Abstract
Separate experiments found that activation of N-methyl-d-aspartate (NMDA) receptors or increased acetylcholine (ACh) efflux in the rat dorsomedial striatum is critical for learning when conditions require a shift in strategies. Increasing evidence indicates that NMDA receptor activity affects cholinergic efflux in the basal ganglia. The present studies determined whether NMDA receptor blockade in the dorsomedial striatum with dl-2-amino-5-phosphonopentanoic acid (AP-5) affects dorsomedial striatal ACh output in a resting condition, as well as during response reversal learning. Experiment 1 investigated the effects of AP-5 (12.5, 25 or 50 µM) infused into the dorsomedial striatum on ACh output in a resting condition. AP-5 infusion at 25 and 50 µM led to a 20% and 40% decrease in dorsomedial striatal ACh output, respectively. AP-5 (12.5 µM) infusion did not change dorsomedial striatal ACh output from basal levels. Experiment 2 determined whether dorsomedial striatal ACh efflux increases during response reversal learning and whether AP-5, at a dose that does not affect basal levels, modifies response reversal learning and ACh efflux. Following acquisition of a response discrimination, rats had microdialysis probes bilaterally inserted into the dorsomedial striatum prior to the reversal learning test. After baseline samples, rats received a response reversal learning test for 30 min. Control rats rapidly improved in the reversal learning session while simultaneously exhibiting an approximately 40% increase in ACh output compared with baseline levels. AP-5 (12.5 µM) treatment during testing significantly impaired response reversal learning while concomitantly blocking an increase in ACh output. These findings suggest that NMDA receptor activation in the dorsomedial striatum may facilitate a shift in response patterns, in part, by increasing ACh efflux.
Keywords: striatum, NMDA, acetylcholine, microdialysis, reversal learning
Several experiments provide evidence that different striatal regions may play distinct roles in learning and memory (Dunnett and Iversen, 1982; Brown and Robbins, 1989; Pisa and Cyr, 1990; Levy et al., 1997; Devan et al., 1999; Eagle et al., 1999; Featherstone and McDonald, 2004a,b, 2005; Palencia and Ragozzino, 2004, 2005; Yin et al., 2004, 2005). In particular, experiments have found that the lateral striatum is critical for the learning of arbitrary stimulus–response associations (Packard and White, 1991; Reading et al., 1991; Jog et al., 1999; Kantak et al., 2001; Featherstone and McDonald, 2004a,b; McDonald and Hong, 2004) or egocentric response information based on proprioceptive feedback (Packard and McGaugh, 1996; Packard, 1999; Chang and Gold, 2004; Yin et al., 2004; Palencia and Ragozzino, 2005). In contrast to the dorsolateral striatum, the dorsomedial striatum does not appear critical for learning stimulus–response associations, but supports learning when conditions require the flexible use of response patterns or a shift in strategies (Kolb, 1977; Livesey and Muter, 1976; Palencia and Ragozzino, 2004; Pisa and Cyr, 1990; Ragozzino et al., 2002a,b; Ragozzino and Choi, 2004; Tzavos et al., 2004). For example, dorsomedial striatal lesions or temporary inactivation does not impair initial discrimination learning, but does impair reversal learning of place, visual cue or response learning (Kirkby, 1969; Kolb, 1977; Pisa and Cyr, 1990; Ragozzino et al., 2002b; Ragozzino and Choi, 2004; Van Golf Racht Delatour and El Massioui, 1999). Electrophysiological evidence also suggests that the dorsomedial striatum may enable learning when conditions require a shift in strategies. Specifically, recordings from dorsomedial striatal neurons indicate that these cells change their correlated firing between light and dark conditions in a spatial test that requires a rat to switch strategies (Mizumori et al., 2000). Taken together, the results support the idea that the dorsomedial striatum is part of a neural system that facilitates a shift in choice patterns.
More recent experiments have investigated the neurochemical mechanisms in the dorsomedial striatum that support learning with a shift in task contingencies. One neurotransmitter that may play a key role in facilitating behavioral flexibility is acetylcholine (ACh). ACh content in the striatum originates almost entirely from interneurons (Woolf and Butcher, 1981; Bolam et al., 1984). Measuring ACh efflux from the dorsomedial striatum during acquisition and reversal learning of a place discrimination revealed that ACh output did not change during place acquisition, but did significantly increase during place reversal learning (Ragozzino and Choi, 2004). These findings suggest that activation of cholinergic neurons in this striatal region facilitates a shift in choice patterns.
Another study demonstrated that N-methyl-d-aspartate (NMDA) receptors in the dorsomedial striatum may also be critical when conditions require a shift in strategies. In particular, this experiment showed that infusions of the competitive NMDA antagonist, 2-amino-5-phosphopentanoic acid (AP-5), into the dorsomedial striatum had no effect on acquisition of a response discrimination, but did impair response reversal learning in a dose-dependant manner (Palencia and Ragozzino, 2004). One possibility is that the effect of NMDA receptor blockade in the dorsomedial striatum may be related to the results indicating ACh activity increases during reversal learning. This is because NMDA receptors are found in high densities on striatal cholinergic neurons (Landwehrmeyer et al., 1995; Morari et al., 1998). Furthermore, activation of NMDA receptors increases striatal ACh release while blockade of NMDA receptors decreases striatal ACh release (Morari et al., 1998; Ikarashi et al., 1998; Knauber et al., 1999). Thus, NMDA receptor blockade in the dorsomedial striatum may attenuate an increase in ACh output during reversal learning which underlies, at least in part, a deficit in shifting choice patterns.
To test this possibility, the present study investigated the effects of NMDA receptor blockade on ACh efflux in the dorsomedial striatum during a resting condition and during response reversal learning. Experiment 1 investigated the effects of AP-5 infused into the dorsomedial striatum on ACh efflux during a resting condition. Experiment 2 determined whether a dose of AP-5 infused into the dorsomedial striatum, that does not affect basal ACh output, alters an increase in striatal ACh efflux and response reversal learning.
EXPERIMENTAL PROCEDURES
Subjects
A total of 32 adult male Long-Evans rats (Charles River Laboratories, Indianapolis, IN, USA) weighing between 310 and 370 g at the beginning of the experiments, served as subjects. Subjects were housed individually in thick transparent plastic cages (54 cm×20 cm×29 cm), inside a temperature- (24 °C) and humidity- (~30%) controlled room. The room was kept on a 12-h light/dark cycle with the lights on at 7 a.m. Animal care and use was in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and was approved by the Institutional Laboratory Animal Care and Use Committee at the University of Illinois at Chicago. Every effort was made to minimize the number of animals used and prevent any suffering.
Handling
One week prior to surgery all rats were food restricted to 85% of their ad libitum weight. Each rat was handled for 10–15 min a day for a total of seven days. During handling rats were allowed to consume Froot Loops cereal (Kellogg’s, Battle Creek, MI, USA).
Surgery
In preparation for surgery, atropine sulfate (0.2 ml of a 250 µg/ml solution) was administered to each rat 15 min before the injection of the general anesthetic (sodium pentobarbital, 50 mg/kg i.p.). After a rat was placed on the stereotaxic apparatus the incisor bar was set to 3.3±0.4 below horizontal zero. The coordinates were based on the atlas of Paxinos and Watson (1996). A midsagittal incision was made to retract the scalp and expose the cranium. Each rat was implanted bilaterally with a 12-mm guide cannula (CMA Microdialysis Inc., North Chelmsford, MA, USA). The stereotaxic coordinates were 0.6mmanterior to bregma, ±2.8 lateral to the midline and 3.8 ventral to dura. The guide cannulas were implanted at a 10° angle. Six jeweler’s screws were positioned in the skull surrounding the guide cannula to serve as anchors. An omega-shaped ring was placed behind the guide cannulas. The omega ring allowed the rat to be connected to the liquid swivel/balance arm by a wire attached with a hook that extended from the liquid swivel. This setup prevented the tubing from being twisted during microdialysis collection. The guide cannulas and omega ring were secured in place with dental acrylic. Following surgery, rats received two separate 3ml injections of saline (s.c.) on the left and right dorsal side of the rat’s body. Following surgery, each rat was fed ground rat chow with sugar that was mixed in water for 1 day.
ACh collection in resting condition
In experiment 1, the effects of AP-5 on dorsomedial striatal ACh output in a resting condition were determined. Prior to the microdialysis procedure rats were acclimated to a round-bottom plastic bowl 60–90 min a day for 5 days. On the last day of this pretest procedure the wire extending from the liquid swivel was attached to the omega ring on top of a rat’s head, but no probes were inserted in the guide cannulas. This allowed a rat to become accustomed to the microdialysis setup. Subjects were placed in a round-bottom plastic bowl, and a 2-mm microdialysis probe was inserted through each guide cannula. The wire extending from the liquid swivel was hooked onto the omega ring. Artificial cerebrospinal fluid (aCSF) was perfused through the microdialysis probes at a rate of 2.0 µl/min. The aCSF contained NaCl (0.128 M), KCl (0.004M), dibasic Na2HPO4 (0.002 M), monobasic Na2HPO4 (0.0006 M), CaCl2 (0.001 M), MgCl2 (0.0009 M) and glucose (0.001 M). The solution was brought to a pH of 7.4 by NaOH. To reliably detect ACh levels, the reversible acetylcholinesterase inhibitor, neostigmine bromide (0.1 µM) was added to the aCSF. The perfusate collected during the first 60 min was not analyzed to allow equilibration between the brain tissue and perfusion solution. Subsequently, five baseline samples were collected at 6-min intervals and analyzed for ACh efflux. After baseline collection, a rat received either AP-5 mixed in the aCSF at 12.5 µM, 25 µM or 50 µM. The treatments were perfused through the microdialysis probes for a total of 30 min. During this reverse dialysis procedure, an additional 5 samples were collected at 6-min intervals.
ACh assay
The same method for analyzing ACh was used in experiments 1 and 2. The perfusate samples (10 µl) were assayed for ACh using high-pressure liquid chromatography with electrochemical detection using BAS® HPLC equipment. The system provides a ≤5 fmol sensitivity. The assay was completed in 12 min. Samples were loaded on a microbore analytical column for separation of ACh and choline. Following separation, an enzymatic post-column reactor containing acetylcholinesterase and choline oxidase converted ACh to choline and acetate and choline to betaine and hydrogen peroxide. Stoichiometric quantities of hydrogen peroxide were produced from the breakdown of ACh and choline. The hydrogen peroxide was further broken down and detected by a glassy carbon-wired electrode coated with horseradish peroxidase operated at +100 mV versus an Ag/AgCl reference electrode (Huang et al., 1995). The mobile phase containing 50 mM Na2HPO4, 0.3 mM EDTA, and 0.005% ProClin (to prevent bacterial growth) was delivered at a rate of 100 µl/min by a solvent delivery system. ACh peaks were quantified by comparison with peak heights of ACh standard solutions.
Apparatus
In experiment 2 a four-arm maze made of 0.6 cm thick black plastic was used for behavioral testing. The maze was placed on a table 72 cm above the floor. Each arm of the maze was 55 cm long×10 cm wide, with walls 15 cm high. Each arm contained a food well (2.3 cm of diameter and 1.6 cm high) placed 3 cm away from the end wall.
Pretest procedure
The pretest procedure began 5–7 days following surgery. Before each session a rat was placed in a round-bottomed plastic bowl for 60–90 min. On the first day, two pieces of Froot Loops cereal were placed in each food well of the cross-maze. A rat was placed in the maze and allowed to explore the maze and consume the cereal pieces. If a rat consumed all the cereal pieces before 15 min expired, then the rat was placed in a holding cage while the food wells were rebaited with two cereal pieces. Subsequently, the rat was placed back in the maze. The session was terminated once 15 min elapsed. In subsequent pretest sessions, two half pieces of cereal were placed in each food well. After a rat ate two half pieces from any arm the rat was picked up and placed in a different start arm. This procedure continued until the rat completed at least four trials in 15 min across two consecutive days. After a rat achieved this criterion, a final pretest session was carried out to determine the turn bias of a rat. Prior to beginning the last pretest session the wire extending from the swivel arm was attached to the omega ring, but no probes were inserted in the cannulas. The purpose was to acclimate a rat to this setup prior to behavioral testing. For the last session, a black plastic block (10 cm wide×13 cm high×2 cm thick) was placed at the entrance of one of the arms, preventing entrance into that arm, and giving the maze a T-shape. Two of the arms were baited with a 1/2 piece of cereal in the food well. A rat was started from the stem arm and allowed to turn either left or right to obtain a half piece of cereal. After consuming cereal from one arm, the rat was placed back in the stem arm and again allowed to enter either of the two choice arms. If a rat turned into the same arm it was placed back in the stem arm and the process was repeated until a rat entered the opposite arm and consumed the second cereal piece. After a rat visited both arms, it was placed in a round plastic bowl. The block was moved to another arm and two other choice arms were baited. This same procedure continued for seven trials. The initial arm choice, left or right, in each trial was recorded. The initial direction that a rat turned for four or more trials was considered a rat’s turn bias. Rats were trained to turn in the opposite direction as their bias during the acquisition phase. The pretest procedure lasted 4–6 days.
Two-choice response discrimination
Behavioral testing involved two phases across two consecutive days: acquisition phase and reversal learning phase. Prior to each session, a rat was hooked up to the liquid swivel wire and placed in the holding chamber. No microdialysis collection was conducted during response acquisition. Therefore, only the wire was attached to the rat without probes inserted. In the acquisition phase, a rat learned to turn in the opposite direction of its turn bias. The maze was arranged in a T-shape such that there was a stem arm and two choice arms. Each rat was started randomly from the “south” or “west” arm. This arrangement gave rats a different pair of choice arms from each start arm and reduced the use of extra-maze cues. A rat had to always turn in the same direction (left or right) to receive 1/2 piece of cereal. If a rat made a correct turn it was allowed to consume the cereal piece in the food well. If a rat made an incorrect choice, it was removed from the non-reinforced arm after reaching the food well. Between trials a rat was placed in the holding chamber, the maze arms were wiped with a sponge moistened with 2% laboratory cleaning solution containing 2-butuxyethanol and sodium dodecylbenzene sulfonate. In the acquisition phase, rats were tested for 24 min. This length of testing was chosen because it was similar to that of a previous study that combined discrimination testing with in vivo microdialysis collection (Ragozzino and Choi, 2004). The trials were separated into four 6-minute blocks in which the number of correct trials and total number of trials was recorded. For each 6-min block, a percent correct score was determined by calculating the number of trials a rat chose the correct arm divided by the total number of trials for that block. Rats were pseudorandomly assigned to the control or AP-5 group in reversal learning such that the groups exhibited similar performance in the last block of acquisition and completed a similar number of trials throughout acquisition testing.
In the reversal learning phase, a rat was placed in the plastic bowl and a microdialysis probe was placed in each guide cannula. The same microdialysis procedure was used as described in experiment 1. The perfusate collected during the first 60 min was not analyzed, to allow equilibration between the brain tissue and perfusion solution. Subsequently, five baseline samples were collected at 6-min intervals. Following baseline collection, a rat was placed in the maze for reversal learning testing. A rat was required to turn in the opposite direction as in acquisition to receive a [1/2] piece of cereal. Each rat was tested for a total of 30 min. A rat’s behavioral performance was separated into 6-min blocks, which corresponded with the collection of microdialysis samples. For each 6-min block, a percent correct score was determined by calculating the number of trials a rat chose the correct arm divided by the total number of trials for that block. Five microdialysis samples were collected concomitantly in 6-min intervals. At the onset of testing, the control group was perfused with aCSF through the microdialysis probes while the drug group was perfused with AP-5 12.5 µM mixed in aCSF through the microdialysis probes. The drug infusion occurred throughout the 30 min of behavioral testing.
Histology
After completion of testing, each rat received a lethal dose of sodium pentobarbital. Just prior to the perfusion procedure a 2-mm microdialysis probe dipped in 2.5% Chicago Blue stain was inserted in each guide cannula to highlight the location of the probe placement. All rats were perfused intracardially with 0.9% phosphate-buffered saline, followed by a 4% formaldehyde solution. Brains were removed and stored in a 4% formaldehyde solution. The brains were frozen and cut in coronal sections (40 µm) on a cryostat. The brain sections were mounted on slides, dried, and examined to determine the spread of the stain. Subsequently, the brain sections were stained with Cresyl Violet to assess the location of the probes.
Statistical analysis
The microdialysis data were analyzed by converting the raw values to percentages from each subject’s baseline output. The baseline output was calculated from the mean of the first five samples for each subject. The percent values were analyzed by a repeated measures ANOVA.
A repeated-measures ANOVA was used to analyze the percent correct scores across blocks for reversal learning between the groups.
RESULTS
Histology
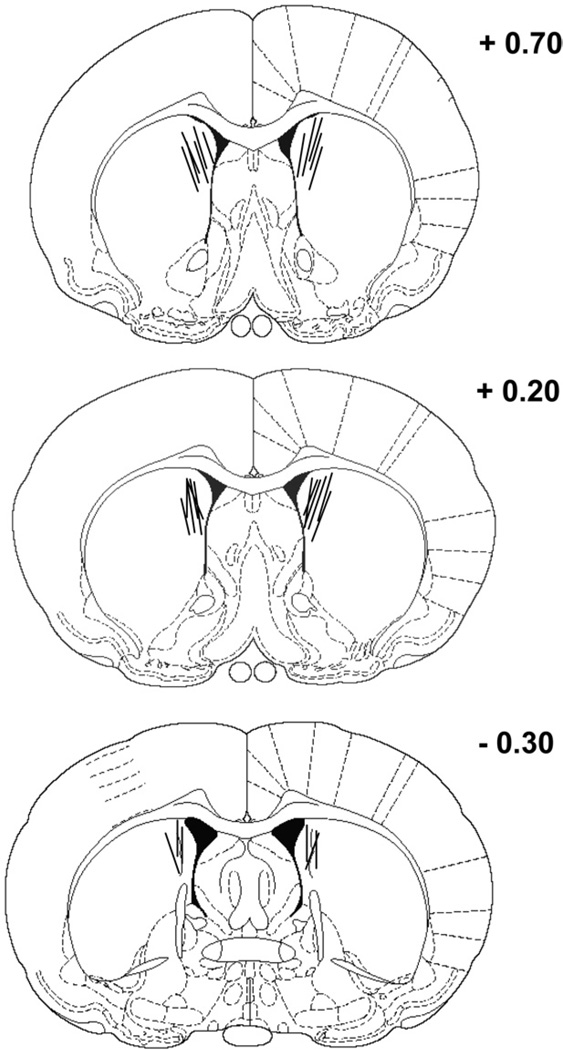
Fig. 1 illustrates the location of the microdialysis probes for experiments 1 and 2. Microdialysis probe location was comparable in both experiments. Specifically, the locations of the microdialysis probes were concentrated in the dorsomedial region of the striatum. In the anterior–posterior plane, the placement of microdialysis probes ranged from 0.7 mm to −0.3 mm relative to bregma. The data from two rats were excluded from the analyses in experiment 1 because of probe misplacements. One had a bilateral placement in the ventral striatum and the other had a unilateral placement in the lateral ventricle. In experiment 2, the data from one rat were excluded from the analyses because of a placement in the ventral striatum.
Fig. 1.
Location of microdialysis probes in the dorsomedial striatum of rats included in the analyses for experiments 1 and 2. The length of the microdialysis probe was 2 mm. The microdialysis probes were concentrated in the dorsomedial striatum ranging from +0.7 mm to −0.3 mm in the anterior–posterior plane relative to bregma. The rat brain sections were modified from the atlas of Paxinos and Watson (1996).
Experiment 1: effect of NMDA receptor blockade in the dorsomedial striatum during a resting condition
ACh output
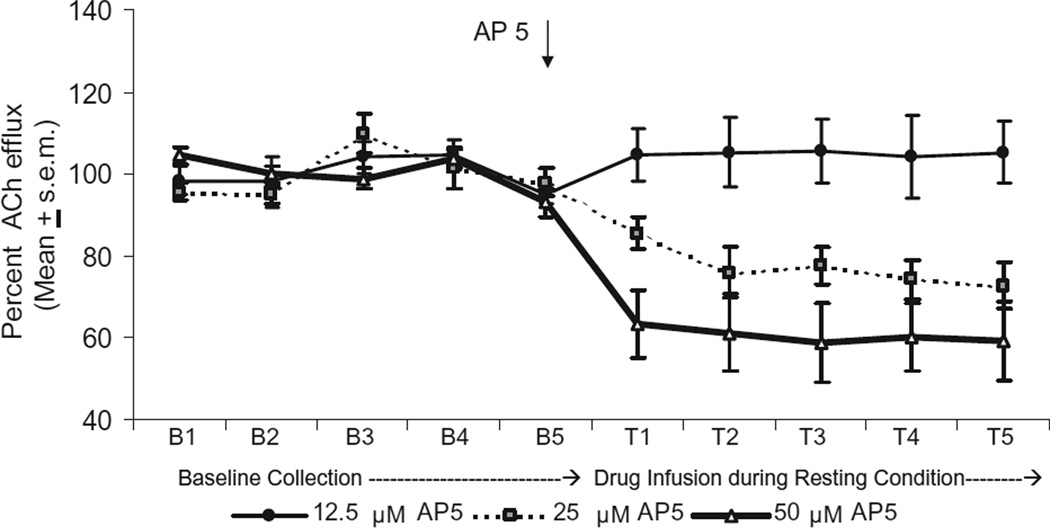
ACh output during the five baseline samples was similar in all the treatment groups. Reverse dialysis infusion of AP-5 50 µM in the dorsomedial striatum significantly decreased ACh efflux by 40% compared with that of basal levels. Perfusions of AP-5 25 µM also reduced ACh output throughout the drug infusion period, but levels were only reduced by approximately 20% compared with that of basal levels. In contrast, an infusion of AP-5 at 12.5 µM into the dorsomedial striatum did not affect ACh output during the sampling period (see Fig. 2). A two-way ANOVA with repeated measures indicated that there was a significant difference in ACh output among the groups [F(2,9)=4.58, P<0.05]. There was also a significant effect of test [F(9,81)=6.27, P<0.01]. Finally, there was a significant group×test interaction [F(18,81)=3.42, P<0.01]. Supplemental analyses were conducted to compare the groups during the drug infusion period using F tests. The difference in ACh output between the AP-5 12.5 µM group and the AP-5 25 µM group was not significant [F(1,6)= 3.56, P>0.05]. However, the difference in ACh output between the AP-5 12.5 µM group and the AP-5 50 µM group was significant [F(1,7)=6.85 P<0.05]. The difference in ACh output between the AP-5 25 µM group and the AP-5 50 µM group was not significant [F(1,5)=1.16, P>0.05]. To determine whether treatment with AP-5 12.5 µM modified ACh output compared with basal levels, Dunnett’s tests were used to compare specific drug samples with the last baseline sample. The findings revealed that there was no significant difference in ACh output between any of the drug infusion samples compared with that of the last baseline sample in the AP-5 12.5 µM group (P’s>0.05). Thus, dorsomedial striatal infusion of AP-5 50 µM produced a significant decrease in striatal ACh output; an infusion of AP-5 25 µM led to a moderate decrease in ACh output and an infusion of AP-5 12.5 µM did not significantly affect basal ACh output.
Fig. 2.
Effect of AP-5 at 50 µM, 25 µM, and 12.5 µM on ACh efflux in the dorsomedial striatum during a resting condition. Treatment groups showed stable baseline levels. Reverse dialysis infusion of AP-5 50 µM significantly decreased ACh efflux to approximately 60% of basal levels. The infusion of AP-5 25 µM reduced ACh efflux to approximately 80% of basal levels. Infusion of AP-5 12.5 µM had no effect ACh output during the sampling period. * P<0.05 vs. baseline ACh efflux levels.
Experiment 2: effect of NMDA receptor blockade in the dorsomedial striatum on ACh efflux during response reversal learning
ACh output
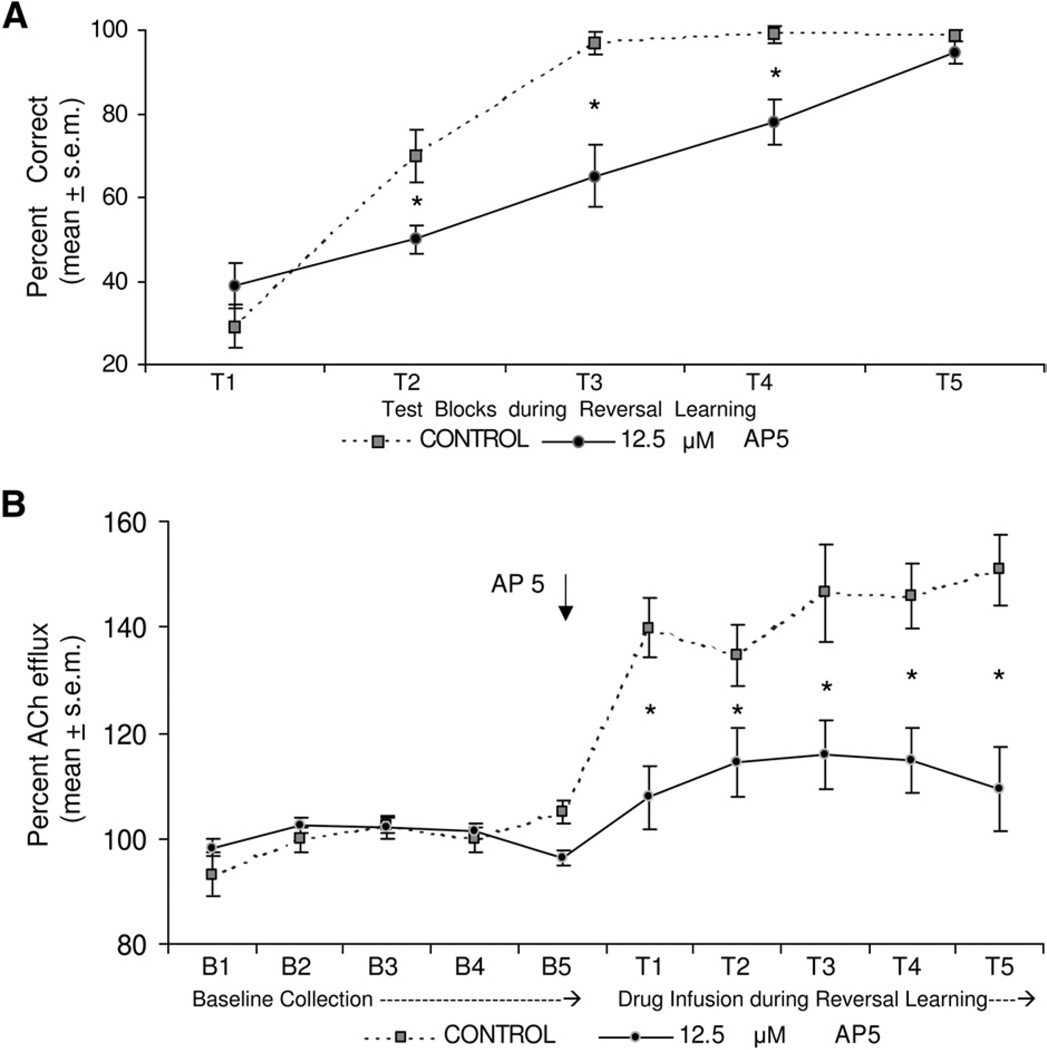
ACh efflux in both groups during baseline collection was stable and comparable across the five sample periods. In the control group, reversal learning testing led to an initial increase in ACh output of ~40% above basal levels that remained elevated throughout testing. In contrast, an infusion of AP-5 12.5 µM attenuated the behaviorally-induced increase in ACh output throughout the test session (see Fig. 3b). A two factor ANOVA with repeated measures was performed to analyze the ACh efflux between the two groups. The analysis indicated that ACh efflux between the two groups was significantly different [F(1,13)=10.320 P<0.05]. The analysis also indicated a significant effect for test block [F(9,117)=17.15 P<0.01]. Finally, there was a significant test×group interaction [F(9,117)=5.80, P<0.01]. Supplemental analyses with F tests comparing the groups during baseline and during reversal learning indicated that there was not a significant different in ACh output between the groups during baseline collection [F(1,13)=2.931, P>0.05]. However, treatment with AP-5 12.5 µM significantly decreased striatal ACh output during reversal learning compared with that of the aCSF group [F(1,13)= 10.345, P<0.01].
Fig. 3.
(a) Mean percent correct across five 6-min test blocks during response reversal learning. The aCSF and AP-5 group had comparable scores in the first block, but AP-5 group had a significantly reduced performance in blocks 2–4. The AP-5 and aCSF groups had a similar level of performance by the last test block. * P<0.05 vs. aCSF group. (b) ACh output during response reversal learning. ACh output increased ~40% above basal levels in controls across testing. AP-5 12.5 µM treatment reduced the increase in ACh output that was comparable to basal levels. * P<0.05 vs. aCSF group.
Behavioral testing
The aCSF and AP-5 (12.5 µM) groups exhibited the same performance at the end of response acquisition testing. More specifically, all rats in each group obtained 100% correct in the last 6-min block of acquisition testing. Thus, both groups achieved the same performance by the end of the acquisition session.
Fig. 3A shows performance during response reversal learning. The results revealed that both groups started below chance (50%) in the first test block. This occurred as rats initially made the turn response that was correct during acquisition. At the second block, rats in the AP-5 group were near chance level (~50%) while the control group increased performance to ~70% correct. The AP-5 group continued to perform below that of controls over the next two test blocks in which the control group achieved over 90% correct by the third block and performed near 100% in the fourth and fifth blocks. By the final test block, the AP-5 group obtained greater than 90% correct. A two-way ANOVA with repeated measures indicated that there was a significant group effect [F(1,13)= 15.47, P<0.01]. The analysis also revealed a significant test effect [F(4,52)=62.71, P<0.01], indicating learning across the test blocks. Finally, there was a significant group×test interaction [F(4,52)=4.96, P<0.01]. Newman-Keuls post hoc tests indicated that the percent correct scores for the AP-5 group was significantly less than that of controls at blocks 2, 3 and 4 (P’s<0.05).
There is the possibility that the effect of AP-5 on striatal ACh output is related to a change in locomotor behavior as opposed to reversal learning. Specifically, the change in ACh output in the dorsomedial striatum may have led to hyperactivity or hypoactivity. If this is the case, then there should be a significant difference between the groups in the number of trials completed during the test session. Therefore, an analysis on the number of trials completed between the groups was carried out. Analyses of the results indicated that the control group had a mean of 36.0±1.3 S.E.M. trials completed. The AP-5 group had a mean of 37.9±1.0 S.E.M. trials completed. The difference in the trials completed between the groups was not significant [t(13)=1.082, P>0.05].
DISCUSSION
Experiment 1 found that a reverse dialysis infusion of AP-5 into the dorsomedial striatum decreased ACh efflux at 25 and 50 µM. In contrast to the 25 and 50 µM concentrations, a 12.5 µM concentration of AP-5 into the dorsomedial striatum did not alter basal ACh output. This is consistent with previous studies demonstrating that AP-5 infusions into the dorsal striatum dose-dependently impair basal ACh output (Knauber et al., 1999). NMDA receptor effects on striatal ACh output are likely due to direction actions on cholinergic interneurons as NMDA receptors are located on striatal cholinergic interneurons (Giovannini et al., 1995; Landwehrmeyer et al., 1995; Ikarashi et al., 1998; Morari et al., 1998).
Although an infusion AP-5 12.5 µM did not affect basal ACh efflux, experiment 2 demonstrated that infusion of AP-5 (12.5 µM) into the dorsomedial striatum concomitantly impaired response reversal learning and attenuated an increase in ACh efflux. These findings indicate that NMDA receptor blockade at this dose does not produce a general decrease in striatal ACh output, but selectively modifies striatal ACh output when cholinergic neurons become activated as in the response reversal learning test. More generally, the results suggest that activation of NMDA receptors in the dorsomedial striatum may facilitate learning when conditions require a shift in strategies, at least in part, by increasing ACh release.
In a previous experiment an increase in dorsomedial striatal ACh output occurred during place reversal learning, but not in place acquisition (Ragozzino and Choi, 2004), suggesting that a change in dorsomedial striatal ACh output observed during response reversal learning is unlikely due to locomotor activity and/or consumption of cereal pieces. In a similar manner, because the AP-5 and control groups completed a comparable number of trials during testing, despite a decrease in ACh output produced by AP-5 treatment, the effect on ACh efflux is unlikely due to a change in locomotor activity and/or motivational factors. Instead, the findings suggest that the attenuation of ACh output produced by NMDA blockade is related to a reversal learning impairment. Furthermore, because an increase in ACh output is observed in place reversal learning, as well as response reversal learning the findings from these studies suggest that cholinergic neurons in the dorsomedial striatum are not activated for a specific type of reversal learning test, but may become activated under a range of conditions that require a shift in choice patterns or strategies.
The importance of ACh actions in the dorsomedial striatum involved in strategy switching is further supported by experiments demonstrating that muscarinic cholinergic receptor blockade in the dorsomedial striatum selectively impairs reversal learning (Ragozzino et al., 2002a; Tzavos et al., 2004). In particular, infusions of the non-specific muscarinic cholinergic antagonist, scopolamine or M1-like antagonist, pirenzepine into the dorsomedial striatum selectively impair reversal learning. Thus, increased cholinergic activity in the dorsomedial striatum may activate muscarinic cholinergic receptors to support reversal learning.
ACh actions in other striatal regions may play a different role than in the dorsomedial striatum. For example, Gold (2003) and Pych et al. (2005) have examined ACh output from the dorsolateral striatum during different learning tests. In the dorsolateral striatum, ACh output gradually increases as a rat develops a response strategy. The maximal increase in dorsolateral striatal ACh output is observed after extensive training when a rat expresses a response strategy (Chang and Gold, 2003; Pych et al., 2005). This increase in ACh output with adaptation of a response strategy suggests that the activation of cholinergic interneurons in this region may support the initial learning of a response strategy. This interpretation is consistent with findings indicating that inactivation with a local anesthetic or NMDA receptor blockade in the dorsolateral striatum impairs initial learning of an egocentric response discrimination, but lesions or NMDA receptor blockade of this area does not affect response reversal learning (Pisa and Cyr, 1990; Chang and Gold, 2004; Palencia and Ragozzino, 2005). The initial increase in ACh efflux from the dorsolateral striatum during discrimination learning was smaller than that observed in the dorsomedial striatum during reversal learning. Furthermore, ACh output in the dorsolateral striatum continued to increase and reach a maximum output at approximately one hour after testing. ACh output in the dorsomedial striatum increased approximately 40% above basal levels within the first 6-min test sample and remained at a similar level throughout testing. Taken together, the results suggest that ACh actions in the dorsolateral and dorsomedial striatum differentially contribute to learning and add further evidence more generally that the dorsomedial and dorsolateral striatum plays a unique role in learning and memory.
Related to the present findings, one question is how does an increase in ACh release in the dorsomedial striatum modify striatal output to support a shift in strategies? Cholinergic interneurons have extensive axonal fields (Izzo and Bolam, 1988) which make them ideally suited to shape patterns of activation in the striatum, as well as the nature of striatal output to other brain regions. At an electrophysiological level, cholinergic interneurons may be the same neurons that exhibit tonic activity (Aosaki et al., 1995). These neurons fire at a tonic rate of 2–10 Hz which is in contrast to the phasic activation of the predominant projection neurons (Wilson et al., 1990). Because these cells are often found at the striosome–matrix border, Graybiel (1995, 1998) and Graybiel et al. (1994) have proposed that tonically active neurons play a key role in integrating activity in different striatal modules that influences learning. Although cholinergic interneurons may not be essential for the expression of a behavioral response, cholinergic activity in the striatum may facilitate the coordination between cortico-striatal modules that leads to an adaptive response (Graybiel, 1998).
As observed with an acute injection of AP-5 into the dorsomedial striatum (Palencia and Ragozzino, 2004), reverse dialysis infusion of AP-5 into this region impaired response reversal learning, by slowing the acquisition of the new contingency. This was the case despite NMDA blockade reducing ACh output throughout the reversal learning session. These findings suggest that NMDA receptors in the dorsomedial striatum play a role in reversal learning, but are not essential when task contingencies require a shift in response patterns. In a similar manner, ACh actions in the dorsomedial striatum are involved in a shift in response patterns, but do no appear essential for the ultimate switch in choice patterns. This pattern of results with ACh output in the dorsomedial striatum is consistent with the idea proposed by Graybiel (1998) in which cholinergic interneurons may be important for facilitating the coordination between different striatal input–output modules, but are not essential for the expression of an adaptive response. Future studies will be critical in determining what mechanisms in the dorsomedial striatum and/or other neural circuitry allow an adaptive response with a change in environmental contingencies.
Abbreviations
- ACh
acetylcholine
- aCSF
artificial cerebrospinal fluid
- AP-5
2-amino-5-phosphopentanoic acid
- NMDA
N-methyl-d-aspartate
REFERENCES
- Aosaki T, Kimura M, Graybiel AM. Temporal and spatial characteristics of tonically active neurons of the primate’s striatum. J Neurophysiol. 1995;3:1234–1252. doi: 10.1152/jn.1995.73.3.1234. [DOI] [PubMed] [Google Scholar]
- Bolam JP, Wainer BH, Smith AD. Characterization of cholinergic neurons in the rat neostriatum. A combination of choline acetyltransferase immunocytochemistry, Golgi-impregnation and electron microscopy. Neuroscience. 1984;12:711–718. doi: 10.1016/0306-4522(84)90165-9. [DOI] [PubMed] [Google Scholar]
- Brown VJ, Robbins TW. Elementary processes of response selection mediated by distinct regions of the striatum. J Neurosci. 1989;9:3760–3765. doi: 10.1523/JNEUROSCI.09-11-03760.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Q, Gold PE. Switching memory systems during learning: changes in patterns of brain acetylcholine release in the hippocampus and striatum in rats. J Neurosci. 2003;23:3001–3005. doi: 10.1523/JNEUROSCI.23-07-03001.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Q, Gold PE. Inactivation of dorsolateral striatum impairs acquisition of response learning in cue-deficient, but not cue-available, conditions. Behav Neurosci. 2004;118:383–388. doi: 10.1037/0735-7044.118.2.383. [DOI] [PubMed] [Google Scholar]
- Devan BD, McDonald RJ, White NM. Effects of medial and lateral caudate-putamen lesions on place- and cue-guided behaviors in the water maze: Relation to thigmotaxis. Behav Brain Res. 1999;100:5–14. doi: 10.1016/s0166-4328(98)00107-7. [DOI] [PubMed] [Google Scholar]
- Dunnett SB, Iversen SD. Neurotoxic lesions of ventrolateral but not anteromedial neostriatum in rats impair differential reinforcement of low rates (DRL) performance. Behav Brain Res. 1982;6:213–226. doi: 10.1016/0166-4328(82)90024-9. [DOI] [PubMed] [Google Scholar]
- Eagle DM, Humby T, Dunnett SB, Robbins TW. Effects of regional striatal lesions on motor, motivational, and executive aspects of progressive-ratio performance in rats. Behav Neurosci. 1999;113:718–731. doi: 10.1037//0735-7044.113.4.718. [DOI] [PubMed] [Google Scholar]
- Featherstone RE, McDonald RJ. Dorsal striatum and stimulus-response learning: Lesions of the dorsolateral, but not dorsomedial, striatum impair acquisition of a stimulus-response-based instrumental discrimination task, while sparing conditioned place preference learning. Neuroscience. 2004a;124:23–31. doi: 10.1016/j.neuroscience.2003.10.038. [DOI] [PubMed] [Google Scholar]
- Featherstone RE, McDonald RJ. Dorsal striatum and stimulus-response learning: Lesions of the dorsolateral, but not dorsomedial, striatum impair acquisition of a simple discrimination task. Behav Brain Res. 2004b;150:15–23. doi: 10.1016/S0166-4328(03)00218-3. [DOI] [PubMed] [Google Scholar]
- Featherstone RE, McDonald RJ. Lesions of the dorsolateral striatum impair acquisition of a simplified stimulus-response dependent conditional discrimination task. Neuroscience. 2005;136:387–395. doi: 10.1016/j.neuroscience.2005.08.021. [DOI] [PubMed] [Google Scholar]
- Giovannini MG, Camilli F, Mundula A, Bianchi L, Colivicchi MA, Pepeu G. Differential regulation by N-methyl-D-aspartate and non-N-methyl-D-aspartate receptors of acetylcholine release from the rat striatum in vivo. Neuroscience. 1995;65:409–415. doi: 10.1016/0306-4522(94)00503-w. [DOI] [PubMed] [Google Scholar]
- Gold PE. Acetylcholine modulation of neural systems involved in learning and memory. Neurobiol Learn Mem. 2003;3:194–210. doi: 10.1016/j.nlm.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Aosaki T, Flaherty AW, Kimura M. The basal ganglia and adaptive motor control. Science. 1994;5180:1826–1831. doi: 10.1126/science.8091209. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. Building action repertoires: memory and learning functions of the basal ganglia. Curr Opin Neurobiol. 1995;6:733–741. doi: 10.1016/0959-4388(95)80100-6. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. The basal ganglia and chunking of action repertoires. Neurobiol Learn Mem. 1998;1–2:119–136. doi: 10.1006/nlme.1998.3843. [DOI] [PubMed] [Google Scholar]
- Huang T, Yang L, Gitzen J, Kissinger PT, Vreeke M, Heller A. Detection of basal acetylcholine in rat brain microdialysate. J Chromatogr B Biomed Appl. 1995;670:323–327. doi: 10.1016/0378-4347(95)00181-6. [DOI] [PubMed] [Google Scholar]
- Ikarashi Y, Yuzurihara M, Takahashi A, Ishimaru H, Shiobara T, Maruyama Y. Direct regulation of acetylcholine release by N-methyl-D-aspartic acid receptors in rat striatum. Brain Res. 1998;795:215–220. doi: 10.1016/s0006-8993(98)00293-5. [DOI] [PubMed] [Google Scholar]
- Izzo PN, Bolam JP. Cholinergic synaptic input to different parts of spiny striatonigral neurons in the rat. J Comp Neurol. 1988;269:219–234. doi: 10.1002/cne.902690207. [DOI] [PubMed] [Google Scholar]
- Jog MS, Kubota Y, Connolly CI, Hillegaart V, Graybiel AM. Building neural representations of habits. Science. 1999;286:1745–1749. doi: 10.1126/science.286.5445.1745. [DOI] [PubMed] [Google Scholar]
- Kantak KM, Green-Jordan K, Valencia E, Kremin T, Eichenbaum HB. Cognitive task performance after lidocaine-induced inactivation of different sites within the basolateral amygdala and dorsal striatum. Behav Neurosci. 2001;115:589–601. doi: 10.1037//0735-7044.115.3.589. [DOI] [PubMed] [Google Scholar]
- Kirkby RJ. Caudate nucleus lesions impair spontaneous alternation. Percept Mot Skills. 1969;29:550. doi: 10.2466/pms.1969.29.2.550. [DOI] [PubMed] [Google Scholar]
- Knauber J, Kischka U, Roth M, Schmidt WJ, Hennerici M, Fassbender K. Modulation of striatal acetylcholine concentrations by NMDA and the competitive NMDA receptor-antagonist AP-5: an in vivo microdialysis study. J Neural Transm. 1999;106:35–45. doi: 10.1007/s007020050139. [DOI] [PubMed] [Google Scholar]
- Kolb B. Studies on the caudate-putamen and the dorsomedial thalamic nucleus of the rat: Implications for mammalian frontal-lobe functions. Physiol Behav. 1977;18:237–244. doi: 10.1016/0031-9384(77)90128-7. [DOI] [PubMed] [Google Scholar]
- Landwehrmeyer GB, Standaert DG, Testa CM, Penney JB, Jr, Young AB. NMDA receptor subunit mRNA expression by projection neurons and interneurons in rat striatum. J Neurosci. 1995;15:5297–5307. doi: 10.1523/JNEUROSCI.15-07-05297.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R, Friedman HR, Davachi L, Goldman-Rakic PS. Differential activation of the caudate nucleus in primates performing spatial and nonspatial working memory tasks. J Neurosci 1997. 1997;17:3870–3882. doi: 10.1523/JNEUROSCI.17-10-03870.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livesey PJ, Muter V. Functional differentiation within the neostriatum of the rat using electrical (blocking) stimulation during discrimination learning. J Comp Physiol Psychol. 1976;90:203–211. doi: 10.1037/h0077200. [DOI] [PubMed] [Google Scholar]
- McDonald RJ, Hong NS. A dissociation of dorso-lateral striatum and amygdala function on the same stimulus-response habit task. Neuroscience. 2004;3:507–513. doi: 10.1016/j.neuroscience.2003.11.041. [DOI] [PubMed] [Google Scholar]
- Mizumori SJ, Cooper BG, Leutgeb S, Pratt WE. A neural systems analysis of adaptive navigation. Mol Neurobiol. 2000;21:57–82. doi: 10.1385/MN:21:1-2:057. [DOI] [PubMed] [Google Scholar]
- Morari M, Sbrenna S, Marti M, Caliari F, Bianchi C, Beani L. NMDA and non-NMDA ionotropic glutamate receptors modulate striatal acetylcholine release via pre- and postsynaptic mechanisms. J Neurochem. 1998;71:2006–2017. doi: 10.1046/j.1471-4159.1998.71052006.x. [DOI] [PubMed] [Google Scholar]
- Packard MG, White NM. Dissociation of hippocampus and caudate nucleus memory systems by posttraining intracerebral injection of dopamine agonists. Behav Neurosci. 1991;105:295–306. doi: 10.1037//0735-7044.105.2.295. [DOI] [PubMed] [Google Scholar]
- Packard MG. Glutamate infused posttraining into the hippocampus or caudate-putamen differentially strengthens place and response learning. Proc Natl Acad Sci U S A. 1999;96:12881–12886. doi: 10.1073/pnas.96.22.12881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG, McGaugh JL. Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiol Learn Mem. 1996;65:65–72. doi: 10.1006/nlme.1996.0007. [DOI] [PubMed] [Google Scholar]
- Palencia CA, Ragozzino ME. The influence of NMDA receptors in the dorsomedial striatum on response reversal learning. Neurobiol Learn Mem. 2004;82:81–89. doi: 10.1016/j.nlm.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Palencia CA, Ragozzino ME. The contribution of NMDA receptors in the dorsolateral striatum to egocentric response learning. Behav Neurosci. 2005;119:953–960. doi: 10.1037/0735-7044.119.4.953. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 3rd ed. Sydney, New South Wales, Australia: Academic Press; 1996. [Google Scholar]
- Pisa M, Cyr J. Regionally selective roles of the rat’s striatum in modality-specific discrimination learning and forelimb reaching. Behav Brain Res. 1990;37:281–292. doi: 10.1016/0166-4328(90)90140-a. [DOI] [PubMed] [Google Scholar]
- Pych JC, Chang Q, Colon-Rivera C, Haag R, Gold PE. Acetylcholine release in the hippocampus and striatum during place and response training. Learn Mem. 2005;6:564–572. doi: 10.1101/lm.33105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Choi D. Dynamic changes in acetylcholine output in the medial striatum during place reversal learning. Learn Mem. 2004;11:70–77. doi: 10.1101/lm.65404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Jih J, Tzavos A. Involvement of the dorsomedial striatum in behavioral flexibility: Role of muscarinic cholinergic receptors. Brain Res. 2002a;953:205–214. doi: 10.1016/s0006-8993(02)03287-0. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Ragozzino KE, Mizumori SJY, Kesner RP. Role of the dorsomedial striatum in behavioral flexibility for response and visual cue discrimination learning. Behav Neurosci. 2002b;116:105–115. doi: 10.1037//0735-7044.116.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reading PJ, Dunnett SB, Robbins TW. Dissociable roles of the ventral, medial and lateral striatum on the acquisition of a complex visual stimulus-response habit. Behav Brain Res. 1991;45:147–161. doi: 10.1016/s0166-4328(05)80080-4. [DOI] [PubMed] [Google Scholar]
- Tzavos A, Jih J, Ragozzino ME. Differential effects of M1 muscarinic receptor blockade and nicotinic receptor blockade in the dorsomedial striatum on response reversal learning. Behav Brain Res. 2004;154:245–253. doi: 10.1016/j.bbr.2004.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Golf Racht Delatour B, El Massioui N. Rule-based learning impairment in rats with lesions to the dorsal striatum. Neurobiol Learn Mem. 1999;72:47–61. doi: 10.1006/nlme.1998.3905. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Chang HT, Kitai ST. Firing patterns and synaptic potentials of identified giant aspiny interneurons in the rat neostriatum. J Neurosci. 1990;10:508–519. doi: 10.1523/JNEUROSCI.10-02-00508.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf NJ, Butcher LL. Cholinergic neurons in the caudate-putamen complex proper are intrinsically organized: a combined Evans blue and acetylcholinesterase analysis. Brain Res Bull. 1981;7:487–507. doi: 10.1016/0361-9230(81)90004-6. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, Balleine BW. Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. Eur J Neurosci. 2004;19:181–189. doi: 10.1111/j.1460-9568.2004.03095.x. [DOI] [PubMed] [Google Scholar]
- Yin HH, Ostlund SB, Knowlton BJ, Balleine BW. The role of the dorsomedial striatum in instrumental conditioning. Eur J Neurosci. 2005;22:513–523. doi: 10.1111/j.1460-9568.2005.04218.x. [DOI] [PubMed] [Google Scholar]