Abstract
We have developed a 2-dimensional gel method for identification of RNA sequences crosslinked by the intercalative drug 4'-hydroxymethyl-4,5',8-trimethylpsoralen (HMT). This method is being used to localize such sites in E. coli ribosomal RNA. We report here the identification of a site for HMT crosslinking within positions 434 and 497 of 16S rRNA. We suggest a likely site for HMT intercalation, in which residues U548 and U473 become crosslinked via the drug.
Full text
PDF





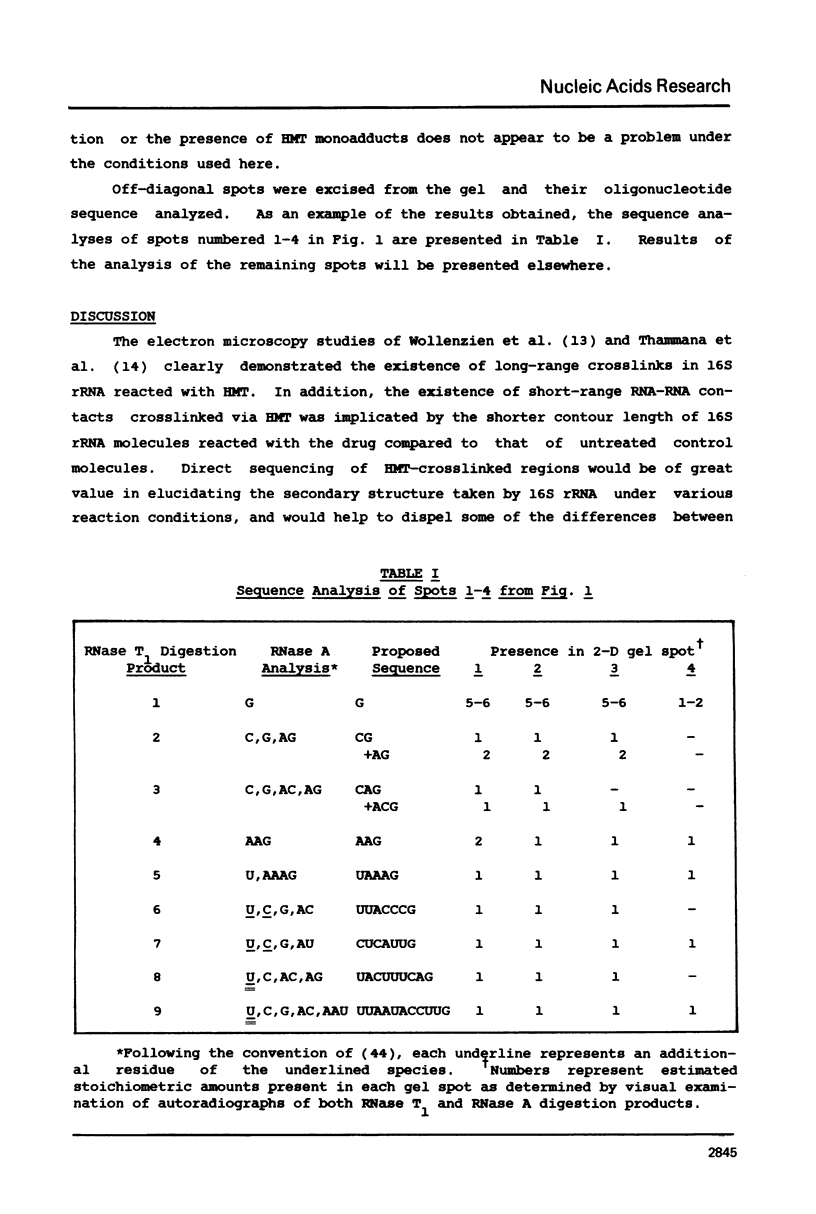

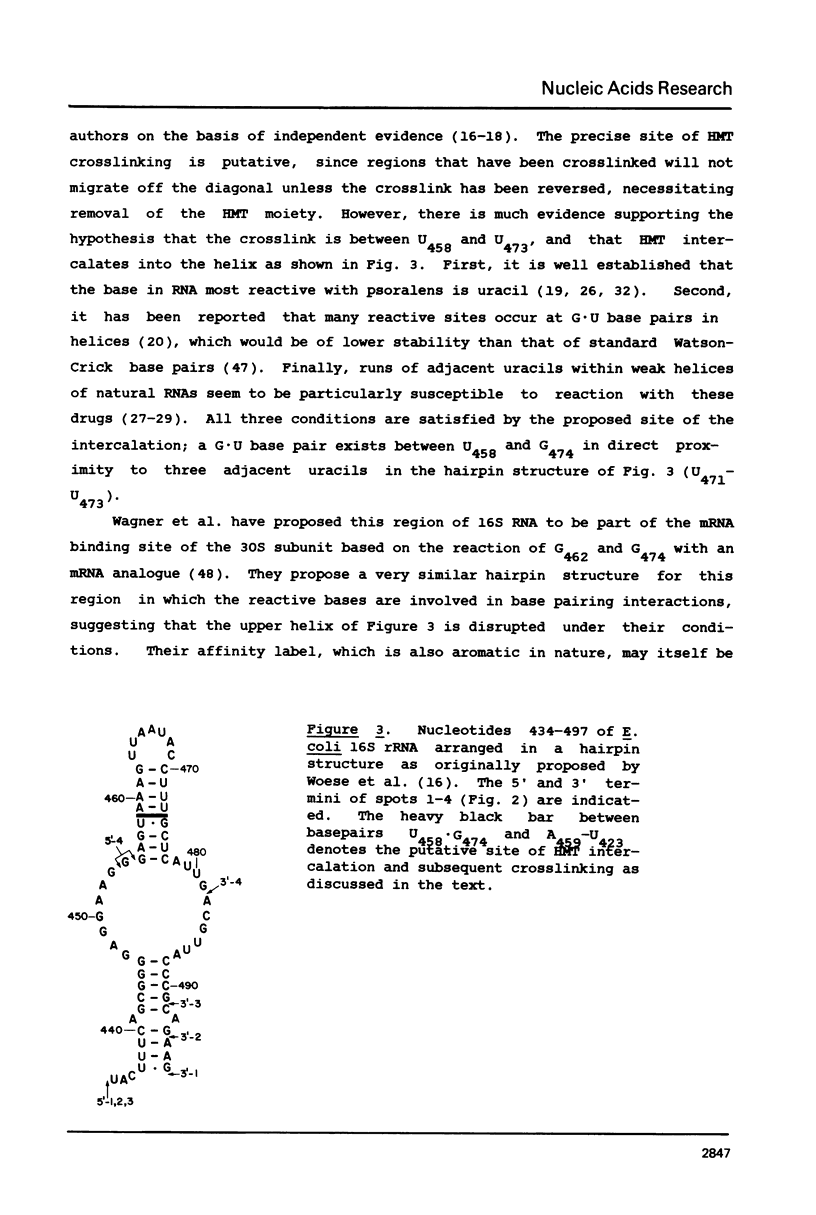


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen S. H., Wong K. P. The hydrodynamic and spectroscopic properties of 16 S RNA from Escherichia coli ribosome in reconstitution buffer. J Biol Chem. 1978 Dec 25;253(24):8759–8766. [PubMed] [Google Scholar]
- Bachellerie J. P., Thompson J. F., Wegnez M. R., Hearst J. E. Identification of the modified nucleotides produced by covalent photoaddition of hydroxymethyltrimethylpsoralen to RNA. Nucleic Acids Res. 1981 May 11;9(9):2207–2222. doi: 10.1093/nar/9.9.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosius J., Palmer M. L., Kennedy P. J., Noller H. F. Complete nucleotide sequence of a 16S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4801–4805. doi: 10.1073/pnas.75.10.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COX R. A., LITTAUER U. Z. Ribonucleic acid from Escherichia coli. III. The influence of ionic strength and temperature on hydrodynamic and optical properties. Biochim Biophys Acta. 1962 Aug 20;61:197–208. [PubMed] [Google Scholar]
- Cantor C. R., Wollenzien P. L., Hearst J. E. Structure and topology of 16S ribosomal RNA. An analysis of the pattern of psoralen crosslinking. Nucleic Acids Res. 1980 Apr 25;8(8):1855–1872. doi: 10.1093/nar/8.8.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbon P., Ehresmann C., Ehresmann B., Ebel J. P. The sequence of Escherichia coli ribosomal 16 S RNA determined by new rapid gel methods. FEBS Lett. 1978 Oct 1;94(1):152–156. doi: 10.1016/0014-5793(78)80926-0. [DOI] [PubMed] [Google Scholar]
- Chapman N. M., Noller H. F. Protection of specific sites in 16 S RNA from chemical modification by association of 30 S and 50 S ribosomes. J Mol Biol. 1977 Jan 5;109(1):131–149. doi: 10.1016/s0022-2836(77)80049-1. [DOI] [PubMed] [Google Scholar]
- Cole R. S. Light-induced cross-linking of DNA in the presence of a furocoumarin (psoralen). Studies with phage lambda, Escherichia coli, and mouse leukemia cells. Biochim Biophys Acta. 1970 Sep 17;217(1):30–39. doi: 10.1016/0005-2787(70)90119-x. [DOI] [PubMed] [Google Scholar]
- Dall'Acqua F., Marciani S., Rodighiero G. Inter-strand cross-linkages occurring in the photoreaction between psoralen and DNA. FEBS Lett. 1970 Jul 29;9(2):121–123. doi: 10.1016/0014-5793(70)80330-1. [DOI] [PubMed] [Google Scholar]
- Dunn J. J., Buzash-Pollert E., Studier F. W. Mutations of bacteriophage T7 that affect initiation of synthesis of the gene 0.3 protein. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2741–2745. doi: 10.1073/pnas.75.6.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn J. M., Wong K. P. Molecular mechanism of in vitro 30 S ribosome assembly. I. Conformational changes of the 16 S RNA. J Biol Chem. 1979 Aug 25;254(16):7705–7711. [PubMed] [Google Scholar]
- Fellner P. Nucleotide sequences from specific areas of the 16S and 23S ribosomal RNAs of E. coli. Eur J Biochem. 1969 Nov;11(1):12–27. doi: 10.1111/j.1432-1033.1969.tb00733.x. [DOI] [PubMed] [Google Scholar]
- Hearst J. E. Psoralen photochemistry. Annu Rev Biophys Bioeng. 1981;10:69–86. doi: 10.1146/annurev.bb.10.060181.000441. [DOI] [PubMed] [Google Scholar]
- Held W. A., Nomura M. Rate determining step in the reconstitution of Escherichia coli 30S ribosomal subunits. Biochemistry. 1973 Aug 14;12(17):3273–3281. doi: 10.1021/bi00741a020. [DOI] [PubMed] [Google Scholar]
- Herr W., Chapman N. M., Noller H. F. Mechanism of ribosomal subunit association: discrimination of specific sites in 16 S RNA essential for association activity. J Mol Biol. 1979 Jun 5;130(4):433–449. doi: 10.1016/0022-2836(79)90433-9. [DOI] [PubMed] [Google Scholar]
- Herr W., Chapman N. M., Noller H. F. Mechanism of ribosomal subunit association: discrimination of specific sites in 16 S RNA essential for association activity. J Mol Biol. 1979 Jun 5;130(4):433–449. doi: 10.1016/0022-2836(79)90433-9. [DOI] [PubMed] [Google Scholar]
- Isaacs S. T., Shen C. K., Hearst J. E., Rapoport H. Synthesis and characterization of new psoralen derivatives with superior photoreactivity with DNA and RNA. Biochemistry. 1977 Mar 22;16(6):1058–1064. doi: 10.1021/bi00625a005. [DOI] [PubMed] [Google Scholar]
- Morris D. R., Dahlberg J. E., Dahlberg A. E. Detection of cation-specific conformational changes in ribosomal RNA by gel electrophoresis. Nucleic Acids Res. 1975 Apr;2(4):447–458. doi: 10.1093/nar/2.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musajo L., Bordin F., Caporale G., Marciani S., Rigatti G. Photoreactions at 3655 Angstrom between pyrimidine bases and skin-photosensitizing furocoumarins. Photochem Photobiol. 1967 Oct;6(10):711–719. doi: 10.1111/j.1751-1097.1967.tb08736.x. [DOI] [PubMed] [Google Scholar]
- Noller H. F., Chaires J. B. Functional modification of 16S ribosomal RNA by kethoxal. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3115–3118. doi: 10.1073/pnas.69.11.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofengand J., Liou R., Kohut J., 3rd, Schwartz I., Zimmermann R. A. Covalent cross-linking of transfer ribonucleic acid to the ribosomal P site. Mechanism and site of reaction in transfer ribonucleic acid. Biochemistry. 1979 Oct 2;18(20):4322–4332. doi: 10.1021/bi00587a010. [DOI] [PubMed] [Google Scholar]
- Ou C. N., Song P. S. Photobinding of 8-methoxypsoralen to transfer RNA and 5-fluorouracil-enriched transfer RNA. Biochemistry. 1978 Mar 21;17(6):1054–1059. doi: 10.1021/bi00599a018. [DOI] [PubMed] [Google Scholar]
- Prince J. B., Hixson S. S., Zimmermann R. A. Photochemical cross-linking of tRNALys and tRNA2Glu to 16 S RNA at the P site of Escherichia coli ribosomes. J Biol Chem. 1979 Jun 10;254(11):4745–4749. [PubMed] [Google Scholar]
- Rabin D., Crothers D. M. Analysis of RNA secondary structure by photochemical reversal of psoralen crosslinks. Nucleic Acids Res. 1979 Oct 10;7(3):689–703. doi: 10.1093/nar/7.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Brownlee G. G., Barrell B. G. A two-dimensional fractionation procedure for radioactive nucleotides. J Mol Biol. 1965 Sep;13(2):373–398. doi: 10.1016/s0022-2836(65)80104-8. [DOI] [PubMed] [Google Scholar]
- Santer M., Shane S. Area of 16S ribonucleic acid at or near the interface between 30S and 50S ribosomes of Escherichia coli. J Bacteriol. 1977 May;130(2):900–910. doi: 10.1128/jb.130.2.900-910.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steitz J. A., Jakes K. How ribosomes select initiator regions in mRNA: base pair formation between the 3' terminus of 16S rRNA and the mRNA during initiation of protein synthesis in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4734–4738. doi: 10.1073/pnas.72.12.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiegler P., Carbon P., Zuker M., Ebel J. P., Ehresmann C. Structural organization of the 16S ribosomal RNA from E. coli. Topography and secondary structure. Nucleic Acids Res. 1981 May 11;9(9):2153–2172. doi: 10.1093/nar/9.9.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sypherd P. S. Ribosomal proteins and the conformation of ribosomal ribonucleic acid. J Mol Biol. 1971 Mar 14;56(2):311–318. doi: 10.1016/0022-2836(71)90466-9. [DOI] [PubMed] [Google Scholar]
- Thammana P., Cantor C. R., Wollenzien P. L., Hearst J. E. Crosslinking studies on the organization of the 16 S ribosomal RNA within the 30 S Escherichia coli ribosomal subunit. J Mol Biol. 1979 Nov 25;135(1):271–283. doi: 10.1016/0022-2836(79)90352-8. [DOI] [PubMed] [Google Scholar]
- Thompson J. F., Wegnez M. R., Hearst J. E. Determination of the secondary structure of Drosophila melanogaster 5 S RNA by hydroxymethyltrimethylpsoralen crosslinking. J Mol Biol. 1981 Apr 15;147(3):417–436. doi: 10.1016/0022-2836(81)90493-9. [DOI] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Traub P., Nomura M. Structure and function of Escherichia coli ribosomes. VI. Mechanism of assembly of 30 s ribosomes studied in vitro. J Mol Biol. 1969 Mar 28;40(3):391–413. doi: 10.1016/0022-2836(69)90161-2. [DOI] [PubMed] [Google Scholar]
- Vasiliev V. D., Selivanova O. M., Koteliansky V. E. Specific selfpacking of the ribosomal 16 S RNA. FEBS Lett. 1978 Nov 15;95(2):273–276. doi: 10.1016/0014-5793(78)81009-6. [DOI] [PubMed] [Google Scholar]
- Wagner R., Gassen H. G. Identification of a 16S rna sequence located in the decoding site of 30S ribosomes. FEBS Lett. 1976 Sep 1;67(3):312–315. doi: 10.1016/0014-5793(76)80554-6. [DOI] [PubMed] [Google Scholar]
- Woese C. R., Magrum L. J., Gupta R., Siegel R. B., Stahl D. A., Kop J., Crawford N., Brosius J., Gutell R., Hogan J. J. Secondary structure model for bacterial 16S ribosomal RNA: phylogenetic, enzymatic and chemical evidence. Nucleic Acids Res. 1980 May 24;8(10):2275–2293. doi: 10.1093/nar/8.10.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollenzien P. L., Youvan D. C., Hearst J. E. Structure of psoralen-crosslinked ribosomal RNA from Drosophila melanogaster. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1642–1646. doi: 10.1073/pnas.75.4.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollenzien P., Hearst J. E., Thammana P., Cantor C. R. Base-pairing between distant regions of the Escherichia coli 16 S ribosomal RNA in solution. J Mol Biol. 1979 Nov 25;135(1):255–269. doi: 10.1016/0022-2836(79)90351-6. [DOI] [PubMed] [Google Scholar]
- Zwieb C., Brimacombe R. Localisation of a series of intra-RNA cross-links in 16S RNA, induced by ultraviolet irradiation of Escherichia coli 30S ribosomal subunits. Nucleic Acids Res. 1980 Jun 11;8(11):2397–2411. doi: 10.1093/nar/8.11.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwieb C., Ross A., Rinke J., Meinke M., Brimacombe R. Evidence for RNA-RNA cross-link formation in Escherichia coli ribosomes. Nucleic Acids Res. 1978 Aug;5(8):2705–2720. doi: 10.1093/nar/5.8.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]