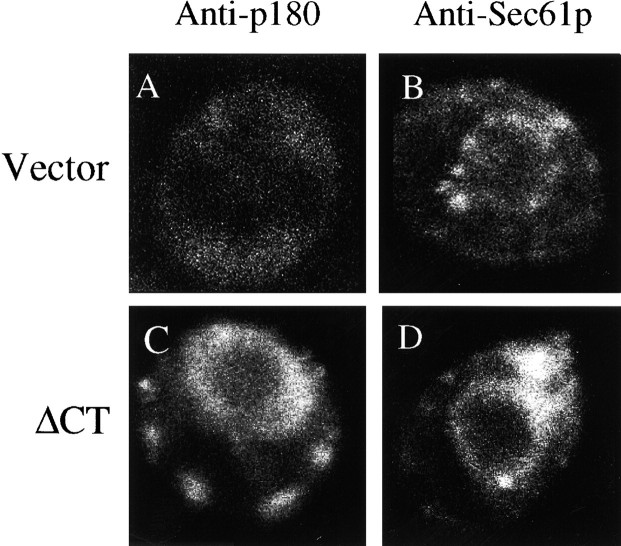
Increased levels of transcription of membrane protein genes translates into increased levels of the proteins they encode (Wanker et al. 1995). To demonstrate that these proteins are incorporated correctly into membranes, immunofluorescent techniques were used. ER-localized Sec61p was detected in the perinuclear membranes of control cells (Fig. 7 B), as is typically observed using anti-ER protein antibodies. Membranes proliferated in response to ΔCT expression show a marked increase in the intensity of anti-Sec61p staining (Fig. 7 D) comparable to the detection of p180 using anti-p180 antibodies (Fig. 7 C). The localization of the fluorescence in the ΔCT strain overlaps nicely with the location of the proliferated membranes seen in the electron microscope (Fig. 6). This result is a good predictor that functional membranes are being produced.
Figure 7.
Expression of ΔCT results in a proliferation of Sec61p-containing membranes. Cells were transfected with pYEX-BX containing the ΔCT version of p180 cDNA. Transformants were selected and grown in Cu2+-containing medium for 5 h before preparation for immunofluorescence microscopy. (A and C) Vector-only and ΔCT-expressing cells stained with anti-p180 antibodies. (B and D) Vector-only and ΔCT-expressing cells stained with anti-Sec61p antibodies.
Rough Membranes Produced by p180 Expression Are Functional
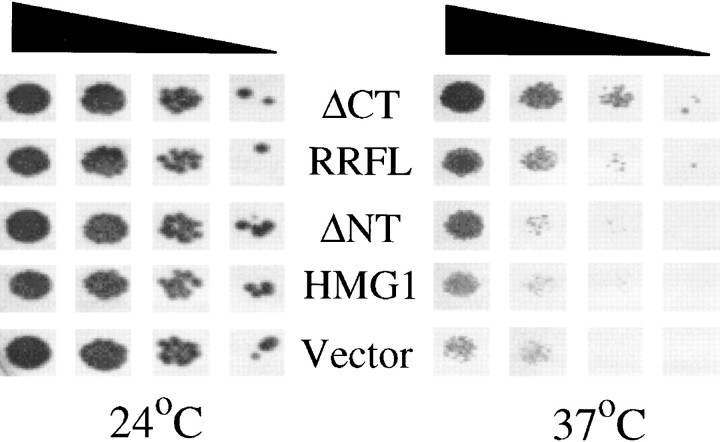
To establish the functionality of the proliferated rough ER, we turned to a genetic approach. We used a previously isolated strain that harbors a temperature-sensitive translocation defect. Originally isolated as ptl1 (Toyn et al. 1988), and subsequently shown to be capable of rescue by a wild-type SEC63 gene (Crowe, J., and D.I. Meyer, unpublished observations), this strain has a decreased ability to translocate nascent secretory proteins in vivo as well as in vitro at the nonpermissive temperature. Although membranes produced through expression of p180 constructs would still have the defective PTL1/SEC63 gene product, compensatory levels of translocation, and hence cell growth, should be observed due to more abundant, albeit defective, rough ER. As can be seen in Fig. 8, ptl1/sec63 cells transfected with vector alone showed a marked reduction in growth at the nonpermissive temperature (37°C). A significant increase in cell growth at the nonpermissive temperature, compared with controls, was observed in the case of cells transfected with p180FL, and an even better rescue was observed in the case of ΔCT expression, in which growth was virtually the same as vector-only at the permissive temperature. In contrast, membrane proliferation alone did not appear to be determinative in rescue, as expression of either HMG1 or ΔNT was not much better than vector-only. From these sets of experiments we conclude that the rough membranes produced in response to ΔCT expression are functional rough ER, and, from the previous data, that membrane composition is at least qualitatively similar to wild-type.
Figure 8.
Induced membrane proliferation rescues a temperature sensitive translocation defect. Cells harboring the sec63/ptl1ts mutation were transformed with the constructs shown in the middle of the figure (RRFL is the same as p180FL). Transformants were serially diluted (10-fold steps) onto Cu2+-containing medium and grown at either the permissive (24°C) or nonpermissive (37°C) temperature. The triangle above the figure indicates decreasing cell concentrations that were plated.
p180 Expression Results in Coordinate Expression of Secretory Pathway Genes
The results presented to this point indicate that functional rough ER is being produced in response to ΔCT expression. Are the more distal aspects of the secretory pathway being induced as well? We answered this question in two ways: by Northern analysis of marker genes for organelles that function post-ER in the secretory pathway, and morphologically using immunofluorescent detection of the Golgi complex. The following markers were examined: Sac1p, which is involved in nucleotide transport and has been localized to ER and to Golgi membranes; Gda1p, which encodes the guanosine diphosphatase required in the Golgi complex for oligosaccharide elaboration; and Sec1p, which is a cytosolic/peripheral membrane protein required in the exocytic fusion of secretory vesicles with the plasma membrane. The results shown in Table demonstrate that transcripts encoding SAC1, GDA1, and SEC1 gene products were all produced at significantly (5–10-fold) higher levels in cells expressing ΔCT compared with controls. In contrast, inducers of smooth membranes, such as the HMG genes or ΔNT expressed these markers at levels close to those of the vector control. Although one cannot test for all genes involved in secretion, these three—as well as the ER markers assessed previously—would likely participate in any process that would increase the secretory capacity of the cell.
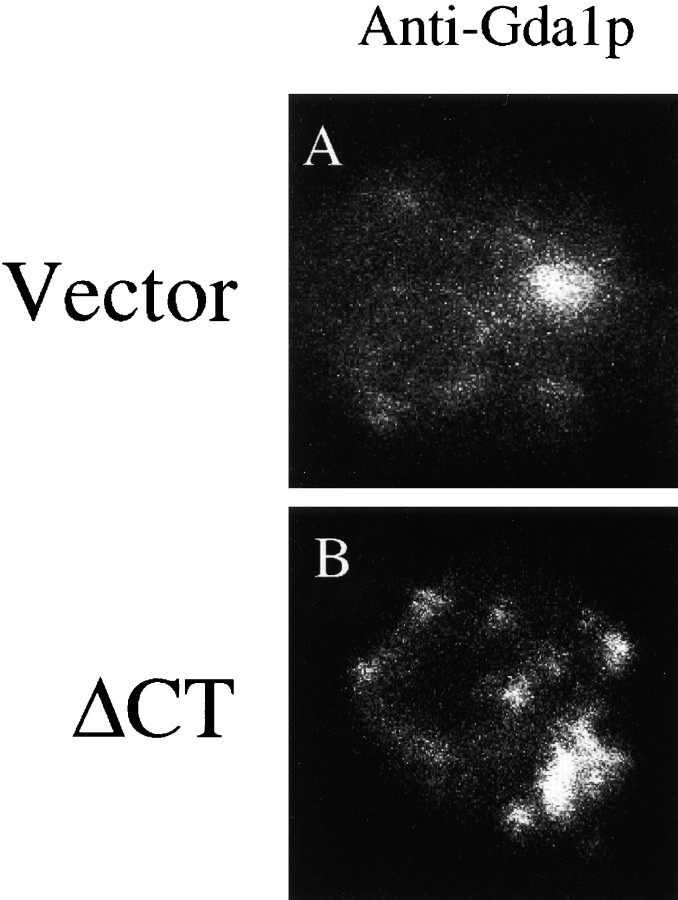
Fluorescence microscopy on control and ΔCT expressing cells was performed to further characterize the expression of post-ER markers. We used an anti-Gda1p antibody to reveal the Golgi complex in these cells. As can be seen in Fig. 9 A, a single Golgi body can be visualized in vector-only cells. In contrast, numerous Golgi appear in ΔCT-expressing cells (Fig. 9 B), some larger and some smaller. Taken together, these data suggest that the entire secretory pathway may be upregulated in response to ΔCT expression.
Figure 9.
Expression of ΔCT results in a proliferation of Gda1p-containing membranes. Cells were grown and prepared as described in Fig. 7. Cells were stained with antibodies against the Golgi marker protein Gda1p.
p180 Expression Results in Increased Secretory Capacity
To get an approximation of the secretory capacity of wild-type yeast, we transfected control strains with a plasmid encoding BPTI. BPTI is a low molecular weight protein that can be easily measured colorimetrically when it appears in the growth medium. The assay is based on the ability of BPTI to inhibit trypsin hydrolysis of an artificial substrate. Accordingly, BPTI was expressed under GAL control in glucose or in galactose-containing medium. Interestingly, once levels of BPTI secretion approach 8–10 μg/ml in wild-type cells grown under standard culture conditions, cell growth is inhibited, ostensibly by blocking or overwhelming the secretory process (Parekh et al. 1995). Fig. 10 shows that vector-only or the smooth membrane-proliferating ΔNT cells were unable to grow when BPTI was expressed through galactose induction for a period of 24 h. On the other hand, cells expressing BPTI were rescued by the expression of p180FL or ΔCT. Our preliminary conclusion was that an increase in secretory capacity enabled toxic quantities of BPTI to be removed from the cells. Recent studies by Wittrup and co-workers (Shusta et al. 1998) identified luminal proteins (Kar2p and PDI) whose increased expression enabled higher levels of single chain antibody secretion. These findings are consistent with the results presented here, as the expression of p180FL or ΔCT induces increases in levels of transcripts encoding these proteins (Table and Becker, F., unpublished results).
Figure 10.
Expression of full-length p180 or ΔCT rescues BPTI-induced growth arrest. Cells transformed with the constructs indicated in the middle of the figure (RRFl is the same as p180FL) were subsequently transformed with a plasmid directing the expression of BPTI under galactose control, and serially diluted (10-fold steps) onto Cu2+-containing growth medium that included either glucose or galactose as the carbon source. Triangle above figure indicates decreasing cell concentrations that were plated.
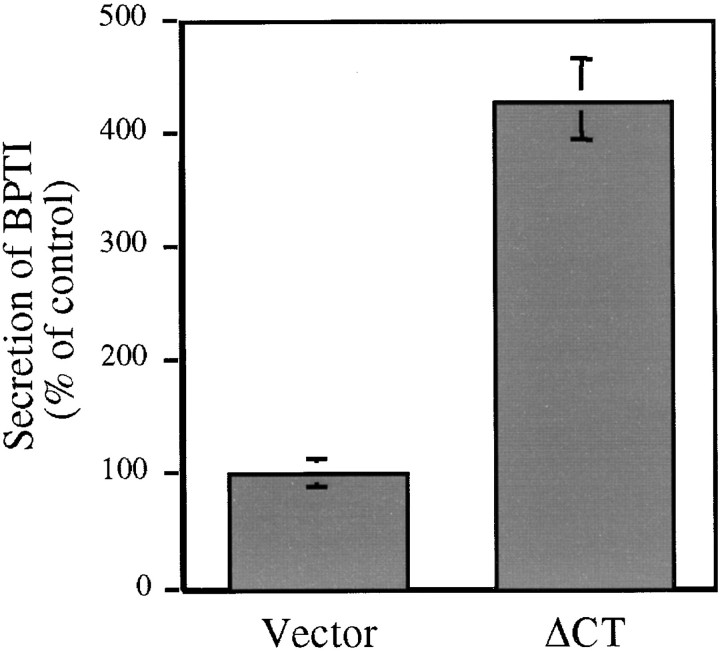
Measuring BPTI accumulation in the medium substantiated the hypothesis that the toxic effect of BPTI was overcome through increased secretion. In the case of cells grown on glucose, BPTI secretion was undetectable. In the case of cells expressing ΔCT and grown on galactose, levels of BPTI that accumulated in the medium during the assay rose >400% compared with control cells (Fig. 11). In contrast, membrane proliferation alone—as observed with ΔNT expression—affected neither rescue from BPTI-mediated growth arrest (Fig. 10), nor BPTI levels in the medium (data not shown). We take this to be direct proof of an increased secretory capacity mediated through the expression of ΔCT.
Figure 11.
Expression of ΔCT significantly increases BPTI secretion. Control and ΔCT-expressing cells, prepared as described in the legend to Fig. 10, were grown on galactose and copper-containing medium. BPTI secreted into the medium was determined by the colorimetric assay described in Materials and Methods.
Discussion
Our findings of an explosive proliferation of functional membranes coupled to an overall increase in secretory capacity in response to the heterologous expression of a single protein raise a number of intriguing questions about the regulation of membrane biogenesis. Previous reports, spanning more than three decades, have documented smooth membrane proliferation as a physiological response to the administration of xenobiotics (Orrenius et al. 1965; McLaughlin et al. 1999) or resulting from the overexpression of specific ER membrane proteins in mammalian cells (Anderson et al. 1983). To a certain extent, these processes have been characterized in molecular detail. In yeast, the overexpression of HMG1 and HMG2 genes in S. cerevisiae produced a number of smooth, closely spaced membranes whose specific morphologies differed depending on the isoform that was overexpressed. In these cases membranes appeared either as karmellae, short karmellae, strips, and/or whorls (Koning et al. 1996). In C. maltosa, the overproduction of cytochrome P450 produced smooth ER tubules as well as karmellae (Ohkuma et al. 1995). Sec61p, a component of the translocation pore, appeared to be a component of the proliferated membranes (Wiedmann et al. 1993; Koning et al. 1996) indicating their derivation from the ER. However, neither an apparent accumulation of rough membranes, nor an increase in secretory activity was observed.
In the case of p180, a strikingly different morphology was observed, depending upon which part of p180 was expressed. Expression of the full-length receptor resulted in a proliferation of widely spaced rough membranes. The 80–100 nm spacing correlated with the presence of the COOH-terminal half of p180, as the ΔNT construct induced smooth membranes with a considerable distance between them. In contrast, expression of ΔCT as well as p1-151 produced rough and smooth membranes, respectively, both possessing a very tightly packed appearance. Based on these data, one could make the prediction that the three separate domains induce three different aspects of rough membrane biogenesis. Production of membranes per se requires the expression of a membrane anchor and some minimum number of cytoplasmically exposed amino acids, e.g., p1-151. Adding on a ribosome binding domain, as in ΔCT, triggers the induction of functional rough ER, while addition of the COOH-terminal domain, as in p180FL and ΔNT, results in the typical uniformly spaced parallel arrays of membranes seen in mammalian secretory tissues (Fawcett 1981).
Parrish et al. 1995 determined that the sequence of Hmg1p that induces karmellae resides in a luminally disposed loop located between two transmembrane helices. It is interesting to note that the primary structure of p180 predicts that fewer than half a dozen amino acids could be located in the lumen of the ER, making a comparable mechanism unlikely. This is similar to the topology of another membrane-inducing protein, cytochrome P450. In this case, there are maximally 15 amino acids that are likely exposed to the lumen (Menzel et al. 1997).
The observation that p180 seems to reach its highest expression levels in tissues with high secretory capacity (Langley et al. 1998) provides a key insight into its probable physiological role: sequestration of ribosomes to the region of the cell where substantial amounts of synthesis and transport are occurring. This hypothesis is consistent with a number of relevant findings. First shown a number of decades ago, and repeatedly observed, is the fact that roughly half of all ribosomes bound to microsomal membranes isolated from secretory tissues can be released by high salt in the absence of the nascent chain release by puromycin (Adelman et al. 1973). The implication is that such ribosomes are not involved in the synthesis of secretory or membrane proteins. p180 is a likely candidate for mediating the attachment of such uninvolved ribosomes to the ER membrane. Additionally, depletion of p180 from reconstituted translocationally competent pancreatic microsomes has a significant effect on protein translocation (Savitz and Meyer 1993), yet it is not required for translocation when only purified components are incorporated into liposomes (Görlich and Rapoport 1993). The high level and efficiency of in vitro protein translocation seen in intact microsomes would be due to the assistance in ribosome binding provided by p180. On the other hand, stabilization of the ribosome-membrane interaction may be transient or nonessential (Nicchitta and Zheng 1997), or may be mediated by the Sec61-containing translocon (Kalies et al., 1994; Powers and Walter 1998) in the highly reconstituted systems.
Why is there no p180 in yeast? The answer to this comes from the same data alluded to above. Yeast cells do not have the secretory capacity of mammalian pancreas or liver. Therefore, just as many mammalian cell types exist with minimal or no expression of p180 (Langley et al. 1998), so can yeast. Ribosome binding to the ER membrane would be achieved through the nascent polypeptide and by interactions between the ribosome and components of the Sec61 complex (Powers and Walter 1998).
Equally if not more intriguing is the question of how the expression of p180 results in the proliferation of membranes in the secretory pathway in a cell in which it is not normally expressed. Hypotheses can be based on the elements of the protein mentioned previously, and their functional properties. It is clear that the membrane anchoring domain plus a few dozen amino acids of the cytosolic domain induces lipid bilayer proliferation. One can expect that the synthetic machinery needed for lipid biosynthesis is switched on in these cases via transcription of the requisite enzymes. This postulate is borne out by studies on other membrane proteins (Menzel et al. 1997). Moreover, our own preliminary results show increased INO1 expression in all of our strains in which membrane proliferation occurred (Block-Alper, L., unpublished observations). However, expression of the membrane anchor domain (p1-151) or ΔNT did not induce SEC61 or any of the other ER resident proteins; their expression correlated with expression of the ribosome binding domain. How then does the addition of this domain make such a difference in the number of genes that are being upregulated, and is the process dependent upon its ability to bind ribosomes with high affinity? It is tempting to speculate that the cell erroneously mobilizes greater secretory capacity due to a loss of free ribosomes from the cytosol, and that sensing this loss is the key step in induction of the relevant genes. If this is true, one could expect that the high level of expression of any secretory or membrane protein would induce the upregulation of secretion as long as it removes ribosomes from the cytosolic pool and directs them to the membrane. This has not yet been systematically investigated. On the other hand, Hanein et al. 1996 have demonstrated that Sec61p complexes form oligomeric rings in the membrane, and that their formation is dependent upon ribosome binding. A recruitment of ribosomes to the membrane could result in the formation of Sec61p complex oligomers and the corresponding decrease in free Sec61p complexes would prompt the upregulation of genes inducing membrane proliferation. These hypotheses form the basis of future experimentation.
Recent studies have elucidated an elaborate signaling pathway in rough ER. In response to a buildup of unfolded or misfolded proteins, a cascade is triggered that results in increased chaperone production (for review see Sidrauski et al. 1998). This unfolded protein response (UPR) is also linked to membrane biogenetic events, including membrane lipid production (Cox et al. 1997). The overexpression in UPR-deficient cells of certain membrane proteins, such as Hmg1p, has been shown to be lethal (Cox et al. 1997). On the other hand, UPR-deficient cells that overexpress cytochrome P450 are viable (Menzel et al. 1997). The p180-induced response also appears independently of the UPR as demonstrated by the fact that both viability and p180-induced membrane proliferation were observed in strains harboring a deletion of the IRE1 gene (Becker, F., unpublished observations).
The work described here makes use of the canine p180 cDNA that was cloned in our laboratory. Extensive data are now available on the human p180 homologue (Basson et al. 1996; Langley et al. 1998), as well as a potential alternatively spliced transcript derived from the p180 gene that was originally characterized in chicken (Krug et al. 1995), but also occurs in humans (Langley et al. 1998). Some of the transcripts processed in this way lack the repeat region entirely, yet retain the membrane anchor and the COOH-terminal region. The overall levels of similarity between canine and human p180s are >90%. The function of the splice variants is still largely speculative. Of interest is the fact that the consensus sequence of the decapeptide repeats is highly conserved, as well the total number, 54 (Wanker et al. 1995; Langley et al. 1998). The report on the human gene also points out that the COOH-terminal domain, for which function has yet to be determined—but from these studies is implied to generate the regular intermembrane spacing observed in secretory tissues—has the hallmarks of forming a coiled-coil structure. Should spacing depend upon specific interactions with elements that organize the cytoplasm, this type of secondary structure of the COOH terminus may be advantageous. On the other hand, the COOH-terminal domain could function in the regulation of ribosome binding to the repeats, or in the spatial arrangement of the molecule on the cytoplasmic surface of the rough ER. It is interesting to note that GST fusions containing the COOH terminus of p180 can bind to columns containing nucleoside triphosphates such as ATP (Savitz, A., and D. Meyer, unpublished observations), consistent with previous observations that p180 is an ATP binding protein (Zimmerman and Walter 1991).
Elucidation of the mechanism of membrane induction by p180 will benefit greatly from the observations made here in the yeast system. Through screening strategies it should be possible to identify genes whose products are capable of stimulating the upregulation of genes essential for membrane biogenesis. Moreover, similar schemes will enable mutant strains to be identified that lack the ability to upregulate membrane biogenesis in response to p180 expression. Identification and characterization of such genes and their products should provide the required toehold for further analysis of this interesting eukaryotic regulatory pathway.
It will also be interesting to analyze rough ER membrane induction by p180 in mammalian cells. Preliminary studies show that increasing cellular levels of p180 significantly stimulates rough membrane induction in nonsecretory cells (Castro-Vargas, E., F. Becker, and D.I. Meyer, unpublished observations). This implies that the expression of p180 may play a central role in the terminal differentiation of secretory tissues. The fact that a similar phenomenon is observed in mammalian cells makes the use of the genetically manipulable yeast model described here especially attractive.
Acknowledgments
We are grateful to Sergey Ryazantsev for his help with preparation of samples for electron microscopy, and to Ed Castro-Vargas and Chien-Min Chen for technical support. Thanks also to Peter Walter (University of California, San Francisco) for ire strains, Randy Schekman for anti-Sec61p, and Greg Payne for anti-Gda1p antibodies. We also thank Maureen Hyde, Ed Castro-Vargas, and members of the Payne group for helpful discussions and criticism.
Frank Becker was supported by a fellowship from the Deutsche Forschungsgemeinschaft (DFG). This work was supported by a grant from National Institutes of Health (GM 38538).
Footnotes
1.used in this paper: BPTI, bovine pancreatic trypsin inhibitor; UPR, unfolded protein response
Frank Becker's present address is Genome Pharmaceutical Corporation AG, Lochhamerstrasse 29, D-82152 Martinsreid, Germany.
References
- Adams A.E.M., Pringle J.R. Relationship of actin and tubulin distribution to bud growth in wild-type and morphogenic-mutant Saccharomyces cerevisiae . J. Cell Biol. 1984;98:934–945. doi: 10.1083/jcb.98.3.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelman M.R., Sabatini D.D., Blobel G. Ribosome-membrane interaction. Nondestructive disassembly of rat liver rough microsomes into ribosomal and membranous components. J. Cell Biol. 1973;56:206–229. doi: 10.1083/jcb.56.1.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R.G., Orci L., Brown M.S., Garcia S.L., Goldstein J.L. Ultrastructural analysis of crystalloid endoplasmic reticulum in UT-1 cells and its disappearance in response to cholesterol. J. Cell Sci. 1983;63:1–20. doi: 10.1242/jcs.63.1.1. [DOI] [PubMed] [Google Scholar]
- Basson C.T., MacRae C.A., Schoenberg-Fejzo M., Morton C.C., Spinner N.B., Genin A., Krug E., Seidman J.G., Seidman C.E. Identification, characterization and chromosomal localization of the human homolog (hES) of ES/130. Genomics. 1996;35:628–631. doi: 10.1006/geno.1996.0413. [DOI] [PubMed] [Google Scholar]
- Borgese N., Mok W., Kreibich G., Sabatini D.D. Ribosomal-membrane interactionin vitro binding of ribosomes to microsomal membranes. J. Mol. Biol. 1974;88:559–580. doi: 10.1016/0022-2836(74)90408-2. [DOI] [PubMed] [Google Scholar]
- Brodsky J.L. Translocation of proteins across the endoplasmic reticulum membrane. Int. Rev. Cytol. 1998;178:277–328. doi: 10.1016/s0074-7696(08)62139-7. [DOI] [PubMed] [Google Scholar]
- Cohen S.N., Chang A.C.Y., Hsu L. Nonchromosomal antibiotic resistance in bacteriagenetic transformation of Escherichia coli by R-factor DNA. Proc. Natl. Acad. Sci. USA. 1972;69:2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J.S., Chapman R.E., Walter P. The unfolded protein response coordinates the production of endoplasmic reticulum protein and endoplasmic reticulum membrane. Mol. Biol. Cell. 1997;8:1805–1814. doi: 10.1091/mbc.8.9.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallner G., Siekevitz P., Palade G.E. Biogenesis of endoplasmic reticulum membranes. I. Structural and chemical differentiation in developing rat hepatocyte. J. Cell Biol. 1966;30:73–96. doi: 10.1083/jcb.30.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett D.W. The Cell 1981. W.B. Saunders Co; Philadelphia, PA: pp. 303–368 [Google Scholar]
- Görlich D., Rapoport T.A. Protein translocation into proteoliposomes reconstituted from purified components of the endoplasmic reticulum membrane. Cell. 1993;75:615–630. doi: 10.1016/0092-8674(93)90483-7. [DOI] [PubMed] [Google Scholar]
- Hanein D., Matlack K.E., Jungnickel B., Plath K., Kalies K.U., Miller K.R., Rapoport T.A., Akey C.W. Oligomeric rings of the Sec61p complex induced by ligands required for protein translocation. Cell. 1996;15:721–732. doi: 10.1016/s0092-8674(00)81391-4. [DOI] [PubMed] [Google Scholar]
- Hollingsworth N.M., Goetsch L., Byers B. The HOP1 gene encodes a meiosis specific component of yeast chromosomes. Cell. 1990;61:73–84. doi: 10.1016/0092-8674(90)90216-2. [DOI] [PubMed] [Google Scholar]
- Hortsch M., Meyer D.I. Transfer of secretory proteins through the membrane of the endoplasmic reticulum. Int. Rev. Cytol. 1986;102:215–242. doi: 10.1016/s0074-7696(08)61276-0. [DOI] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J. Bacteriol. 1983;153:163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalies K.U., Görlich D., Rapoport T.A. Binding of ribosomes to the rough endoplasmic reticulum mediated by the Sec61p-complex. J. Cell Biol. 1993;126:925–934. doi: 10.1083/jcb.126.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koning A.J., Roberts C.J., Wright R.L. Different subcellular localization of Saccharomyces cerevisiae HMG-CoA reductase isozyems at elevated levels corresponds to distinct endoplasmic reticulum membrane proliferations. Mol. Biol. Cell. 1996;7:769–789. doi: 10.1091/mbc.7.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug E.L., Rezaee M., Isokawa K., Turner D.K., Litke L.L., Wunsch A.M., Bain J.L., Riley D.A., Capeheart A.A., Markwald R.R. Transformation of cardiac endothelium into cushion mesenchyme is dependent on ES/130temporal, spatial, and functional studies in the early chick embryo. Cell. Mol. Biol. Res. 1995;41:263–277. [PubMed] [Google Scholar]
- Langley R., Leung E., Morris C., Berg R., McDonald M., Weaver A., Parry D.A., Ni J., Su J., Gentz R. Identification of multiple forms of 180-kDa ribosome receptor in human cells. DNA Cell Biol. 1998;17:449–460. doi: 10.1089/dna.1998.17.449. [DOI] [PubMed] [Google Scholar]
- Leung E., Print C.G., Parry D.A., Closey D.N., Lockhart P.J., Skinner S.J., Batchelor D.C., Krissansen G.W. Cloning of novel kinectin splice variants with alternative C-terministructure distribution and evolution of mouse kinectin. Immunol. Cell Biol. 1996;74:421–433. doi: 10.1038/icb.1996.72. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Fritsch E.F., Sambrook J. Molecular CloningA Laboratory Manual 1982. Cold Spring Harbor Laboratory, Cold Spring Harbor; NY: 43–7.45. pp. 7 [Google Scholar]
- McLaughlin L., Burchell B., Pritchard M., Wolf C.R., Friedberg T. Treatment of mammalian cells with the endoplasmic reticulum-proliferator compactin strongly induces recombinant and endogenous xenobiotic metabolizing enzymes and 3-hydroxy-methylglutaryl CoA reductase in vitro. J. Cell Sci. 1999;112:515–523. doi: 10.1242/jcs.112.4.515. [DOI] [PubMed] [Google Scholar]
- Menzel R., Vogel F., Keargel E., Schunck W.H. Inducible membranes in yeastrelation to the unfolded-protein-response pathway. Yeast. 1997;13:1121–1129. doi: 10.1002/(SICI)1097-0061(199710)13:13<1211::AID-YEA168>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Nicchitta C.V., Zheng T. Regulation of the ribosome-membrane junction at early stages of presecretory protein translocation in the mammalian endoplasmic reticulum. J. Cell Biol. 1997;139:1697–1708. doi: 10.1083/jcb.139.7.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkuma M., Park S.M., Zimmer T., Menzel R., Vogel F., Schunck W.H., Ohta A., Takagi M. Proliferation of intracellular membrane structures upon homologous overproduction of cytochrome P450 in Candida maltosa . Biochim. Biophys. Acta. 1995;1236:163–169. doi: 10.1016/0005-2736(95)00040-a. [DOI] [PubMed] [Google Scholar]
- Orrenius S., Ericsson J.L., Ernster L. Phenobarbital-induced synthesis of the microsomal drug-metabolizing enzyme system and its relationship to the proliferation of endoplasmic membranes. A morphological and biochemical study. J. Cell Biol. 1965;25:627–639. doi: 10.1083/jcb.25.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh R., Forrester K., Wittrup D. Multicopy overexpression of bovine pancreatic trypsin inhibitor saturates the protein folding and secretory capacity of Saccharomyces cerevisiae . Protein Expr. Purif. 1995;6:534–545. doi: 10.1006/prep.1995.1071. [DOI] [PubMed] [Google Scholar]
- Parrish M.L., Sengstag C., Rine J.D., Wright R.L. Identification of the sequences in HMG-CoA reductase required for karmellae assembly. Mol. Biol. Cell. 1995;6:1535–1547. doi: 10.1091/mbc.6.11.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pictet R.L., Clark W.R., Williams R.H., Rutter W.J. An ultrastructural analysis of the developing embryonic pancreas. Dev. Biol. 1972;29:436–467. doi: 10.1016/0012-1606(72)90083-8. [DOI] [PubMed] [Google Scholar]
- Powers T., Walter P. A ribosome at the end of the tunnel. Science. 1998;278:2072–2073. doi: 10.1126/science.278.5346.2072. [DOI] [PubMed] [Google Scholar]
- Pringle J.R., Adams A.E.M., Drubin D.G., Haarer B.K. Immunofluorescence methods for yeast. Methods Enzymol. 1991;194:565–602. doi: 10.1016/0076-6879(91)94043-c. [DOI] [PubMed] [Google Scholar]
- Rosbash M., Penman S. Membrane-associated protein synthesis of mammalian cellsthe two classes of membrane-associated ribosomes. J. Mol. Biol. 1971;59:227–241. doi: 10.1016/0022-2836(71)90048-9. [DOI] [PubMed] [Google Scholar]
- Rothblatt J., Novick P., Stevens T.H. Guidebook to the Secretory Pathway 1994. Oxford University Press; Oxford, United Kingdom: pp. 31–39 [Google Scholar]
- Savitz A.J., Meyer D.I. Identification of a ribosome receptor in the rough endoplasmic reticulum. Nature. 1990;346:540–544. doi: 10.1038/346540a0. [DOI] [PubMed] [Google Scholar]
- Savitz A.J., Meyer D.I. 180-kD ribosome receptor is essential for both ribosome binding and protein translocation. J. Cell Biol. 1993;120:853–863. doi: 10.1083/jcb.120.4.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohat M., Janossy G., Dourmashkin R.R. Development of rough endoplasmic reticulum in mouse splenic lymphocytes stimulated by mitgens. Eur. J. Immunol. 1973;3:680–687. doi: 10.1002/eji.1830031106. [DOI] [PubMed] [Google Scholar]
- Shusta E.V., Raines R.T., Pluckthun A., Wittrup K.D. Increasing the secretory capacity of Saccharomyces cerevisiae for production of single-chain antibody fragments. Nat. Biotechnol. 1998;16:773–777. doi: 10.1038/nbt0898-773. [DOI] [PubMed] [Google Scholar]
- Sidrauski C., Chapman R., Walter P. The unfolded protein responsean intracellular signaling pathway with many surprising features. Trends Cell Biol. 1998;8:245–249. doi: 10.1016/s0962-8924(98)01267-7. [DOI] [PubMed] [Google Scholar]
- Silberstein S., Collins P.G., Kelleher D.J., Rapiejko P.J., Gilmore R. The alpha subunit of the Saccharomyces cerevisiae oligosaccharyltransferase complex is essential for vegetative growth of yeast and is homologous to mammalian ribophorin I. J. Cell Biol. 1995;128:525–536. doi: 10.1083/jcb.128.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyn J., Hibbs A.R., Sanz P., Crowe J., Meyer D.I. In vivo and in vitro analysis of ptll, a yeast ts mutant with a membrane associated defect in protein translocation. EMBO (Eur. Mol. Biol. Org.) J. 1988;7:4347–4353. doi: 10.1002/j.1460-2075.1988.tb03333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergères G., Yen T.S., Aggeler J., Lausier J., Waskell L. A model system for studying membrane biogenesis. Overexpression of cytochrome b5 in yeast results in marked proliferation of the intracellular membrane. J. Cell Sci. 1993;106:249–259. doi: 10.1242/jcs.106.1.249. [DOI] [PubMed] [Google Scholar]
- Vogel J.P., Misra L.M., Rose M.D. Loss of BiP/GRP78 function blocks translocation of secretory proteins in yeast. J. Cell Biol. 1990;110:1885–1895. doi: 10.1083/jcb.110.6.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanker E.E., Sun Y., Savitz A.J., Meyer D.I. Functional characterization of the 180-kD ribosome receptor in vivo. J. Cell Biol. 1995;130:29–39. doi: 10.1083/jcb.130.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels N.K., Evans J. Ultrastructural studies of early morphogenesis and cytodifferentiation in the embryonic mammalian pancreas. Dev. Biol. 1968;17:413–446. doi: 10.1016/0012-1606(68)90073-0. [DOI] [PubMed] [Google Scholar]
- Wiedmann B., Silver P., Schunck W.H., Wiedmann M. Overexpression of the ER membrane protein P450 CYP52A3 mimics sec mutant characteristics in Saccharomyces cerevisiae . Biochim. Biophys. Acta. 1993;1153:267–276. doi: 10.1016/0005-2736(93)90415-v. [DOI] [PubMed] [Google Scholar]
- Wilsbach K., Payne G.S. Vps1p a member of the dynamin GTPase family, is necessary for Golgi membrane protein retention in Saccharomyces cerevisiae . EMBO (Eur. Mol. Biol. Org.) J. 1993;12:3049–3059. doi: 10.1002/j.1460-2075.1993.tb05974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright R., Basson M., D'Ari L., Rine J. Increased amounts of HMG CoA reductase induce “karmellae”a proliferation of stacked membrane pairs surrounding the yeast nucleus. J. Cell Biol. 1988;107:101–114. doi: 10.1083/jcb.107.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman D.L., Walter P. An ATP-binding membrane protein is required for protein translocation across the endoplasmic reticulum membrane. Cell Regul. 1991;2:851–859. doi: 10.1091/mbc.2.10.851. [DOI] [PMC free article] [PubMed] [Google Scholar]