Abstract
The present studies determined whether blockade of M1-like muscarinic or nicotinic cholinergic receptors in the dorsomedial striatum affects acquisition or reversal learning of a response discrimination. Testing occurred in a modified cross-maze across two consecutive sessions. In the acquisition phase, a rat learned to turn to the left or to the right. In the reversal learning phase, a rat learned to turn in the opposite direction as required during acquisition. Experiment 1 investigated the effects of the M1-like muscarinic receptor antagonist, pirenzepine infused into the dorsomedial striatum on acquisition and reversal learning. Experiment 2 examined the effects of the nicotinic cholinergic antagonist, mecamylamine injected into the dorsomedial striatum on acquisition and reversal learning. Bilateral injections of pirenzepine at 10 µg, but not 1 µg, selectively impaired reversal learning. Analysis of the errors indicated that pirenzepine treatment did not impair the initial shift, but increased reversions back to the original response choice following the initial shift. Bilateral injections of mecamylamine, 6 or 18 µg, did not affect acquisition or reversal learning. The results suggest that activation of M1 muscarinic cholinergic receptors, but not nicotinic cholinergic receptors, in the dorsomedial striatum is important for facilitating the flexible shifting of response patterns.
Keywords: Striatum, Learning, Acetylcholine, Muscarinic receptors, Basal ganglia, Nicotinic receptors, Pirenzepine, Mecamylamine
1. Introduction
There is accumulating evidence that the dorsomedial striatum plays a critical role in learning when conditions demand the flexible use of response patterns [8,10–12,19,21,31,33,34,39,43]. In discrimination learning, several studies revealed that lesions, temporary inactivation or electrical stimulation of the dorsomedial striatum do not impair initial learning of various discrimination tasks, however, these manipulations do produce reversal learning deficits [19,21,23,27,31,33,34]. Learning impairments are observed in conditions that demand a shift in specific choices as with reversal learning, e.g. choose object A but not object B, now choose object B but not A. Deficits in learning are also exhibited in conditions that demand inhibiting the use of one type of attribute information, i.e. visual cue, while learning to use a different type of attribute information, i.e. egocentric response [31]. Thus, the dorsomedial striatum, may be part of a larger neural system, that enables flexibly adaptive responses in a variety of conditions that demand a shift in behavioral patterns.
Recent studies have focused on the neurochemical mechanisms in the dorsomedial striatum that support the flexible shifting of response patterns [33,34]. These investigations revealed that acetylcholine actions in this area may facilitate learning under changing task contingencies. The almost exclusive source of striatal acetylcholine originates from cholinergic interneurons [3]. Measuring acetylcholine efflux from the dorsomedial striatum during the acquisition and reversal learning of a place discrimination indicated that acetylcholine efflux does not change from basal levels during place acquisition, but increases during place reversal learning [34]. Furthermore, an increase in acetylcholine output occurred as a rat was beginning to learn a new choice pattern and returned near basal levels when a rat was reliably executing the new choice pattern. The pattern of change in acetylcholine efflux suggests that this neurotransmitter in the dorsomedial striatum may be important for the flexible shifting of response patterns.
Further support for the idea that acetylcholine actions in the dorsomedial striatum support learning when conditions require a shift in choice patterns comes from a study examining the effects of muscarinic cholinergic receptor blockade in reversal learning. In particular, infusions of the muscarinic cholinergic antagonist, scopolamine do not impair acquisition of a response discrimination, but impair response reversal learning [33]. The findings suggest that activation of muscarinic cholinergic receptors in the dorsomedial striatum may enable learning with changes in task demands.
Although this study suggests an important role of muscarinic cholinergic receptors in the dorsomedial striatum for the flexible shifting of response patterns, scopolamine is a non-specific muscarinic cholinergic antagonist. The striatum is known to contain several different muscarinic receptor subtypes [17,45]. Unknown is whether specific muscarinic receptor subtypes in the dorsomedial striatum contribute to the flexible shifting of response patterns. The M1 muscarinic receptor subtype is one subtype found at high levels in the striatum [1,17,45]. M1 muscarinic receptors are predominantly located postsynaptic on the dendrites of medium spiny neurons, the main output neuron, and to some degree on axon terminals of striatal afferents [1,17,45]. This contrasts with M2 muscarinic receptors which are found predominantly, if not exclusively, on cholinergic interneurons [1,17,45]. One possibility is that specific muscarinic receptor subytypes in the dorsomedial striatum contribute to learning when conditions demand a shift in response patterns. As an initial attempt to explore this possibility, Experiment 1 investigated whether infusions of the M1-like antagonist, pirenzepine into the dorsomedial striatum affect the acquisition and/or reversal learning of a response discrimination.
Acetylcholine also binds to nicotinic receptors and the striatum contains a moderate density of nicotinic receptors [7]. Although the results described above suggest that muscarinic receptors in the dorsomedial striatum are important, unknown is whether nicotinic receptors in this region contribute to learning during a switch in task contingencies. Past experiments showed that nicotine treatment enhances attention and memory in rats and mecamyalmine treatment, a nicotinic antagonist, impairs attention and memory [4,16,20,35]. To determine whether nicotinic cholinergic receptors in the dorsomedial striatum affect learning, Experiment 2 examined the effects of mecamylamine infused into the dorsomedial striatum on the acquisition and reversal learning of a response discrimination.
2. Materials and methods
2.1. Experiment 1
2.1.1. Subjects
Male Long–Evans rats (Charles River Laboratories, Indianapolis, IN) weighing between 300 and 350 g at the start of the experiment served as subjects. Rats were housed individually in plastic cages (26.5 cm wide × 50 cm long × 20 cm high) in a temperature controlled room. Subjects were kept on a 12:12 h light–dark cycle (lights on 7:00 h). Throughout the experiment, all rats were food restricted to maintain their weight at about 85% of their ad libitum weight with continual access to water. The experiments were conducted in accordance with the United States government principles for the utilization and care of vertebrate animals used in testing, research and training.
2.1.2. Apparatus
A four-arm cross maze made of 0.6 cm thick black plastic was used for all behavioral testing. The maze was placed on a table that was 72 cm above the floor. Each arm was 55 cm long × 10 cm wide. The height of the arm walls was 15.0 cm. Each arm contained a food well hole that was 2.3 cm in diameter and 1.6 cm high. The food well hole was surrounded by a wall 1.6 cm high and the food well was 3 cm away from the end wall.
2.1.3. Surgery
Each rat received stereotaxic surgery to bilaterally implant cannula into the dorsomedial striatum. Rats were first injected with atropine sulfate (0.2 ml of a 250 µg/ml solution, i.p.). Ten minutes after the injection of atropine, the general anesthetic, sodium pentobarbital (50 mg/kg, i.p.) was administered. A midsaggital incision was made and the scalp retracted. Each rat was bilaterally implanted with an 8 mm stainless steel guide cannula (Plastics One, Roanoke, VA) into the dorsomedial striatum. The stereotaxic coordinates were 1.1 mm anterior to bregma, ±2.8 mm lateral to the midline and 3.5 mm ventral to dura. The incisor bar was lowered to 3.3 ± 0.4 below horizontal zero to equal the height of bregma and lambda. Cannulas were implanted at a 10° angle. The coordinates were based on the atlas of Paxinos and Watson [26]. Four jeweler’s screws were positioned in the skull surrounding the cannulas. The cannulas were secured in place with dental acrylic (Stoelting, Wood Dale, IL). Stylets were secured in the guide cannulas after the dental acrylic dried. Following surgery, rats received 6 ml of saline subcutaneously. Each rat was fed ground rat chow with sugar that was mixed in water for 1 day.
2.1.4. Habituation
After surgery, rats were allowed to recover 5–7 days before beginning habituation sessions in the cross maze. On the first day of habituation, three half pieces of Froot Loops cereal (Kelloggs, Battle Creek, MI) were placed in each arm of the maze. Two pieces were placed in each food well, and one was placed outside the food well. A rat was placed in the maze and allowed to explore and consume cereal pieces. If a rat consumed all cereal pieces before 15 min elapsed, then a rat was placed in a holding cage, the maze was reloaded with Froot Loops, and a rat was placed back in the maze. Once a rat ate all the cereal pieces from the food wells and at least 15 min elapsed the session was terminated. If 20 min elapsed and cereal pieces were still in the maze a rat was removed from the maze and the session terminated. Thus, each habituation session lasted between 15 and 20 min.
On subsequent habituation days, only two half pieces of Froot Loops cereal were placed inside each well. After eating two half pieces of cereal from any food well, a rat was picked up and placed into a different start arm. This acclimated the rat to being picked up in the maze after consuming cereal as would occur in the test procedure. Placing a rat in a different arm each time also balanced the different locations being used as a “start” arm and “goal” arm reducing the potential of a bias for a turn pattern or particular spatial location to develop before testing. This procedure was continued until a rat completed four trials in 15 min or less across 2 consecutive days. On the last day of habituation the turn bias for each rat was determined. The maze was arranged such that a black Plexiglas block (9 cm wide × 13 cm high × 1.0 cm thick) was placed at the entrance of one arm so that it prevented entry into that arm, giving the maze a T-shape. A rat was started from the stem arm and allowed to turn left or right to obtain a 1/2 piece of cereal. After this initial choice, a rat was picked up and placed back into the stem arm and allowed to make a second choice. If a rat chose the same arm as the initial choice, it was returned to the stem arm until it chose the other arm and consumed the cereal piece. After a rat chose both arms the rat was placed back in the holding cage, the block was moved to a different location and the two choice arms baited. This procedure continued for seven trials. The initial arm choice, either right or left, was recorded toward the turn bias of the rat. The direction a rat turned four or more times was considered its turn bias. A rat was trained to turn in the opposite direction of its turn bias during the acquisition phase of testing. After completing the last habituation session, the stylets of the rat were removed, and the injection needle was placed into the cannula for 1 min to prevent clogging of the microinfusions on test days. The habituation procedure lasted 5–8 days. Rats were assigned to the different treatment groups to match the amount of habituation among the groups.
2.1.5. Microinfusion
Bilateral infusions into the dorsomedial striatum were made through an inner cannula (28 gauge) that extended 1.0 mm below the guide cannula. The inner cannula was attached to a polyethylene tube (PE-20) connected to a 10 µl Hamilton syringe. The syringe was driven by a microinfusion pump (74900 Series, Cole-Parmer, Vernon Hills, IL). Solutions were infused at a rate of 0.5 µl per 2 min as described previously [28–30,32,34]. The total volume injected on each side was 0.5 µl. Following the injection, the inner cannula remained in the guide cannula for approximately 1 min to allow diffusion. Rats received either saline or pirenzepine (1 or 10 µg per side) into the dorsomedial striatum. Pirenzepine was mixed into saline. The doses were based on previous studies in which comparable doses produced a behavioral impairment when infused intracranially [13,18,41].
2.1.6. Two-choice response discrimination testing procedure
Behavioral testing involved an acquisition phase and reversal learning phase across 2 consecutive days. In the acquisition phase, each rat was required to turn in the opposite direction of its turn bias. For example, if a rat had a right turn bias, then during the acquisition phase the rat was required to always turn left to receive one half piece of Froot Loops cereal in the food well. Between trials a rat was placed in a holding cage, which sat on a table next to the maze. Subsequently, the maze arms were wiped down by a sponge moistened with an ammonium chloride solution. The intertrial interval was approximately 10 s. To minimize the use of intramaze cues every four trials the maze was turned 90° clockwise relative to the experimenter. A rat reached criterion when it made 10 consecutive correct choices. On the second day of testing (reversal learning phase) a rat was required to turn in the opposite direction as on the first day of testing. The same criterion of 10 consecutive correct choices was used.
Additional measures were analyzed on the reversal learning phase to determine whether a treatment altered perseveration or reversions back to the previously correct response pattern after perseveration had ceased. Perseveration involved continuing to make the same egocentric response as required on the acquisition phase. Perseveration was defined as entering the incorrect arm in three or more trials in consecutive blocks of four trials each. After a rat stopped perseverating, that is once a rat made less than three errors in a block for the first time, all subsequent errors were no longer counted as perseverative errors. From that point on, the number of errors were counted as regressive errors. This allowed a measure of the ability to maintain a new choice after initially shifting away from the previously learned choice pattern.
Five minutes prior to each test session, a rat received a microinfusion. Each rat was randomly assigned to one of five treatment groups. Group assignment was determined by the treatment administered during each phase: (1) acquisition—saline and reversal learning—saline (n = 7); (2) acquisition—saline and reversal learning—pirenzepine 1 µg (n = 7); (3) acquisition—saline and reversal learning—pirenzepine 10 µg (n = 8); (4) acquisition—pirenzepine 1 µg and reversal learning—saline (n = 5); (5) acquisition—pirenzepine 10 µg and reversal learning—saline (n = 5). Group 1 served as the control group. Groups 2 and 3 determined whether pirenzepine infusions into the dorsomedial striatum altered reversal learning. Groups 4 and 5 determined whether pirenzepine infusions into the dorsomedial striatum modified initial learning.
2.1.7. Histology
After completion of behavioral testing, rats received a lethal dose of sodium pentobarbital followed by a 0.5 µl injection of 2.5% Chicago blue stain through each guide cannula, as used previously to highlight the location of the cannulas [28–34]. Rats were perfused intracardially with 0.9% phosphate-buffered saline followed by a 4% formaldehyde solution. Brains were removed and stored in a 4% formaldehyde solution. The brains were frozen and cut in coronal sections (40 µm) on a cryostat. The brain sections were mounted on slides, dried, and examined to determine the spread of the stain. Subsequently, the brain sections were stained with cresyl violet to assess the location of the cannula tips.
2.1.8. Statistical analysis
An analysis of variance was used to determine whether the groups differed on the number of trials to criterion for the acquisition and reversal learning phases. Separate ANOVA tests were employed to examine perseverative and regressive errors among the groups.
2.2. Experiment 2
The materials and methods used in Experiment 2 were the same as in Experiment 1 with the following exceptions described in the following sections.
2.2.1. Microinfusion
Rats received a bilateral injection of either saline or mecamylamine hydrochloride (6 µg or 18 µg/0.5 µl) into the dorsomedial striatum. The doses of mecamylamine selected were based on previous studies indicating that comparable doses affect learning and memory when infused intracranially [19,38].
2.2.2. Two-choice response discrimination test procedure
Five minutes prior to each test session, rats received a microinfusion. Each rat was randomly assigned to one of the five treatment groups. Group assignment was determined by the treatment administered during each phase: (1) acquisition—saline and reversal learning—saline (n = 6); (2) acquisition—saline and reversal learning—mecamylamine 6 µg (n = 6); (3) acquisition—saline and reversal learning—mecamylamine 18 µg (n = 6); (4) acquisition—mecamylamine (6 µg) and reversal learning—saline (n = 5); (5) acquisition—mecamylamine (18 µg) and reversal learning—saline (n = 5). Group 1 served as the control group. Groups 2 and 3 determined whether blockade of nicotinic cholinergic receptors, at different doses, altered reversal learning. Groups 4 and 5 determined whether, mecamylamine, at different doses, modified initial learning.
3. Results
3.1. Experiment 1
3.1.1. Histology
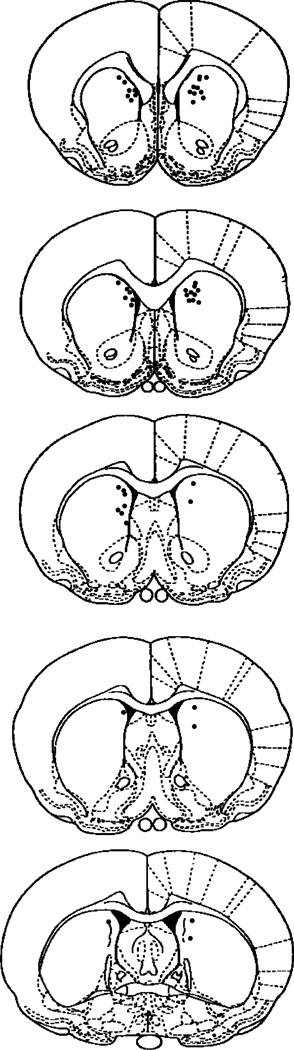
Fig. 1 illustrates the location of the cannula tips in the dorsomedial striatum for Experiment 1. The cannula tips were concentrated in the medial striatum, while some were found more lateral in the central region of the striatum. In the anterior–posterior plane the cannula tips were located at the level of the genu of the corpus callosum. In no cases did the dye extend into the nucleus accumbens. In five cases, a cannula placement was found either unilaterally or bilaterally in the lateral ventricles. The data from these rats were excluded from the behavioral analyses.
Fig. 1.
Illustration of the cannula tip placements in the dorsomedial striatum for rats included in the behavioral analyses in Experiment 1. Cannula tips were concentrated in the dorsomedial striatal region ranging from −0.26 to 1.7 anterior to bregma. The number of circles does not match the total number of rats included in the behavioral analyses because certain cannula placements were overlapping to such a large extent that a single circle represents more than one cannula placement. Rat brain sections were modified from the atlas of Paxinos and Watson [26].
3.1.2. Response acquisition and reversal learning
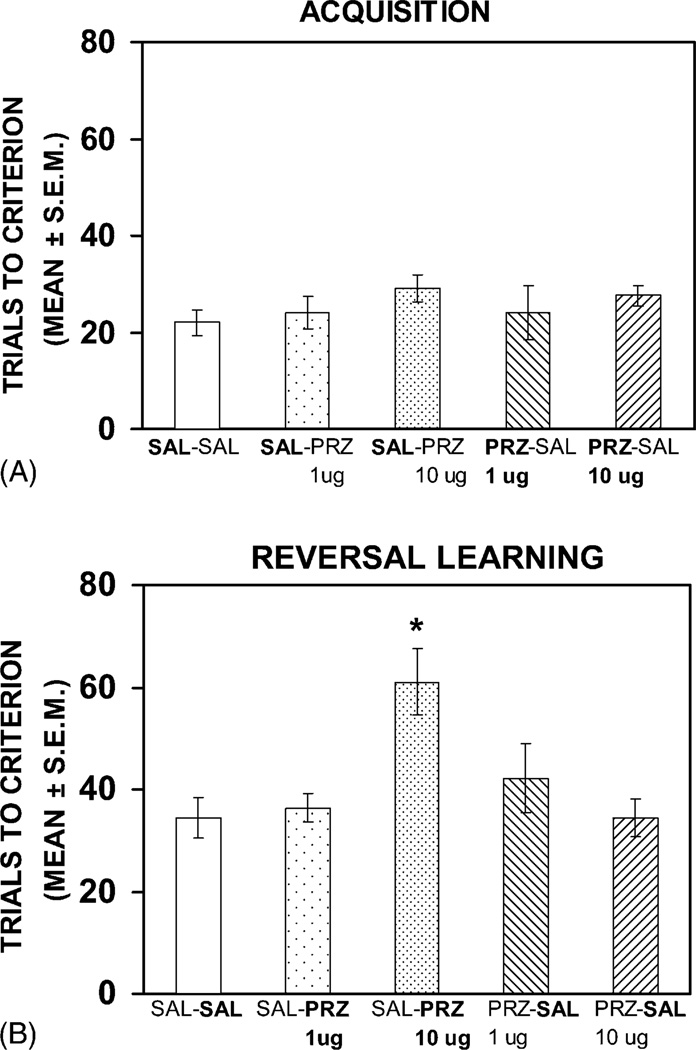
Fig. 2 illustrates the results on acquisition and reversal learning of the response discrimination. All the groups achieved criterion in approximately 25–30 trials. The difference in trials to criterion among the groups was not significant (F(4, 27) = 0.64, P > 0.05). In contrast, there was a significant difference among the groups in reaching criterion during reversal learning (F(4, 27) = 4.75, P < 0.01). Newman–Keuls post hoc tests indicated that the saline–pirenzepine 10 µg group required significantly more trials to reach criterion in the reversal learning phase compared to any of the other four treatment groups (Ps < 0.05). Individual group comparisons in the difference for trials to criterion during reversal learning among the saline–saline, pirenzepine 1 µg–saline, pirenzepine 10 µg–saline and saline–pirenzepine 1 µg groups were not significant (Ps > 0.05).
Fig. 2.
(A) Mean trials to criterion on acquisition of the response discrimination after bilateral infusions of saline or pirenzepine (1 or 10 µg) into the dorsomedial striatum. The findings indicate that pirenzepine injections did not impair acquisition compared to that of saline infusions. The treatment received on this test is in bold for each group. SAL, saline; PRZ, pirenzepine. (B) Mean trials to criterion during reversal learning of the response discrimination after saline or pirenzepine infusions into the dorsomedial striatum. Pirenzepine infusions at the 10 µg dose, but not 1 µg dose, significantly increased the trials to criterion compared to that of saline injections (*P < 0.05). The treatment received on this test is in bold for each group.
3.1.3. Perseverative and regressive errors during reversal learning
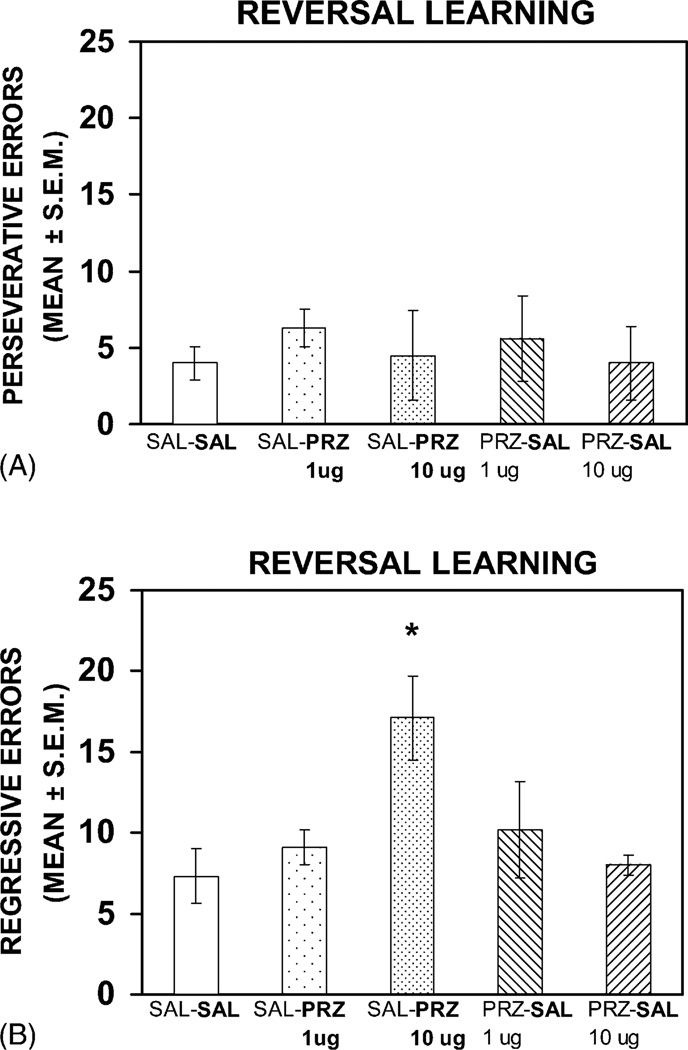
An analysis on the type of errors made during reversal learning revealed that an infusion of pirenzepine (10 µg) into the dorsomedial striatum led to a comparable amount of perseveration as that of the other treatment groups (see Fig. 3A). An ANOVA indicated that the groups did not differ significantly in the number of perseverative errors during reversal learning (F(4, 27) = 0.20, P > 0.05). However, the difference in regressive errors among the groups was significant (F(4, 27) = 3.62, P < 0.05). A post hoc analyses showed that the saline–pirenzepine 10 µg group made significantly more regressive errors than rats from either the saline–saline, saline–pirenzepine 1 µg, pirenzepine 1 µg–saline or pirenzepine 10 µg–saline group (Ps < 0.05, see Fig. 3B). Individual comparisons for the difference in regressive errors among the saline–saline, saline–pirenzepine 1 µg, pirenzepine 1 µg–saline or pirenzepine 10 µg–saline were not significant (Ps > 0.05).
Fig. 3.
(A) Mean number of perseverative errors during reversal learning of the response discrimination following saline or pirenzepine infusions. Pirenzepine infusions did not increase the number of perseverative errors compared to saline infusions. The treatment received on this test is in bold for each group. SAL, saline; PRZ, pirenzepine. (B) Mean number of regressive errors in reversal learning following saline or pirenzepine infusions. Infusion of pirenzepine (10 µg) significantly increased regressive errors compared to that of saline infusions and pirenzepine (1 µg) infusion (*P < 0.05). The treatment received on this test is in bold for each group.
3.2. Experiment 2
3.2.1. Histology
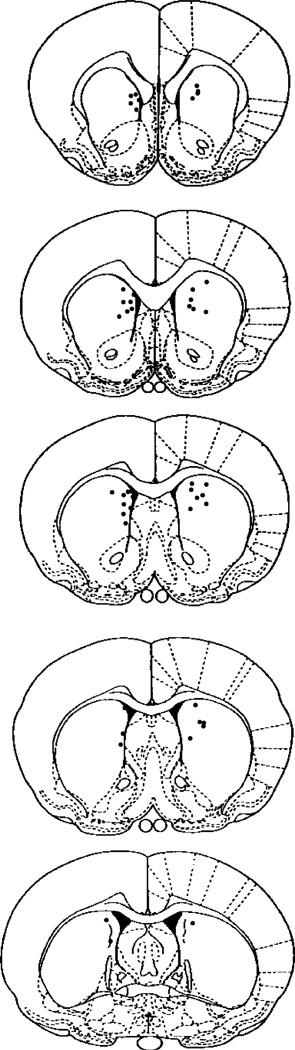
The location of the cannula placements in the dorsomedial striatum for rats included in the behavioral analyses is shown in Fig. 4. As in Experiment 1, the cannula tips were concentrated in the anterior region of the dorsomedial striatum. Four rats were excluded from the behavioral analyses because of misplacements. Two rats had a unilateral cannula placement in the lateral ventricles and two rats had a unilateral placement in the corpus callosum.
Fig. 4.
Illustration of the cannula tip placements in the dorsomedial striatum for rats included in the behavioral analyses in Experiment 2. Cannula tips were concentrated in the dorsomedial striatal region ranging from −0.26 to 1.7 anterior to bregma. The number of circles does not match the total number of rats included in the behavioral analyses because certain cannula placements were overlapping to such a large extent that a single circle represents more than one cannula placement. Rat brain sections were modified from the atlas of Paxinos and Watson [26].
3.2.2. Response acquisition and reversal learning
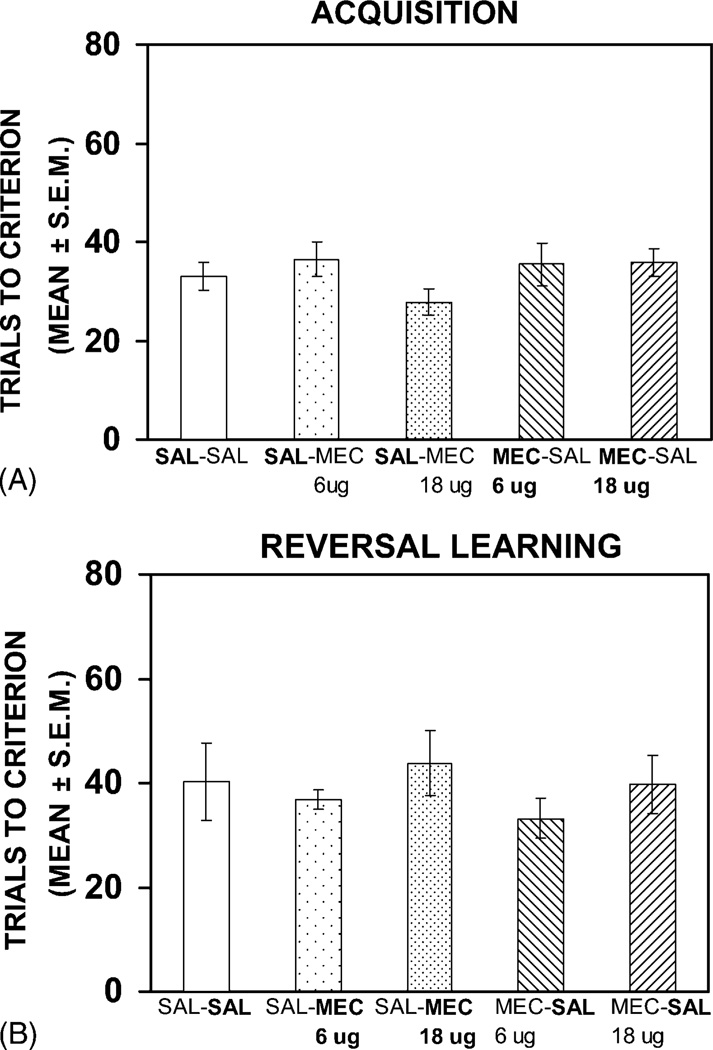
Mecamylamine treatment did not affect acquisition of the response discrimination (see Fig. 5A). The difference in trials to criterion among the groups was not significant (F(4, 23) = 0.58, P > 0.05). Mecamylamine treatment at the 6 or 18 µg dose did not alter reversal learning performance compared to that following saline treatment (see Fig. 5B). The difference in trials to criterion among the groups was not significant (F(4, 23) = 0.42, P > 0.05).
Fig. 5.
(A) Mean trials to criterion on acquisition of the response discrimination after bilateral infusions of saline or mecamylamine (6 or 18 µg) into the dorsomedial striatum. The findings indicate that mecamylamine injections at either dose did not impair acquisition compared to that of saline infusions. The treatment received on this test is in bold for each group. SAL, saline; MEC, mecamylamine. (B) Mean trials to criterion during reversal learning of the response discrimination after saline or mecamylamine infusions into the dorsomedial striatum. Mecamylamine infusions at either dose did not affect trials to criterion on reversal learning. The treatment received on this test is in bold for each group.
3.2.3. Perseverative and regressive errors during reversal learning
An analysis of the errors during reversal learning indicated that there was no significant difference among the groups in the number of perseverative errors (F(4, 23) = 0.24, P > 0.05). In addition, the difference in regressive errors among the group was not significant (F(4, 23) = 0.98, P > 0.05).
4. General discussion
The findings from Experiment 1 indicate that pirenzepine infusions into the dorsomedial striatum selectively impair response reversal learning. Because pirenzepine infusions only affect performance during reversal learning, the deficit is unlikely due to a general impairment in learning or any motivational or motor side effects. These findings are consistent with previous experiments demonstrating that lesions of the dorsomedial striatum do not impair initial learning of different discriminations tasks, but do produce deficits in reversal learning [19,21,23,27,33,34]. The pirenzepine-induced deficit is also comparable to the findings indicating that scopolamine injections into the dorsomedial striatum impair response reversal learning, but not acquisition of a response discrimination [33]. Unlike scopolamine which is a non-selective muscarinic antagonist, pirenzepine shows a high affinity for M1 muscarinic receptors [9]. This suggests that the response reversal learning deficit may arise due to blockade of M1 muscarinic receptors. Thus, specific muscarinic receptor subtypes in the dorsomedial striatum may contribute to learning when conditions demand the shifting of response patterns.
Although this initial study suggests a role for M1 muscarinic receptors in the dorsomedial striatum for the flexible shifting of response patterns, pirenzepine also shows an affinity for M4 muscarinic receptors [9]. Eventhough pirenzepine has a greater affinity of M1 versus M4 muscarinic receptors, this raises the possibility that the reversal learning deficit following pirenzepine infusions into the dorsomedial striatum may be due to blockade of M4 muscarinic receptors or a combination of M1 and M4 muscarinic receptors. Furthermore, M2 muscarinic cholinergic receptors are found on the axons of striatal cholinergic interneurons where they act as autoreceptors [1,17,45]. Unknown is whether M2 muscarinic cholinergic receptors in the dorsomedial striatum may also play a role in reversal learning. Future studies using more selective agents and/or other pharmacological tools will be important in more fully characterizing what muscarinic receptor subtypes in the dorsomedial striatum are critical for learning when conditions demand the flexible shifting of response patterns.
Analysis of the errors during reversal learning revealed that pirenzepine infusions specifically increase regressive errors but not perseverative errors. This pattern of errors indicates that pirenzepine infusions into the dorsomedial striatal do not prevent the initial shift away from the previously relevant response pattern, but increase reversions back to the previously correct response pattern. A selective increase in regressive errors was also observed following either infusions of bupivacaine or scopolamine into the dorsomedial striatum [33,34]. Thus, the pattern of regressive errors following pirenzepine infusions into the dorsomedial striatal is not unique to this type of manipulation. Furthermore, the present results are consistent with the idea that the striatum facilitates the execution of effective response patterns for a particular behavioral context by reinforcing the correct choice pattern when generated [44].
The present findings suggest that while the dorsomedial striatum may be critical for response selection, it may not play a general role in response selection. This is because pirenzepine, as well as other pharmacological manipulations of the dorsomedial striatum do not impair initial learning in a variety of discrimination tasks [19,21,27,31,33]. In both the acquisition and reversal learning phase of the discrimination task a selection between two alternative choice patterns is required. However, the key difference is that the reversal learning phase requires inhibition of a previously reinforced choice pattern while learning to execute the opposite choice pattern, but in the acquisition phase a rat must select between two different choices that were not differentially reinforced prior to this test phase. Thus, in reversal learning a rat must inhibit the execution of a recently formed habit and now form a new habit. The present results suggest that activation of M1 muscarinic receptors in the dorsomedial striatum are critical for conditions like reversal learning in which a previously relevant response pattern must be inhibited and a new response pattern must be learned.
Another possible interpretation of the reversal learning deficit produced by pirenzepine infusions is that the impairment is due, at least in part, to a working memory impairment causing rats to make an increased number of regressive errors. In this case, a rat under pirenzepine who makes an incorrect or correct choice on a trial may not be able to retain that information in short-term memory, thereby being less likely to make the correct choice on the following trial. Past investigations have demonstrated that lesions or pharmacological manipulations of the dorsomedial striatum can produce working memory deficits [11,38]. However, working memory tasks routinely have a strategic component that requires the flexible use of choice patterns, e.g. delayed match-to-sample or nonmatch-to-sample. Because the findings indicated that pirenzepine treatment did not impair acquisition a parsimonious explanation is that the reversal learning impairment results from a deficit in inhibiting a previously learned choice pattern while learning to execute a new, relevant choice pattern.
The increase in regressive errors observed in the present study is also comparable to results showing that Parkinson’s disease patients were able to learn a sequence of movements and when required to shift to a new sequence of movements could initially switch to a different movement pattern, but had a deficit in maintaining the new sequence pattern [14,36]. Previous studies also indicate that alterations in muscarinic cholinergic receptors within the caudate may contribute to the cognitive flexibility deficits observed in Parkinson’s disease patients [2]. Examination of the striatum in Parkinson’s disease patients revealed that there is a decrease in muscarinic receptors in the anterior caudate [15]. Furthermore, administration of different muscarinic cholinergic antagonists, i.e. scopolamine, to Parkinson’s patients does not impair learning, but does impair task switching ability [2]. In contrast, low doses of muscarinic cholinergic antagonists administered to patients with Alzheimer’s disease impaired memory, but did not affect cognitive flexibility [40]. Alzheimer’s disease commonly alters cortical cholinergic receptors, but leaves striatal cholinergic receptors intact [37]. The dissociation in the effects of muscarinic cholinergic antagonists in Parkinson’s and Alzheimer’s disease patients suggests that muscarinic cholinergic receptors in the striatum may play a central role in facilitating cognitive flexibility.
The pattern of results in the response discrimination test following pirenzepine injections into the dorsomedial striatum are also comparable to the results measuring acetylcholine efflux from the dorsomedial striatum during the acquisition and reversal learning of a place discrimination [34]. As described earlier, acetylcholine efflux selectively increases during place reversal learning. However, acetylcholine efflux did not initially change when a rat was executing the previously learned choice pattern. Acetylcholine efflux did significantly increase as a rat began to learn the new relevant choice pattern and returned near basal levels when a rat reliably executed the new choice pattern. Taken together with the present findings, these results suggest that increases in acetylcholine output in the dorsomedial striatum may activate M1 muscarinic cholinergic receptors to enable learning under changing environmental demands.
At a cellular level, striatal muscarinic cholinergic receptors have been found to influence striatal synaptic plasticity [5,6]. For example, scopolamine blocks striatal long-term potentiation produced by high frequency stimulation of corticostriatal fibers [6]. In a similar fashion, the muscarinic M1 antagonist, pirenzepine also blocks long-term potentiation in the striatum suggesting that specifically, M1 muscarinic receptors may be critical [6]. However, scopolamine or pirenzepine do not alter baseline synaptic potentials. These findings raise the possibility that blockade of muscarinic cholinergic receptors in the dorsomedial striatum may prevent LTP-like synaptic plasticity that results in reversal learning deficits as observed in the present study.
To examine whether acetylcholine actions at nicotinic cholinergic receptors in the dorsomedial striatum influences learning under changing environmental demands, Experiment 2 examined the effects of the nicotinic antagonist, mecamylamine infused into the dorsomedial striatum on response reversal learning. Mecamylamine treatment at either dose did not affect response acquisition or reversal learning. The findings suggest that nicotinic cholinergic receptors in the dorsomedial striatum are not critical for the flexible shifting of response patterns. Thus, acetylcholine may selectively act at muscarinic cholinergic receptors in the dorsomedial striatum to facilitate learning when conditions demand the switching of response patterns.
Mecamylamine is reported to act as a channel blocker and a specific antagonist for nicotinic acetylcholine receptors containing α3β4 subunits [42]. Nicotinic antagonists acting at other nicotinic acetylcholine receptors containing different subunits, e.g. α4β2 or α7 subunits, have differentially modified various measures of striatal activity compared to that of mecamylamine [22,24,25,46]. Thus, there is a possibility that specific nicotinic acetylcholine receptors in the dorsomedial striatum play a role in learning with changes in task demands. Exploring the behavioral effects of other nicotinic antagonists, that target different nicotinic receptor subunits, infused into the dorsomedial striatum can help clarify this issue.
In summary, pirenzepine infusions in the dorsomedial striatum selectively impaired response reversal learning. In contrast, mecamylamine infusions into the dorsomedial striatum did not affect acquisition or reversal learning of a response discrimination. The findings suggest that activation of M1 muscarinic cholinergic receptors, but not nicotinic cholinergic receptors, in the dorsomedial striatum are important for the flexible shifting of response patterns. Because pirenzepine infusions into the dorsomedial striatum caused an increase in regressive errors during reversal learning M1 muscarinic cholinergic receptors in this region may facilitate behavioral switching by influencing the reliable execution of effective response patterns.
Acknowledgements
This research was supported by NIH grants MH61889 and NS043283. We thank Sara Yap and Quintino Mano for their excellent technical assistance.
References
- 1.Alcantara AA, Mrzljak L, Jakab RL, Levey AI, Hersch SM, Goldman-Rakic PS. Muscarinic m1 and m2 receptor proteins in local circuit and projection neurons of the primate striatum: anatomical evidence for cholinergic modulation of glutamatergic prefronto-striatal pathways. J Comp Neurol. 2001;434:445–460. doi: 10.1002/cne.1186. [DOI] [PubMed] [Google Scholar]
- 2.Bedard MA, Pillon B, Dubois B, Duchesne N, Masson H, Agid Y. Acute and long-term administration of anticholinergics in Parkinson’s disease: specific effects on the subcortico-frontal syndrome. Brain Cogn. 1999;40:289–313. doi: 10.1006/brcg.1999.1083. [DOI] [PubMed] [Google Scholar]
- 3.Bolam JP, Ingham CA, Smith AD. The section-Golgi-impregnation procedure. 3. Combination of Golgi impregnation with enzyme histochemistry and electron microscopy to characterize acetylcholinesterase-containing neurons in the rat neostriatum. Neuroscience. 1984;12:687–709. doi: 10.1016/0306-4522(84)90164-7. [DOI] [PubMed] [Google Scholar]
- 4.Brown RW, Beale KS, Frye GDJ. Mecamylamine blocks enhancement of reference memory but not working memory produced by post-training injection of nicotine in rats tested on the radial arm maze. Behav Brain Res. 2002;134:259–265. doi: 10.1016/s0166-4328(02)00047-5. [DOI] [PubMed] [Google Scholar]
- 5.Calabresi P, Centonze D, Gubellini P, Pisani A, Bernardi G. Endogenous ACh enhances striatal NMDA-responses via M1-like muscarinic receptors and PKC activation. Eur J Neurosci. 1998;10:2887–2895. doi: 10.1111/j.1460-9568.1998.00294.x. [DOI] [PubMed] [Google Scholar]
- 6.Calabresi P, Centonze D, Gubellini P, Pisani A, Bernardi G. Acetylcholine-mediated modulation of striatal function. Trends Neurosci. 2000;23:120–126. doi: 10.1016/s0166-2236(99)01501-5. [DOI] [PubMed] [Google Scholar]
- 7.Clarke PBS, Pert CB, Pert A. Autoradiographic distribution of nicotinic receptors in the rat brain. Brain Res. 1984;323:390–395. doi: 10.1016/0006-8993(84)90320-2. [DOI] [PubMed] [Google Scholar]
- 8.Cory-Slechta DA, O’Mara DJ, Brockel BJ. Learning versus performance impairments following regional administration of MK-801 into nucleus accumbens and dorsomedial striatum. Behav Brain Res. 1999;102:181–194. doi: 10.1016/s0166-4328(99)00015-7. [DOI] [PubMed] [Google Scholar]
- 9.Dorje F, Wess J, Lambrecht G, Tacke R, Mutschler E, Brann MR. Antagonist binding profiles of five cloned human muscarinic receptor subtypes. J Pharmacol Exp Ther. 1991;256:727–733. [PubMed] [Google Scholar]
- 10.Dunnett SB, Iversen SD. Learning impairments following selective kainic acid-induced lesions within the neostriatum of rats. Behav Brain Res. 1981;2:189–209. doi: 10.1016/0166-4328(81)90055-3. [DOI] [PubMed] [Google Scholar]
- 11.Dunnett SB, Nathwani F, Brasted PJ. Medial prefrontal and neostriatal lesions disrupt performance in an operant delayed alternation task in rats. Behav Brain Res. 1999;106:13–28. doi: 10.1016/s0166-4328(99)00076-5. [DOI] [PubMed] [Google Scholar]
- 12.Eagle DM, Humby T, Dunnett SB, Robbins TW. Effects of regional striatal lesions on motor. Behav Neurosci. 1999;113:718–731. doi: 10.1037//0735-7044.113.4.718. [DOI] [PubMed] [Google Scholar]
- 13.Ferreira AR, Furstenau L, Blanco C, Kornisiuk E, Sanchez G, Daroit D, et al. Role of hippocampal M1 and M4 muscarinic receptor subtypes in memory consolidation in the rat. Pharmacol Biochem Behav. 2003;74:411–415. doi: 10.1016/s0091-3057(02)01007-9. [DOI] [PubMed] [Google Scholar]
- 14.Flowers KA, Robertson C. The effect of Parkinson’s disease on the ability to maintain a mental set. J Neurol Neurosurg Psychol. 1985;48:517–529. doi: 10.1136/jnnp.48.6.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griffiths PD, Perry RH, Crossman AR. A detailed anatomical analysis of neurotransmitter receptors in the putamen and caudate in Parkinson’s disease and Alzheimer’s disease. Neurosci Lett. 1994;169:68–72. doi: 10.1016/0304-3940(94)90358-1. [DOI] [PubMed] [Google Scholar]
- 16.Grottick AJ, Higgins GA. Assessing a vigilance decrement in aged rats: effects of pre-feeding, task manipulation, and psychostimulants. Psychopharmacology. 2000;164:33–41. doi: 10.1007/s00213-002-1174-3. [DOI] [PubMed] [Google Scholar]
- 17.Hersch SM, Gutekunst CA, Rees HD, Heilman CJ, Levey AI. Distribution of m1–m4 muscarinic receptor proteins in the rat striatum: light and electron microscopic immunocytochemistry using subtype-specific antibodies. J Neurosci. 1994;14:3351–3363. doi: 10.1523/JNEUROSCI.14-05-03351.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kikusui T, Aoyagi A, Kaneko T. Spatial working memory is independent of hippocampal CA1 long-term potentiation. Behav Neurosci. 2000;114:700–706. [PubMed] [Google Scholar]
- 19.Kirkby RJ. Caudate nucleus lesions and perseverative behavior. Physiol Behav. 1969;4:451–454. [Google Scholar]
- 20.Kim JS, Levin ED. Nicotinic, muscarinic and dopaminergic actions in the ventral hippocampus and the nucleus accumbens: effect on spatial working memory in rats. Brain Res. 1996;725:231–240. doi: 10.1016/0006-8993(96)00213-2. [DOI] [PubMed] [Google Scholar]
- 21.Kolb B. Studies on the caudate-putamen and the dorsomedial thalamic nucleus of the rat: implications for mammalian frontal-lobe functions. Physiol Behav. 1977;18:237–244. doi: 10.1016/0031-9384(77)90128-7. [DOI] [PubMed] [Google Scholar]
- 22.Lecca D, Shim I, Costa E, Javaid JI. Striatal application of nicotine, but not of lobeline, attenuates dopamine release in freely moving rats. Neuropharmacology. 2000;39:88–98. doi: 10.1016/s0028-3908(99)00085-4. [DOI] [PubMed] [Google Scholar]
- 23.Livesey PJ, Muter V. Functional differentiation within the neostriatum of the rat using electrical (blocking) stimulation during discrimination learning. J Comp Physiol Psych. 1976;90:203–211. doi: 10.1037/h0077200. [DOI] [PubMed] [Google Scholar]
- 24.Marchi M, Risso F, Viola C, Cavazzani P, Raiteri M. Direct evidence that release-stimulating α7 nicotinic cholinergic receptors are localized on human and rat brain glutamatergic axon terminals. J Neurochem. 2002;80:1071–1078. doi: 10.1046/j.0022-3042.2002.00805.x. [DOI] [PubMed] [Google Scholar]
- 25.Nayak SV, Dougherty JJ, McIntosh JM, Nichols RA. Ca2+ changes induced by different presynaptic nicotinic receptors in separate populations of individual striatal nerve terminals. J Neurochem. 2001;76:1860–1870. doi: 10.1046/j.1471-4159.2001.00197.x. [DOI] [PubMed] [Google Scholar]
- 26.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 3rd ed. Sydney: Academic Press; 1996. [DOI] [PubMed] [Google Scholar]
- 27.Pisa M, Cyr J. Regionally selective roles of the rat’s striatum in modality specific discrimination learning and forelimb reaching. Behav Brain Res. 1990;37:281–292. doi: 10.1016/0166-4328(90)90140-a. [DOI] [PubMed] [Google Scholar]
- 28.Ragozzino ME, Kesner RP. The effects of muscarinic cholinergic receptor blockade in the rat anterior cingulate and prelimbic/infralimbic cortices on spatial working memory. Neurobiol Learn Mem. 1998;69:241–257. doi: 10.1006/nlme.1998.3823. [DOI] [PubMed] [Google Scholar]
- 29.Ragozzino ME, Detrick S, Kesner RP. Involvement of the prelimbic–infralimbic areas of the rodent prefrontal cortex in behavioral flexibility for place and response learning. J Neurosci. 1999;19:4585–4594. doi: 10.1523/JNEUROSCI.19-11-04585.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ragozzino ME, Wilcox C, Raso M, Kesner RP. Involvement of rodent prefrontal cortex subregions in strategy switching. Behav Neurosci. 1999;113:32–41. doi: 10.1037//0735-7044.113.1.32. [DOI] [PubMed] [Google Scholar]
- 31.Ragozzino ME, Ragozzino KE, Mizumori SJY, Kesner RP. The role of the dorsomedial stratum in behavioral flexibility for response and visual cue discrimination learning. Behav Neurosci. 2002;116:105–115. doi: 10.1037//0735-7044.116.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ragozzino ME. The effects of dopamine D1 receptor blockade in the prelimbic–infralimbic areas on behavioral flexibility. Learn Mem. 2002;9:18–28. doi: 10.1101/lm.45802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ragozzino ME, Jih J, Tzavos A. Involvement of the dorsomedial striatum in behavioral flexibility: role of muscarinic cholinergic receptors. Brain Res. 2002;953:205–214. doi: 10.1016/s0006-8993(02)03287-0. [DOI] [PubMed] [Google Scholar]
- 34.Ragozzino ME, Choi D. Dynamic changes in acetylcholine output in the medial striatum during place reversal learning. Learn Mem. 2004;11:70–77. doi: 10.1101/lm.65404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rezvani AH, Bushnell PJ, Levin ED. Effects of nicotine and mecamylamine on choice accuracy in an operant visual signal detection task in female rats. Psychopharmacology. 2002;164:369–375. doi: 10.1007/s00213-002-1221-0. [DOI] [PubMed] [Google Scholar]
- 36.Robertson C, Flowers KA. Motor set in Parkinson’s disease. J Neurol Neurosurg Psych. 1990;53:1149–1161. doi: 10.1136/jnnp.53.7.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodriguez-Puertas R, Pascual J, Vilaro T, Pazos A. Autoradiographic M1, M2, M3, and M4 muscarinic receptor subtypes in Alzheimier’s disease. Synapse. 1997;26:341–350. doi: 10.1002/(SICI)1098-2396(199708)26:4<341::AID-SYN2>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 38.Schildein S, Huston JP, Schwarting RK. Open field habituation learning is improved by nicotine and attenuated by mecamylamine administered posttrial into the nucleus accumbens. Neurobiol Learn Mem. 2002;77:277–290. doi: 10.1006/nlme.2001.4017. [DOI] [PubMed] [Google Scholar]
- 39.Smith-Roe SL, Sadeghian K, Kelley AE. Spatial learning and performance in the radial arm maze is impaired after N-methyl-d-aspartate (NMDA) receptor blockade in striatal subregions. Behav Neurosci. 1999;113:703–717. doi: 10.1037//0735-7044.113.4.703. [DOI] [PubMed] [Google Scholar]
- 40.Sunderland T, Tariot PN, Cohen RM, Weingartner H, Mueller EA, Murphy DL. Anticholinergic sensitivity in patients with dementia of the Alzheimer type and age-matched controls. Arch Gen Psychiat. 1987;44:418–426. doi: 10.1001/archpsyc.1987.01800170032006. [DOI] [PubMed] [Google Scholar]
- 41.Wall PM, Messier C. Infralimbic kappa opioid and muscarinic M1 receptor interactions in the concurrent modulation of anxiety and memory. Psychopharmacology. 2002;160:233–244. doi: 10.1007/s00213-001-0979-9. [DOI] [PubMed] [Google Scholar]
- 42.Webster JC, Francis MM, Porter JK, Robinson G, Stokes C, Horenstein B, et al. Antagonist activities of mecamylamine and nicotine show reciprocal dependence on beta subunit sequence in the second transmembrane domain. Br Pharmacol. 1999;127:1337–1348. doi: 10.1038/sj.bjp.0702686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whishaw IQ, Mittleman G, Bunch ST, Dunnett SB. Impairments in the acquisition, retention and selection of spatial navigation strategies after medial caudate-putamen lesions in rats. Behav Brain Res. 1987;24:125–138. doi: 10.1016/0166-4328(87)90250-6. [DOI] [PubMed] [Google Scholar]
- 44.Wise SP, Murray EA, Gerfen CR. The frontal cortex–basal ganglia system in primates. Crit Rev Neurobiol. 1996;10:317–356. doi: 10.1615/critrevneurobiol.v10.i3-4.30. [DOI] [PubMed] [Google Scholar]
- 45.Yan Z, Flores-Hernandez J, Surmeier DJ. Coordinated expression of muscarinic receptor messenger RNAs in striatal medium spiny neurons. Neuroscience. 2001;103:1017–1024. doi: 10.1016/s0306-4522(01)00039-2. [DOI] [PubMed] [Google Scholar]
- 46.Zhou FM, Liang Y, Dani JA. Endogenous nicotinic cholinergic activity regulates dopamine release in the striatum. Nat Neurosci. 2001;4:1224–1229. doi: 10.1038/nn769. [DOI] [PubMed] [Google Scholar]