Abstract
Peptidoglycan glycosyltransferases (PGTs) are highly conserved enzymes that catalyze the polymerization of Lipid II to form the glycan strands of bacterial murein. Because they play a key role in bacterial cell wall synthesis, these enzymes are potentially important antibiotic targets; however, their mechanisms are not yet understood. One longstanding question about these enzymes is whether they elongate glycan chains by adding subunits to the anomeric (reducing) end or to the 4-hydroxyl (non-reducing) end. We have developed an approach to test the direction of chain elongation that involves the use of nascent peptidoglycan chains which are blocked at their non-reducing ends. In the presence of the PGT domains of Staphylococcus aureus PBP2, Aquifex aeolicus PBP1A, Escherichia coli PBP1A or Escherichia coli PBP1B, these blocked substrates react with Lipid II to form longer glycan chains. These results establish that PGTs elongate nascent peptidoglycan chains by the addition of disaccharide subunits to the anomeric (reducing) end of the growing polymer.
Peptidoglycan glycosyltransferases (PGTs) are highly conserved bacterial enzymes that catalyze the polymerization of a disaccharide called Lipid II (Figure 1) to form the glycan strands of peptidoglycan. PGTs are regarded as desirable antibiotic targets because they are extracellular, they do not have eukaryotic counterparts, and they play an essential role in a validated therapeutic pathway.1 Although their importance has been appreciated for decades, the mechanism of glycan chain polymerization is not yet understood. One aspect of the PGT reaction that is central to defining the mechanism is the direction of glycan chain elongation (Figure 2A).2 Here, we describe an approach to test the direction of glycan chain growth. We have applied this strategy to four different PGTs from both Gram-negative and Gram-positive organisms, including two PGTs for which crystal structures were recently reported.3 The results show that all these PGTs polymerize Lipid II by the addition of new disaccharide units to the anomeric (diphospholipid) end of the elongating polymer (called the “reducing end” by convention, although not a lactol).
Figure 1.
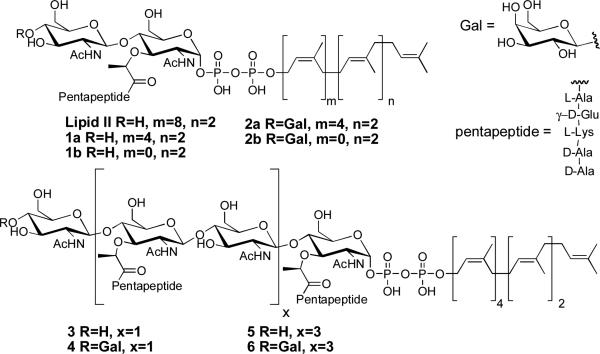
Natural Lipid II as well Lipid II (1), Lipid IV (3), and Lipid VIII (5) substrate analogs and their Gal-labeled versions (2, 4, and 6).
Figure 2.
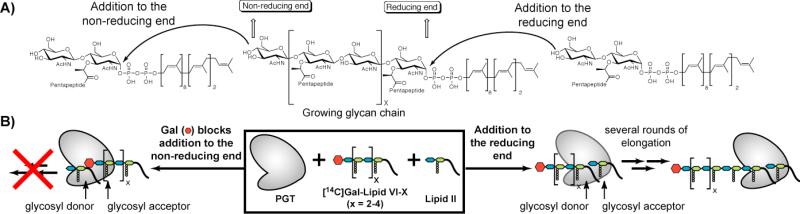
Models for the direction of glycan polymerization. A) Scheme showing that a glycan strand can be extended by adding new units to the non-reducing end (left) or to the reducing end (right). B) Scheme showing our strategy to test the direction of chain elongation. If the PGT is incubated with Lipid II and a polymer substrate blocked at the non-reducing end with [14C]Gal, then the polymer will only be extended if new units add to the reducing end.
Our strategy to determine the direction of glycan polymerization, illustrated in Figure 2B, relies on the synthesis of nascent peptidoglycan chains that are blocked at their non-reducing ends (Figure 2B). If elongation occurs by reaction at the reducing end of the growing polymer, PGTs should elongate these substrates in the presence of Lipid II (Figure 2B, right). If elongation occurs in the other direction, then these blocked oligomers will not be substrates for PGTs (Figure 2B, left).
To enable our strategy for probing the direction of chain elongation, we required a facile method to selectively modify the non-reducing ends of the nascent glycan chains. We chose to utilize β-1,4-galactosyltransferase (GalT), an enzyme that transfers galactose (Gal) from UDP-Gal to the C4-OH of terminal N-acetylglucosamine (GlcNAc) residues on a wide variety of molecules.4 Incubation of synthetic Lipid II substrate analog 1b with GalT in the presence of UDP-Gal resulted in disappearance of 1b and the concomitant appearance of a new compound with a mass consistent with the structure of the Gal-labeled analog 2b (expected and observed: m/z 1491.6). To verify that Gal-labeling prevents reaction at the non-reducing end, we prepared heptaprenyl-[14C]Gal-Lipid II (2a) and [14C]Gal-Lipid IV (4) by treating heptaprenyl-Lipid II (1a)5a or heptaprenyl-Lipid IV (3)5b with UDP-[14C]Gal and GalT. No radiolabeled self-condensation products were detected when these substrate analogs were incubated with the PGT domain of A. aeolicus PBP1A. In the presence of Lipid II, however, the Gal-labeled substrates formed radiolabeled polymeric products (Figs. S1 and S2). These experiments demonstrate that Gal labeling of the non-reducing end of the Lipid II or Lipid IV substrates blocks this end from serving as a glycosyl acceptor.
The experiments described above show that the Gal-labeled substrates can react at their reducing ends but not their non-reducing ends; however, they do not establish the direction of glycan chain polymerization because 1a and 3 self-condense. Since these substrates are capable of reacting at both ends, their Gal-labeled analogs cannot be used to discriminate between the two possible mechanisms of elongation (see Figure S3). In order to establish the direction of chain elongation, we needed to identify peptidoglycan substrates that can be elongated by Lipid II but do not react with themselves. Nascent chains containing three or more disaccharide subunits meet this criterion.6
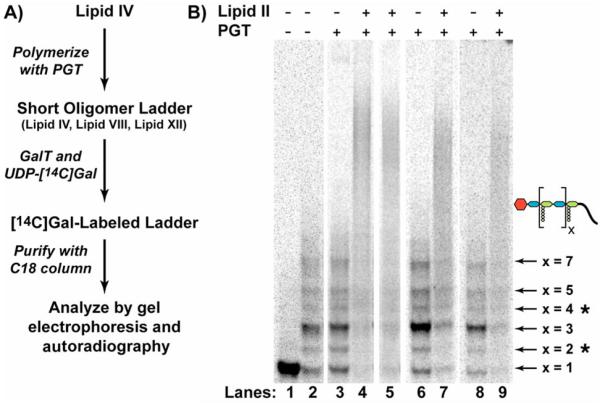
To synthesize the longer Gal-blocked substrates (Figure 3A) required to test the direction of chain elongation, we incubated compound 3 with a high concentration of E. coli PBP1A. Under these conditions, 3 reacted to give a mixture of products in which the major product, identified by its exact mass, was the octasaccharide Lipid VIII (5, Figure 1; Table S1). The non-reducing ends of the oligomer mixture were derivatized with [14C]Gal by treatment with GalT. The resulting blocked oligomers were purified by C18 chromatography and analyzed by gel electrophoresis (Figure 3B, Lane 2).7 Assignment of oligomer sizes was based on exact mass measurements of the Lipid IV polymerization reactions (Table S1) and on the electrophoretic mobilities of Gal-labeled oligomers (Figure S2).
Figure 3.
Gel electrophoresis assays to determine the direction of elongation. A) Scheme for the synthesis of the Gal-oligomer substrates. B) Gel electrophoresis of PGT reaction mixtures. Each lane contains either [14C]-Gal-Lipid IV (Lane 1) or the [14C]-Gal-oligomer mix (Lanes 2-9) incubated in the presence or absence of PGT and Lipid II. The PGTs investigated include E. coli PBP1A (Lanes 3-4), E. coli PBP1B (Lane 5), A. aeolicus PBP1A (Lane 6-7), and S. aureus PBP2 (Lane 8-9). The identities of the Gal-labeled oligomers, as assessed by electrophoretic mobilities (Figure S2)6 and MS analysis of the unlabeled reaction mixture (Table S1), are indicated. Asterisked products7 arise late in the reaction, presumably as a result of hydrolysis or transglycosylation with diphospholipid. They do not affect the analysis.
To assess the direction of chain elongation, the Gal-oligomers were incubated with four PGTs, including E. coli PBP1A, E. coli PBP1B, A. aeolicus PBP1A, and S. aureus PBP2,3a, 5b, 8 in the presence and absence of Lipid II. The reactions were analyzed using a recently developed gel electrophoresis assay.3a, 6 In the absence of Lipid II, the distribution of oligomers was unchanged (compare Lanes 3, 6, and 8 to Control Lane 2, Figure 3B). In the presence of Lipid II, the oligomers disappeared and a smear of radioactivity appeared higher up in the gel (lanes 4, 5, 7, and 9, Figure 3B), indicating that longer peptidoglycan products were formed. Since Gal-labeling prevents substrates from reacting at their non-reducing ends these experiments demonstrate that the glycan polymers grow by addition of disaccharide subunits to the reducing end of the growing chain.
Knowing the direction of chain elongation is fundamental to understanding the mechanism of any polymerase. In the case of the PGTs, the direction of glycan chain polymerization is important for thinking about how the bacterial cell wall is built. Accordingly, some of the earliest experiments carried out to understand the process of peptidoglycan synthesis addressed the direction in which the glycan chains elongate.9 It was suggested that the chains grow from their reducing ends, although the generality of the finding was questioned.2 Two crystal structures of PGTs have presented models for the polymerization reaction in which the glycan chains were proposed to grow from their reducing ends,3 but no biochemical support for the models was provided. Since the only experiments addressing directionality involved crude membrane preparations, and since similar experiments with other glycan polymerases have been shown to give conflicting, and sometimes erroneous, results,10 we have assessed the direction of chain elongation using pure PGTs as well as a new experimental strategy. Our results have established that four different PGTs from a diverse set of Gram-negative and Gram-positive organisms catalyze the addition of Lipid II to the reducing end of the growing peptidoglycan polymer. Given the structural homology of this enzyme family, we propose that the entire class uses a reducing end mechanism for elongation.
Supplementary Material
Acknowledgement
This research was supported by the National Institutes of Health (GM076710 to SW and DK, and a postdoctoral fellowship for D.L.P.). We thank Y. Yuan for providing purified PGTs.
Footnotes
Supporting Information Available: Experimental procedures, Supplemental Figs. S1, S2 and S3, and Tables S1 and S2.
REFERENCES
- 1.a Ostash B, Walker S. Curr. Opin. Chem. Biol. 2005;9:459–466. doi: 10.1016/j.cbpa.2005.08.014. [DOI] [PubMed] [Google Scholar]; b Walsh C. Antibiotics: actions, origins, resistance. ASM Press; Washington, D. C.: 2003. pp. 23–49. [Google Scholar]
- 2.van Heijenoort J. Glycobiology. 2001;11:25R–36R. doi: 10.1093/glycob/11.3.25r. [DOI] [PubMed] [Google Scholar]
- 3.a Yuan YQ, Barrett D, Zhang Y, Kahne D, Sliz P, Walker S. Proc. Natl. Acad. Sci. U. S. A. 2007;104:5348–5353. doi: 10.1073/pnas.0701160104. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Lovering AL, de Castro LH, Lim D, Strynadka NCJ. Science. 2007;315:1402–1405. doi: 10.1126/science.1136611. [DOI] [PubMed] [Google Scholar]
- 4.a Boeggeman E, Ramakrishnan B, Kilgore C, Khidekel N, Hsieh-Wilson LC, Simpson JT, Qasba PK. Bioconjug. Chem. 2007;18:806–14. doi: 10.1021/bc060341n. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Khidekel N, Ficarro SB, Clark PM, Bryan MC, Swaney DL, Rexach JE, Sun YE, Coon JJ, Peters EC, Hsieh-Wilson LC. Nat. Chem. Biol. 2007;3:339–48. doi: 10.1038/nchembio881. [DOI] [PubMed] [Google Scholar]; c Roquemore EP, Chou TY, Hart GW. Methods Enzymol. 1994;230:443–60. doi: 10.1016/0076-6879(94)30028-3. [DOI] [PubMed] [Google Scholar]; d Schindler M, Mirelman D, Schwarz U. Eur. J. Biochem. 1976;71:131–134. doi: 10.1111/j.1432-1033.1976.tb11098.x. [DOI] [PubMed] [Google Scholar]
- 5.a Ye X, Lo M, Brunner L, Walker D, Kahne D, Walker S. J. Am. Chem. Soc. 2001;123:3155–3156. doi: 10.1021/ja010028q. [DOI] [PubMed] [Google Scholar]; b Zhang Y, Fechter EJ, Wang TSA, Barrett D, Walker S;, Kahne DE. J. Am. Chem. Soc. 2007;129:3080–3081. doi: 10.1021/ja069060g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barrett D, Wang TSA, Yuan Y, Zhang Y, Kahne D, Walker S. J. Biol. Chem . 2007 doi: 10.1074/jbc.M705440200. online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.In addition to Lipid IV and its coupling products, we observe two other products in the polymerization reaction (* in Figure 3B), the smaller of which was determined by exact mass analysis to be Lipid VI (Table S1). The electrophoretic mobility of their Gal-labeled analogs is consistent with the assignments indicated (Figure S2).
- 8.a Chen L, Walker D, Sun B, Hu Y, Walker S, Kahne D. Proc. Natl. Acad. Sci. USA. 2003;100:5658–5663. doi: 10.1073/pnas.0931492100. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Barrett D, Chen L, Litterman N, Walker S. Biochemistry. 2004;43:12375–12381. doi: 10.1021/bi049142m. [DOI] [PubMed] [Google Scholar]; c Barrett D, Leimkuhler C, Chen L, Walker D, Kahne D, Walker S. J. Bacteriol. 2005;187:2215–2217. doi: 10.1128/JB.187.6.2215-2217.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Leimkuhler C, Chen L, Barrett D, Panzone G, Sun B, Flacone B, Oberthur M, Donadio S, Walker S, Kahne D. J. Am. Chem. Soc. 2005;127:3250–3251. doi: 10.1021/ja043849e. [DOI] [PubMed] [Google Scholar]
- 9.a Ward J, Perkins H. Biochem. J. 1973;135:721–728. doi: 10.1042/bj1350721. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Weston A, Ward JB, Perkins HR. J. Gen. Microbiol. 1977;99:171–181. [Google Scholar]
- 10.Bodevin-Authelet S, Kusche-Gullberg M, Pummill PE, DeAngelis PL, Lindahl U. J. Biol. Chem. 2005;280:8813–8818. doi: 10.1074/jbc.M412803200. and refs therein. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.