Abstract
Previous investigations examining the rat prefrontal cortex subregions in attentional-set shifting have commonly employed two-choice discriminations. To better understand how varying levels of difficulty influence the contribution of the prefrontal cortex to learning, the present studies examined the effects of orbitofrontal cortex inactivation in a two- or four-choice odor reversal learning test. Long–Evans rats were trained to dig in cups that contained distinct odors. In the two-choice odor discrimination, one odor cup was always associated with a cereal reinforcement in acquisition while the opposite odor cup was associated with a cereal reinforcement in reversal learning. In the four-choice odor discrimination, an additional two cups containing distinct odors were used that were never associated with reinforcement in acquisition or reversal learning. Bilateral infusions of the GABA-A agonist, muscimol (0.5 μg) into the orbitofrontal cortex did not impair acquisition of either the two- or four-choice discrimination task. However, muscimol infusions into the orbitofrontal cortex impaired two- and four-choice reversal learning. In the two-choice odor reversal, muscimol treatment selectively increased perseverative errors. In the four-choice odor reversal, muscimol treatment increased perseverative, regressive, as well as irrelevant errors. These findings suggest that the orbital prefrontal cortex not only enables task switching by supporting the initial inhibition of a previously relevant choice pattern, but under increasing task demands also enables the reliable execution of a new choice pattern and reduction of interference to irrelevant stimuli.
Keywords: Prefrontal cortex, Learning, Memory, Muscimol, Attention and perseveration
1. Introduction
There is accumulating evidence that separate prefrontal cortex regions differentially contribute to learning when a switch in strategies is required (Birrell & Brown, 2000; Chudasasma, Bussey, & Muir, 2001; Chudasasma & Robbins, 2003; McAlonan & Brown, 2003; Ragozzino, Detrick, & Kesner, 1999a; Ragozzino, Kim, Hassert, Minniti, & Kiang, 2003). In particular, lesions, temporary inactivation, or targeted pharmacological manipulations centered in the rat prelimbic area do not impair the initial acquisition or reversal learning of different discrimination tasks, i.e., a two-choice spatial discrimination (Birrell & Brown, 2000; Chudasasma et al., 2001; Ragozzino et al., 1999a, 2003), but do impair learning in extra-dimensional shifts when a rat must shift from using one type of attribute information to using a different type of attribute information (Birrell & Brown, 2000; deBruin, Sanchez-Santed, Heinsbroek, Donker, & Postmes, 1994; Ragozzino et al., 1999a, 2003; Ragozzino, Wilcox, Raso, & Kesner, 1999b; Ragozzino, 2002; Stefani, Groth, & Moghaddam, 2003). The pattern of results suggests that the rat prelimbic area, located in the medial prefrontal cortex, supports learning when conditions demand complete inhibition of responding to stimuli in a particular dimension while learning what stimulus in a different dimension is critical for correct responding. However, the prelimbic area does not support learning when conditions demand a shift in specific exemplars within a dimension as required in reversal learning.
In contrast to the prelimbic area, the orbitofrontal cortex plays a critical role in reversal learning. Several experiments indicate that lesions of the lateral prefrontal cortex, centered in the orbitofrontal cortex, impair odor, tactile or visual cue reversal learning (Bohn, Giertler, & Hauber, 2003; Chudasasma & Robbins, 2003; Ferry, Lu, & Price, 2000; McAlonan & Brown, 2003; Schoenbaum, Nugent, Saddoris, & Setlow, 2002). A recent investigation further demonstrated that while orbitofrontal cortex lesions impair different types of reversal learning, the lesions do not impair extra-dimensional shifts (McAlonan & Brown, 2003). Thus, the orbitofrontal cortex and prelimbic area may play distinct roles in facilitating learning when task contingencies change.
Another important issue to investigate is how changes in task difficulty influence orbitofrontal cortex involvement in task switching. This is because previous results indicate that the prefrontal cortex, including the orbito-frontal cortex, is sensitive to changes in attentional demands or task difficulty (Bussey, Muir, Everitt, & Robbins, 1997; Gill, Sarter, & Givens, 2000; Granon & Poucet, 1995; Otto & Eichenbaum, 1992; Williams, Mohler, & Givens, 1999). Therefore, the orbitofrontal cortex may be important in reducing interference to irrelevant stimuli in a task, even stimuli that were not previously relevant in a different phase of learning. However, to date, there have been no investigations of how varying the level of difficulty in a reversal learning task may affect the contribution of the orbital prefrontal cortex to shifting of response patterns.
To investigate this issue, the present experiments examined the effects of orbitofrontal cortex inactivation on the acquisition and reversal learning of a two-choice, as well as a four-choice odor discrimination.
2. Materials and methods
2.1. Subjects
Male Long–Evans rats (Charles River Laboratories, Indianapolis, Indiana) weighing between 350 and 375 g at the beginning of the experiment served as subjects. Rats were housed individually in plastic cages (26.5 cm wide × 50 cm long × 20 cm high) located in a temperature controlled room (24 °C) that was maintained at 20–40% humidity. The rats were kept on a 12 h light–dark cycle (lights on 07:00 h). All rats were food restricted to maintain their weight at approximately 85% of their ad libitum weight but had free access to water throughout the experiment.
2.2. Apparatus
A rectangular-shaped maze made of 0.6 cm black plastic was used in both experiments. The maze was placed on a table that was elevated 75 cm above the floor. The two side walls were each 76 cm × 32 cm. The front and back walls were each 50 cm × 32 cm.
2.3. Surgery
Rats received atropine sulfate (0.2 ml of a 250 μg/ml solution, i.p.) 10 min before administering the general anesthetic (sodium pentobarbital, 50 mg/kg, i.p.). A midsagittal incision was made, and the scalp was retracted. Each rat received a bilateral implant of an 8 mm stainless steel guide cannula (22 gauge; Plastics One, Roanoke, VA) aimed toward the lateral orbitofrontal area. The stereotaxic coordinates were 4.0 anterior to bregma, ± 3.2 lateral to the midline, and 4.4 mm ventral to dura. The incisor bar was lowered to 3.3 ± 0.2 below horizontal zero so that the heights of bregma and lambda were equal. The cannulas were inserted at a 10° angle. The coordinates were based on the atlas of Paxinos and Watson (1996). Six jeweler's screws were placed in the skull surrounding the cannulas. The cannulas were secured in place with dental acrylic (Plastics One). Stylets were secured in the guide cannulas after the dental acrylic dried. After surgery, rats received ground rat chow mixed in water for 1 day.
2.4. Microinfusion
Bilateral injections into the orbitofrontal cortex were made through an inner cannula (28 gauge) that extended 1.0 mm below the guide cannula. The inner cannula was attached by a polyethylene tube (PE-20) to a 10 μl Hamilton syringe. The syringe was driven by a microinfusion pump with solutions infused in a volume of 0.5 μl per side for 2 min. The inner cannula was left in place for 1 min after completion of the infusion to allow for diffusion. Rats received either the GABA-A agonist, muscimol 0.5 μg per side (Sigma–Aldrich, St. Louis, MO) or saline. The same concentration of muscimol has been infused in other brain regions to examine its effect on learning and memory (Corcoran & Maren, 2001; Souza et al., 2002).
2.5. Pretraining procedure
Rats were allowed 7–10 days to recover from surgery before the pretraining procedure commenced. Two days after surgery rats were food restricted to 85% of their original ad libitum weight. During food restriction rats were also handled for 10 min/day. On the first day of pre-training in the two-choice odor discrimination experiment, two round stainless steel bowls (9 cm diameter and 3.2 cm deep) were filled with 100 g sterile sand and placed near one end of the maze. The bowls were kept 5 cm apart. Two 1/2 pieces of Froot Loops cereal (Kelloggs, Battle Creek, MI) were placed on top of the sand. A rat was placed at the opposite end of the maze and allowed to navigate to the bowls and consume the cereal pieces. After a rat consumed all the cereal pieces a rat was placed back in a holding cage. The sand bowls were baited again and a rat was returned to the maze. On subsequent days, only one 1/2 piece of cereal was placed in each bowl. The pieces were increasingly buried under the sand until the cereal was completely buried and a rat had to dig in the sand to retrieve the cereal piece. When a rat retrieved cereal pieces that were completely buried for two consecutive days it received one final day of pre-training. In the final pretraining session, a piece of cereal was placed in only one of the bowls. The cereal piece was pseudorandomly placed such that it was buried an equal amount of times in each bowl for the session. A rat was allowed to dig in each bowl until it found the buried cereal piece. This procedure was carried out because a pilot study indicated this eliminated a rat from ceasing to run and dig for the cereal after making several consecutive incorrect choices. Each pretraining session lasted 15 min. Following the final pretraining session, a rat's stylets were removed from the guide cannulas and an injection cannula was inserted for 1 min. There was no solution injected at this time. This procedure was performed to prevent clogging of the microinfusion on test days. Behavioral testing was started the next day.
The pretraining procedure in the four-choice odor discrimination was similar as the two-choice procedure except that four sand bowls were used instead of two. Each bowl was placed near the back wall of the maze and spaced 5 cm apart. As in the two-choice procedure, when a rat retrieved cereal pieces that were completely buried for two consecutive days a rat received one final day of pretraining. In the final pretraining session, the number of bowls containing a cereal piece was varied from one to four. The trials consisting of one, two, three or four cereal pieces were randomly presented across the session. The cereal pieces were pseudorandomly placed such that cereal was buried an equal amount of times in each bowl for the session. A rat was allowed to dig in each bowl until it found all of the buried cereal piece(s).
2.6. Odor discrimination test procedure
In the test procedure each bowl was filled with 100 g of sand with 1.25 g of either cumin, cinnamon, nutmeg or curry mixed in. In the two-choice odor discrimination the following four spice pairings were used: cinnamon–cumin, cinnamon–curry, nutmeg–cumin, and nutmeg–curry. The spice pairings used for a test were pseudorandomly chosen such that each pair was used a similar amount among the rats. Each test consisted of an acquisition phase and a reversal learning phase. In the acquisition phase, one spice was designated the positive odor and the other spice was designated the negative odor. One half piece of cereal was always buried in the bowl containing the positive odor. For each spice pair, a particular spice was designated a positive odor in acquisition or reversal learning a similar frequency among the rats. The bowls were randomly switched between spatial locations across trials. A rat was allowed to dig in only one bowl per trial. If a rat dug in the positive odor bowl, then a rat was allowed to consume the cereal piece. If a rat dug in the negative odor bowl, then a rat was removed from the maze. Between trials a rat was placed in a holding cage, which sat on table next to the maze. Subsequently, the maze floor was wiped with a sponge. The sponge was moistened with a 0.1% ammonium chloride solution. The intertrial interval was 10–15 s. A rat reached criterion when it made 10 consecutive correct choices. This is the same criterion as used in previous studies (Ragozzino, 2002; Ragozzino, Jih, & Tzavos, 2002a; Ragozzino, Ragozzino, Mizumori, & Kesner, 2002b; Ragozzino et al., 2003).
On the following day, the reversal learning phase was started. The procedure was identical to the acquisition phase except that the odor-reinforcement contingency was reversed. Additional measures were analyzed on the reversal learning phase to determine whether treatments altered perseveration or reversions back to the previously correct choice after perseveration had ceased. Perseveration involved continuing to choose the odor that was designated positive in the acquisition phase. Perseveration was defined as digging in the incorrect bowl for three or more trials in consecutive blocks of 4 trials each. This is a similar criterion as used in past studies (Ragozzino et al., 1999a, 2002b, 2003). Once a rat made less than three errors in a block the first time all subsequent errors were no longer counted as perseverative errors. From that point on, the number of errors was counted as regressive errors. This allowed a measure of the ability to maintain a new choice after initially shifting away from the previously correct choice.
A similar test procedure was used in the four-choice odor discrimination as in the two-choice odor discrimination. In this experiment, the four spices cumin, cinnamon, nutmeg, and curry were used. A rat had to discriminate among four odor bowls. In the acquisition phase, one spice was designated the positive odor and the other spices were designated the negative odors. One half piece of cereal was always buried in the bowl containing the positive odor. The bowls were randomly switched among spatial locations across trials. A rat was allowed to dig in only one bowl per trial. A rat reached criterion when it made 10 consecutive correct choices.
In reversal learning, one of the three negative odors now became the positive odor. The positive odor in acquisition now became a negative odor in reversal learning along with the other two odors. Thus, in this procedure two of the odors were never associated with reinforcement in the acquisition or reversal learning phase. The spices used for the positive odor in acquisition, the positive odor in reversal learning, as well as the negative odors in both phases were pseudorandomly chosen such that each spice was used in each condition a similar amount among the rats. Criterion was obtained when a rat made 10 consecutive correct choices. As in Experiment 1, measures of perseverative and regressive errors were obtained. In this experiment a rat could also make an error by choosing one of the odor bowls that was not reinforced in acquisition or reversal learning. Because a rat could not know that these choices were initially incorrect in reversal learning, the number of times a rat made one of these choices after the initial choice was counted. The total of the two choices was calculated and counted as irrelevant errors.
Five minutes before each test session rats received a microinfusion. In the two-choice odor discrimination, each rat was randomly assigned to one of the three treatment groups. Group assignment was determined by which treatment was administered during each version: (1) acquisition—saline and reversal learning—saline (n = 6); (2) acquisition—saline and reversal learning—muscimol 0.5 μg (n = 6); and (3) acquisition—muscimol 0.5 μg and reversal learning—saline (n = 6). The same experimental design was used in the four-choice odor discrimination as in the two-choice odor discrimination. The groups were as follows: acquisition—saline and reversal learning—saline (n = 8); (2) acquisition—saline and reversal learning—muscimol 0.5 μg (n = 8); and (3) acquisition—muscimol 0.5 μg and reversal learning—saline (n = 7).
2.7. Histology
After completion of behavioral testing in each experiment, rats received a lethal dose of sodium pentobarbital followed by a 0.5 μl injection of 2.5% Chicago blue stain through each guide cannula. As in previous experiments (Ragozzino et al., 1999a, 1999b; Ragozzino, 2002) the stain was used to highlight the location of the cannula tip. Rats were perfused intracardially with 0.9% saline followed by a 4% formaldehyde solution. Brains were removed and stored in a 4% formaldehyde solution. The brains were frozen and cut in coronal sections (40 μm) on a cryostat. The sections were mounted on slides, dried, and examined to determine the location of the cannula tips.
2.8. Statistical analysis
In each experiment, a separate analysis of variance (ANOVA) was used in the acquisition and reversal learning phase to determine a significant difference among the groups in achieving trials to criterion. ANOVA tests were also used to assess differences in perseveration, regressive, and irrelevant errors among the groups.
3. Results
3.1. Experiment 1: The effect of orbitofrontal cortex inactivation on acquisition and reversal learning of a two-choice odor discrimination
3.1.1. Histology
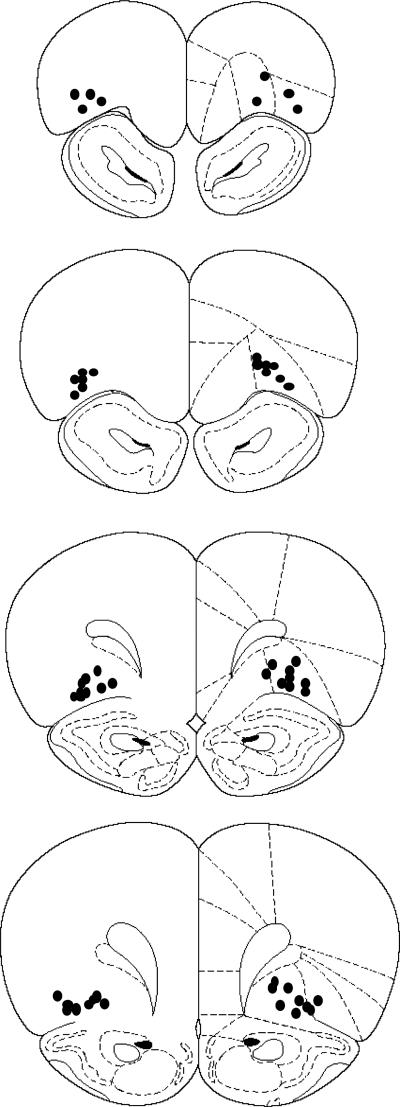
The location of the cannula tips in the orbitofrontal cortex for Experiments 1 and 2 is shown in Fig. 1. The analysis indicated that the injection tips were located mainly in the lateral orbitofrontal cortex. However, cannula tips were also found in the dorsolateral orbitofrontal cortex for the most anterior placements located 4.7 mm anterior to bregma and in the ventral orbitofrontal cortex for the most posterior placements found 3.2 mm anterior to bregma.
Fig. 1.
Placement of the injection cannula tips in the orbitofrontal cortex for rats included in the behavioral analyses in Experiments 1 and 2. Location of the cannula tips in the orbitofrontal cortex ranged from 3.2 to 4.7 anterior to bregma. The number of circles does not match the total number of rats included in the behavioral analyses because some cannula tip placements were overlapping to such a large extent that a single circle represents more than one cannula tip placement. Modified from the The rat brain in stereotaxic coordinates, 3rd ed., Paxinos and Watson (1996).
The data from one rat were excluded from the behavioral analyses because of a cannula misplacement. This rat had a bilateral cannula placement in the overlying forceps minor of the corpus callosum.
3.1.2. Acquisition and reversal learning
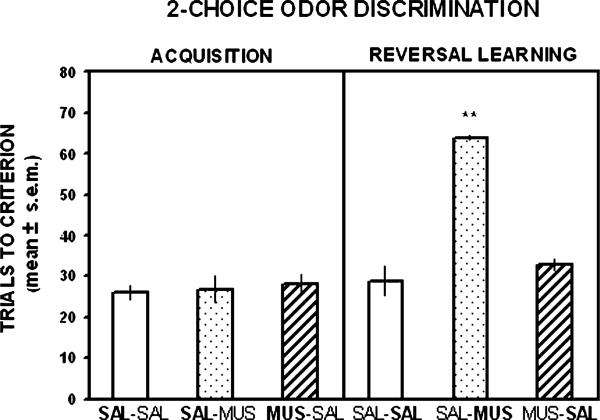
The findings on the trials to criterion for odor discrimination acquisition are shown in Fig. 2. The three groups took approximately 25–30 trials to reach criterion. The analysis indicated that there was not a significant difference among the groups for initial learning of the odor discrimination, (F2,15 = 0.12, p > .05). Fig. 2 illustrates the results on the trials to criterion for reversal learning of the odor discrimination. In reversal learning, the difference in trials to criterion among the groups was significant (F2,15 = 48.52, p < .01). Newman–Keuls tests indicated that the group receiving muscimol treatment in reversal learning required significantly more trials to reach criterion compared to that of the groups that received saline treatment in reversal learning (p's < .01).
Fig. 2.
Mean (±SEM) trials to criterion on acquisition and reversal learning of a two-choice odor discrimination. Each rat received a bilateral infusion of saline (SAL) or muscimol 0.5 μg (MUS) into the orbitofrontal cortex 5 min prior to each test session. Muscimol treatment did not impair acquisition, but significantly increased the trials to criterion in reversal learning compared with saline treatment. The treatment received on this test is shown in bold for each group. *p < .01 versus saline-injected controls.
Data excluded from the behavioral analyses of the one rat because of a cannula misplacement was in the saline-muscimol treatment group. Despite receiving muscimol during reversal learning this rat only needed 23 trials to achieve learning criterion.
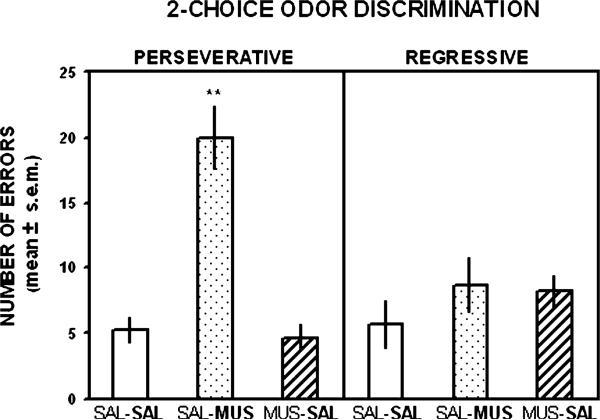
Fig. 3 illustrates the findings on the number of perseverative and regressive errors made in the reversal of the two-choice odor discrimination. An analysis of the errors during reversal learning of the odor discrimination revealed there was a significant difference among the groups in the number of perseverative errors (F2,15 = 26.13, p < .01). Newman–Keuls tests indicated that the group receiving muscimol treatment in reversal learning committed significantly more perseverative errors than either group that received saline treatment in reversal learning (p's < .01). In contrast, the difference in regressive errors among the groups was not significant (F2,15 = 0.22, p > .05).
Fig. 3.
Mean (±SEM) number of perseverative and regressive errors in the two-choice odor reversal learning test. Muscimol treatment significantly increased the number of perseverative errors, but not regressive errors compared to that following saline treatment. The treatment received on this test is shown in bold for each group. *p < .01 versus saline-injected controls.
3.2. Experiment 2: The effect of orbitofrontal cortex inactivation on acquisition and reversal learning of a four-choice odor discrimination
3.2.1. Histology
The location of the cannula tips in the orbitofrontal cortex is shown in Fig. 1. The location of the injection tips was similar to that as described in Experiment 1. There were five rats excluded from the behavioral analyses because of cannula misplacement. One of the cannula misplacements was due to a unilateral placement in the overlying forceps minor of the corpus callosum. Two rats had a misplacement due to a bilateral cannula placement in the forceps minor of the corpus callosum. Two other rats had misplacements due to a unilateral cannula placement in the dorsal part of the anterior olfactory nucleus.
3.2.2. Acquisition and reversal learning
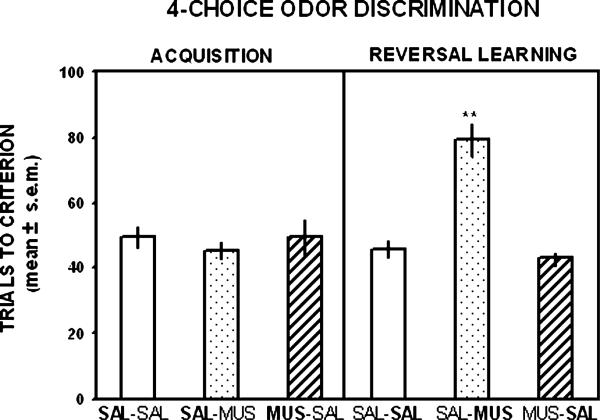
The findings on the trials to criterion for acquisition and reversal learning of the four-choice odor discrimination are shown in Fig. 4. The three groups took approximately 45–50 trials to reach criterion. The analysis indicated that there was not a significant difference among the groups for initial learning of the odor discrimination, (F2,20 = 0.32, p > .05). In reversal learning, the difference in trials to criterion among the groups was significant (F2,20 = 26.26, p < .01). Newman–Keuls tests indicated that the group receiving muscimol treatment in reversal learning required significantly more trials to reach criterion compared to that of the groups that received saline treatment in reversal learning (p's < .01).
Fig. 4.
Mean (±SEM) trials to criterion in acquisition and reversal learning of a four-choice odor discrimination. Each rat received a bilateral infusion of saline (SAL) or muscimol 0.5 μg (MUS) into the orbitofrontal cortex 5 min prior to each test session. Muscimol treatment did not impair acquisition, but significantly increased the trials to criterion in reversal learning compared with saline treatment. The treatment received on this test is shown in bold for each group. *p < .01 versus saline-injected controls.
Two of the five rats in which their behavioral data were excluded from the statistical analyses because of a cannula misplacement were in the saline–muscimol treatment group. The cannula placement for both rats was located in the forceps minor of the corpus callosum dorsal to the orbitofrontal cortex. In the reversal learning phase, one rat required 47 trials and the other rat required 43 trials to achieve learning criterion. One other rat excluded from the analyses was in the saline–saline group and had a unilateral placement in the forceps minor of the corpus callosum. This rat required 49 trials to reach criterion in reversal learning. The other two rats excluded from the behavioral analyses were in muscimol–saline group and both had unilateral placements in the anterior olfactory nucleus. One rat required 65 trials to reach criterion and the other rat required 42 trials to reach criterion in reversal learning.
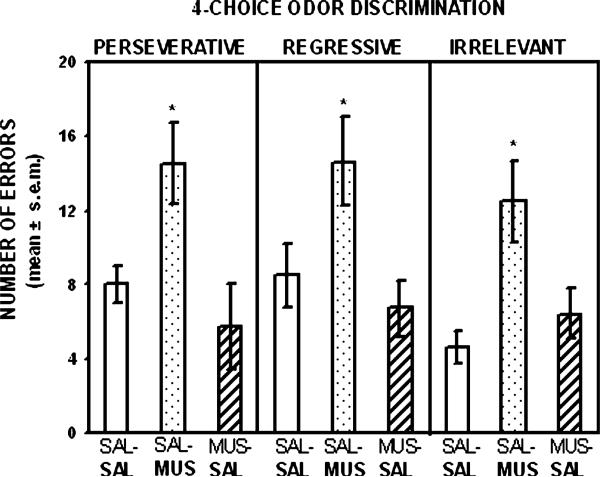
Fig. 5 illustrates the findings on the number of perseverative, regressive, and irrelevant errors made in reversal of the four-choice odor discrimination. An analysis of the errors during reversal learning of the odor discrimination revealed there was a significant difference among the groups in the number of perseverative errors (F2,20 = 5.02, p < .01). Newman–Keuls tests indicated that the group receiving muscimol treatment in reversal learning committed significantly more perseverative errors than either group that received saline treatment in reversal learning (p's < .05). The difference in regressive errors among the groups was also significant (F2,20 = 4.13, p < .05). Newman–Keuls tests indicated that the group receiving muscimol treatment in reversal learning committed significantly more regressive errors than either group that received saline treatment in reversal learning (p's < .05). There was also a significant difference among the groups in the number of irrelevant errors committed (F2,20= 6.41, p < .01). Newman–Keuls tests indicated that the group receiving muscimol treatment in reversal learning committed significantly more irrelevant errors than the two groups that received saline treatment in reversal learning (p's < .05).
Fig. 5.
Mean (±SEM) number of perseverative, regressive and irrelevant errors in the four-choice odor reversal learning test. Muscimol treatment significantly increased the number of perseverative, regressive and irrelevant errors compared to that following saline treatment. The treatment received on this test is shown in bold for each group. *p < .05 versus saline-injected controls.
4. Discussion
These experiments demonstrate that orbitofrontal cortex inactivation impairs reversal learning in a two-and four-choice odor discrimination test. The observed reversal learning deficits are consistent with previous studies indicating that lesions of the orbitofrontal cortex impair reversal learning in a variety of discrimination tests (Berlin, Rolls, & Kischka, 2004; Bohn et al., 2003; Chudasasma & Robbins, 2003; Dias, Robbins, & Roberts, 1997; Ferry et al., 2000; Jones & Mishkin, 1972; McAlonan & Brown, 2003; Schoenbaum et al., 2002). The present pattern of results is also comparable to findings in which orbitofrontal cortex lesions fail to decrease responding to a conditioned stimulus following devaluation of the reinforcer (Gallagher, McMahan, & Schoenbaum, 1999; Pickens et al., 2003). In devaluation paradigms a subject first learns to respond to a cue that is associated with presentation of a food reinforcer, e.g., orienting to a light stimulus when presented. After the reinforcer is devalued by pairing the food with illness, this new association leads a normal rat to decrease responding to the previously associated cue, but orbitofrontal cortex lesioned rats do not adjust their responding to the cue (Gallagher et al., 1999; Pickens et al., 2003). Thus, the orbitofrontal cortex may support learning in a variety of conditions in which there is a change in reinforcement contingencies.
One rat in the two-choice discrimination and two rats in the four-choice discrimination received muscimol treatment during reversal learning, but had a cannula tip placement in the forceps minor of the corpus callosum. All three rats performed similar to that as controls in the respective reversal learning tests. Although only based on three rats, the lack of deficit observed in rats with dorsal cannula placement suggests that the muscimol-induced deficit observed in the reversal learning tests is not due to diffusion dorsal to the orbitofrontal cortex. Furthermore, the reversal learning deficits following orbitofrontal inactivation are not due to a general learning impairment as a muscimol injection into the orbitofrontal cortex does not impair acquisition of either a two- or four-choice odor discrimination. This is similar to findings in which orbitofrontal cortex lesions do not impair initial learning in discrimination tasks (Chudasasma & Robbins, 2003; Ferry et al., 2000; McAlonan & Brown, 2003; Schoenbaum et al., 2002). The selective reversal learning deficit following orbitofrontal cortex inactivation suggests that the orbitofrontal cortex enables behavioral flexibility when a rat has to inhibit a previously learned choice pattern while learning a new relevant choice pattern.
A novel finding in this study was revealed when examining the error pattern following muscimol injections into the orbitofrontal cortex in a two-choice versus four-choice odor reversal learning test. Orbitofrontal cortex inactivation selectively increased perseverative errors in the two-choice odor reversal. These results are consistent with previous studies demonstrating that orbitofrontal cortex lesions increase perseveration in two-choice reversal learning tests (Chudasasma & Robbins, 2003; Dias et al., 1997; Jones & Mishkin, 1972). This pattern suggests that the orbitofrontal cortex is critical for the initial inhibition of a previously learned response pattern and/or the generation of a new response pattern. However, because orbitofrontal cortex inactivation did not increase regressive errors the Wnding suggests that this area is not involved in maintaining or learning a new choice pattern following the initial shift away from the previously relevant choice pattern.
In the four-choice odor reversal, orbitofrontal cortex inactivation significantly increased perseverative, regressive, and irrelevant errors. Because previous studies have only found orbitofrontal cortex lesions increase perseverative errors in two-choice reversal learning tests, the error pattern in the four-choice reversal learning test following orbitofrontal cortex inactivation represents a unique set of results. The findings in the four-choice reversal taken together with those in the two-choice reversal suggest that as task demands increase in conditions that require the flexible use of information orbitofrontal cortex supports multiple processes to enable the learning of a different response pattern. Specifically, the findings in the four-choice reversal learning test suggest that under these conditions the orbitofrontal cortex is not only critical for initial inhibition of a previously relevant choice pattern and/or generation of a new choice pattern, but is also critical for reliably executing a new response pattern following the initial shift, as well as reducing interference to irrelevant stimuli. The latter finding is particularly interesting because a significant increase in irrelevant errors suggests that orbitofrontal inactivation does not impair reversal learning solely due to an inability to inhibit the previously reinforced choice whether it be in the initial stage of reversal learning as measured by perseveration or at a subsequent stage as measured by regressive errors. Because irrelevant choices are never-reinforced in acquisition or reversal learning these choices serve as “distracter” choices and increase the interference in discriminating which odor stimulus is the correct choice. Thus, the pattern of errors in the four-choice reversal learning task suggests that the orbitofrontal cortex is critical for inhibiting a previously relevant response pattern, as well as reducing interference from irrelevant or distracting stimuli.
The present findings suggest that the orbitofrontal cortex can facilitate a switch in response patterns by influencing multiple processes. In humans, a switch from a two-choice to a four-choice task leads to even greater activation of the orbitofrontal cortex than in a two-choice task (Elliot, Dolan, & Frith, 2000). One possibility is that greater task demands, such as in a four-choice reversal versus a two-choice reversal, lead to a different dynamic state of orbitofrontal activation which underlies multiple behavioral processes to facilitate reversal learning. In general, understanding the full complement of processes that are mediated by the orbitofrontal cortex to a shift in response patterns can only be revealed by extending the use of tests beyond a standard two-choice reversal learning paradigm.
In contrast to orbitofrontal cortex inactivation, inactivation of the prelimbic–infralimbic areas of the medial prefrontal cortex does not impair two- or four-choice odor reversal learning (Ragozzino et al., 2003). However, prelimbic inactivation does impair learning when rats must fiexibly shift between using odor and place information. As described earlier, orbitofrontal cortex lesions do not impair an extra-dimensional shift involving different type of stimulus information (McAlonan & Brown, 2003). Similar to the effects of orbitofrontal cortex inactivation in a two-choice odor reversal learning test, prelimbic inactivation impairs a two-choice extra-dimensional shift by increasing perseveration (Ragozzino et al., 1999a, 2003). Taken together, the findings might suggest that the prefrontal cortex, as a whole, is critical for the initial inhibition of a previously learned strategy and/or generation of new strategies. Although specific prefrontal cortex subregions may enable the ability to shift to new choice patterns dependent on the specific task demands.
One interpretation of a reversal learning deficit following orbitofrontal cortex damage is that this area enables learning when stimulus-reward contingencies change requiring a shift in goal-directed behavior (Bohn et al., 2003; Pickens et al., 2003; Rolls, Hornak, Wade, & McGrath, 1994; Schoenbaum et al., 2002). In a related view, Wise, Murray, and Gerfen (1996) propose that the primate orbitofrontal cortex supports lower order rules for the shifting of specific choices or exemplars. More specifically, a lower order process allows the approach to and avoidance of a particular stimulus. Thus, when a positive or negative valence can be attached to stimuli the orbitofrontal cortex supports learning with a change in task contingencies.
Any change in reward contingencies that requires a shift in goal-directed behavior does not necessarily recruit the orbitofrontal cortex. For example in an extra-dimensional shift, a subject must first perform a compound discrimination learning what stimulus within a specific dimension leads to a reinforcement while learning what dimension is not relevant. Subsequently, a subject must shift to inhibiting a choice based on the previously relevant dimension and now choose based on the previously irrelevant dimension to receive a reinforcement. As described above, orbitofrontal cortex lesions do not impair learning in extra-dimensional shifts (Dias et al., 1997; McAlonan & Brown, 2003). However, in these cases, learning must go beyond simply attaching a positive or negative valence to stimuli within a particular mode or dimension and instead require attention to components of a stimulus. Wise and colleagues (1996) suggest that extra-dimensional shifts are representative of a higher order rule that requires taking a fundamentally new approach to solving a task. This entails using a new strategy or type of information and not simply changing the valence of stimuli within a particular dimension. Thus, the orbitofrontal cortex may be critical for adapting a behavioral response under conditions in which a reward value can be applied to a stimulus, but when a condition also requires the fiexible use of stimulus components or modes other prefrontal cortex areas besides the orbitofrontal cortex may be critical (Elliot et al., 2000; Wise et al., 1996).
Although the orbitofrontal cortex may be involved in certain reversal learning conditions this frontal area may not facilitate reversal learning in all conditions. For example, orbitofrontal cortex damage in non-human primates and rats does not impair spatial reversal learning (Corwin, Fussinger, Meyer, King, & Reep, 1994; Meunier, Bachevalier, & Mishkin, 1997; Nonneman, Voigt, & Kolb, 1974). To date, the evidence indicates that the orbitofrontal cortex supports reversal learning for odor, tactile or visual cue information (Bohn et al., 2003; Chudasasma & Robbins, 2003; Ferry et al., 2000; McAlonan & Brown, 2003; Rolls et al., 1994; Meunier et al., 1997; Schoenbaum et al., 2002). It has been proposed that the medial prefrontal cortex in primates supports reversal learning for spatial information (Wise et al., 1996). Therefore, separate prefrontal cortex subregions may differentially enable the shifting of response patterns in reversal learning based on the type of stimulus or attribute information to be used. This is consistent with the attribute model of prefrontal cortex functioning which asserts that different prefrontal cortex areas play distinct roles in learning based on the attribute information to be used (Kesner, 2000).
A different set of findings suggest that the orbitofrontal cortex supports learning involving a particular behavior-guiding process, e.g., a lower order process that allows a switch in specific choice patterns, but only for specific types of attribute information. One possibility is that more broadly the prefrontal cortex is functionally organized such that separate prefrontal cortex subregions differentially enable fiexible forms of learning that involve both a specific type of behavior-guiding process, as well as a specific type of attribute information.
Acknowledgment
This work was supported by NIH Grant NS043283.
References
- Berlin HA, Rolls ET, Kischka U. Impulsivity, time perception, emotion and reinforcement sensitivity in patients with orbitofrontal cortex lesions. Brain. 2004;127:1108–1126. doi: 10.1093/brain/awh135. [DOI] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set-shifting in the rat. Journal of Neuroscience. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn I, Giertler C, Hauber W. Orbital prefrontal cortex and guidance of instrumental behaviour in rats under reversal conditions. Behavioural Brain Research. 2003;143:49–56. doi: 10.1016/s0166-4328(03)00008-1. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Muir JL, Everitt BJ, Robbins TW. Triple dissociation of anterior cingulate, posterior cingulate, and medial prefrontal cortices on visual discrimination using a touchscreen testing procedure for the rat. Behavioral Neuroscience. 1997;111:920–936. doi: 10.1037//0735-7044.111.5.920. [DOI] [PubMed] [Google Scholar]
- Chudasasma Y, Bussey TJ, Muir JL. Effects of selective thalamic and prelimbic cortex lesions on two types of visual discrimination and reversal learning. European Journal of Neuroscience. 2001;14:1009–1020. doi: 10.1046/j.0953-816x.2001.01607.x. [DOI] [PubMed] [Google Scholar]
- Chudasasma Y, Robbins TW. Dissociable contributions of the orbitofrontal and infralimbic cortex to Pavlovian autoshaping and discrimination reversal learning: Further evidence for the functional heterogeneity of the rodent frontal cortex. Journal of Neuroscience. 2003;23:8771–8780. doi: 10.1523/JNEUROSCI.23-25-08771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Maren S. Hippocampal inactivation disrupts contextual retrieval of fear memory after extinction. Journal of Neuroscience. 2001;21:1720–1726. doi: 10.1523/JNEUROSCI.21-05-01720.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin JV, Fussinger M, Meyer RC, King VR, Reep RL. Bilateral destruction of the ventrolateral orbital cortex produces allocentric but not egocentric spatial deficits in rats. Behavioural Brain Research. 1994;61:79–86. doi: 10.1016/0166-4328(94)90010-8. [DOI] [PubMed] [Google Scholar]
- deBruin JPC, Sanchez-Santed F, Heinsbroek RPW, Donker A, Postmes P. A behavioural analysis of rats with damage to the medial prefrontal cortex using the Morris water maze: Evidence for behavioural flexibility, but not for impaired spatial navigation. Brain Research. 1994;652:323–333. doi: 10.1016/0006-8993(94)90243-7. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Dissociable forms of inhibitory control within prefrontal cortex with an analog of the Wisconsin Card Sort Test: Restriction to novel situations and independence from “on-line” processing. Journal of Neuroscience. 1997;17:9285–9297. doi: 10.1523/JNEUROSCI.17-23-09285.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliot R, Dolan RJ, Frith CD. Dissociable functions in the medial and lateral orbitofrontal cortex: Evidence from human neuroimaging studies. Cerebral Cortex. 2000;10:308–317. doi: 10.1093/cercor/10.3.308. [DOI] [PubMed] [Google Scholar]
- Ferry AT, Lu XCM, Price JL. Effects of excitotoxic lesions in the ventral striatopallidal–thalamocortical pathway on odor reversal learning: Inability to extinguish an incorrect response. Experimental Brain Research. 2000;131:320–335. doi: 10.1007/s002219900240. [DOI] [PubMed] [Google Scholar]
- Gallagher M, McMahan, Schoenbaum G. Orbitofrontal cortex and representation of incentive value in associative learning. Journal of Neuroscience. 1999;19:6610–6614. doi: 10.1523/JNEUROSCI.19-15-06610.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill TM, Sarter M, Givens B. Sustained visual attention performance-associated prefrontal neuronal activity: Evidence for cholinergic modulation. Journal of Neuroscience. 2000;20:4745–4757. doi: 10.1523/JNEUROSCI.20-12-04745.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granon S, Poucet B. Medial prefrontal lesions in the rat and spatial navigation: Evidence for impaired planning. Behavioral Neuroscience. 1995;109:474–484. doi: 10.1037//0735-7044.109.3.474. [DOI] [PubMed] [Google Scholar]
- Jones B, Mishkin M. Limbic lesions and the problem of stimulus–reinforcement associations. Experimental Neurology. 1972;36:362–377. doi: 10.1016/0014-4886(72)90030-1. [DOI] [PubMed] [Google Scholar]
- Kesner RP. Subregional analysis of mnemonic functions of the prefrontal cortex in the rat. Psychobiology. 2000;28:219–228. [Google Scholar]
- McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behavioural Brain Research. 2003;146:97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Meunier M, Bachevalier J, Mishkin M. Effects of orbital frontal and anterior cingulate lesions on object and spatial memory in rhesus monkeys. Neuropsychologia. 1997;35:999–1015. doi: 10.1016/s0028-3932(97)00027-4. [DOI] [PubMed] [Google Scholar]
- Nonneman AJ, Voigt J, Kolb BE. Comparisons of behavioral effects of hippocampal and prefrontal cortex lesions in the rat. Journal of Comparative and Physiological Psychology. 1974;87:249–260. doi: 10.1037/h0036864. [DOI] [PubMed] [Google Scholar]
- Otto T, Eichenbaum H. Complementary roles of the orbital prefrontal cortex an the perirhina-l-entorhinal cortices in an odor-guided delayed-nonmatching-to-sample task. Behavioral Neurosciences. 1992;106:762–775. doi: 10.1037//0735-7044.106.5.762. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 3rd ed. Academic Press; Sydney: 1996. [Google Scholar]
- Pickens CL, Saddoris MP, Setlow B, Gallagher M, Holland PC, Schoenbaum G. Different roles for orbitofrontal cortex and basolateral amygdala in a reinforcer devaluation task. Journal of Neuroscience. 2003;23:11078–11084. doi: 10.1523/JNEUROSCI.23-35-11078.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Detrick S, Kesner RP. Involvement of the prelimbic–infralimbic areas of the rodent prefrontal cortex in behavioral flexibility for place and response learning. Journal of Neuroscience. 1999a;19:4585–4594. doi: 10.1523/JNEUROSCI.19-11-04585.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Wilcox C, Raso M, Kesner RP. Involvement of rodent prefrontal cortex subregions in strategy switching. Behavioral Neuroscience. 1999b;113:32–41. doi: 10.1037//0735-7044.113.1.32. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME. The effects of dopamine D1 receptor blockade in the prelimbic–infralimbic areas on behavioral flexibility. Learning & Memory. 2002;9:18–28. doi: 10.1101/lm.45802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Jih J, Tzavos A. Involvement of the dorsomedial striatum in behavioral flexibility: role of muscarinic cholinergic receptors. Brain Research. 2002a;953:205–214. doi: 10.1016/s0006-8993(02)03287-0. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Ragozzino KE, Mizumori SJY, Kesner RP. The role of the dorsomedial striatum in behavioral flexibility for response and visual cue discrimination learning. Behavioral Neuroscience. 2002b;116:105–115. doi: 10.1037//0735-7044.116.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Kim J, Hassert D, Minniti N, Kiang C. The contribution of the rat prelimbic–infralimbic areas to different forms of task switching. Behavioral Neuroscience. 2003;117:1054–1065. doi: 10.1037/0735-7044.117.5.1054. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Hornak J, Wade D, McGrath J. Emotion related learning in patients with social and emotional changes associated with frontal lobe damage. Journal of Neurology, Neurosurgery and Psychiatry. 1994;57:1518–1524. doi: 10.1136/jnnp.57.12.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Nugent SL, Saddoris ML, Setlow B. Orbitofrontal lesions in rats impair reversal but not acquisition of go, no-go odor discriminations. Neuroreport. 2002;13:885–890. doi: 10.1097/00001756-200205070-00030. [DOI] [PubMed] [Google Scholar]
- Souza MM, Souza T, Vinade ER, Rodrigues C, Choi HK, Silva TL, et al. Melloe Dedavide effects of posttraining treatments in the posterior cingulate cortex on short- and long-term memory for inhibitory avoidance in rats. Neurobiology of Learning and Memory. 2002;77:202–210. doi: 10.1006/nlme.2001.4009. [DOI] [PubMed] [Google Scholar]
- Stefani MR, Groth K, Moghaddam B. Glutamate receptors in the rat medial prefrontal cortex regulate set-shifting ability. Behavioral Neuroscience. 2003;117:728–737. doi: 10.1037/0735-7044.117.4.728. [DOI] [PubMed] [Google Scholar]
- Williams JM, Mohler EG, Givens B. The role of the medial prefrontal cortex in attention: altering predictability of task difficulty. Psychobiology. 1999;27:462–469. [Google Scholar]
- Wise SP, Murray EA, Gerfen CR. The frontal cortex–basal ganglia system in primates. Critical Reviews in Neurobiology. 1996;10:317–356. doi: 10.1615/critrevneurobiol.v10.i3-4.30. [DOI] [PubMed] [Google Scholar]